SUMMARY
In social interactions among mammals, individuals are recognized by olfactory cues, but identifying the key signals among thousands of compounds remains a major challenge. To address this need, we developed a new technique, Component-Activity Matching (CAM), to select candidate ligands that “explain” patterns of bioactivity across diverse complex mixtures. Using mouse urine from eight different sexes and strains, we identified 23 components to explain firing rates in seven of eight functional classes of vomeronasal sensory neurons. Focusing on a class of neurons selective for females, we identified a novel family of vomeronasal ligands, steroid carboxylic acids. These ligands accounted for much of the neuronal activity of urine from some female strains, were necessary for normal levels of male investigatory behavior of female scents and sufficed to trigger mounting behavior. CAM represents the first step towards an exhaustive characterization of the molecular cues for natural behavior in a mammalian olfactory system.
Graphical Abstract
INTRODUCTION
Mammals explore the chemical world with several olfactory modalities (Ma, 2007). The accessory olfactory system (AOS) emphasizes the detection of social cues, sometimes called pheromones, that regulate behavior among members of the same species (Dulac and Torello, 2003; Halpern and Martinez-Marcos, 2003). One of the great attractions of the AOS is the opportunity to explore the molecular, cellular, and circuit underpinnings of behavior in a genetically tractable mammal. Perhaps the most substantial current barrier to exploit this promise is our incomplete understanding of the natural cues that the AOS detects. Knowing more about the nature of the stimuli will enable sophisticated studies of sensory coding, circuit function, and behavior.
Several scent sources are known to be involved in chemical communication in mammals: secretory glands (lacrimal, salivary, and preputial), urine, and feces (Halpern and Martinez-Marcos, 2003; Kimoto et al., 2005). Mouse urine excites widespread activity among vomeronasal sensory neurons (VSNs) (He et al., 2008; Holy et al., 2000; Stowers et al., 2002; Tolokh et al., 2013) and is the best behaviorally characterized source of chemical cues for mammalian social communication (Halpern and Martinez-Marcos, 2003). Mouse urine conveys information about the sex and strain (Brennan and Keverne, 1997; Kimchi et al., 2007; Leypold et al., 2002; Pankevich et al., 2004; Stowers et al., 2002). Although progress has been made recently toward identifying the molecular nature of pheromone cues by purifying individual ligands from mouse urine (Chamero et al., 2007; Hsu et al., 2008; Nodari et al., 2008), the identities of olfactory ligands inside urine cues are largely unknown; there is not even an estimate of how many distinct compounds comprise the “olfactory identity” of an individual.
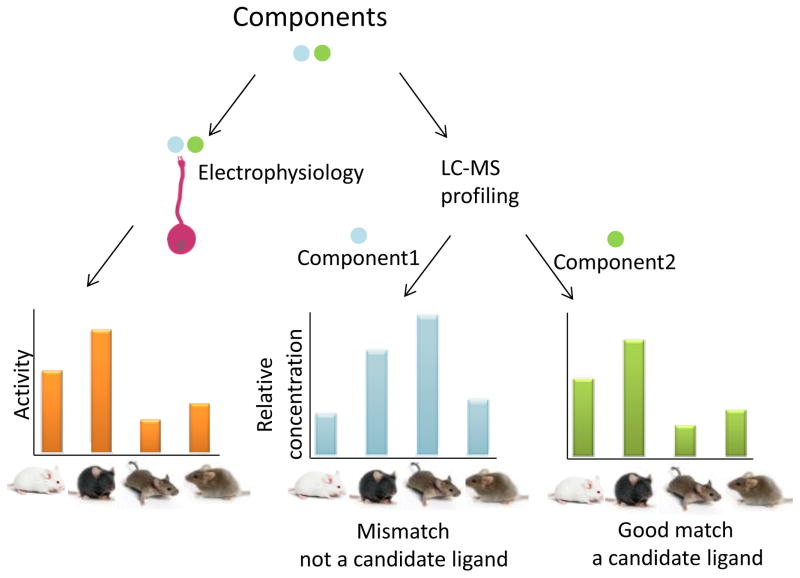
Here, we developed an approach, Component-Activity Matching (CAM) (Figure 1), with the aim of systematically and exhaustively defining a “short list” of candidate vomeronasal ligands that encode identity. This approach exploits the striking differences among natural stimuli across different sexes and strains of mice (Tolokh et al., 2013), which here we use as a proxy for the variability that one might find at the individual level in natural, genetically diverse, populations of mice. With CAM, we performed a forward screen for compounds that may drive activity in vomeronasal neurons, without the need for laborious sample purification. Using a combination of physiological recording and quantitative liquid chromatography-mass spectrometry (LC-MS) (Figure 1), we identified a small set of constituents whose concentrations match a pattern of neuronal responsiveness across samples.
Figure 1.
Component-Activity Matching (CAM) for ligand identification. A diverse collection of samples (here, urine extracts from male and female mice of different strains) are analyzed to extract the relative concentrations of each component by LC-ESI mass spectrometry and subjected to an assay of activity (left, by recordings of spiking responses). For a selected neuron, physiological responses (neuronal firing rate, orange bars) to the different stimuli are compared to the abundances of individual components (blue and green bars). Component 1 (blue bars) is not distributed in a manner that could explain the firing rate responses, and is therefore an implausible candidate. In contrast, component 2 (green bars) has an across-sample distribution consistent with the measured neuronal firing rates, and is thus a plausible candidate to explain the response.
Using this approach, we focused on a class of neurons that responded to urine from females of all strains, but not to any male strains. We purified and structurally identified the two best CAM candidates to explain this pattern of neuronal activity. These two candidates are carboxylic-acid steroid metabolites, which we call “cortigynic acid” and “corticosteronic acid.” Cortigynic acid accounts for one-fourth of neuronal activity of female C57BL/6J mouse urine. Cortigynic acid was seven-fold more abundant in the urine of gonadally intact adult female mice than in juvenile mice, thirty-fold higher than in an ovariectomized female, and was not detectable in male mice. When added to the urine of male mice, cortigynic acid induced additional exploratory behavior as observed previously with whole female urine (Pankevich et al., 2004; Holy and Guo, 2005; Guo and Holy, 2007). Removing cortigynic acid from the female urine extract significantly decreased male’s investigation time towards the “deficient” female urine extract. Finally, we found that the combination of cortigynic acid and corticosteronic acid is sufficient to promote male-mounting behavior.
This manuscript describes the first steps in a comprehensive analysis of the molecular cues for identity in a mammalian olfactory system. We applied this approach to a long-standing mystery (Dixon and Mackintosh, 1975) and discovered the chemical identity of female sex pheromones for the mouse.
RESULTS
A new strategy for identifying vomeronasal ligands from complex mixtures
In urine, much of the activity that drives firing in VSNs originates from multiple compounds with similar physico-chemical properties (Nodari et al., 2008). This similarity poses a major challenge to traditional methods for identifying the source of neuronal activity from complex mixtures. To overcome the difficulties, we developed a new strategy based on the observation that urine from different sexes and strains excites differing patterns of activity in vomeronasal neurons (Tolokh et al., 2013). We hypothesized that these different neuronal patterns could be caused by different ligands present in one or more of urine samples. We reasoned that the molecular correlates of identity might, therefore, be discoverable by seeking compounds whose abundance could “explain” these patterns of physiological responses. By picking out compounds whose concentrations across samples matched the pattern of neuronal firing (activity), we were able to identify plausible VSN candidate ligands without any preliminary purification (Figure 1).
Diverse vomeronasal responses to urine samples from different sexes and strains
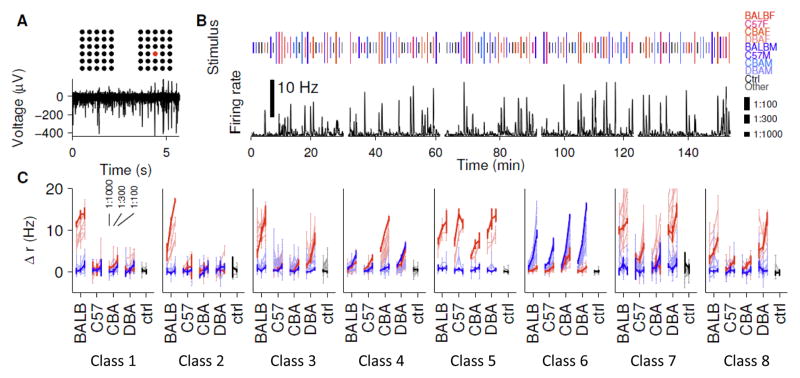
Inspired by the genetic differences among individuals present in natural populations, we selected mice of four laboratory strains (BALB/c, C57BL/6J, CBA, and DBA, Figure 1) and tested their C18 urine extracts for their ability to drive spiking responses in mouse vomeronasal neurons. We used a multi-electrode array (Nodari et al., 2008; Arnson et al., 2010; Holy et al., 2000) to record spiking responses of large numbers of VSNs simultaneously from 13 intact vomeronasal neuroepithelia isolated from B6D2F1 male mice (Figure 2A). To reduce potential confounds from saturation, each of these eight urine extracts was tested at three different dilutions, 1:100, 1:300, and 1:1000. A total of 488 single neurons were isolated from these spike recordings. To test the reproducibility of the responses, each stimulus was presented once in each of five cycles, each cycle delivering stimuli and negative controls alternating with flush solution in pseudo-random order. This stimulation paradigm resulted in a very large number—a total of 130—stimulus trials for each neuron in each preparation (Figure 2A). Responses were quantified in terms of the mean change in firing rate, Δr, upon stimulation (Figure 2B).
Figure 2.
Categorization of VSN responses. (A) Electrophysiological recording with a multielectrode array. The voltage signal recorded from a representative electrode (indicated by red color) is illustrated. (B) Experimental design: firing rate vs. time of one neuron, across 5 cycles of stimulus presentation. A collection of extracts from males (M) and females (F) of four strains, encoded by color, are presented at three different concentrations (encoded by stimulus bar height). Stimulus order is randomized across cycles, and each stimulus is presented once per cycle. Note that collecting this number of sensory responses required more than two and a half hours of continuous recording. (C) Overlays of all neurons assigned to one of eight repeatedly identified response patterns. Single exemplar neurons are shown in bold colors, other neurons of similar functional type are shown in faint colors. Red indicates responses to female urine, blue to male, and responses to all three dilutions are shown. The primary differentiator of the first two classes is their concentration-dependence. Error bars represent firing rate s.e.m. See also Figure S1.
Of 488 single-units, 121 responded (Δr > 5 Hz and distinguishable from the response to the standard at a level of p < 0.05, t-test) to at least one stimulus (Figure S1). No neuron passing this test responded significantly to the independent negative control (Figure S1). Individual neurons showed a diversity of responses; some showed specificity to a single sample, others responded to a few strains from the same sex, and yet others responded selectively for one or the other sex but were relatively insensitive to strain. Examination of the entire population of recorded neurons revealed that functionally similar neurons were observed repeatedly across different preparations (Figure 2C). Of 121 responsive single units, we manually collected 57 of the cells into 8 functional classes, based on specificity, sensitivity, and repeated observation of patterns across multiple single units. Of the remaining (not categorized) units, most were weakly-responsive, and the rest exhibited idiosyncratic responses that did not, on their own, appear to clearly justify formation of a class (Figure S1). These classes served to select particularly clear “exemplar” neurons, which we temporarily assume to represent the entire class, for the purpose of identifying the ligands that drive them.
Chemical profiles across sex and strain
These neurophysiological responses are presumably attributable to the presence of particular ligands in one or more of these samples, each at a specific concentration. If a response is driven by a single ligand, its pattern should be predictable from the concentration of the ligand across samples (Figure 1)—samples containing higher concentrations of this ligand should drive more activity. We reasoned that the molecular correlates of identity might, therefore, be discoverable by seeking compounds whose abundance could “explain” these patterns of physiological response.
To quantify the relative abundance of the individual molecular components, these same urine extracts were profiled with LC-MS. To achieve high sensitivity and specificity, we performed negative-ion nanoLC-MS using a water/acetonitrile gradient for the LC and high mass resolving power (to enable mass accuracy of a few ppm). It is apparent (Figure S2A) that these samples show high chemical diversity, consistent with the differences observed electrophysiologically. When viewed at high mass resolving power, individual constituents were readily isolated from one another (Figure S2A&B). In some cases, multiple compounds with the same mass-to-charge (m/z) ratio eluted at different times. We used a combination of automated and manual algorithms to segment individual peaks (see Experimental Procedures), focusing on those representing components with reasonably high ion counts in at least one sample. Altogether, we quantified the integrated ion counts for components represented by 1,634 different peaks with 862 distinct m/z values. In terms of total ion count, these components spanned more than four orders of magnitude in abundance (Figure S2C), thus, representing a “deep” profile of the individual constituents of these samples.
Molecular correlates of neuronal responses
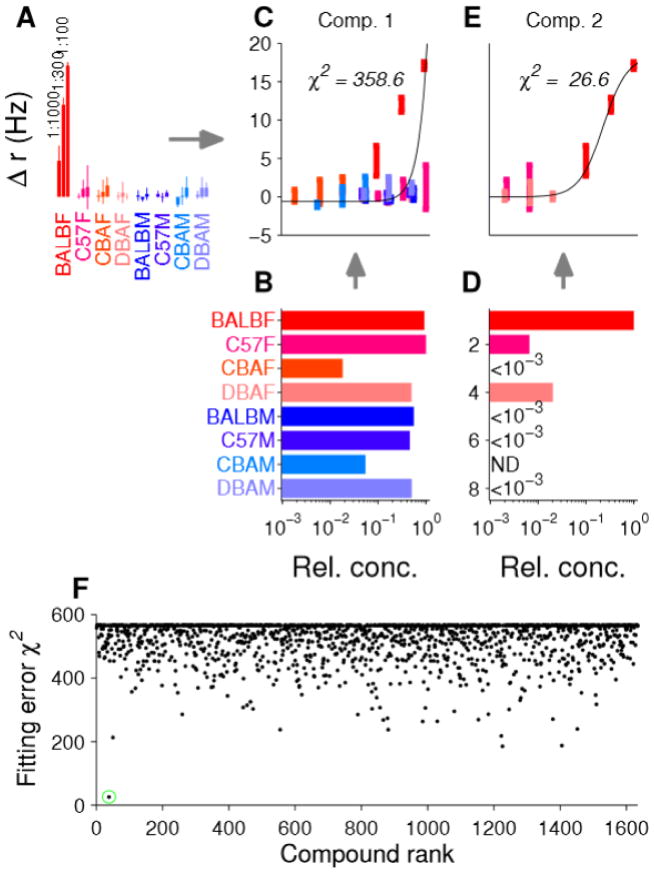
The central hypothesis of CAM is that individual compounds, present in one or more of the urine samples, might be the source of individual VSN responses. To test this hypothesis, we combined the relative abundance of a single peak across samples with the dilution factor during stimulus delivery, thereby generating an “effective concentration” of this putative ligand across all samples tested electrophysiologically. We then examined the extent to which the pattern of concentrations across samples could explain a VSN’s responses (Figure 1). We fit the responses to the Hill equation (Hill, 1938; Arnson and Holy, 2013) quantifying the fitting error by χ2 (see Experimental Procedures). Thus, each VSN/component pair yielded a single χ2 measuring the degree to which a particular ligand might “explain” this neuron’s responses. Collectively, taking one exemplar from each of the eight neuronal classes (Figure 2C) led to 13,072 distinct measures of goodness of fit.
An example class 2 (Figure 2C, marked with yellow arrow in Figure S1) neuron (shown in Figure 3) exhibited a higher firing rate to BALB/c females, even at 1:1000 dilution, than to any other sample (Figure 3A). This implies that any ligand explaining this pattern of firing must have at least ten-fold higher concentration in this sample than in any other sample. Most compounds did not fit this pattern. To illustrate, we choose one example, a compound giving an ion of m/z = 336.0723 eluting at 34–37% acetonitrile (Figure 3B) and exhibiting relatively little selectivity across samples. Correspondingly, when the concentration of this component, incorporating the dilution factor, was paired with firing rate, the resulting pattern of firing across samples was a poor fit to this model (Figure 3C, χ2 = 352.6) and could be confidently excluded (p < 10−50, 21 d.o.f.). In contrast, this constraint was met for a component giving an ion of m/z = 427.1793 eluting at 52–56% acetonitrile (Figure 3D), and the firing pattern was well-fit by the Hill equation with χ2 = 26.6 (Figure 3E; p = 0.18, meaning that the fit cannot be excluded as an explanation of this pattern of firing).
Figure 3.
Matching component concentrations to single-neuron activity patterns. (A) Firing rate of a neuron from the second class in Figure 2C, marked with a yellow arrow in Figure S1. (B) Relative abundance across samples of a component with m/z 336.0723 eluting between 34–37% acetonitrile. (C) Incorporation of the abundance with the dilution factor for each stimulus leads to a putative dose-response curve as a function of the concentration of this particular component. Fitting the points to a Hill model leads to a goodness-of-fit measured by χ2. This example represents a very poor fit (p < 10−50). (D) Another component with m/z 427.1793 eluting between 52–56% acetonitrile is most abundant in BALB/c female urine. (E) The dose-response curve from this second component fits well (p = 0.18). (F) χ2 for a class 1 neuron (Figure 2C, marked with a green arrow in Figure S1) for each of 1,634 components. Only a single component (marked with a green circle), the one in D&E, represents a good fit (χ2 < 75). See also Figure S2.
We performed this analysis for all eight “exemplar” neurons (one per class), each with all 1,634 components. For the exemplar class 1 (Figure 2C, marked with green arrow in Figure S1) neuron, the fitting error to all components is shown in Figure 3F. Remarkably, only one gave a plausible fit; namely, the compound giving an ion of m/z = 427.1793 shown in Figure 3D and 3E. This accurate mass is consistent with a compound, 4-pregnene-11,20,21-triol-3-one 21-sulfate (Figure 3F marked with a green circle), which we previously identified from BALB/c female mouse urine (Hsu et al., 2008; Nodari et al., 2008).
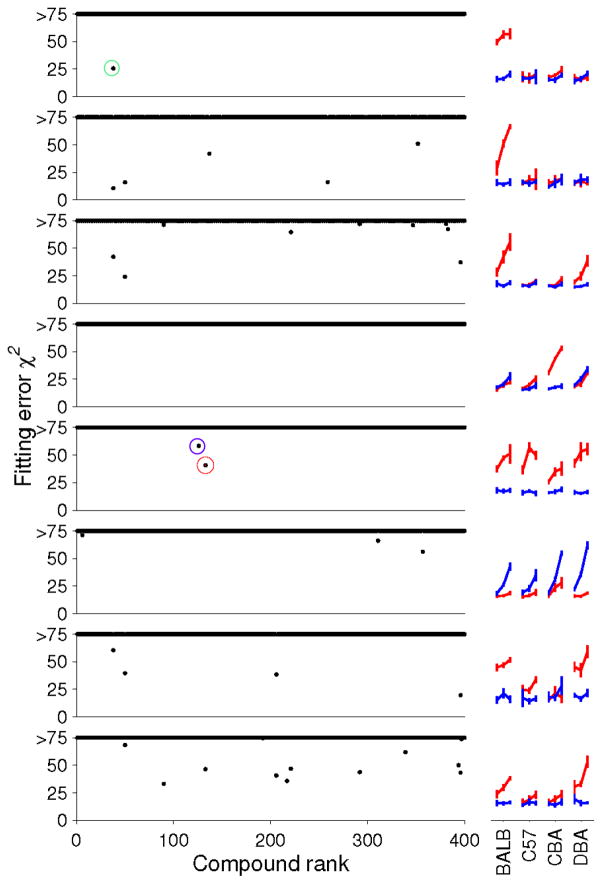
This result is a striking demonstration of CAM’s ability to identify plausible candidate ligands among the thousands of compounds in mouse urine. To analyze all eight classes of physiological responses (Figure 2C), we set a generous cutoff of χ2 < 75 and focused on components represented by 400 peaks with highest ion count, as these were most likely to be reliably quantified across samples and purified in sufficient amounts for further experiments. For 7/8 sensory neuron classes, we obtained one or more plausible candidate ligands (Figure 4; with 1, 5, 9, 0, 2, 3, 4 and 12 candidates for classes 1–8, respectively). Altogether, this approach identified 23 unique candidate ligands to explain these seven patterns of response.
Figure 4.
Candidate ligands for each class of VSN response. Goodness-of-fit plotted for the 400 most abundant components for each neuron shown at right. In each case, at most a handful of components represent plausible ligands. For each candidate with χ2 < 75, m/z and elution data are listed in Table S1. Circles highlight previously-identified CAM ligand m/z 427.1793 (4-pregnene-11,20,21-triol-3-one 21-sulfate, green) and the two novel CAM candidates to explain a pan-female response (class 5), m/z 377.1971 (red) and m/z 361.2018 (purple). See also Figure S3 and Table S1.
The entire list of candidates is presented in Table S1. These 23 components of urine represent a first short list of molecular cues encoding an individual animal’s “vomeronasal identity.”
Isolation of CAM Candidate Ligands for “Pan-female” Neuron Response Type
To test whether CAM successfully identified novel ligands, we decided to focus on a class of neuronal response lacking any known ligands. Among mice, pheromones and other social odor cues convey information about sex, social status, and identity. We selected the “pan-female” response type (the fifth class in Figure 2C), as no VSN ligand for “femaleness” has yet been identified. This pattern of neuronal activity had just two likely candidates, of which the best match was a compound that we preliminarily denoted as M377; the component elutes at 44–46% acetonitrile and gives by mass spectrometry an anion of m/z 377.1971 (presumably [M – H]−, marked with a red circle in Figure 4) in the negative-ion mode. Direct inspection of the nanoLC-MS profiles confirmed that this compound was found specifically in female mouse urine (Figure S3). This class of neurons has just one other CAM candidate shown in Figure 4, a compound we called M361, eluting at 54–58% acetonitrile with a m/z 361.2018 (marked with a purple circle in Figure 4).
The LC data indicated that these two compounds co-elute with many others, but that these contaminants are less severe in samples from C57BL/6J females. Therefore, we chose this strain for further purification. We fractionated 100 mL of C57BL/6J female mouse urine extract by HPLC (see Experimental Procedures), using mass spectrometry to identify fractions containing ions of the anticipated m/z 377 and 361. The corresponding HPLC fractions were further purified by thin-layer chromatography (TLC) to obtain a sample of high purity.
M377 accounts for approximately one-fourth of the neuronal response to urine from C57BL/6J females
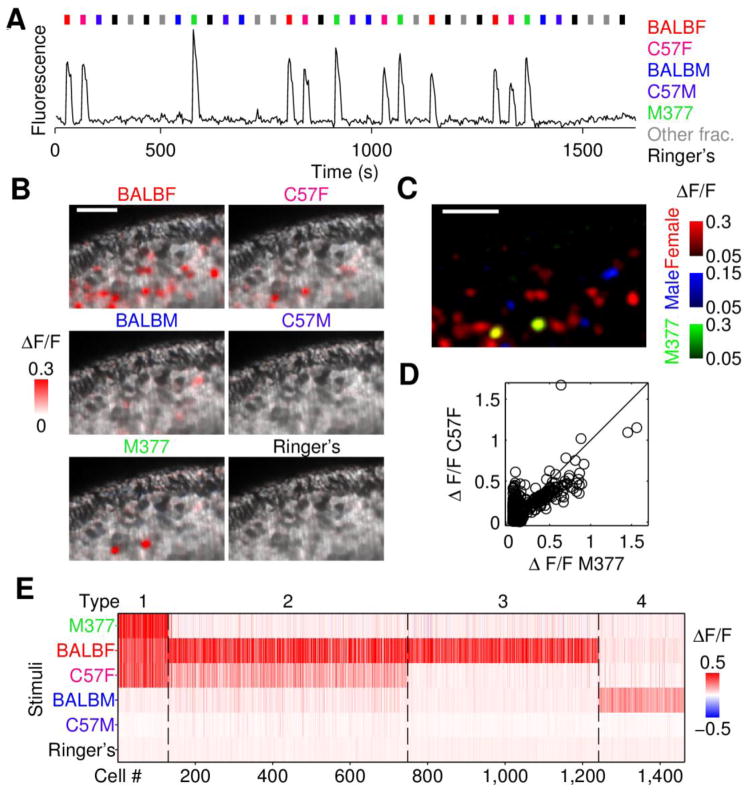
To determine whether M377 is the source of activity for all “class 5” neurons (Figure 4) as predicted by CAM, we used OCPI/light sheet fluorescence microscopy (Holekamp et al., 2008) and calcium imaging to monitor neuronal activity in VSNs. Using male mice expressing GCaMP2 in VSNs (He et al., 2008), we recorded responses in the intact whole-mount preparation (Turaga and Holy, 2012; Xu and Holy, 2013). We presented urine extracts from BALB/c and C57BL/6J males and females, as well as purified M377 matching its endogenous concentration in C57BL/6J female urine extract. These stimuli were presented in four repeated trials, randomizing the order of stimuli within each cycle (Figure 5A). We recorded from two epithelial imaging volumes, containing an estimated 20,000 vomeronasal neurons (Turaga and Holy, 2012).
Figure 5.
M377 excites female-selective neurons. (A) Experimental paradigm for calcium imaging experiments. Each stimulus presentation is marked by a color-coded bar, each repeated four times. GCaMP2 fluorescence intensity for a particular neuron is shown. (B) Single optical sections of the whole-mount VNO, with the dendritic knob layer near the top of the image. ΔF/F is encoded voxel-wise using a red color scale. Female extracts excite more neuronal activity than male extracts; M377 potently excites a subset of neurons. Scale bar: 50 μm. (C) Overlay of ΔF/F responses across stimuli. Note responses to M377 overlap responses to those cells activated by female cues. (D) Comparison of response amplitudes to C57BL/6J female extract and purified M377. Each neuron sensitive to C57BL/6J female extract is shown as a separate circle. Diagonal line represents equal response to the two stimuli. (E) Heat map of all urine- or M377-responsive neurons imaged in two male VNOs. Each cell is represented as a single column, and cells with similar response patterns were grouped. The first three groups correspond to three different types of female-selective responses. Note that all neurons showing roughly equal responsiveness to both BALB/c and C57BL/6J female urine are also responsive to M377. See also Figure S4.
We identified 1,463 cells as responding robustly to at least one of the urine extracts. The largest numbers of neurons were activated by urine from females, consistent with electrophysiological recordings (Figure S1). Individual neurons were largely sex specific, and displayed a range of strain selectivity. We also observed 130 neurons responsive to purified M377, demonstrating that this compound indeed activates a subset of neurons in the vomeronasal organ (VNO). Moreover, neurons responsive to M377 invariably were also responsive to urine from C57BL/6J and BALB/c females, but did not respond to urine from males (Figure 5B, 5C and 5E).
To investigate quantitatively the relationship between responses to M377 and mouse urine, for each M377-responsive neuron, we compared the intensity of its ΔF/F to both M377 and C57BL/6J female mouse urine. The response amplitudes were closely matched over the entire range of response intensities (Figure 5D), indicating a 1:1 relationship between these neurons’ responses to C57BL/6J female mouse urine and purified M377. Consequently we conclude that these neurons’ urine responses are caused by M377.
The characteristics of the M377-responsive neurons shown in Figure 5E—approximate equal sensitivity to BALB/c and C57BL6 female urine—are consistent with class 5 neurons (Figures 2C and 4). Two other types of female-responsive neurons, labeled types 2 and 3 (Figure 5E), are consistent with classes 7–8 and 1–3 (Figure 4), respectively; these neurons were unresponsive to M377.
Structural characterization of M377
Based on accurate mass measurement, M377 is ionized to an [M – H]− chemical formula of C21H29O6−, indicating that it is a novel ligand. Following determination of its chemical formula, we solved its structure by using a combination of MS-based methods, including high mass resolving power, tandem MS (MS2 and MS3), HD exchange (to count exchangeable hydrogens), and a chemical reaction with periodic acid followed by MS detection. The details of structure elucidation are provided in the Supplementary Information and Figures S4-S5. As a result of these analyses, the proposed structure of M377 is 16-hydroxycorticosterone 20-hydroxy-21-acid.
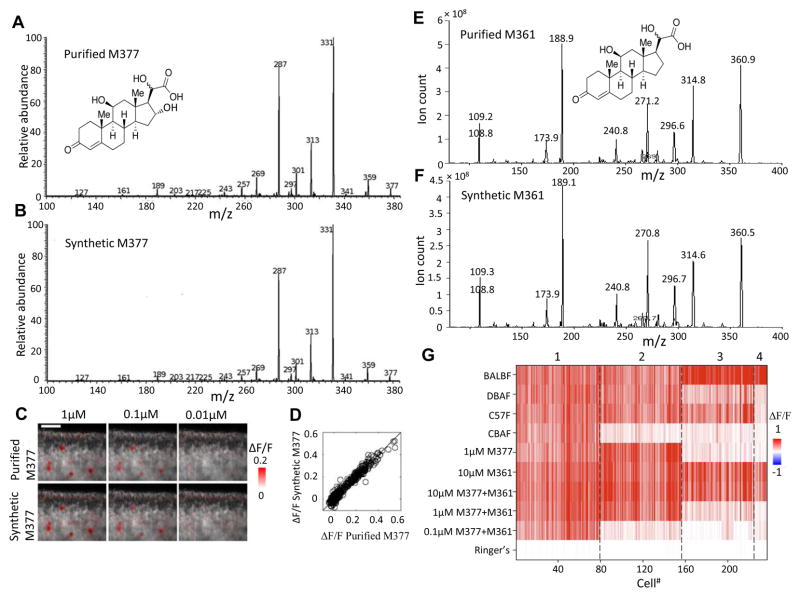
To test the validity of this structural interpretation, we commissioned a custom synthesis of this proposed compound, as an epimeric mixture at carbon 20. Urine-derived M377 and synthetic 16-hydroxycorticosterone-20-hydroxy-21-acid were indistinguishable by thin-layer chromatography (TLC) and reversed-phase HPLC (Figure S4A). Further, product-ion mass spectra of the synthetic and endogenous compounds were nearly indistinguishable (Figure 6A and 6B), indicating identical structures of the two. Fragmentations by MS3 of major MS2 fragment ions from both compounds are also consistent (data not shown).
Figure 6.
Structural validation of M377 and M361. (A) Proposed chemical structure of purified M377 and its MS2 product-ion spectrum. (B) Structure of synthetic cortigynic acid and its MS2 spectrum. (C) Calcium imaging to purified and synthetic M377 over a range of matched concentrations. (D) Response amplitude of each neuron to purified and synthetic M377, pooled across all concentrations. Average response across four trials by each neuron to each matched-concentration stimulus combination shown as a circle. (E-F) Chemical structure of purified and synthetic M361 and the MS2 product-ion spectrum. (G) Combinatorial coding of female-specific cues. Neuronal responses to hundred-fold dilute urine stimuli, M377, M361, and their mixture at three different concentrations. See also Figure S5 and Table S2.
We then asked whether synthetic M377 (16-hydroxycorticosterone 20-hydroxy-21-acid) showed the same neuronal activity as endogenous M377. The two stereoisomeric components of the synthetic epimeric mixture were quantified by HPLC as having a concentration ratio of 10:1; of which the less abundant component matched the endogenous compound in elution time (Figure S4A). Synthetic 16-hydroxycorticosterone-20-hydroxy-21-acid and endogenous M377 were presented at three different concentrations (1 μM, 0.1 μM, and 0.01 μM) in four repeated trials, randomizing the order of stimuli within each cycle. VSNs responding to the synthetic compound also responded to endogenous M377 with identical concentration dependence (Figure 6C). These results conclusively demonstrate that M377 is 16-hydroxycorticosterone-20-hydroxy-21-acid.
This structure has several distinctive features. To our knowledge, biological activity of a steroid carboxylic acid has not been previously reported. As a 21-carbon steroid with a hydroxyl group at the 11th position, this structure likely derives from metabolism of a glucocorticoid (Wang et al., 2010). However, the hydroxyl group at position 16 is a motif characteristic of a female sex steroid, estriol, which in humans is only produced in significant amounts during pregnancy (Raju et al., 1990). We therefore named this new VSN ligand “cortigynic acid.”
Structural characterization of M361
Based on accurate mass measurement, M361 gives an [M – H]− chemical formula of C21H29O5−, indicating that it is a novel ligand. By comparing its chemical formula with that of cortigynic acid (C21H29O6−), we deduced that this new unknown compound might be an immediate metabolic precursor of cortigynic acid. We hypothesized that the distinctive C-16 hydroxyl group of M377 might be added as the last step, and, therefore, we proposed that M361 is 20-dihydro-corticosterone-21-carboxylic acid. To test our proposed structure for M361, we also commissioned a custom synthesis of this second compound. Urine-derived M361 and synthetic 20-dihydro-corticosterone-21-carboxylic acid were indistinguishable by thin-layer chromatography (TLC) (data not shown) and reversed-phase HPLC (Figure S4B). MS/MS analysis demonstrated that the product-ion mass spectra of the synthetic and endogenous compounds are identical (Figure 6E-F). These results suggest that M361 is 20-dihydro-corticosterone-21-carboxylic acid, which we will also refer to as corticosteronic acid.
Combinatorial coding of female-specific ligands
To learn more about how these ligands are encoded by VSNs, we examined responses to a stimulus battery that included cortigynic acid and corticosteronic acid, together with hundred-fold diluted urine samples from all four strains (BALB/c, DBA, C57BL/6J and CBA) of females. Cortigynic acid and corticosteronic acid were presented individually at their endogenous concentrations in whole, undiluted urine and in mixtures of the two spanning two orders of magnitude in concentration, the lowest of these approximately matching their concentrations in the diluted urine extract samples. Using male mice expressing GCaMP3 in VSNs, we recorded responses in the intact whole-mount preparation (Turaga and Holy, 2012; Xu and Holy, 2013) by OCPI microscopy (Holekamp et al., 2008).
From three epithelial imaging volumes, we identified 236 cells responding robustly to either cortigynic acid or corticosteronic acid. Using a clustering algorithm, we identified four types of responses. The first type was highly responsive to all four strains of females (the signature of class 5 neurons, Figure 2C and Figure 4), and were exquisitely sensitive to both cortigynic acid (M377) and corticosteronic acid (M361), exhibiting responses at sub-micromolar concentrations as present in the diluted urine samples. With the exhaustive sampling of OCPI microscopy and expanded range of stimulus concentrations, we also identified three other clusters responsive to at least one of these compounds and a subset of the mouse strains. One of these was strongly activated by BALB/c, DBA, and C57BL6, and perhaps weakly by CBA females; these neurons were responsive to high concentrations of M377 and M361, but only weakly responsive at the lowest concentration. As CBA females have the lowest endogenous concentration of M377 (by a factor of approximately 2, see Figure S3), it seems likely that the partial strain-selectivity of this class of neurons stems from a threshold effect at the tested concentrations. The two other clusters responded to a subset of strains and were not activated by M377, but were activated by M361. Consequently, multiple classes of VSNs (likely expressing different vomeronasal receptor genes) participate in a combinatorial code for the presence and concentration of these two ligands (Figure 6G).
Cortigynic acid is specific to sexually-intact adult female mice
Cortigynic acid is expressed in urine of all tested adult female strains at a concentration near 1 μM, and is essentially absent from the urine of adult males (Figure S3). To further explore the factors that control its expression, we collected urine from estrus and non-estrus females, ovariectomized females and juvenile males and females (see Experimental Procedures). Quantitative LC-MS of these samples showed that cortigynic acid was seven-fold less-concentrated in the urine of P21 juvenile females, and more than 30-fold lower in the urine of ovariectomized females (data not shown). There was no cortigynic acid detected in urine from adrenalectomized males and females (data not shown), indicating that its synthesis is affected by both the gonads and the hypothalamic/pituitary/adrenal axis. Somewhat surprisingly, the concentration of cortigynic acid was only modestly (at most ~30%) modulated by the estrous cycle. To determine whether cortigynic acid is species-specific, we also examined hamster and rabbit urine from both sexes. No cortigynic acid was detected in these two species, suggesting that cortigynic acid is a female-specific cue for mice.
Cortigynic acid increases investigatory behavior of male mice
Male mice investigate urine from females significantly longer than urine from males, in a manner that depends on direct physical contact and an intact vomeronasal system (Pankevich et al., 2004). This behavior depends on a positive cue in female urine, rather than a negative cue in male mouse urine: mixtures of the two stimuli cause similar investigatory behavior to that triggered by female urine alone, when presented at matching concentration (Guo and Holy, 2007).
We used an optical beam-break detector to record bouts of voluntary investigation by untrained, freely behaving male mice (Figure 7A). As cortigynic acid, corticosteronic acid and female urine extract are not volatile and, therefore, would attract little notice by themselves, we separately added them to whole male mouse urine. We compared investigatory behavior to a swab with just male mouse urine against one with male mouse urine doped with 10 μM cortigynic acid, cortigynic acid + corticosteronic acid, female urine extract and female urine. Each male was presented with each swab, plus a blank in between, in sessions of 120 s, spaced over a period of approximately 1 h. The order of presentation of the stimuli was counterbalanced across mice, and the experimenter was blinded to the identity of the stimuli.
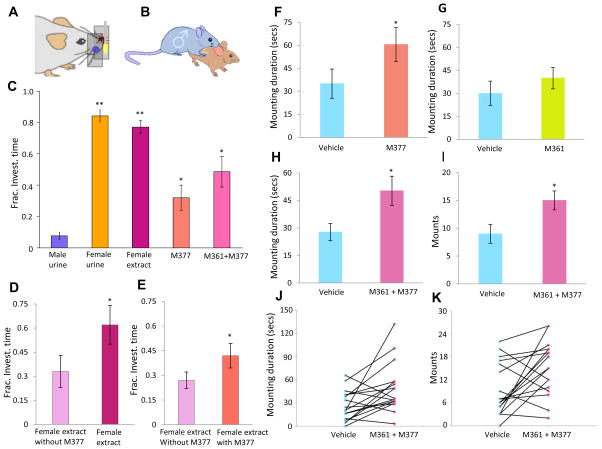
Figure 7.
Male investigatory and mounting behavior. (A–B) Schematic of the experiment. Male “sniff time” to stimuli presented on a cotton swab is measured by an optical beam-break detector; male mounting behavior was scored manually. (C) Mean and s.e.m. of the fraction of time spent investigating the swab after initial contact. Males spent longer investigating the sample doped with M377, M377 + M361, female extract or female urine (*p < 0.001, **p < 0.00001, n = 11, t -test). (D) Removing M377 from female urine extract significantly reduced wild type males’ investigation time (*p < 0.01, n = 12, t –test). (E) Adding M377 back to the “deficient” female urine extract robustly increased male’s investigation time (*p < 0.05, n = 12, t –test). (F) Duration of mounting toward females painted with vehicle (light blue) and M377 (salmon) (*p < 0.05, n = 12, Mann-Whitney test). (G) Duration of mounting toward females painted with vehicle (light blue) and M361 (lime). (H) Duration of mounting toward females painted with vehicle (light blue) and M361 + M377 (pink) (*p < 0.02, n = 17, Mann-Whitney test). (I) Frequency of mounting toward females painted with vehicle (light blue) and M361 + M377 (pink) (*p < 0.02, n = 17, Mann-Whitney test). (J) Mounting duration of individual males toward females painted with vehicle (light blue) and M377 + M361 (pink). (K) Mounting frequency of individual males toward females painted with vehicle (light blue) and M377 + M361 (pink). See also Figure S6.
Males investigated female mouse urine for durations that were many times longer than exhibited for male mouse urine. Extracts of female-mouse urine were not significantly different (p > 0.1) from whole female mouse urine, demonstrating that their particular interest in this stimulus is largely triggered by the non-volatile components (Figure 7C; **p < 0.00001, n = 11).
The addition of cortigynic acid alone to male mouse urine also triggered a large increase in behavioral investigation (Figure 7C; *p < 0.001, n = 11). The addition of corticosteronic acid to cortigynic acid did not significantly increase the male investigation time compared with that caused by M377 on its own (Figure 7C; p > 0.1, n = 11). To determine whether this effect is simply a consequence of novelty, we also tested whether a structurally-similar compound, 1,4-pregnandien-11β, 17, 20-triol-3-one-21-carboxylic acid (P0750) exhibited a similar effect. Using the same testing procedure, we found no difference between male urine and male urine doped with P0750 (Figure S6C; p > 0.7, n = 10), indicating that increased investigation is not a general reaction to any steroid carboxylic acid.
Transient receptor potential channels (TRPC2) play a fundamental role in detecting pheromonal and other chemical social recognition signals (Stowers et al.,2002; Leypold et al., 2002; Omura and Mombaerts, 2014). In TrpC2−/− mice, cortigynic acid did not increase the investigation time (Figure S6D).
To complement the gain of function observed when adding cortigynic acid to male mouse urine, we asked whether cortigynic acid is necessary by removing it from female mouse urine. Female urine extract was separated by HPLC using a methanol/water gradient and then recombined, omitting the two fractions (out of 100) containing M377 (Figure S6B). In behavioral testing, male mice significantly decreased their investigation of the “deficient” female urine extract compared to the whole female urine extract (Figure 7D; *p < 0.01, n = 12). Adding cortigynic acid back to the “deficient” female urine extract significantly increased male investigation time (Figure 7E; *p < 0.05, n = 12). These results demonstrate that cortigynic acid plays a major and specific role in attracting male investigation.
Cortigynic acid and corticosteronic acid are sufficient to promote mounting behavior
Classic experiments demonstrated that female mouse urine contains one or more pheromones that stimulate male mounting behavior (Dixon and Mackintosh, 1975; Haga-Yamanaka et al., 2014); however, the chemical identity of the cue(s) has never been established. To determine whether cortigynic acid and corticosteronic acid might be the missing cue(s), we painted the anogenital area of ovariectomized females with these compounds and exposed them to sexually-naive males. Cortigynic acid alone significantly increased the total amount of time spent mounting (Figure 7F; p < 0.05, n = 12), but exhibited only a trend-level increase on the total number of mounting episodes (data not shown). In contrast, females painted with corticosteronic acid alone did not undergo significantly more mounting than those painted with water (Figure 7G; p > 0.05, n = 12). Interestingly, the combination of cortigynic acid and corticosteronic acid induced the most robust mounting activity (Figure 7H-K; p < 0.02, n = 17). Thus, individually cortigynic acid is sufficient to induce mounting, but it exhibits a synergistic effect with corticosteronic acid.
DISCUSSION
To elucidate the vomeronasal code for identity, we developed CAM to perform a broad screen for ligands that “explain” the response of VSNs. This strategy exploits natural diversity to identify many candidate ligands from a single round of bioassays, and is intrinsically parallelizable to multiple sensory neuron types. CAM’s only requirements—functionally-diverse samples and a richly-quantifiable bioassay—may be met in other studies, making the technique broadly applicable.
Without requiring any purification, CAM identified a single compound, out of 1,634 possibilities, to explain responses of a neuronal type selective for urine from BALB/c females (Figure 3). This single compound had been previously identified from BALB/c female-mouse urine (Nodari et al., 2008), and this finding demonstrated that CAM can identify a small handful of plausible ligands from hundreds or thousands of candidates. We organized neuronal responses into eight reproducibly identified classes, of which seven had one or more CAM candidates. Explaining these responses would seemingly require a minimum of seven distinct ligands; our statistical analysis selected a set of 23 plausible candidates. It is also worth noting that some of these 23 candidate ligands apply to more than one class of neuron (Table S1). Indeed, our detailed analysis of the class 5 (Figure 2C and Figure S1) candidate ligands (M377 and M361) demonstrated that four distinct classes of neurons responded to these two ligands. These results suggest that vomeronasal coding may be organized around many receptor genes that are tuned with differing specificities and sensitivities to a much smaller number of behaviorally-relevant ligands. This interpretation is consistent with the much larger number of receptor genes (~300) than candidate ligands (23) in urine. Moreover, the structural similarity of cortigynic acid and corticosteronic acid, like that found in an earlier ligand discovery (Nodari et al., 2008), suggests an emerging general pattern: that the vomeronasal system detects multiple intermediates in the same metabolic pathway, using multiple receptor genes as a combinatorial code (Figure 6G) to provide robust information about concentration and identity (Arnson and Holy, 2013).
Neuronal responses to mouse urine were previously demonstrated to exhibit sex-selectivity (He et al., 2008; Holy et al., 2000; Tolokh et al., 2013). However, no urinary ligand exhibiting a pattern of sex-, but not strain-, specific distribution had yet been reported. Cortigynic acid and corticosteronic acid are the first potential cues for encoding “sex” from urinary cues for the mouse vomeronasal system. Cortigynic acid was present at high concentration only in intact adult female mice urine, and was relatively constant across strains (Figure S3).
In behavioral tests, cortigynic acid substantially and significantly increased male investigatory and mounting behavior. When added to male urine, this one ligand increased the duration of investigation bouts by more than two-fold. Conversely, removing cortigynic acid from female urine extract dramatically reduced male investigatory time (Figure 7D). Most remarkably, painting ovariectomized females with M377, alone or especially in conjunction with M361, significantly increased male mounting behavior. Based on results with a close structural analog, we speculate that the specific interest in this compound integrates multiple structural features, including the 16-hydroxyl functional group specific to female mice.
Previous results (Dixon and Mackintosh, 1975; Haga-Yamanaka et al., 2014) demonstrated that estrous urine, but not diestrous urine, was effective in promoting mounting behavior towards ovariectomized females. Here, we observed a robust increase in mounting with compounds whose concentration was at most modestly (~30%) influenced by the estrous cycle. There appear to be several possible interpretations of this result. First, it is possible that even a modest modulation of concentration is sufficient to lead to estrous-dependent behavior. Second, it is possible that estrous is signaled by the presence of cortigynic/corticosteronic acid together with the absence of one or more (currently unknown) diestrous-specific cue(s). In such a scenario, the presence of cortigynic/corticosteronic acid would mimic estrous even though these compounds do not exhibit dramatic cycling. Third, to ensure that we would not miss a positive effect, in mounting assays cortigynic acid and corticosteronic acid were used at approximately tenfold excess concentration compared to their natural concentrations; it is possible that these ligands cross-react to a receptor that is more sensitively tuned to a different, estrous-specific ligand. The conditions under which our urine samples were collected, with five animals per cage, were not optimal for studying the role of estrous; we anticipate that this will be an important topic for future studies.
Together with the identification of receptors, the identification of VSN ligands establishes a molecular foundation for understanding how sensory inputs are used in recognition and behavior. Although receptors tend to fall into gene families and can be exhaustively mapped via genome sequencing and homology analysis, organic ligands are diverse and have heretofore been discovered idiosyncratically. In this study, we developed a robust method that enabled us to systematically pick out plausible ligands for vomeronasal neurons. These results take a major step towards a comprehensive understanding of the molecular code for identity in the vomeronasal system.
EXPERIMENTAL PROCEDURES
Mice
Male B6D2F1 mice 8–12 weeks of age were used in all electrophysiology experiments. tetO-GCaMP2/OMP-IRES-tTA mice (He et al., 2008) and Ai38(GCaMP3)/OMP-Cre male mice aged 8–12 weeks were used for imaging by Objective-Coupled Planar Illumination (OCPI) microscopy (Holekamp et al., 2008). Prior to dissection, mice were anesthetized with carbon dioxide and decapitated. All procedures were approved by the Washington University Animal Studies Committee. Sexually naive three-month-old male B6D2F1 mice were used to examine mounting behaviors. Overiectomization of two-month-old C57BL/6J females was performed by a veterinary technician from Department of Comparative Medicine at Washington University in St. Louis.
Electrophysiological recording and calcium imaging
Dissection and recording procedures were performed as previously described (Arnson, et al., 2010; Turaga and Holy, 2012; Xu and Holy, 2013); briefly, intact B6D2F1 male vomeronasal epithelia were isolated and mounted on a multi-electrode array for electrophysiological recording, and adhered onto a nitrocellulose membrane (0.45μm, Millipore Co., MA, USA) for calcium imaging. Before recording, the VNO was acclimated by superfusing with Ringer’s for at least 30 min. The order of stimuli together with Ringer’s control was randomized across multiple repeated trials. Further details about stimulus preparation, recording method, and data analysis can be found in Extended Experimental Procedures.
Component-activity matching, purification, structural identification, and removal of M377 from urine extracts
See Extended Experimental Procedures.
Investigation behavior
Male mouse chemosensory investigation was recorded as previously described (Guo and Holy, 2007; Holy and Guo, 2005). Periods in which the swab was investigated were extracted algorithmically from the voltage signal from an optical beam-break detector. Significance was assessed by paired t-test. Further detail is presented in Extended Experimental Procedures.
Mounting behavior
Individual B6D2F1 males were singly-housed for at least two weeks without bedding change prior to the mounting test. Assays were performed in their home cages. Videos were scored manually using Interact Mangold software (Mangold International, Germany). Statistical significance was assessed by the Mann-Whitney test. Further detail is presented in Extended Experimental Procedures.
Supplementary Material
Highlights.
CAM is an approach to match candidate ligands and specific patterns of bioactivity
23 compounds in mouse urine explain firing rates in 7 classes of vomeronasal neurons
Steroid carboxylic acids are a new family of vomeronasal ligands
Steroid carboxylic acids are female sex pheromones for the mouse
Acknowledgments
We thank Amber Tyler for helping collect estrus female mice urine, and Petland St. Louis (south city store) for helping collect hamster and rabbit urine. We thank members of the Holy and Gross labs, as well as the anonymous referees, for many constructive comments. This work was supported by NIH/NIDCD R01 DC005964, NIH/NIMS/NIAAA R01 NS068409, and NIH/NIDCD R01 DC010381 (T.E.H.) and NIH/NIGMS P41 GM103422-36 (M.L.G.).
Footnotes
AUTHOR CONTRIBUTIONS
X.F. and T.E.H. designed the project. X.F. performed electrophysiology experiments and analysis. I.G. performed LC-MS profiling. T.E.H. developed the CAM method and analyzed the data. X.F. purified M377 and M361. Y.Y., X.F., and M.L.G. performed the structural analysis. S.P.X. and X.F. performed calcium imaging and analysis of data. X.F. and W.C. performed the behavior tests. T.E.H. and X.F. did the analysis. X.F. and T.E.H. wrote the manuscript with contributions from all authors.
Supplementary information is available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnson HA, Fu X, Holy TE. Multielectrode array recordings of the vomeronasal epithelium. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnson HA, Holy TE. Robust encoding of stimulus identity and concentration in the accessory olfactory system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13388–13397. doi: 10.1523/JNEUROSCI.0967-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Progress in neurobiology. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Comaniciu D, Meer P. Mean shift: A robust approach toward feature space analysis. Ieee T Pattern Anal. 2002;24:603–619. [Google Scholar]
- Dixon AK, Mackintosh JH. The relationship between the physiological condition of female mice and the effects of their urine on the social behaviour of adult males. Animal Behaviour. 1975;23:513–520. doi: 10.1016/0003-3472(75)90128-1. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature reviews Neuroscience. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Hostetler LD. Estimation of Gradient of a Density-Function, with Applications in Pattern-Recognition. Ieee T Inform Theory. 1975;21:32–40. [Google Scholar]
- Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chemical senses. 2007;32:463–473. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife (Cambridge) 2014;3:e0302. doi: 10.7554/eLife.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Progress in neurobiology. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–538. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and dynamics constants of muscles. Proc R Soc Lond B (London: Royal Society) 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Holekamp TF, Turaga D, Holy TE. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron. 2008;57:661–672. doi: 10.1016/j.neuron.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS biology. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FF, Nodari F, Kao LF, Fu X, Holekamp TF, Turk J, Holy TE. Structural characterization of sulfated steroids that activate mouse pheromone-sensing neurons. Biochemistry. 2008;47:14009–14019. doi: 10.1021/bi801392j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MH. Encoding olfactory signals via multiple chemiosensory systems. biochemistry and molecular biology. Biochemistry and molecular biology. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- Marre O, Amodei D, Deshmukh N, Sadeghi K, Soo F, Holy TE, Berry MJ. Mapping a complete Neural Population in the Retina. Journal of Neuroscience. 2012;32:14859–14873. doi: 10.1523/JNEUROSCI.0723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Arnson HA, Holy TE. Representation and transformation of chemosensory information in the mouse accessory olfactory system. Nature Neuroscience. 2010;13:723–730. doi: 10.1038/nn.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshiro M, Lakso M, Kawajiri K, Negishi M. Rip locus: regulation of female-specific isozyme (I-P-450(16 alpha) of testosterone 16 alpha-hydroxylase in mouse liver, chromosome localization, and cloning of P-450 cDNA. Biochemistry. 1988;27:6434–6443. doi: 10.1021/bi00417a035. [DOI] [PubMed] [Google Scholar]
- Omura M, Mombaerts P. Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Reports. 2014;8:583–595. doi: 10.1016/j.celrep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Paatero P, Tapper U. Positive Matrix Factorization - a Nonnegative Factor Model with Optimal Utilization of Error-Estimates of Data Values. Environmetrics. 1994;5:111–126. [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju U, Bradlow HL, Levitz M. Estriol-3-Sulfate in Human Breast Cyst Fluid - Concentrations, Possible Origin, and Physiological Implications. Annals of the New York Academy of Sciences. 1990;586:83–87. doi: 10.1111/j.1749-6632.1990.tb17793.x. [DOI] [PubMed] [Google Scholar]
- Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Tolokh II, Fu X, Holy TE. Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13903–13913. doi: 10.1523/JNEUROSCI.0037-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turaga D, Holy TE. Organization of vomeronasal sensory coding revealed by fast volumetric calcium imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1612–1621. doi: 10.1523/JNEUROSCI.5339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Shah YM, Matsubara T, Zhen Y, Tanabe T, Nagano T, Fotso S, Krausz KW, Zabriskie TM, Idle JR, et al. Control of steroid 21-oic acid synthesis by peroxisome proliferator-activated receptor alpha and role of the hypothalamic-pituitary-adrenal axis. The Journal of biological chemistry. 2010;285:7670–7685. doi: 10.1074/jbc.M109.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PS, Holy TE. Whole-mount imaging of responses in mouse vomeronasal neurons. Methods in molecular biology. 2013;1068:201–210. doi: 10.1007/978-1-62703-619-1_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.