Abstract
Several bifunctional peptides were synthesized and characterized based on the pentapeptide-derived ligand NP30 (1: Tyr-DAla-Gly-Phe-Gly-Trp-O-[3’,5’-Bzl(CF3)2]). Modification and truncation of amino acid residues were performed, and the tripeptide-derived ligand NP66 (11: Dmt-DAla-Trp-NH-[3',5'-(CF3)2-Bzl]) was obtained based on the overlapping pharmacophore concept. The Trp3 residue of ligand 11 works as a message residue for both opioid and NK1 activities. The significance lies in the observation that the approach of appropriate truncation of peptide sequence could lead to a tripeptide-derived chimeric ligand with effective binding and functional activities for both mu and delta opioid and NK1 receptors with agonist activities at mu and delta opioid and antagonist activity at NK1 receptors, respectively.
Keywords: multifunctional ligands, opioid receptor agonists, neutokinin-1 receptor antagonists, Truncation of peptide sequence
Graphical abstract
The opioid receptors have been a target for study due to their direct antinociceptive properties and have generated a wealth of information concerning ligand design elements.1 In doing this, the desire for specificity of agonists has been examined in order to decrease the side effects associated with known therapies. While this strategy has yielded promising results, the eradication of the side effects has remained an elusive goal. Subsequently, agonists affecting both the delta opioid receptor (DOR) and mu opioid receptor (MOR) have been explored in order to exploit the desired characteristics of each of these receptors. The MOR has been the traditional target of drug research because of its dominating efficacy in antinociception. However, this receptor has undesirable affects such as dependence, depressed respiratory response, dysphoria and constipation. Moreover, while ligands affecting specifically the DOR show decreased respiratory depression, dependency or dysphoria, they also have decreased efficacy in pain management. In exploring chimeric ligands that bind to both the MOR and DOR, it may be possible that a ligand designed for the delta/mu specificity might produce a highly efficacious compound which will have decreased dependency and dysphoria, as was found for biphalin (H-Tyr-DAla-Gly-Phe-NH-NH-Phe-Gly-DAla-Tyr-NH2).2 In general design principles, the presence of a Tyr and Phe in the message region accompanied by constraints have proven to have antinociceptive properties which are highly selective and efficacious.3 Interestingly, prolonged exposure to morphine enhanced release of substance P.4 Combining the agonist effects at opioid receptors together with blocking signals through the neurokinin 1 (NK1) receptors has shown enhanced antinociceptive effect in acute pain animal models and has prevented the opioid-induced tolerance in chronic trials.5
While signaling through the opioid receptor is involved in the mitigation of pain, other receptors are also involved in pain pathways such as the NK1. An important common endogenous ligand for the NK1 receptor is the 11 amino acid peptide substance P (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2), which causes an excitatory response in pain pathways. The mechanism for this involves the release of substance P when an external nociceptive stimulus is applied. The release of substance P causes the reduction in the transport properties of the potassium channels which in turn makes the nerve cell more responsive.6 Therefore, agonists for the NKl receptor are involved in systemic pain signaling. Thus in theory, the administration of an NK1 antagonist should regulate pain by shutting down pain signaling pathways before reaching the specific site of brain. However, administration of such antagonist has not proven effective as a stand-alone therapy in clinical trials against acute pain.7
Presently, opioids are the primary drugs for the treatment of neuropathic pain, and an increased dosage is needed to combat neuropathic pain which inevitably leads to tolerance and dependence. Therefore, we have proposed that a chimeric ligand involving opiate mu and delta agonist activity with an NK1 receptor antagonist can produce pain relief, including neuropathic pain, without opiate-induced hyperalgesia and associated antinociceptive tolerance.8,9 It has been postulated that different receptor systems are responsible for the regulation of pain in the different types of pain, and that substance P (NKl) antagonists can regulate neuropathic pain. We have been pursuing this approach and have recently designed peptidomimetic ligands that have potent delta/mu agonist activity and potent NK1 receptor antagonist activity all in a single molecule, including conformational analysis, to obtain insight into the conformational properties critical for the bioactivity profile and to cross the blood brain barrier.8,9
The desirable pharmacological activities of our ligand would include potent analgesic affects in both acute pain and in neuropathic pain states without the development of tolerance. In fact, our lead compound TY005 (Tyr1-DAla2-Gly3-Phe4-Met5-Pro6-Leu7-Trp8-O-[3’,5’-Bzl(CF3)2]) and TY027 (Tyr1-DAla2-Gly3-Phe4-Met5-Pro6-Leu7-Trp8-NH-[3’,5’-Bzl(CF3)2]) have been shown to reverse neuropathic pain in a rodent model, no sign of opioid-induce tolerance, and no development of reward liability, validating our hypothesis that a single compound possessing opioid agonist/NK1 antagonist activities could be an effective treatment against neuropathic pain.10 An additional advantage of this hybrid approach is that administration of one compound instead of a specific ratio of two or three separate ligands will provide synergy in bioactivity. While it is possible to administer two separate peptides, the pharmacokinetic distribution for these two ligands generally will result in two different bioavailabilities.
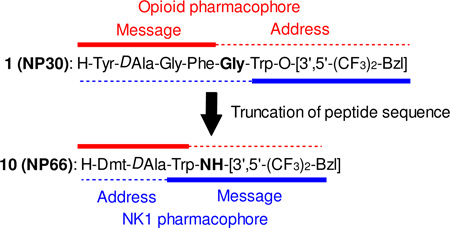
The design of a bifunctional ligand relies on the correct placement and attachment of the message and address moieties of the various pharmacophore structures.8,9 The importance of this concept is that the message-address region must be placed and oriented correctly in the receptor binding pocket for proper signaling to occur. While this is primarily controlled within the ligand itself, the role of the linker residue, which is located between the two message pharmacophores, is also an important consideration for the design of multivalent ligands. In fact, our previous study clearly demonstrated that the linker works as address region for both pharmacophores. Moreover, opioid agonist pharmacophore works as an address region for NK1 antagonist activity, and vice versa (Fig 1).9
Figure 1.
Sequences of multivalent ligands.
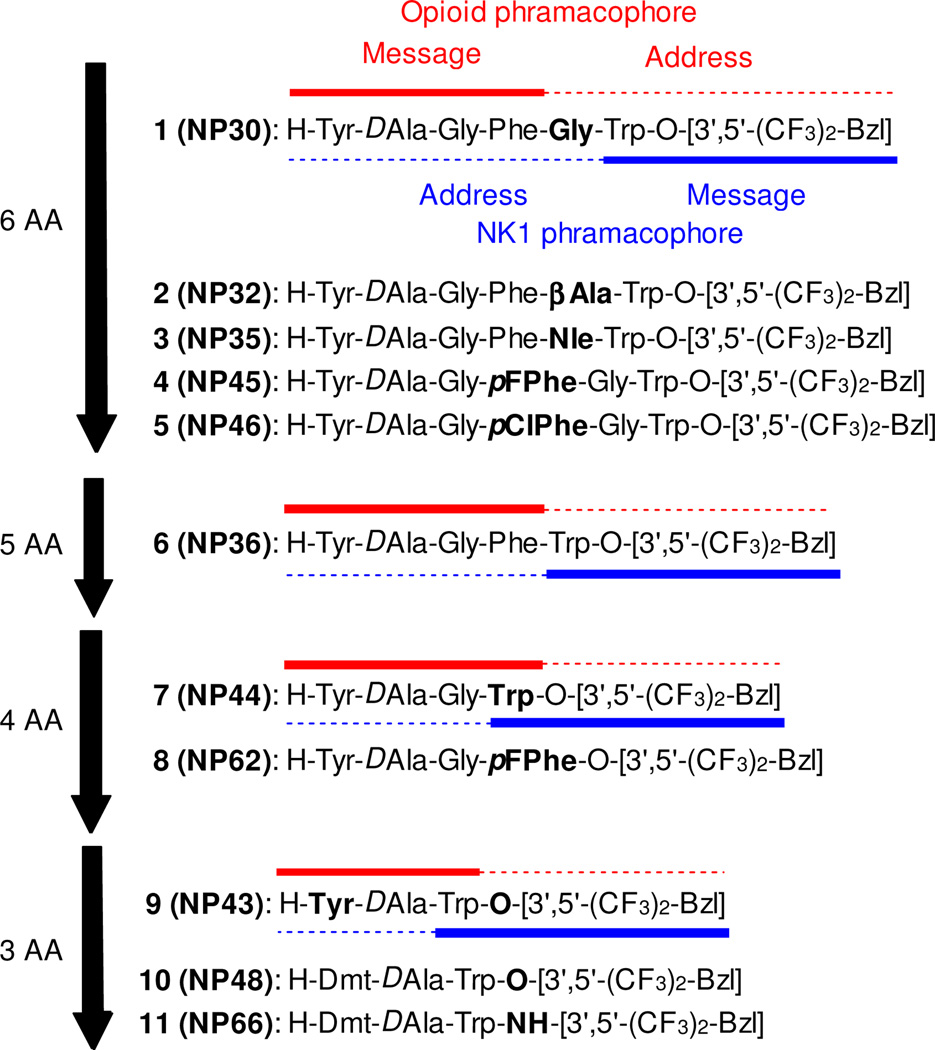
Previously, we reported the truncation of peptide sequence of octapeptide-derived TY005, and hexapeptide-derivative NP30 (1: Tyr-DAla-Gly-Phe-Gly-Trp-O-[3’,5’-Bzl(CF3)2]) was obtained with balanced functional activities as delta/mu opioid agonist and NK1 antagonist, and with significant antinociceptive and antihypersensitive activities.11 In this paper, we would like to discuss further contraction of the peptide sequence of 1. The short peptides have several advantages over long peptides for easier synthesis, lower preparative cost, and being the better template to be orally-available small molecule peptide mimetics.11, 12 To this end peptides were synthesized with the specific intention of evaluating the linker residue or the overlap of two message pharmacophores along with the truncation, and the conformation it induces in the peptide.
Our design of bifunctional ligands is based on a simple concept of conjoining two message pharmacophores, namely the opioid and the NK1 pharmacophores. Residues such as Gly, β-Ala and Nle were further incorporated between the message pharmacophores to investigate any distance-based effects that the two respective messaging pharmacophores may produce. DADLE (H-Tyr-DAla-Gly-Phe-DLeu) or biphalin-derived modified enkephalin sequence Tyr-DAla-Gly-Phe was chosen as the opioid message pharmacophore. Trp-O-[3’,5’-Bzl(CF3)2] was chosen as the NK1 receptor antagonist which is a simple but optimized aromatic ester of tryptophan highly optimized with respect to its structure and its potent nature in in-vitro13
As reported previously, 1 (Figure 1) has Gly as the residue to conjoin the minimal opioid and NKl message pharmacophores. Compound 2 shares common pharmacophores except that β-Ala replaces the Gly, while in 3 we used Nle as the linker. These changes in structures were made to evaluate the effect of a linker on the bioactivities of the different pharmacophore structures. These changes have proven to give interesting results as shown in Table 1. Compound 2 showed a greater than 5-fold decreased binding to the DOR and MOR receptors compared to 1, with similar results in GTPγS binding assay. In the case of the rat NK1 receptor (rNK1), the binding affinity improved from 4.2 nM (Gly linker) to 1.6 nM (β-Ala linker). In contrast, the binding affinity in the human NK1 receptor (hNK1) decreased from 0.0057 nM (Gly residue) to 0.057 nM (β-Ala residue). This demonstrates that the rodent and human NK1 receptors possess variations which partly affect the binding of the ligands. Interestingly, when Nle is used as the linker residue (3) binding at the hNK1 receptors is nearly identical to the one at the rNK1 receptor. Comparing the binding affinity of the ligands with the MOR vs DOR by changing the linkers causes changes in the specificity for the opioid receptor. Compound 1 and 2 have selectivity for the MOR whereas for the 3 the selectivity is for the DOR. In all these cases, it should be remembered that the actual message pharmacophore moieties for the ligands have not been changed, only the linker residue, which works as the address region for both pharmacophores. The GTPγS binding assays were performed to see if the change in the linkers led to changes in second messenger functions. For compound 1, GTPγS binding was 27 and 10 nM, respectively, at the DOR and MOR whereas changing the linker to β-Ala (2) led to decreased GTPγS binding for both the MOR (59 nM) and the DOR (160 nM) (Table 1). However, changing the linker to Nle caused a 70-fold increase in the GTPγS for the DOR (0.38 nM) and only a 2-fold decrease at the MOR (25 nM) compared to 1. In the isolated tissue-based assays 3 showed consistent opioid agonist activities in the GPI and MVD assays (IC50 = 6.0 and 1100 nM, respectively) with the 13 nM Ke value for the GPI assay for substance P stimulation.
Table 1.
Binding affinities and functional activities of bifunctional peptide derivatives at δ/μ opioid receptors and NK1 receptors
| Opioid agonist activities | NK1 antagonist activities | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| radioligand binding assays | [35S]GTPγS binding assays | MVD (δ) | GPI (μ) | radioligand binding assays | GPI | ||||||||
| hDORa,b | rMORa, c | Ki(μ) /Ki(ν) |
hDORa | rMORa | Opioid agonist | hNK1d, e | rNK1d, f | Ki(rNK1) /Ki(hNK1) |
Substance P antagonist |
||||
| no | Ki (nM)g |
Ki (nM)g |
EC50 (nM)h | Emax (%)i |
EC50 (nM)h | Emax (%)i |
IC50 (nM)j |
IC50 (nM)j |
Ki (nM)g |
Ki (nM)g |
Ke (nM)k |
||
| 1l | 4.7 | 0.29 | 0.062 | 27 | 87 | 10 | 36 | 21 | 26 | 0.0057 | 4.2 | 740 | 59 |
| 2 | 58 | 11 | 0.19 | 160 | 87 | 59 | 70 | 13 | 430 | 0.050 | 1.6 | 32 | 250 |
| 3 | 1.6 | 33 | 21 | 0.38 | 77 | 25 | 38 | 6.0 | 1100 | 0.34 | 0.58 | 1.7 | 13 |
| 4 | 4.5 | 0.050 | 0.011 | 3.4 | 68 | 5.5 | 58 | 9.5 | 60 | 0.0040 | 0.042 | 11 | 10 |
| 5 | 11 | 0.20 | 0.018 | 25 | 41 | 41 | 16 | 33 | 120 | 0.47 | 4.9 | 10 | 30 |
| 6 | 35 | 37 | 1.1 | 150 | 14 | 100 | 14 | 43 | 520 | 0.12 | 0.15 | 1.3 | 38 |
| 7 | 310 | 49 | 0.16 | 280 | 33 | 23 | 34 | 300 | 840 | 0.030 | 15 | 500 | 5.0 |
| 8 | 56 | 3.2 | 0.057 | 6.7 | 78 | 7.1 | 58 | 180 | 210 | 0.20 | 230 | 1200 | 52 |
| 9 | 100 | 180 | 1.8 | 91 | 36 | 170 | 26 | 5.5% 1μM | 3.3% 1μM | 0.0040 | 2.9 | 730 | 49 |
| 10 | 0.80 | 0.30 | 0.38 | 12 | 27 | 1.6 | 49 | 400 | 20% 1μM | 0.016 | 0.28 | 18 | 110 |
| 11 | 64 | 2.4 | 0.038 | 24 | 50 | 5.2 | 50 | 41 | 120 | 0.85 | 92 | 110 | 22 |
| Biphalinm | 2.6 | 1.4 | 0.54 | 1.1 | 83 | 2.7 | 8.8 | ||||||
| DAMGOn | 37 | 150 | |||||||||||
| L-732,138o | 0.73 | 130 | 180 | 250 | |||||||||
Competition analyses were carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the DOR and MOR.
[3H]DPDPE; Kd = 0.45 ± 0.1 nM.
[3H]DAMGO; Kd = 0.50 ± 0.1 nM.
Competition analyses were carried out using membrane preparations from transfected CHO cells that constitutively expressed rat or human NK1 receptors.
[3H]Substance P; Kd = 0.16 ± 0.03 nM.
[3H]Substance P; Kd = 0.40 ± 0.17 nM.
The data were collected from at least two independent experiments performed in duplicate. The Ki values are calculated using the Cheng and Prusoff equation to correct for the concentration of the radioligand used in the assay.
The EC50 values were determined from the non-linear regression analysis of data collected from at least two independent experiments performed in duplicate.
[Total bound − Basal]/[Basal − Non-specific] × 100.
Concentration at 50% inhibition of muscle contraction at electrically stimulated isolated tissues (n = 4).
Inhibitory activity against the substance P induced muscle contraction in the presence of 1 μM naloxone, Ke: concentration of antagonist needed to inhibit substance P to half its activity (n = 4).
Reference 11.
Reference 15.
Reference 9a.
Reference 9c.
Next, we examined the role of the Phe in the opioid moiety. This Phe has been well established as an important pharmacophore moiety in opioid ligands. Therefore, changes in the Phe residue of this peptide would be expected to modify the binding affinity towards the opioid receptors. In this series, the Phe residue was substituted with a pF-Phe and pCI-Phe, both of which can enhance opioid binding affinity. Changing the Phe residue to a pF-Phe 4 increased the mu opioid potency. The GTP functional assay values for 4 also show an increase in GTPγS binding and at both the MOR and DOR. Interestingly, the binding affinity for the rNK1 also increased 100-fold with only a slight effect on the hNK1 receptor, compared to those in 1. However, one can say that binding affinity of the ligand based solely on these residues enhanced the rNK1 receptor binding. The Ke value in isolated tissue-based assay against substance P stimulation was increased from those of 1 (10 nM). When the Phe residue was altered to pCl-Phe (5), its biological properties did not significantly change as compared to those of 1. Therefore, changes in the binding affinity due to halogenation of the Phe residue seem to be dependent on the electronegativity of the halogen atom. It should be mentioned that changing this one residue has had a global effect on the entire multimeric ligand, namely both for opioid and NK1 antagonist activities.
Because of the large effects on binding at both opioid and NK1 receptors a ligand was prepared in which the linker was removed. Thus a compound with the most important features of the two address pharmacophores was incorporated designing towards a shorter sequence of the pentapeptide-derived compound 6. Compound 6 showed decreased binding affinities and functional activities for opioid and NK1 receptors compared to those of 1. Further truncation was made to design the tetrapeptide-derivative 7 with deletion of Phe residue which is an important address pharmacophore of opioid antagonist activity. As expected, the opioid agonist affinities and activities of 7 were drastically decreased from those of 1 or 6, especially at the DOR. This result suggested that this Phe residue is indispensable for the opioid agonist activity, and Trp could not work as its substituting residue in this case. While, 7 displayed good affinity at the hNK1 receptor and excellent antagonist activity in GPI assay using substance P stimulation (Ki = 0.030 nM and Ke = 5.0 nM, respectively), indicating this truncation provide minor influence on human and guinea pig NK1 affinities. In another tetrapeptide-derivative 8, pF-Phe was used as overlapping message residue for both opioid and NK1 pharmacophores. Interestingly, 8 maintained antagonist activity against substance P stimulation in the GPI assay compared to 1, indicating that pF-Phe works as the replacement of Trp residue for guinea pig NK1 receptor. However, 8 has decreased affinities for hNK1 and rNK1 receptors as well as for DOR and MOR.
Tripeptide based enkephalins have been known to not only bind MOR and DOR but also exhibit potent functional agonism.14 Thus the most optimizable short sequence required for the opioid ligand would be to eliminate the Gly spacer from the tetrapeptide. Since DAla is required and crucial to maintain the conformation of the two aromatic residues, respectively, for Tyr and Phe. Therefore compound 9 was synthesized in order to see what the absence of the Gly linker from ligand 7, and also Gly between the DAla and Phe would have on pharmacological properties. Results show that the binding affinity across both opioid receptor systems decreased with the negligible agonism in MVD and GPI isolated tissue-based assays. While the binding affinity improved at the NK1 receptors with slightly higher substance P antagonist activity in the GPI assay than those of 1.
Both the Phe4 and Tyr1 residues are critical for the binding and second messenger activities of the enkephalins. Therefore, the final residue studied in this series was the Tyr which was replaced with 2’,6’-dimethyltyrosine (DMT).9i This residue has been shown to increase the binding and signaling of many enkephalin derivatives, and therefore would be a good candidate here to enhance these activities in our multivalent ligands. An added critical property of lipophilicity is also introduced by incorporating DMT. Through our discussions here the above designed ligands have been systematically optimized rationally by introducing linker residues then increasing electronegative moieties on the Phe residue followed by deletion of Gly residue. Following the same strategy one can modify the Tyr residue and also eliminate the fluoro-Phe residue, which leads to design of compound 10. This design is plausible because of its integrated features that are maintained and required as part of the pharmacophore features. The Trp-O-[3’,5’-Bzl(CF3)2] moiety satisfies the stand alone NK1 pharmacophore feature, while Dmt and Trp of Trp-O-[3’,5’-Bzl(CF3)2] serves as the new and modified pharmacophore for the opioid receptor. Compound 10 shows a 6-fold increase in the binding to the DOR with negligible change in binding to the MOR. Furthermore, this ligand shows enhanced binding at the rNK1 receptor while only slightly decreased binding at the hNK1 receptor. The second messenger function of 10 shows improvement of the GTPγS binding at the DOR going to 12 nM as compared to 27 nM for 1, but the Emax drops from 87% to 27%. The significance in these values lies in the fact that two Gly and a Phe were eliminated from the ligand 1. As stated, the Phe residue also is needed for the proper signal transduction of the enkephalins. Therefore the fact that there is significant binding when there is no Phe present might be due to the Trp functioning at the opioid receptor. This means that the Trp serves as a pharmacophore element for both the NK1 receptor and the opioid receptors. Using one residue to serve in two separate receptors (overlapping pharmacophores), one can design smaller and more intricate multimeric ligands which would be simpler to synthesize, and might be a good template to design orally available small molecules.
However, compared to the excellent affinities and functional activities for 10 in vitro, the results of functional activities using isolated tissue-based assays were dramatically decreased from those of 1. The IC50 value was 400 nM in the MVD. Only 20% inhibition was observed in GPI for mu opioid agonism at 1 μM. The GPI assay against substance P stimulation gave Ke value of 110 nM. We presumed that this decrease came from the enzymatic cleavage of ester bond of the benzyl derivative at the C-teminus in the isolated organs, and this replacement of the ester to an amide bond to avoid the degradation was examined.9 The resulting peptide 11 showed nanomolar range binding affinity at the MOR (2.4 nM), and its EC50 value was improved from that of 1 in the GTPγS binding assay (5.2 nM). IC50 value of 11 in the GPI assay for mu opioid agonism improved to 120 nM. For DOR, the affinity of 11 was relatively low with a Ki value of 64 nM. However, its second messenger function was equipotent to that of 1 (EC50 = 24 nM), and functional activity in the MVD showed an IC50 value of 41 nM, which was still in the same range with the ligand 1. The binding affinity for hNK1 of 11 was in the subnanomolar range, and the Ke value in GPI assay against substance P stimulation was improved from 1. It should be stressed again that 11 has no crucial Phe residue for opioid activity, but showed effective opioid agonist function in second messenger assay as well as in the isolated tissues. These results clearly indicated that Trp functions as the message region not only for the NK1 pharmacophore, but also for the opioid pharmacophore. These results demonstrated that a truncated multifunctional peptide-derivative with only three amino acid residues can have effective functional activities for all three receptors.
In conclusion, we have been able to design, synthesize and characterize several truncated multifunctional peptides based on pentapeptide-derived ligand 1. Modification and truncation of amino acid residues were performed, and the most interesting result is that the impact of changing one residue can have a significant effect on the affinities and activities for both mu and delta opioid and NK1 receptors. The contraction of peptide chain based on overlapping pharmacophore concept was successfully provided a tripeptide-derived ligand 11, whose Trp3 residue works as a message residue for both opioid and NK1 activities. The significance lies in the approach of appropriate truncation of peptide sequence that leads to tripeptide-derived chimeric ligand with effective function for both opioid and NK1 receptors. Ligand 11 could be an interesting research tool for developing novel analgesic drugs, and could be used as a good template to design novel orally-available peptide-mimetic small molecules.
Acknowledgments
The work was supported by grants from the USDHS, National Institute on Drug Abuse, DA-13449 and DA-06284. We thank Dr. Yeon Sun Lee and Dr. Eva Varga for scientific discussion and Ms. Magdalena Kaczmarska for culturing cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Schiller PW. The AAAPS J. 2007;7(3):E560. [Google Scholar]; (b) Janecka A, Fichna J, Janecki T. Cur. Top.Med. Chem. 2004;4:1. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- 2.Horan PJ, Mattia A, Bilsky EJ, Weber SJ, Davis TP, Yamamura HI, Malatynska E, Applyard SM, Slaninova J, Misicka A, Lipkowski AW, Hruby VJ, Porreca F. J Pharmacol. Exp. Ther. 1993;265:1446. [PubMed] [Google Scholar]
- 3.(a) Aubry A, Sakarellos C. Biopolymers. 1989;28:27. doi: 10.1002/bip.360280106. [DOI] [PubMed] [Google Scholar]; (b) Keys C, Payne P, Amsterdam P, Toll L, Lowe G. Mol. Pharacol. 1987;33:528. [PubMed] [Google Scholar]; (c) Hruby VJ, Gehrig CA. Med Res. Rev. 1989;9:343. doi: 10.1002/med.2610090306. [DOI] [PubMed] [Google Scholar]
- 4.King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Neurosignals. 2005;14:194. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- 5.(a) King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Pain. 2005;116:276. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ma W, Zheng WH, Kar S, Quirion R. Neuroscience. 2000;99:529. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]; (c) Powell KJ, Quirion R, Jhamandas K. Eur. J. Neurosci. 2003;18:1572. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- 6.Young JK, Anklin C, Hicks RP. Biopolymers. 1994;34(11):1449. doi: 10.1002/bip.360341102. [DOI] [PubMed] [Google Scholar]
- 7.Hill R. Trends Pharmacol Sci. 2000;21(7):244. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 8.(a) Hruby VJ, Porreca F, Yamamura HI, Tollin G, Agnes RS, Lee YS, Cai M, Alves I, Cowell S, Varga E, Davis P, Salamon Z, Roeske W, Vanderah T, Lai J. Am. Assoc. Pharm. Sci. J. 2006;3:E450. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hruby VJ. J. Org. Chem. 2009;74:9245. doi: 10.1021/jo901767e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Yamamoto T, Nair P, Davis P, Ma SW, Navratilova E, Moye M, Tumati S, Vanderah TW, Lai J, Porreca F, Yamamura HI, Hruby VJ. J. Med. Chem. 2007;50:2779. doi: 10.1021/jm061369n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yamamoto T, Nair P, Vagner J, Davis P, Ma S-W, Navratilova E, Moye M, Tumati S, Vanderah TW, Lai J, Porreca F, Yamamura HI, Hruby VJ. J. Med. Chem. 2008;51:1369. doi: 10.1021/jm070332f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yamamoto T, Nair P, Jacobsen NE, Davis P, Ma SW, Navratilova E, Lai J, Yamamura HI, Vanderah TW, Porreca F, Hruby VJ. J. Med. Chem. 2008;51:6334. doi: 10.1021/jm800389v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yamamoto T, Nair P, Jacobsen NE, Vagner J, Kulkarni V, Davis P, Ma SW, Navratilova E, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. J. Med. Chem. 2009;52:5164. doi: 10.1021/jm900473p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nair P, Yamamoto T, Kulkarni V, Moye S, Navratilova E, Davis P, Largent T, Ma SW, Yamamura HI, Vanderah T, Lai J, Porreca F, Hruby VJ. Adv. Exp. Med. Biol. 2009;611:537. doi: 10.1007/978-0-387-73657-0_235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yamamoto T, Nair P, Ma SW, Davis P, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. Bioorg. Med. Chem. 2009;17(20):7337. doi: 10.1016/j.bmc.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yamamoto T, Nair P, Jacobsen NE, Vagner J, Kulkarni V, Davis P, Ma SW, Navratilova E, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. J. Med. Chem. 2009;52(16):5164. doi: 10.1021/jm900473p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yamamoto T, Nair P, Jacobsen NE, Kulkarni V, Davis P, Ma SW, Navratilova E, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. J. Med. Chem. 2010;53(15):5491. doi: 10.1021/jm100157m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yamamoto T, Nair P, Largent-Milnes TM, Jacobsen NE, Davis P, Ma SW, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. J. Med. Chem. 2011;54(7):2029. doi: 10.1021/jm101023r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Largent-Milnes TM, Yamamoto T, Nair P, Moulton JW, Hruby VJ, Lai J, Porreca F, Vanderah TW. Br. J. Pharmacol. 2010;161(5):986. doi: 10.1111/j.1476-5381.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Largent-Milnes TM, Brookshire SW, Skinner DP, Hanlon KE, Giuvelis D, Yamamoto T, Davis P, Campos CR, Nair P, Deekonda S, Bilsky EJ, Porreca F, Hruby VJ, Vandearh TW. J Pharmacol Exp Ther. 2013;347(1):7. doi: 10.1124/jpet.113.205245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair P, Yamamoto T, Largent-Milnes TM, Cowell S, Kulkarni V, Moye S, Navratilova E, Davis P, Ma SW, Vanderah TW, Lai J, Porreca F, Hruby J. Bioorg. Med. Chem. Lett. 2013;23(17):4975. doi: 10.1016/j.bmcl.2013.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Hruby VJ, Nair P, Yamamoto T. 8026218. U.S. Patent. 2007; (b) Ballet S, Feytens D, Buysse K, Chung NN, Lemieux C, Tumati S, Keresztes A, Van Duppen J, Lai J, Varga E, Porreca F, Schiller PW, Vanden Broeck J, Tourwé D. J. Med. Chem. 2011;54(7):467. doi: 10.1021/jm1016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Cascieri MA, Macleod AM, Underwood D, Shiao LL, Ber E, Sadowski S, Yu H, Merchant KJ, Swain CJ, Strader CD, Fong TM. J. Biol. Chem. 1994;269:6587. [PubMed] [Google Scholar]; (b) MacLeod AM, Merchant KJ, Cascieri MA, Sadowski S, Ber E, Swain CJ, Baker RJ. Med. Chem. 1993;36:2044. doi: 10.1021/jm00066a015. [DOI] [PubMed] [Google Scholar]; (c) Millet R, Goossens L, Goossens JF, Chavatte P, Bertrand-Caumont K, Houssin R, Henichart JP. J. Pept. Sci. 2001;7:323. doi: 10.1002/psc.326. [DOI] [PubMed] [Google Scholar]
- 14.Vavrek RJ, His LH, York EJ, Hall ME, Stewart JM. Peptides. 1981;2(3):303. doi: 10.1016/s0196-9781(81)80124-6. [DOI] [PubMed] [Google Scholar]
- 15.Lipkowski AW, Misicka A, Davis P, Stropova D, Janders J, Lachwa M, Porreca F, Yamamura HI, Hruby VJ. Bioorg. Med. Chem. Lett. 1999;9:2763. doi: 10.1016/s0960-894x(99)00464-3. [DOI] [PubMed] [Google Scholar]