Abstract
Serotonergic hallucinogens, such as (+)-lysergic acid diethylamide, psilocybin, and mescaline, are somewhat enigmatic substances. Although these drugs are derived from multiple chemical families, they all produce remarkably similar effects in animals and humans, and they show cross-tolerance. This article reviews the evidence demonstrating the serotonin 5-HT2A receptor is the primary site of hallucinogen action. The 5-HT2A receptor is responsible for mediating the effects of hallucinogens in human subjects, as well as in animal behavioral paradigms such as drug discrimination, head twitch response, prepulse inhibition of startle, exploratory behavior, and interval timing. Many recent clinical trials have yielded important new findings regarding the psychopharmacology of these substances. Furthermore, the use of modern imaging and electrophysiological techniques is beginning to help unravel how hallucinogens work in the brain. Evidence is also emerging that hallucinogens may possess therapeutic efficacy.
Keywords: psychedelic, 5-HT2A receptor, head twitch, prefrontal cortex, visual effects
1. Introduction
Hallucinogenic drugs have been used by humans for thousands of years, but western scientists only became interested in these substances beginning in the late 1800s. These agents produce profound changes in consciousness. Because other drug classes can sometimes produce effects that overlap with those of the hallucinogens, it has been important to develop a formal definition for these compounds. This has turned out to be a difficult and contentious task. Hallucinogens have been defined as agents that alter thought, perception, and mood without producing memory impairment, delirium, or addiction (Hollister, 1968; Grinspoon and Bakalar, 1979). However, this definition is overly broad because it fails to exclude a wide-range of agents that are generally not classified as hallucinogens, such as cannabinoids and NMDA antagonists. It is now recognized that hallucinogens produce similar discriminative stimulus effects (Glennon et al., 1982) and act as agonists of the serotonin-2A (5-HT2A) receptor (Glennon et al., 1983). Therefore, it has been proposed (Glennon, 1999) that in addition to having the characteristics listed above, hallucinogens should also bind to the 5-HT2A receptor and produce full substitution in animals trained to discriminate the prototypical hallucinogen 2,5-dimethoxy-4-methylamphetamine (DOM). For this reason, hallucinogens are often categorized as classical hallucinogens or serotonergic hallucinogens. This article will review the pharmacology of hallucinogens, including their mechanism-of-action, their effects in animals and humans, and recent findings regarding how they interact with specific brain regions.
2. Pharmacology of hallucinogens
2.1. Receptor interactions
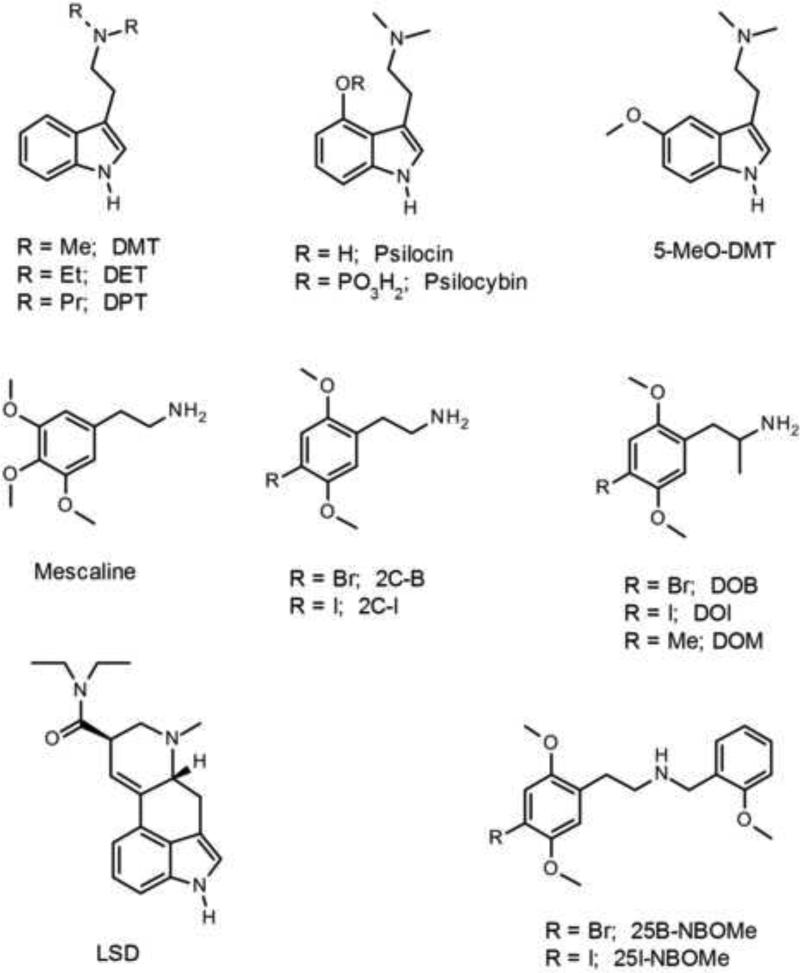
Classical hallucinogens can be divided into two main structural classes: indoleamines and phenylalkylamines (Nichols, 2012). Indoleamines include the tetracyclic ergoline (+)-lysergic acid diethylamide (LSD) and the chemically simpler indolealkylamines, which includes N,N-dimethyltryptamine (DMT), N,N-dipropyltryptamine (DPT), 5-methoxy-DMT (5-MeO-DMT), and psilocybin (4-phosphoryloxy-DMT) and its active O-dephosphorylated metabolite psilocin (4-hydroxy-DMT). DMT is found in several hallucinogenic snuffs used in the Caribbean and in South America. It is also a component of ayahuasca, an infusion or decoction prepared from DMT-containing plants in combination with species of Banisteriopsis containing β-carboline alkaloids that act as monoamine oxidase inhibitors (McKenna et al., 1984). Psilocybin and its metabolite psilocin are the active components of hallucinogenic teonanácatl mushrooms belonging to the genus Psilocybe.
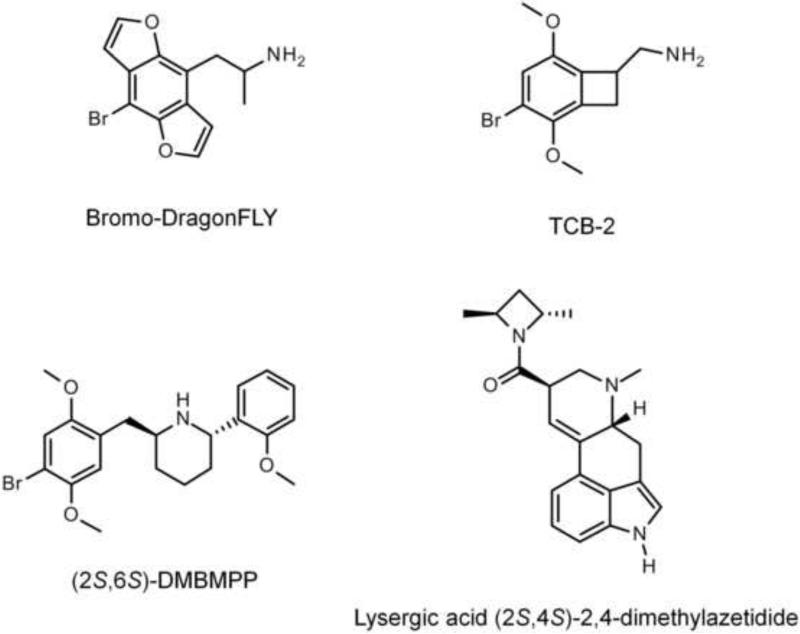
The phenylalkylamines can be subdivided into phenethylamines, such as mescaline from the peyote cactus (Lophophora williamsii), 2,5-dimethoxy-4-bromophenethylamine (2C-B), and 2,5-dimethoxy-4-iodophenethylamine (2C-I); and phenylisopropylamines (“amphetamines”), including DOM, 2,5-dimethoxy-4-iodoamphetamine (DOI), and 2,5-dimethoxy-4-bromoamphetamine (DOB). Although N-alkyl substituted phenylalkylamines are usually inactive as hallucinogens, the addition of a N-benzyl group to phenethylamines can dramatically increase their activity, and N-benzylphenethylamines are a new class of potent hallucinogenic compounds (Braden et al., 2006). Examples of N-benzylphenethylamine hallucinogens include N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine (25B-NBOMe). The chemical structures of many of these hallucinogens are illustrated in Figure 1. Nichols and colleagues have also developed conformationally-restricted derivatives of phenylalkylamine hallucinogens: bromo-DragonFLY (1-(8-bromobenzo[1,2-b;4,5-b]difuran-4-yl)-2-aminopropane; Parker et al., 1998); TCB-2 (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine; McLean et al., 2006); and 2S,6S-DMBMPP ((2S,6S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine; Juncosa et al., 2012). Likewise, lysergic acid 2,4-dimethylazetidide was developed as a rigid analogue of LSD that shows similar in vivo potency (Nichols et al., 2002). Figure 2 shows examples of rigid hallucinogen analogues.
Figure 1.
Chemical structures of indolealkylamine, phenylalkylamine, and ergoline hallucinogens.
Figure 2.
Chemical structures of conformationally-restricted hallucinogens.
Phenylalkylamine hallucinogens are selective for 5-HT2 receptors, including 5-HT2A, 5-HT2B, and 5-HT2C sites (Titeler et al., 1988; Leysen, 1989; Pierce and Peroutka, 1989). The indolealkylamines, by contrast, bind non-selectively to 5-HT receptors. Certain indolealkylamines, most notably DMT and some of its derivatives, bind to σ1 receptors (Fontanilla et al., 2009) and the trace amine receptor (Bunzow et al., 2001), and are substrates for the 5-HT transporter (SERT) (Nagai et al., 2007; Cozzi et al., 2009). However, compared with σ1 and SERT, tryptamines are more potent at 5-HT1A and 5-HT2A receptors by several orders of magnitude, so the former sites probably do not contribute to the hallucinogenic response. LSD and other ergoline hallucinogens display high affinity for 5-HT receptors, as well as dopaminergic and adrenergic receptors (reviewed by: Halberstadt and Geyer, 2011; Nichols, 2012).
2.2. Pharmacology of the 5-HT2A receptor
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT, see Fig. 3) has potent contractile effects upon smooth muscle, especially rat uterus and guinea pig ileum. The first indication that there are multiple 5-HT receptor subtypes came from studies conducted by Gaddum and Picarelli (1957). They reported that treatment with either dibenzyline or morphine alone could only partially block the effect of 5-HT on guinea pig ileum. However, in tissue exposed to dibenzyline for 30 min, morphine markedly antagonized 5-HT-induced contraction, and dibenzyline acted as a full 5-HT antagonist in tissue previously exposed to morphine. These findings demonstrated that 5-HT was acting through two different receptor classes (type D and type M) to induce contraction of guinea pig ileum.
Figure 3.
Structure of serotonin.
Soon after the development of radioreceptor techniques to demonstrate receptor binding, this methodology was applied to the investigation of 5-HT receptors. The first radioligands utilized were [3H]LSD and [3H]5-HT (Bennett and Snyder, 1975, 1976). Both of those radioligands bind to rat brain membranes with high-affinity in a reversible, saturable, and stereoselective manner, suggesting they are interacting with specific recognition sites. After introduction of the dopamine antagonist radioligand [3H]spiperone, it was recognized that [3H]spiperone binds to 5-HT receptors distinct from the sites labeled by [3H]5-HT (Peroutka and Snyder, 1979). The sites labeled by [3H]5-HT and [3H]spiperone were designated as 5-HT1 and 5-HT2 receptors, respectively, and it was recognized that [3H]LSD labeled both sites. The D receptor was eventually shown to be equivalent to the 5-HT2 receptor, whereas the M receptor is pharmacologically distinct from 5-HT1 sites and was later classified by Bradley and coworkers (Bradley et al., 1986) as the 5-HT3 receptor. The 5-HT2 receptor class was later reorganized to include three subtypes: 5-HT2A (equivalent to the site known historically as the 5-HT2 receptor or the D receptor), 5-HT2B (formerly known as the 5-HT2F receptor), and 5-HT2C (formerly known as the 5-HT1C receptor) (Hoyer et al., 1994).
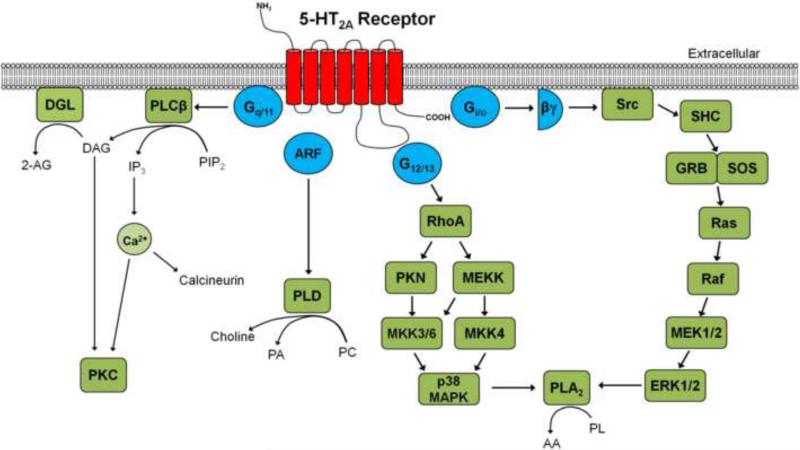
The 5-HT2A receptor couples to Gq and activates phospholipase Cβ (PLCβ) signaling, resulting in the hydrolysis of membrane phospholipids to inositol triphosphate (IP3) and diacylglycerol, and mobilization of intracellular Ca2+ (see Fig. 4). There is evidence that 5-HT2A is coupled to several non-canonical signaling pathways, including β-arrestin-2, Src (potentially involving Gi/o-associated Gβγ subunits), extracellular-regulated kinase (ERK), p38 mitogen-activated protein (MAP) kinase, phospholipase A2 (downstream from ERK 1,2 and p38 MAP kinase), Akt, and phospholipase D (dependent on the small G protein ADP-ribosylation factor-1 (ARF1)) (Kurrasch-Orbaugh et al., 2003a; González-Maeso et al., 2007; Schmid and Bohn, 2010; Barclay et al., 2011). However, the signaling pathways responsible for mediating the characteristic effects of hallucinogens have not been conclusively identified. Activation of the canonical Gq-PLCβ signaling pathway is apparently not sufficient to produce hallucinogen-like behavioral effects in animal models (Rabin et al., 2002; Kurrasch-Orbaugh et al., 2003b; González-Maeso et al., 2007). Multiple signaling pathways may be involved because the behavioral response to DOI is partially blunted in Gq knockout mice (Garcia et al., 2007). Schmid and colleagues have reported that β-arrestin-2 is not required for the behavioral effects of DOI and 5-MeO-DMT (Schmid et al., 2008; Schmid and Bohn, 2010). There also does not appear to be a direct relationship between phospholipase A2 activation and generation of hallucinogen effects (Kurrasch-Orbaugh et al., 2003b).
Figure 4.
Signaling pathways coupled to the 5-HT2A receptor. Abbreviations: AA, arachidonic acid; 2-AG, 2-arachidonoylglycerol; ARF, ADP-ribosylation factor-1; DAG, diacylglycerol; DGL, diacylglycerol lipase; ERK1/2, extracellular-regulated kinases 1 and 2; GRB, growth factor receptor-bound protein 2; IP3, inositol triphosphate; p38 MAPK, p38 mitogen-activated protein kinase; MEK1/2, mitogen/extracellular signal-regulated kinases 1 and 2; MKK3/6, MAPK kinases 3and 6; MKK4, MAPK kinase 4; MEKK, MAPK kinase kinase; PA, phosphatidic acid; PC, phosphatidyl choline; PIP2, phosphatidylinositol 4,5-biphosphate; PKC, protein kinase C; PKN, protein kinase N; PL, phospholipids; PLCβ, phospholipase Cβ; PLD, phospholipase D; SHC, Src homology 2 domain containing transforming factor; SOS, son of sevenless homolog.
3. Evidence that serotonergic hallucinogens belong to a unitary class
3.1. Subjective effects
Despite having different chemical structures, phenylalkylamine, tryptamine, and ergoline hallucinogens produce remarkably similar subjective effects (Isbell, 1959; Hollister, 1961; Wolbach et al., 1962a,b; Hollister and Hartman, 1962; Rosenberg et al., 1964; Abramson and Rolo, 1967; Hollister et al., 1969). It is very difficult for hallucinogen-experienced subjects to distinguish between psilocybin and LSD if those substances are administered in a blinded fashion, with the only apparent difference being the duration of action (Abramson and Rolo, 1967). Similar findings have been reported when mescaline, LSD, and psilocybin are compared in the same subjects (Hollister and Hartman, 1962; Wolbach et al., 1962a,b). By contrast, the effects of hallucinogens can be distinguished from those of other drug classes. The effects of classical hallucinogens and anticholinergic agents are qualitatively distinct (Lebovits et al., 1960; Hollister et al., 1960). Studies using the Addiction Research Center Inventory (ARCI) instrument (Haertzen et al., 1963) have confirmed that the effects of LSD are dissimilar from those of (+)-amphetamine (Rosenberg et al., 1963) and Δ9-tetrahydrocannabinol (Isbell and Jasinski, 1969). The ARCI can also distinguish between the subjective responses to 20 mg (+)-amphetamine and an ayahuasca preparation containing the equivalent of a 1 mg/kg dose of DMT (Dos Santos et al., 2011). Although it does not appear that any studies have directly compared the experiences produced by classical hallucinogens and the κ-opioid receptor agonist salvinorin A from Salvia divinorum, there is evidence that the phenomenology of salvinorin A is unique (Albertson and Grubbs, 2009), and the ARCI is relatively insensitive to the effects of salvinorin A (MacLean et al., 2013).
Several recent studies have compared the effects of hallucinogens and other drug classes using psychometrically-validated instruments. One instrument that has been widely-used to assess the subjective response to hallucinogens is the Altered States of Consciousness Questionnaire (APZ), as well as well as APZ variants such as the APZ-OAV and the 5D-ASC. These rating scales are designed to assess altered states of consciousness independent of their etiology (Dittrich, 1975, 1998). The APZ and APZ-OAV include three core dimensions: Oceanic Boundlessness (OB), Anxious Ego Dissolution (AED) and Visionary Restructuralization (VR). The OB dimension reflects a pleasant state of depersonalization and derealization, the AED dimension measures dysphoric effects such as ego disintegration, delusions, loss of self-control, thought disorder, and anxiety, and the VR dimension involves elementary and complex visual hallucinations and perceptual illusions (see Table 1). Mescaline. psilocybin, and DMT produce profound increases in OB, AED and VR scores (Hermle et al., 1992; Vollenweider et al., 1997; Dittrich et al., 1998; Gouzoulis-Mayfrank et al., 1999b; Grob et al., 2011). Another instrument is the Hallucinogen Rating Scale (HRS), which was specifically designed to measure the effects of parenteral DMT (Strassman et al., 1994) Double-blind studies have confirmed the APZ and the HRS can distinguish the effects of psilocybin and mescaline from those of (+)-methamphetamine, methylphenidate, and 3,4-methylenedioxyethylamphetamine (Hermle et al., 1992; Gouzoulis-Mayfrank et al., 1999b; Griffiths et al., 2006). Ayahuasca also elicited significantly greater effects than (+)-amphetamine on 4 of 6 subscales of the HRS (Dos Santos et al., 2011).
Table 1.
Core dimensions of the APZ (Dittrich, 1998)
| Dimension | Symptoms Assessed |
|---|---|
| Oceanic Boundlessness (OB) | Positive derealization |
| Positive depersonalization | |
| Altered sense of time | |
| Positive mood | |
| Mania-like experience | |
| Anxious Ego Dissolution (AED) | Anxious derealization |
| Thought disorder | |
| Delusion | |
| Fear of loss of control | |
| Visionary Restructuralization (VR) | Elementary hallucinations |
| Visual pseudohallucinations | |
| Synesthesia | |
| Changed meaning of percepts | |
| Facilitated recollection | |
| Facilitated imagination | |
A double-blind crossover study comparing DMT and the NMDA antagonist (S)-ketamine found DMT produces effects that more closely resemble the positive symptoms of schizophrenia, whereas the effects of (S)-ketamine are more similar to the negative and catatonic symptoms of schizophrenia (Gouzoulis-Mayfrank et al., 2005). Subjects experienced vivid visual hallucinations after treatment with DMT but not with (S)-ketamine; this difference was reflected by scores in the VR dimension of the APZ-OAV, which was more strongly affected by DMT than by (S)-ketamine. Another notable difference between ketamine and serotonergic hallucinogens is that ketamine does not produce mystical experiences (Lofwall et al., 2006), whereas hallucinogens induce these states with some reliability (Pahnke, 1969; Griffiths et al., 2006, 2008, 2011; Lyvers and Meester, 2012).
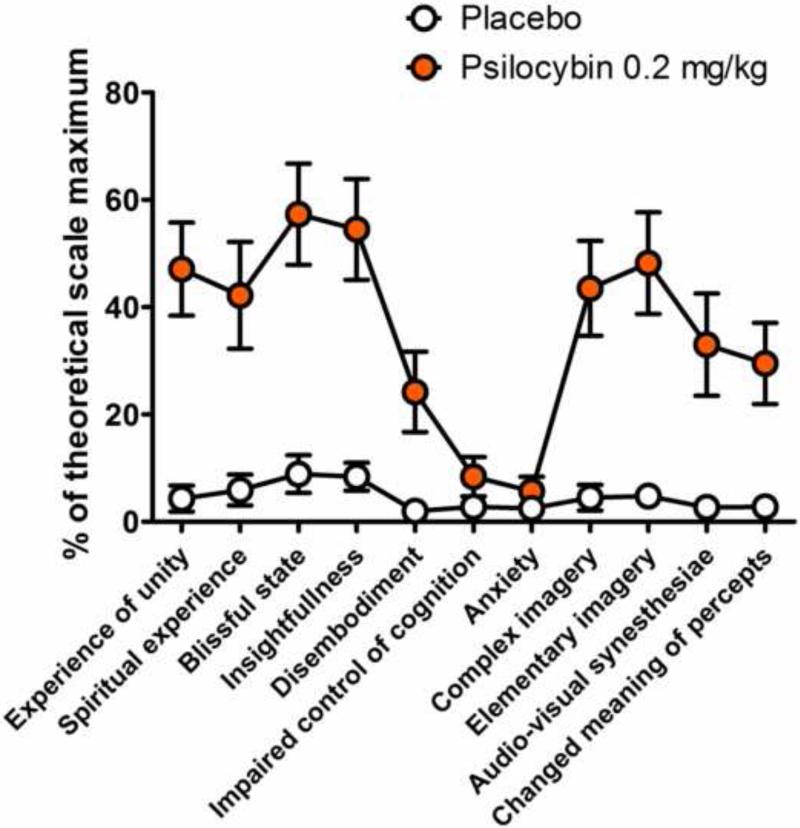
Vollenweider and colleagues have conducted a psychometric assessment of APZ-OAV data pooled from 43 studies with psilocybin, (S)-ketamine, and the entactogen 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) (Studerus et al., 2010). Examination of the factorial structure of the APZ-OAV revealed the OB, AED and VR scales are multidimensional, and Vollenweider et al. were able to extract 11 new homogenous APZ-OAV scales that are very effective at differentiating the subjective effects of psilocybin, (S)-ketamine, and MDMA. There are clear differences in the relative magnitude of drug effects on several of the new scales; for example MDMA has strong effects on blissful state, (S)-ketamine produces the largest increase in disembodiment, and complex imagery and elementary imagery are most strongly influenced by psilocybin Figure 5 compares the effects of psilocybin and placebo on the new homogeneous APZ-OAV subscales. In summary, even though there are some similarities between the subjective effects of serotonergic hallucinogens, NMDA antagonists, psychostimulants, and entactogens, the effects produced by the latter three drug classes are clearly distinct from those elicited by classical hallucinogenic drugs.
Figure 5.
Subjective effects of psilocybin as measured by the 5-Dimension Altered States of Consciousness instrument (5D-ASC). The values reported by Grob et al. (2011) were reanalyzed using the 11 new homogenous APZ subscales developed by Studerus et al. (2010). Values are the mean (SEM) percentages of the total possible score. The placebo was niacin.
3.2. Tolerance and cross-tolerance
Tachyphylaxis (tolerance) develops rapidly to the effects of classical hallucinogens. If LSD and DOM are administered repeatedly at daily intervals tolerance is observed after 1-3 days and there is eventually nearly a complete loss of response (Abramson et al., 1956; Isbell et al., 1956, 1961; Angrist et al., 1974). Tolerance occurs with a variety of phenylalkylamine, indolealkylamine, and ergoline hallucinogens, and compounds from these classes exhibit symmetrical cross-tolerance (Abramson et al., 1958, 1960; Balestrieri and Fontanari, 1959; Isbell et al., 1961; Wolbach et al., 1962a; Abramson and Rolo, 1967; Hollister et al., 1969). Importantly, cross-tolerance does not occur between LSD and (1) (+)-amphetamine (Rosenberg et al., 1963), (2) the anticholinergic N-methyl-3-piperidyl benzilate (Balestrieri, 1960), or (3) Δ9-tetrahydrocannabinol (Isbell and Jasinski, 1969). Similar findings have been reported by parallel studies in laboratory animals (Appel and Freedman, 1968; Teresa et al., 1968; Winter, 1971; Wallach et al., 1974; Colasanti and Khazan, 1975; Schlemmer and Davis, 1986). The fact that serotonergic hallucinogens produce similar experiences and induce cross-tolerance indicates that these compounds share a common mechanism of action.
4. Involvement of the 5-HT2A receptor in hallucinogen effects
4.1. Evidence from human studies
Multiple, converging lines of evidence point to 5-HT2A receptor activation as the unitary mechanism responsible for mediating hallucinogenesis. Indoleamine and phenylalkylamine hallucinogens bind to 5-HT2 sites with moderate to high affinity (Shannon et al., 1984; Lyon et al., 1988; Sadzot et al., 1989; McKenna et al., 1990). Although indoleamine hallucinogens show relatively promiscuous binding profiles, phenylisopropylamine hallucinogens such as DOM and DOB are highly-selective for 5-HT2 receptors (Titeler et al., 1988; Pierce and Peroutka, 1989) and therefore it is likely that their effects are mediated by a member of the 5-HT2 family. Additionally, there is a very strong correlation (r = 0.90–0.97) between 5-HT2A receptor affinity and human hallucinogenic potency (Glennon et al., 1984; Titeler et al., 1988; Sadzot et al., 1989). Another compelling finding is that 5-HT2A receptor blockade ameliorates most of the effects of psilocybin in human subjects. A series of studies conducted by Franz Vollenweider and colleagues at the University Hospital of Psychiatry in Zürich have shown that the effects of psilocybin (215-260 μg/kg, p.o.) on the OB, AED, and VR dimensions of the APZOAV and 5D-ASC are completely blocked by pretreatment with either the 5-HT2A/2C antagonist ketanserin or the mixed 5-HT2A/D2 antagonist risperidone (Vollenweider et al., 1998; Carter et al., 2005, 2007; Kometer et al., 2012, 2013; Quednow et al., 2012). By contrast, pretreatment with the dopamine D2 antagonist haloperidol had no effect on psilocybin-induced VR scores and actually intensified the effect of psilocybin on scores in the AED dimension (Vollenweider et al., 1998). Ketanserin also blocks the effects of psilocybin on a variety of neurophysiological measures in humans, including tests of spatial working memory (Vollenweider et al., 1998), prepulse inhibition of acoustic startle (Quednow et al., 2012), N170 visual-evoked potentials (Kometer et al., 2013), semantic interference in the Stroop test (Quednow et al., 2012), and recognition of emotional facial cues in a go/nogo task (Kometer et al., 2012). Furthermore, a positron emission tomography (PET) study with the 5-HT2A radiotracer [18F]altanserin has shown that the intensity of the response to psilocybin is directly correlated with the level of 5-HT2A occupation (Quednow et al., 2010).
4.2. Evidence from animal behavioral models
Because of regulatory constraints on human studies, animal behavioral models are the primary methodology used to study hallucinogens in vivo. Although it has been difficult to develop appropriate models of hallucinogenic activity because of the variability and complexity of their effects, several animal models have made important contributions to our understanding of hallucinogen pharmacology. Importantly, although there are some exceptions, almost all the behavioral effects of hallucinogens studies in laboratory animals are mediated by the 5-HT2A receptor.
4.2.1. Drug discrimination
Laboratory animals can be trained to discriminate hallucinogens from saline using operant conditioning techniques. Rats are the species most commonly employed, although mice and monkeys have also been used. Many classical hallucinogens have been used as training drugs, including LSD, mescaline, DOM, DOB, DOI, psilocybin, 5-MeO-DMT, DMT, and DPT (Hirschhorn and Winter, 1971; Glennon et al., 1979, 1982, 1987; Young et al., 1981; Glennon, 1986; Smith et al., 2003; Benneyworth et al., 2005; Winter et al., 2007; Fantegrossi et al., 2008; Li et al., 2008; Gatch et al., 2011). All of these hallucinogens produce cross-generalization, suggesting that they evoke similar interoceptive stimulus cues. By contrast, drugs from other pharmacological classes do not produce hallucinogen-like stimulus effects (Glennon et al., 1982; Appel and Cunningham, 1986; Li et al., 2008). There is a great deal of evidence that the discriminative stimulus effects of hallucinogens are mediated by the 5-HT2A receptor. For example, Glennon and colleagues conducted substitution tests with 22 hallucinogens in rats trained to discriminate 1 mg/kg DOM from saline and found that the ED50 values for stimulus generalization are highly correlated (r = 0.938) with 5-HT2A binding affinity (Glennon et al., 1984). Another study with 18 hallucinogens found a strong correlation (r = 0.90) between ED50 values for stimulus generalization to 1 mg/kg DOM and affinity at 5-HT2A receptors labeled with [3H]DOB (Titeler et al., 1988). The stimulus effects of hallucinogens can be blocked by the selective 5-HT2 antagonists ketanserin and pirenperone (Colpaert et al., 1982; Glennon et al., 1983; Glennon, 1986; Cunningham and Appel, 1987; Appel and Callahan, 1989). Blockade by ketanserin and pirenperone, however, does not eliminate the possibility of 5-HT2C receptor involvement because those antagonists are relatively nonselective for 5-HT2A versus 5-HT2C sites. Importantly, M100907, a 5-HT2A antagonist with high selectivity versus the 5-HT2C receptor, blocks stimulus control in animals trained with DOI (Schreiber et al., 1994; Smith et al., 1998, 1999, 2003), DOM (Li et al., 2008; May et al., 2009), R-(–)-DOM (Eckler et al., 2003), LSD (Winter et al., 2004; Benneyworth et al., 2005; Marona-Lewicka et al., 2005; Gresch et al., 2007), and psilocybin (Winter et al., 2007). Conversely, neither the selective 5-HT2C antagonist SB 242,084 nor the mixed 5-HT2C/2B antagonists SB 200,646A and SB 206,553 block stimulus control induced by DOI, LSD, or psilocybin (Smith et al.,1998, 1999; Schreiber et al.,1994; Gresch et al., 2007; Winter et al., 2007). Furthermore, Fiorella et al. (1995a) tested eleven 5-HT2 antagonists and found the rank order of potencies for blocking R-(–)-DOM substitution in LSD-trained rats parallels their affinities for 5-HT2A (r = 0.95) but not for 5-HT2C (r = -0.29).
Although most phenalkylamines are relatively nonselective for 5-HT2A versus 5-HT2C, 2S,6S-DMBMPP displays 124-fold selectivity for 5-HT2A receptors (Juncosa et al., 2012). Although racemic trans-DMBMPP is less selective, it still shows 98-fold higher affinity for 5- HT2A over 5-HT2C receptors. Importantly, trans-DMBMPP fully substitutes in rats trained to discriminate 0.08 mg/kg LSD. By contrast, several studies have demonstrated that 5-HT2C agonists fail to mimic the hallucinogen discriminative stimulus. Neither 1-(3-trifluoromethylphenyl)piperazine (TFMPP) nor m-chlorophenylpiperazine (mCPP) substitute for DOM, DOI, or LSD (Glennon and McKenney, 1985; Glennon et al., 1986; Appel and Cunningham, 1986). These findings demonstrate that 5-HT2A activation is sufficient to produce hallucinogen-like stimulus effects. Furthermore, 5-HT2C activation does not play a role in mediating the hallucinogen discriminative stimulus cue. The available data provide strong support for the conclusion that hallucinogens evoke a uniform discriminative stimulus cue that is mediated by the 5-HT2A receptor.
Although it is clear that the 5-HT2A receptor is primarily responsible for generating hallucinogen-induced stimulus control, interactions with other receptors may contribute to or modify the stimulus effects of hallucinogens. This appears to be especially true for indoleamines, which are much less selective than phenylalkylamines for 5-HT2A sites. For example, there appears to be a time-dependent dopaminergic component to the LSD discriminative stimulus in rats (Marona-Lewicka and Nichols, 2007; Marona-Lewicka et al., 2009). There is evidence that the 5-HT1A receptor also contributes to the discriminative stimulus effects of LSD. 5-HT1A agonists such as 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and ipaspirone produce partial substitution in rats and mice trained with LSD (Winter and Rabin, 1988; Arnt, 1989; Benneyworth et al., 2005; Reissig et al., 2005). The 5-HT1A antagonist WAY-100635 does not alter LSD discrimination in rats (Appel et al., 2004; Reissig et al., 2005; Gresch et al., 2007), but the 5-HT1A receptor may make an more prominent contribution to the LSD cue in mice because discrimination can be partially blocked by administration of either WAY-100635 or M100907 (Benneyworth et al., 2005). However, the ability of R-(–)-DOB to substitute for LSD in mice is completely blocked by M100907 but not by WAY-100635, demonstrating the stimulus element generated by 5-HT1A is a non-essential component of the LSD cue and not a shared aspect of hallucinogen pharmacology. Although certain indolealkylamines produce compound stimulus cues involving both 5-HT1A- and 5-HT2A-mediated components (Glennon et al., 1988; Winter et al., 2000; Fantegrossi et al., 2008), 5-HT1A receptors do not play a role in the interoceptive effects of psilocybin (Winter et al., 2007) or 5-methoxy-N,N-diisopropyltryptamine (Fantegrossi et al., 2006).
A potential confound associated with drug discrimination studies is the possibility of “false positive” results. False-positives occur where an animal trained to discriminate a hallucinogen generalizes to a drug that is known to be non-hallucinogenic in humans. Lisuride is one example of drug that can produce false-positive results. Lisuride is an isolysergic acid derivative that is structurally similar to LSD (see Fig. 6), and acts as an agonist at a variety of serotonergic, dopaminergic, and adrenergic receptors (Leysen, 1989; Piercey et al,. 1996; Egan et al., 1998; Marona-Lewicka et al., 2002; Millan et al., 2002; Nichols et al., 2002). Despite the fact that lisuride has high affinity for the 5-HT2A receptor and acts as an agonist (Egan et al. 1998; Kurrasch-Orbaugh et al., 2003b; Cussac et al., 2008), it is not hallucinogenic in humans (Herrmann et al., 1977; Verde et al., 1980; Raffaelli et al., 1983; Beneš et al., 2006) and has been used clinically to treat migraine and Parkinson's disease. Some studies have found that lisuride produces full substitution in rats trained with either LSD, DOI, or DOM (White and Appel, 1982; Glennon and Hauck, 1985; Fiorella et al., 1995b; Appel et al., 1999), but in other studies it produced only partial substitution (Holohean et al.,1982; Marona-Lewicka et al., 2002). Although clearly some degree of similarity exists between the stimulus cues evoked by lisuride and classical hallucinogens, there are also subtle differences because rats can be trained to discriminate between lisuride and LSD using three-choice (drug-drug-vehicle) discrimination procedures (Callahan and Appel, 1990). Discrimination studies where animals are trained to discriminate between LSD and another drug such as pentobarbital or cocaine also appear to be less sensitive to lisuride-induced false-positive responses (Appel et al., 1999).
Figure 6.
Chemical structure of lisuride.
González-Maeso et al. (2007) have proposed that the behavioral differences between LSD and lisuride are due to 5-HT2A functional selectivity. They found LSD and lisuride both activate Gq/11 signaling via the 5-HT2A receptor, but only LSD increases the cortical expression of the immediate early genes egr-1 and egr-2 by activating Gi/o and Src (González-Maeso et al. 2007). Therefore, they hypothesized that LSD is hallucinogenic because it is capable of activating specific signaling mechanisms that are not recruited by lisuride. Alternatively, the reason why lisuride fails to recruit Gi/o may have nothing to do with functional selectivity, and could be a consequence of its low intrinsic efficacy at 5-HT2A (Rabin et al., 2002; Kurrasch-Orbaugh et al., 2003b; Cussac et al., 2008). Although animals trained with DOM will generalize to lisuride (Glennon and Hauck 1985; Fiorella et al. 1995b), the response to DOM is attenuated when it is co-administered with lisuride (Glennon, 1991). The fact that lisuride induces a response when administered alone but act as an antagonist in the presence of a full agonist (DOM) is consistent with the behavior of a partial agonist..
4.2.2. Head twitch response
Many mammalian species display a paroxysmal rotational shaking of the head in response to mechanical or chemical irritation of the pinna. Mice show a similar behavior, known as the head twitch response (HTR), after administration of hallucinogens (Corne and Pickering, 1967;Silva and Calil, 1975; Darmani et al., 1990). Hallucinogens also induce head twitches in rats, but in that species the behavior often involves both the head and the trunk (Yamamoto and Ueki, 1975; Bedard and Pycock, 1977). The responses made by rats are sometimes called wet-dog shakes because they resemble the behavior of a dog drying itself after emerging from the water. It is important to recognize that the HTR can occur in response to administration of 5-HT precursors (e.g., l-tryptophan and l-5-hydroxytryptophan) and drugs that increase 5-HT release (e.g., fenfluramine and p-chloroamphetamine), and therefore the behavior is not specific to hallucinogens (Corne et al., 1963; Matthews and Smith, 1980; Singleton and Marsden, 1981; Yamaguchi et al., 1987). Nonetheless, the HTR has gained prominence as a behavioral proxy in rodents for human hallucinogen effects because the HTR is one of only a few behaviors that can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A agonists (González-Maeso et al., 2007). Indeed, even high doses of lisuride fail to induce the HTR in mice (González-Maeso et al., 2007; Halberstadt and Geyer, 2013).
It is well-established that phenylisopropylamine and indoleamine hallucinogens induce the HTR (reviewed by: Halberstadt and Geyer, 2011), but the literature is less clear with regard to phenethylamine hallucinogens. Many studies have demonstrated that mescaline produces head twitch behavior in rats and mice (Silva and Calil, 1975; Yamamoto and Ueki, 1975; González-Maeso et al., 2007). It has also been reported that the hallucinogen 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7) induces the HTR in mice (Fantegrossi et al., 2005). Studies in rats, however, have shown 2C-I, 2C-B, and 2,5-dimethoxy-4-methylphenethylamine (2C-D) do not induce the HTR (Moya et al., 2007). In contrast to those findings, we recently reported 2C-I and the N-benzyl derivatives 25I-NBOMe and N-(2,3-methylenedioxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBMD) produce dose-dependent increases in HTR behavior in C57BL/6J mice (Halberstadt and Geyer, 2014). 25BNBOMe also induces the HTR in mice (Ettrup et al., 2013). The discrepant findings with regard to 2C-I and other phenethylamines may reflect the fact that mice are more sensitive than rats to the HTR induced by 5-HT2A partial agonists. 2C-I has relatively low intrinsic activity at the 5-HT2A receptor (Parrish et al., 2005; Moya et al., 2007), and it may not have sufficient efficacy to provoke head twitches in rats. Nevertheless, we are not aware of any serotonergic hallucinogens that do not produce the HTR in mice.
The kinematics of the HTR induced by DOI have been characterized in C57BL/6J mice and Sprague-Dawley rats (Halberstadt and Geyer, 2013). When mice make a head twitch, the head rapidly twists from side-to-side. Each HTR consists of 5–11 head movements, with the head movements occurring at 78–98 Hz (i.e., each head movement lasts approximately 11 msec). The behavior is similar in rats but in that species the frequency of head movement is lower. One drawback to traditional HTR studies is that they require direct behavioral observation that can be extremely time-consuming. However, as we have recently demonstrated, it is possible to detect the behavior with a head-mounted magnet and a magnetometer coil, providing a highly-sensitive, semi-automated assessment of the behavior (Halberstadt and Geyer, 2013, 2014).
The HTR induced by hallucinogens and other 5-HT agonists is closely linked to 5-HT2A activation. It was proposed in 1982 that the mescaline-induced HTR is mediated by the 5-HT2A receptor, based on the fact that the relative potency of 5-HT antagonists to block the behavior is correlated (r = 0.875) with their 5-HT2A affinity (Leysen et al., 1982). Similar findings were later reported for the HTR induced by DOI (Schreiber et al., 1995; Dursum and Handley, 1996). Numerous studies have shown M100907 blocks the HTR induced by hallucinogens (Table 2). For example, we found M100907 blocks the HTR induced by the hallucinogen 25I-NBOMe with an ID50 = 6.2 μg/kg (Fig. 7; Halberstadt and Geyer, 2014). Based on ex vivo binding data it is unlikely M100907 produces any appreciable occupation of 5-HT2C receptors at that dose level (Smith et al., 1995). Studies have also demonstrated that the highly-selective 5-HT2A antagonist MDL 11,939 blocks the HTR induced by DOI and TCB-2 in mice (Fox et al., 2009; Dougherty and Aloyo, 2011). Mice lacking the 5-HT2A receptor gene do not produce head twitches in response to mescaline, DOI, DOM, LSD, DMT, 5-MeO-DMT, psilocin, or 1-methylpsilocin (González-Maeso et al., 2007; Keiser et al., 2009; Halberstadt et al., 2011), although the response can be rescued by selectively restoring the 5-HT2A receptor gene to cortical regions (González-Maeso et al., 2007). By contrast, 1 mg/kg DOI produces a significant (albeit somewhat blunted) HTR in 5-HT2C knockout mice (Canal et al., 2010). The fact that DOI can provoke head twitches in 5-HT2C knockout mice but not in 5-HT2A knockout mice strongly indicates the 5-HT2A receptor is the member of the 5-HT2 family responsible for mediating the HTR. Similarly, there is a consensus in the literature that the ability of DOI to induce the HTR is not blocked by selective 5-HT2C antagonists or mixed 5-HT2C/2B antagonists (Kennett et al., 1994; Schreiber et al., 1995; Wettstein et al., 1999; Vickers et al., 2001; Fantegrossi et al., 2010).
Table 2.
The selective 5-HT2A antagonist M100907 blocks the head twitch response induced by hallucinogens in rats and mice.
| Hallucinogen | M100907 | Species | Reference | ||||
|---|---|---|---|---|---|---|---|
| Drug | Dose | Route1 | Potency2 | Effective dose3 | Route1 | ||
| 5-MeO-DMT | 30 mg/kg | IP | ID50 = 0.03 | IP | Mouse | Kenne et al., 1996 | |
| 5-MeO-DMT | 10 mg/kg | IP | 0.05 mg/kg | IP | Mouse | Schmid & Bohn, 2010 | |
| DPT | 3 mg/kg | IP | 0.01 mg/kg | IP | Mouse | Fantegrossi et al., 2008 | |
| DOI | 2.5 mg/kg | IP | ID50 = 0.005 | 0.04 mg/kg | SC | Rat | Schreiber et al., 1995 |
| DOI | 3 mg/kg | IP | 1 mg/kg | IP | Rat | Wettstein et al., 1999 | |
| R-(-)-DOI | 3 mg/kg | IP | ID50 = 0.01 | 0.1 mg/kg | SC | Mouse | Bartoszyk et al., 2003 |
| DOI | 2.5 mg/kg | IP | 0.25 mg/kg | IP | Mouse | Garcia et al., 2007 | |
| DOI | 2 mg/kg | IP | 0.3 mg/kg | IP | Mouse | Jennings et al., 2008 | |
| DOI | 1 mg/kg | IP | 0.05 mg/kg | IP | Mouse | Schmid et al., 2008 | |
| DOI | 1 mg/kg | IP | 0.25 mg/kg | SC | Mouse | Canal et al., 2010 | |
| DOI | 1 mg/kg | IP | 0.025 mg/kg | SC | Mouse | Canal et al., 2013 | |
| 2C-I | 3 mg/kg | SC | ID50 = 0.0045 | 0.1 mg/kg | SC | Mouse | Halberstadt & Geyer, 2014 |
| 25I-NBOMe | 0.3 mg/kg | SC | ID50 = 0.0062 | 0.1 mg/kg | SC | Mouse | Halberstadt & Geyer, 2014 |
| 25I-NBMD | 3 mg/kg | SC | ID50 = 0.0015 | 0.1 mg/kg | SC | Mouse | Halberstadt & Geyer, 2014 |
IP, intraperitoneal; SC, subcutaneous.
ID50 = inhibitory dose50 in mg/kg.
Dose of M100907 that produced 90–100% blockade of the head twitch response.
Figure 7.
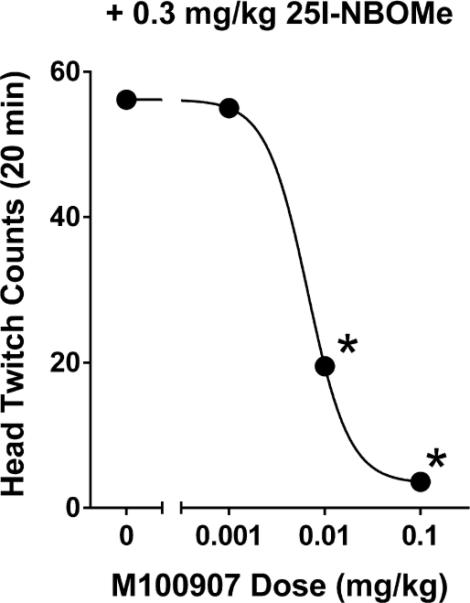
Effect of pretreatment with the selective 5-HT2A antagonist M100907 on the head twitch response induced by 0.3 mg/kg 25I-NBOMe in C57BL/6J mice. Data are presented as group means ± SEM for 20-min test sessions. **p < 0.01, significant difference from 25I-NBOMe alone. Data from: Halberstadt and Geyer, 2014.
Although it has been conclusively established that the 5-HT2C receptor is not required for generation of the HTR, there is some evidence that 5-HT2C sites may play a modulatory role. 5-HT2 agonists that are selective for 5-HT2C sites, such as (S)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine (Ro 60-0175), 6-chloro-2-(1-piperazinyl)pyrazine (MK-212), and mCPP, do not induce the HTR in rats unless administered in combination with the 5-HT2C antagonist SB 242,084 (Vickers et al., 2001). There is also evidence that the ability of DOI to induce the HTR is significantly attenuated by pretreatment with selective 5-HT2C agonists, including Ro 60-0175, CP-809,101, and mCPP (Schreiber et al., 1995; Siuciak et al., 2007; Fantegrossi et al., 2010; Canal et al., 2013). These findings indicate 5-HT2C activation suppresses expression of the HTR. Likewise, DOI produces a biphasic dose-response curve in NIH Swiss and Swiss-Webster mice, and SB 242084 reportedly shifts the descending arm of the DOI response to the right (Fantegrossi et al., 2010). Here again there is evidence that the 5-HT2C receptor can inhibit the HTR. On the other hand, as was noted above, Canal and colleagues have reported that 5-HT2C knockout mice show a blunted HTR to 1 mg/kg DOI (Canal et al., 2010). Furthermore, in contrast to many other reports, the same investigators found pretreatment with SB 242,084 or SB 206,553 diminished the magnitude of the HTR induced by 1 mg/kg DOI in C57BL/6J and DBA/2J mice (Canal et al., 2010, 2013). It is not clear why the 5-HT2C receptor attenuates the HTR in certain studies and augments the response in others, but Fantegrossi et al. (2010) have argued these differences may be strain dependent. For example, there are strain differences in the editing of 5-HT2C mRNA (Calcagno and Invernizzi, 2010; Hackler et al., 2006). Since 5-HT2C editing can influence the downstream coupling of the receptor (Burns et al., 1997), the nature of the interactions between 5-HT2A and 5-HT2C could potentially vary by mouse strain.
4.2.3. Prepulse inhibition of Startle
Prepulse inhibition (PPI) refers to the phenomenon where a weak prestimulus presented prior to a startling stimulus will attenuate the startle response; PPI is often used as an operational measure of sensorimotor gating, and reflects central mechanisms that filter out irrelevant or distracting sensory stimuli (Swerdlow and Geyer, 1998). Rats treated with DOI (Sipes and Geyer, 1994; Padich et al., 1996), DOB (Johansson et al., 1995), LSD (Ouagazzal et al., 2001; Halberstadt and Geyer, 2010), mescaline (Pálenícek et al., 2008), and 2C-B (Páleníček et al., 2013) show reductions in PPI. These effects can be blocked by M100907 and MDL 11,939 (Sipes and Geyer, 1995; Padich et al., 1996; Ouagazzal et al., 2001; Halberstadt and Geyer, 2010). By contrast, neither SB 242,084 nor the 5-HT2C/2B antagonist SER-082 are effective. Although one study found haloperidol can block the PPI disruption produced by hallucinogens (Sipes and Geyer, 1994), this was not replicated by subsequent investigations (Varty and Higgins, 1995; Ouagazzal et al., 2001). Lisuride also disrupts PPI in rats, but this effect is blocked by the D2/3 antagonist raclopride and not by MDL 11,939 (Halberstadt and Geyer, 2010).
4.2.4. Interval timing
Temporal perception can be markedly altered by hallucinogens. Subjects under the influence of mescaline and LSD often report that their sense of time appears to speed up or slow down, or they may experience a sensation of timelessness (Serko, 1913; Hoch et al., 1952; Bercel et al., 1956; Aronson et al., 1959; Kenna and Sedman, 1964). Psilocybin also alters performance on laboratory measures of timing (Wittmann et al., 2007).
Temporal perception can be assessed in rodents using interval timing paradigms. For example, in the free-operant psychophysical task, animals are trained to respond on two levers, and they must respond on one lever during the first half of the trial and on the other lever during the second half (Stubbs, 1980). In the discrete-trials task, animals are trained to press one lever in response to short duration stimuli and another lever in response to long duration stimuli, and are then challenged with a variety of stimulus durations (Body et al., 2002). DOI disrupts the performance of rats in both of these tasks (Body et al., 2003, 2006; Asgari et al., 2006). Although DOI affects performance in the discrete trials task, it does not affect performance in a similar task where rats have to discriminate different light intensities, indicating that DOI is specifically influencing temporal perception and not disrupting stimulus control or attentional processes (Hampson et al., 2010). The effect of DOI in the discrete-trials task and that free-operant task are blocked by ketanserin and M100907 (Asgari et al., 2006; Body et al., 2006), demonstrating the involvement of 5-HT2A.
4.2.5. Exploratory and investigatory behavior
Measures of locomotor activity are often used to characterize the effects of psychoactive drugs on exploratory behavior. Locomotion alone, however, is not necessarily a reliable measure of exploration because it includes does not distinguish specific exploratory responses to environmental stimuli from other types of motor activity (Hughes, 1972). Given the complexity of hallucinogen effects, it is not surprising that hallucinogens cannot be distinguished from other drug classes using traditional open field locomotor measures (Silva and Calil, 1975). However, multivariate assessment methods have been more successful. One example is the Behavioral Pattern Monitor (BPM), which combines features from activity chambers and holeboards and provides quantitative as well as qualitative measures of the spatial and temporal structure of activity (Geyer et al., 1986; Paulus and Geyer, 1991). BPM studies have shown hallucinogens produce a very characteristic profile of behavioral effects. When rats are tested in unfamiliar BPM chambers after administration of hallucinogens (including mescaline, DOM, DOI, LSD, DMT, 5-MeO-DMT, and psilocin), the animals display reduced amounts of locomotor activity, rearings, and holepokes at the beginning of the test session, and avoidance of the center of the BPM chamber is increased (Adams and Geyer, 1985a,b; Wing et al., 1990; Krebs-Thomson et al., 2006). Most of these effects are markedly diminished in animals habituated to the BPM chambers, indicating that hallucinogens act by enhancing neophobia. The ability of hallucinogens to increase the avoidance of novel (and potentially threatening) test chambers by rats may be analogous to the enhanced sensitivity and reactivity to environmental stimuli that occurs in humans (Salvatore and Hyde, 1956).
Extensive testing has confirmed this pattern of effects in the BPM is highly specific to hallucinogens (Geyer et al., 1986, 1987; Mittman and Geyer, 1989; Callaway et al., 1990; Lehmann-Masten and Geyer, 1991). For example, although 8-OH-DPAT and other selective 5-HT1A agonists reduce locomotor activity, rearings, and holepokes in rats, these effects are not influenced by environmental familiarity and hence are likely to reflect sedation (Mittman and Geyer, 1989). When Adams and Geyer (1985c) compared lisuride and LSD in the BPM, they found the two compounds produce markedly different patterns of effects. Lisuride produces effects that are similar to those of apomorphine and other dopamine agonists, with sedative effects occurring at low doses and perseverative patterns of hyperactivity occurring at higher doses.
The 5-HT2A receptor is responsible for mediating most of the effects of hallucinogens in the rat BPM. It was first shown that ritanserin and ketanserin block the effects of mescaline, DOM, and DOI in the BPM, indicating 5-HT2 involvement (Wing et al., 1990). Later studies demonstrated that the effects of DOI are blocked by M100907 but not by SER-082 (Krebs-Thomson et al., 1998), confirming mediation by 5-HT2A. The action of indoleamine hallucinogens in the BPM is more complex mechanistically, with 5-HT1A and 5-HT2A receptors contributing to the effects of LSD and 5-MeO-DMT (Mittman and Geyer, 1991; Krebs-Thomson and Geyer, 1996; Krebs-Thomson et al., 2006; Halberstadt et al., 2008).
Hallucinogens have also been tested in a version of the BPM designed for mice (Tanaka et al., 2012). In contrast to rats, phenylalkylamine and indolealkylamine hallucinogens produce disparate effects on exploratory and investigatory behavior in C57BL/6J mice. Phenylalkylamines, including DOI, mescaline, and TCB-2, inhibit investigatory behavior and alter locomotor activity in a dose-dependent manner, increasing activity at low to moderate doses and reducing activity at high doses (Halberstadt et al., 2009, 2013). Other groups have reported similar findings with DOM and DOI in mice (Yamamoto and Ueki, 1975; Darmani, 1996; Brookshire and Jones, 2009; Carlsson et al., 2011). The increase in locomotor activity induced by 1 mg/kg DOI, 25 mg/kg mescaline, or 3 mg/kg TCB-2 is blocked by low doses of M100907 and is absent in 5-HT2A knockout mice. By contrast, the reduction of locomotor activity induced by 10 mg/kg DOI is attenuated by SER-082. Taken together, it appears that 5-HT2A and 5-HT2C receptors have countervailing effects on locomotor activity, with 5-HT2A activation increasing activity and 5-HT2C activation reducing activity. Administration of psilocin and 5-MeO-DMT to C57BL/6J mice reduces locomotor activity and investigatory behavior (Halberstadt et al., 2011). These effects are blocked by WAY-100635 but are unaffected by SB 242,084 or by 5-HT2A gene deletion. Similarly, 5-MeO-DMT has no effect on activity in 5-HT1A knockout mice (van den Buuse et al., 2011). Hence, whereas the phenylalkylamines act through 5-HT2 sites to alter behavior in the mouse BPM, indoleamine hallucinogens appear to act via the 5-HT1A receptor.
4.3. Tolerance studies
As noted in section 3.2, serotonergic hallucinogens produce a profound degree of tolerance and cross-tolerance in animals and humans. Although very little is known about the mechanisms leading to the development of tolerance to hallucinogens in humans, there is evidence in animals that tolerance is linked to 5-HT2A downregulation. Rats treated repeatedly with DOM, LSD, or psilocin show a significantly lowered density of 5-HT2A receptors in several brain regions (Leysen et al., 1989; Buckholtz et al., 1989, 1990). Binding to 5-HT1A, 5-HT1B, α2, β1, or D2 receptors is unaffected. Another study demonstrated that treatment with 1 mg/kg DOI for 8 days produced a significant reduction in the density of 5-HT2A receptors in the cortex, but there was no change in 5-HT2C receptor expression (Smith et al., 1999). An identical treatment regimen caused tolerance to develop in rats trained to discriminate DOI. Likewise, there is a significant reduction of 5-HT2A-stimulated [35S]GTPγS binding in the medial prefrontal cortex (mPFC) and anterior cingulate cortex in rats treated with LSD (0.13 mg/kg/day) for 5 days (Gresch et al., 2005); this indicates tolerance to LSD is associated with a reduction of 5-HT2A signaling.
Although most hallucinogens produce tolerance in humans, DMT seems to be the exception. It has been reported that DMT does not evoke tolerance in man, even after an intramuscular (IM) dosage regimen of 0.7 mg/kg twice daily for five days (Gillin et al., 1976). More recently, Strassman et al. (1996) found there was no tolerance to the subjective effects of DMT in volunteers who received four intravenous (i.v.) injections of 0.3 mg/kg at 30 minute intervals. In vitro experiments have shown that exposure to LSD or DOI desensitizes 5-HT2A and 5-HT2C receptors in transfected cell lines (Smith et al., 1998; Roth et al., 1995). However, after exposure to DMT, 5-HT2C receptors showed desensitization but there was no change in the response to 5-HT2A activation (Smith et al., 1998). These observations suggest that DMT fails to induce tolerance because it does not desensitize the 5-HT2A receptor.
5. Hallucinogen effects on neuronal activity
5.1. Locus coeruleus
The locus coeruleus (LC), located in the dorsal pons, is the source of almost all noradrenergic projections in the CNS. LC neurons are responsive to sensory stimuli, especially of a novel or arousing nature, and the firing of LC neurons is markedly increased by noxious stimulation (reviewed by: Singewald and Philippu, 1998). Intravenous administration of mescaline (2 mg/kg), LSD (5–10 μg/kg), DOM (20–80 μg/kg), DOB (50–100 μg/kg), or DOI (16–50 μg/kg) profoundly enhances the responses of LC neurons to sensory stimuli while simultaneously depressing their spontaneous firing (Aghajanian, 1980; Rasmussen and Aghajanian, 1986; Gorea and Adrien, 1988; Chiang and Aston-Jones, 1993). After administration of hallucinogens, the enhancement of responsiveness is so pronounced that even innocuous sensory stimuli normally ineffective at driving LC cell firing will evoke a response (Aghajanian, 1980). The ability to produce opposite effects upon spontaneous and sensory-evoked LC firing is a specific property of LSD-like drugs, as other pharmacological agents that alter the basal activity of LC cells (e.g., (+)-amphetamine, clonidine, desipramine, or idazoxan) do not alter evoked LC firing (Aghajanian, 1980; Rasmussen and Aghajanian, 1986; Chiang and Aston-Jones, 1993). The observation that hallucinogens decrease the spontaneous activity of LC cells is supported by the work of Done and Sharp (1992) who found that DOI and DOB lower the concentration of NE in hippocampal dialysates, which indicates those compounds decrease tonic NE release from LC projections.
The effects of hallucinogens upon LC unit activity appear to be mediated by 5 HT2A receptors. The 5-HT2 antagonists ketanserin and ritanserin have been shown to block the actions of hallucinogens in the LC (Rasmussen and Aghajanian, 1986; Gorea and Adrien, 1988). Furthermore, Szabo and Blier (2001) found that the ability of DOI to alter the activity of LC neurons is abolished by M100907. Nonetheless, 5-HT2A receptors are sparsely distributed within the LC (e.g., Cornea-Hébert et al., 1999), and application of the 5-HT2A/5-HT3 agonist quipazine or hallucinogens such as DOI directly into the LC does not mimic the effects of their systemic administration (Rasmussen and Aghajanian, 1986; Gorea and Adrien, 1988; Gorea et al., 1991; Chiang and Aston-Jones, 1993). Intravenous administration of mescaline and LSD also had no effect on the ability of locally applied acetylcholine, glutamate (Glu), or substance P to excite LC neuronal activity (Aghajanian, 1980). Presumably then, hallucinogens act upon LC afferents, altering the firing of LC cells indirectly by modulating the activity of one or more input pathways.
Chiang and Aston-Jones (1993) reported that the decrease in LC spontaneous firing induced by DOI could be blocked by the GABAA receptor antagonists bicuculline and picrotoxin, whereas the ability of DOI to enhance sensory-evoked LC responses was blocked by the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid but not by the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Thus, hallucinogens appear to tonically activate GABAergic input to LC and concomitantly facilitate glutamatergic sensory input. It is likely that the nucleus prepositus hypoglossi (PrH), an area known to provide direct GABAergic inhibitory input into the LC (Ennis and Aston-Jones, 1989a,b), mediates the hallucinogen-induced inhibition of spontaneous LC activity. Although one group reported that microinjection of quipazine directly into the PrH did not alter LC unit activity in the rat (Gorea et al., 1991), subsequent work confirmed that DOI depolarizes PrH neurons (Bobker, 1994). Moreover, electrolytic lesions of PrH significantly attenuate the ability of systemic quipazine injections to reduce the frequency of LC unit discharge (Gorea et al., 1991). This strongly implicates the PrH or one of its afferents as the site through which 5-HT2A agonists modulate spontaneous LC firing. The identity of the specific LC afferent(s) responsible for the hallucinogen-induced facilitation of LC glutamatergic sensory input is currently unknown. Although the nucleus paragigantocellularis in the ventrolateral rostral medulla is a major source of excitatory input into the LC (Ennis and Aston-Jones, 1986; Chiang and Aston-Jones, 1993), the ability of somatosensory stimuli to excite the LC is unaffected by lesions of nucleus paragigantocellularis (Rasmussen and Aghajanian, 1989). The LC also receives excitatory input from the prefrontal cortex (PFC), both directly and indirectly (Sesack et al., 1989; Jodo and Aston-Jones, 1997; Jodo et al., 1998), and the excitatory effects of hallucinogens on the LC may be mediated by those pathways. As will be discussed below in section 5.2, hallucinogens increase the firing of PFC projection neurons.
The LC projects heavily to cortex, where there is overlap between the distribution of α1-adrenoceptors and 5-HT2A receptors (Palacios et al., 1987). Interestingly, in the PFC, α1-adrenoceptors and 5-HT2A receptors have similar effects on the activity of layer V pyramidal neurons (Marek and Aghajanian, 1999). Hallucinogens increase the intensity of sensory experiences and affective responses, and it is tempting to speculate that the LC may contribute to these effects. Indeed, the ability of LSD to potentiate neophobia in rats in the Behavioral Pattern Monitor is diminished by depletion of norepinephrine from LC projections (Geyer et al., 1985).
5.2. Prefrontal cortex (PFC)
5.2.1. Effects on PFC network activity in vitro
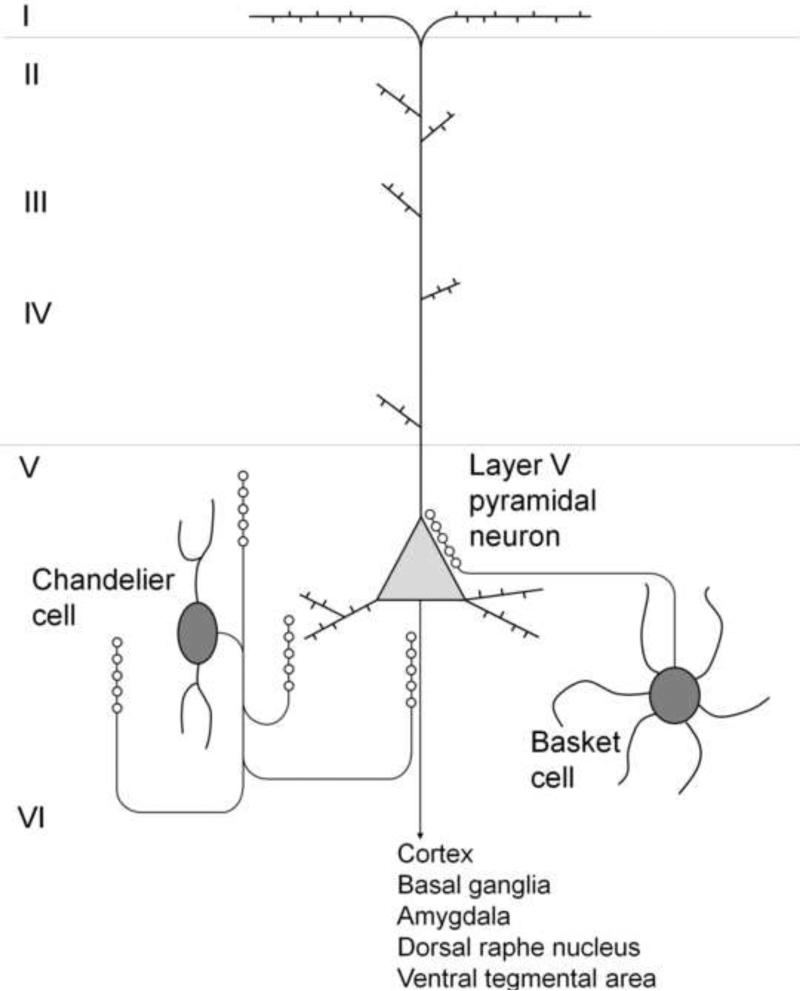
It is now recognized that the PFC is an important site of action for hallucinogens. The 5-HT2A receptor is expressed heavily in the PFC and adjacent cortical regions, particularly in lamina V (Mengod et al., 1990; Lopez-Gimenez et al., 1997; Jakab and Goldman-Rakic, 1998; Cornea-Hébert et al., 1999). In situ hybridization histochemistry has confirmed that most of the cells in monkey and human PFC express 5-HT2A mRNA (De Almeida and Mengod, 2007). Likewise, in rats, a large percentage of the cells in the superficial, middle, and deep layers of the secondary motor, anterior cingulate (ACA), prelimbic (PrL), and infralimbic (IL) areas express 5-HT2A mRNA (Amargos-Bosch et al., 2004; Santana et al., 2004). Almost all prefrontal pyramidal neurons express the 5-HT2A receptor, with the receptor localized primarily to the proximal apical dendrites (Willins et al., 1997; Jakab and Goldman-Rakic, 1998; Cornea-Hébert et al., 1999; Xu and Pandey, 2000). In addition to pyramidal neurons, 5-HT2A receptors are also expressed by subsets of parvalbumin- and calbindin-positive interneurons (Willins et al., 1997; Jakab and Goldman-Rakic, 2000; Santana et al., 2004; De Almeida and Mengod, 2007; Puig et al., 2010; Weber and Adrade, 2010). Approximately 20–25% of the glutamic acid decarboxylase-positive cells in PFC express 5-HT2A mRNA (De Almeida and Mengod, 2007). From their morphology these interneurons appear to be basket cells and chandelier cells (Jakab and Goldman-Rakic, 2000). GABAergic interneurons expressing parvalbumin and calbindin are sources of perisomatic inhibition that synchronize the oscillatory firing of large ensembles of pyramidal neurons (Bartos et al., 2007; Freund and Katona, 2007; Buzsaki and Wang, 2012). Therefore, 5-HT2A receptors are likely to have direct and indirect effects on the activity of pyramidal cells (see Fig. 8).
Figure 8.
Distribution of 5-HT2A receptors in neurons in layer V of the prefrontal cortex. 5-HT2A receptors are expressed by glutamatergic pyramidal neurons and GABAergic basket cells and chandelier cells. Hallucinogens increase the frequency of spontaneous EPSCs and IPSCs in layer V pyramidal neurons by enhancing recurrent glutamatergic and GABAergic network activity.
Electrophysiological studies have shown that 5-HT2A activation (with DOB or DOI) produces several effects on the membrane properties of layer V pyramidal neurons: there is a moderate depolarization, spike-frequency accommodation is reduced, and the afterhyperpolarization (AHP) that normally follows a burst of spikes is replaced by a slow depolarizing afterpotential (sADP)(Araneda and Andrade, 1991; Tanaka and North, 1993; Arvanov et al., 1999). The effect on AHP is mediated by activation of PLCβ signaling, which inhibits one of the currents (IsAHP) underlying the AHP (Villalobos et al., 2005, 2011); the induction of sADP is probably a consequence of activating a Ca2+-dependent nonselective cation channel (ICAN). Both of these effects increase the excitability of pyramidal neurons (Zhang and Arsenault, 2005). DOI also produces a 5-HT2A-dependent inhibition of voltage-dependent Na+-currents and L-type Ca2+-currents in PFC pyramidal cells via the PLCβ–IP3–protein kinase C and PLCβ–IP3–calcineurin signaling cascades, respectively, effects that would likely influence dendritic integration (Carr et al., 2002; Day et al., 2002).
Hallucinogens have profound effects on excitatory and inhibitory transmission in medial PFC (mPFC) in vitro. Recordings from brain slices have shown that DOI and other 5-HT2A agonists produce a marked enhancement of the frequency and amplitude of spontaneous excitatory postsynaptic potentials/currents (EPSPs/EPSCs) in most layer V pyramidal neurons in mPFC (Aghajanian and Marek, 1997; Zhou and Hablitz, 1999; Klodzinska et al., 2002). These effects are mediated by an increase in Glu release and subsequent activation of postsynaptic AMPA receptors (Aghajanian and Marek, 1997; Zhang and Marek, 2008). Because these studies failed to locate any glutamatergic mPFC neurons that were driven to fire action potentials by 5-HT2A activation, it was initially thought that the increase in Glu release was caused by local activation of the terminals of glutamatergic thalamocortical afferents (Lambe and Aghajanian, 2001; Marek et al., 2001). However, although the ability of 5-HT to induce EPSCs is lost after deletion of the 5-HT2A gene (htr2A−/− mice), the effect can be rescued by selective restoration of 5-HT2A receptors to pyramidal neurons in the forebrain (Weisstaub et al., 2006). The htr2A−/−mice used by Weisstaub et al. were generated by inserting a floxed Neo-stop cassette between the promoter and the coding region, so the gene could be rescued by crossing the mice with Emx1-Cre+/− mice (which selectively expresses Cre recombinase in the forebrain). The fact that the EPSCs were rescued in htr2A−/− × Emx1-Cre+/− mice shows that projections from thalamus and other subcortical structures are not being directly excited by 5-HT2A receptors. More recent work has identified a subpopulation of pyramidal neurons in mPFC deep layer V that are depolarized and excited by DOI (Béïque et al., 2007), indicating hallucinogens induce spontaneous EPSCs by increasing recurrent glutamatergic network activity. 5-HT2A receptor activation also increases the frequency of spontaneous IPSCs in pyramidal neurons (Zhou and Hablitz, 1999), an effect that is mediated by activation of neighboring GABAergic interneurons (Weber and Andrade, 2010; Zhang et al., 2010). Therefore, it appears hallucinogens recruit glutamatergic and GABAergic neurons, which produces a marked enhancement of excitatory and inhibitory recurrent network activity in mPFC (Lambe and Aghajanian, 2006, 2007). This conclusion is supported by microdialysis data showing that hallucinogens increase extracellular levels of Glu (Scruggs et al. , 2003; Muschamp et al. , 2004; Mocci et al., 2014) and GABA (Abi-Saab et al., 1999) in mPFC.
There is evidence that enhancement of glutamatergic activity in mPFC plays an important role in mediating the effects of hallucinogens. Manipulations that suppress the facilitation of recurrent glutamatergic network activity, including the use of mGlu2/3 agonists, μ-opioid agonists, adenosine A1 agonists, and AMPA antagonists (Marek and Aghajanian, 1998; Marek et al. , 2000; Stutzmann et al., 2001; Klodzinska et al., 2002; Zhai et al., 2003; Benneyworth et al., 2007), block many of the neurochemical and behavioral effects of hallucinogens. These interactions have been demonstrated most extensively for the HTR (see Table 3), a 5-HT2A-mediated behavior that can be provoked by infusion of DOI directly into the mPFC (Willins and Meltzer, 1997; Ciccocioppo et al., 1999). Likewise, the discriminative stimulus effects of LSD are attenuated by the mGlu2/3 agonist LY379268 and augmented by the mGlu2/3 antagonist LY341495 (Winter et al., 2004), and there is evidence that the LSD stimulus cue is mediated by activation of 5-HT2A receptors in the ACA (Gresch et al., 2007). Another example is the ability of DOI to increase impulsive responding in rats, which is attenuated by administration of LY379268 systemically or directly into mPFC (Wischhof et al., 2011; Wischhof and Koch, 2012). In addition to 5-HT2A antagonists, mGlu2/3 agonists and AMPA antagonists also block the ability of DOI to increase cortical expression of BDNF and the immediate-early genes c-fos, erg-2, and Arc (Scruggs et al., 2000; Gewirtz et al., 2002; Zhai et al., 2003; Pei et al., 2004; Gonzalez-Maseo et al., 2008; Wischhof and Koch, 2012). Evidence has emerged that mGlu2 and 5-HT2A receptors can form heteromeric complexes in cortex (Gonzalez-Maeso et al., 2008; Moreno et al., 2012), and these complexes may mediate the crosstalk that occurs between these receptors. It is important to note, however, that it has not been conclusively demonstrated that the heterodimers are responsible for the interactions between 5-HT2A and mGlu2 (Delille et al., 2012, 2013), and it is possible the crosstalk is purely functional and occurs at the circuit level. mGlu2 receptors function predominantly as presynaptic autoreceptors (Schoepp, 2001), so mGlu2 activation could potentially suppress 5-HT2A-induced spontaneous EPSCs by reducing Glu release from axon terminals.
Table 3.
Receptor agonists and antagonists that modulate the electrophysiological effects of 5-HT2A activation in the mPFC also alter the head twitch response in rats and mice.
CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; DAMGO, [D-Ala2, N-MePhe4, Gly-ol5]-enkephalin; DNQX, 6,7-dinitroquinoxaline-2,3-dione; NBQX, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide; sEPSCs, spontaneous excitatory postsynaptic currents.
The specified ligand reduces the response (↓), has no effect (Ø), or enhances the response (↑).
5.2.2 Effects on PFC network activity in vivo
Recent studies have examined the effects of hallucinogens on PFC activity in vivo. Extracellular recordings from anesthetized rats have shown that DOI (0.05–0.8 mg/kg, i.v.) and 5-MeO-DMT (0.1 mg/kg, i.v., in combination with the monoamine oxidase inhibitor clorgyline) produce a net excitatory effect on pyramidal neurons in the PrL, IL, and ACA regions of mPFC (Puig et al., 2003; Wang et al., 2009; Riga et al., 2014). Individual pyramidal neurons are either excited (38–53%), inhibited (27–35%), or show no response. It appears that these effects are mediated by recruitment of glutamatergic and GABAergic neurons because the excitatory response to DOI is blocked by LY379268 and the inhibitory response is blocked by the GABAA antagonist picrotoxinin (Puig et al., 2003; Wang et al., 2009). These effects are also blocked by 5-HT2A antagonists. In contrast to those findings, another group has reported that higher doses of DOI (3–5 mg/kg, i.p.) tend to inhibit the firing of pyramidal cells in ACA and the ventral, dorsolateral, and lateral orbitofrontal cortices of behaving rats (Wood et al., 2012).
Despite the discrepant findings outlined above, hallucinogens produce strikingly similar effects on cortical network activity in anesthetized and freely-moving rats. Under anesthesia or during slow-wave sleep, cortical networks display slow (0.5–1 Hz) and delta (1–4 Hz) oscillations (Steriade et al., 1993a,b; Steriade, 1997) that reflect alternations between periods of silence (DOWN states) and periods of depolarization with repetitive spiking (UP states). This contrasts with the active waking state, which is characterized by fast rhythms in the gamma range (30–80 Hz) that play a putative role in a multitude of perceptual and cognitive functions (Tallon-Baudry et al., 1998; Fries et al., 2001; Herrmann et al., 2004; Schroeder and Lakatos, 2009; Roux and Uhlhaas, 2014). Recordings of local field potentials (LFPs) from the PFC have shown DOI reduces low-frequency oscillations in anesthetized rats (Celada et al., 2008), and dampens gamma oscillations in freely-moving rats (Wood et al., 2012). DOI also desynchronizes the firing of pyramidal neurons so that their activity is no longer coupled to LFPs (Celada et al., 2008; Wood et al., 2012). 5-MeO-DMT has similar effects on low-frequency PFC network activity in anesthetized rats (Riga et al., 2014). Taken together, these findings demonstrate that hallucinogens disrupt the oscillatory activity of cortical networks and reduce the likelihood that individual pyramidal neurons will fire in synchrony.
Similar to the LFP data in rats, magnetoencephalographic (MEG) recordings in humans have shown that psilocybin (2 mg, i.v.) produces broadband reductions in cortical oscillatory power (Muthukumaraswamy et al., 2013). Dynamic causal modeling of the MEG data indicates that psilocybin reduces cortical synchrony by increasing the excitability of deep-layer pyramidal neurons. Likewise, electroencephalographic studies have reported that ayahuasca (containing the equivalent of 0.85 mg/kg DMT) reduces cortical oscillatory power across multiple frequency bands (Riba et al., 2002, 2004). Since cortical oscillations play a fundamental role in a diverse set of mental processes and are required for the coordination of neural processing (Gray et al., 1989; Singer, 1999; Buzsaki and Draguhn, 2004; Sejnowski and Paulsen, 2006; Fries, 2009; Klimesch, 2012), it is tempting to speculate that the reduction of neuronal synchrony by hallucinogens could be responsible for mediating many of their effects on perception and cognition. Along these lines, there is evidence that schizophrenia patients show deficits of gamma oscillations and synchrony (Spencer et al., 2003; Cho et al., 2006; Minzenberg et al., 2010; Uhlhaas and Singer, 2010) and reductions in slow-wave sleep (Yang and Winkelman, 2006), and it has been hypothesized that these abnormalities play an important role in the pathophysiology of psychosis.
As was noted earlier, neuroimaging studies have demonstrated that hallucinogens alter activity in the frontal cortex. Studies using PET and single-photon emission computed tomography (SPECT) have consistently found that hallucinogens produce frontal hyperactivity. Administration of mescaline sulfate (500 mg, p.o.) produces a hyperfrontal metabolic pattern, especially in the right hemisphere (Hermle et al., 1992). PET studies with [18F]fluorodeoxyglucose ([18F]FDG) have shown that psilocybin (0.20–0.36 mg/kg, p.o.) also produces a hyperfrontal pattern, with robust metabolic increases in frontolateral and frontomedial cortical regions and ACA (Vollenweider et al., 1997; Gouzoulis-Mayfrank et al., 1999a). Similar patterns of brain activation were found in subjects administered ayahuasca as part of a SPECT study (Riba et al., 2006). By contrast, it has been argued, based on functional MRI (fMRI) data, that psilocybin reduces resting-state brain activity (Carhart-Harris et al., 2012a). In that study, volunteers received 2 mg i.v. psilocybin and regional blood flow and venous oxygenation were assessed using arterial spin labeling and blood-oxygen level-dependent (BOLD) fMRI scans. Psilocybin reduced blood flow and BOLD signal in ACA and mPFC, and there was evidence of reduced coupling between mPFC and the posterior cingulate cortex. Based on those results, Carhart-Harris, Nutt, and colleagues concluded that psilocybin reduces activity and connectivity in core nodes of the default-mode network, brain regions that are active during the resting state and potentially involved in introspective processes (for more information, see: Raichle et al., 2001; Mason et al., 2007; Buckner et al., 2008). It remains to be determined why psilocybin produces such discrepant effects in PET and fMRI studies. One potential explanation is that the hemodynamic responses measured by fMRI are actually better correlated with cortical oscillatory activity than with neuronal firing (Mathiesen et al., 1998; Logothetis et al., 2001; Niessing et al., 2005; Nir et al., 2007; Viswanathan and Freeman, 2007). Indeed, recent work by Artigas and co-workers confirms the decoupling of BOLD measures and spiking in rats (Riga et al., 2014). According to their report, 5-MeO-DMT (0.1 mg/kg, i.v.) increased the firing rate of mPFC pyramidal cells by 215%, but significantly reduced the BOLD signal (measured by fMRI) and the power of low-frequency oscillations (measured by LFP recordings). Therefore, PET and fMRI studies may be assessing different types of neurophysiological responses to psilocybin, with PET measuring effects on neuronal firing (reflected by changes in metabolic activity and [18F]FDG uptake) and fMRI measuring effects on cortical oscillatory activity. Alternatively, it is possible that the hemodynamic changes induced by psilocybin are unrelated to its hallucinogenic effects. Psilocybin and its O-dephosphorylated metabolite psilocin activate the 5-HT1A receptor in vivo (Halberstadt et al., 2011; Halberstadt and Geyer, 2011), and 5-HT1A agonists are known to alter hemodynamic measures in cingulate cortex and other brain regions (Mueggler et al., 2011).
5.2.3. Interactions of the PFC with other structures: cortical and subcortical sites
Since most of the projections from PFC to cortical and subcortical regions originate from pyramidal neurons in deep layers V–VI, hallucinogens could potentially profoundly alter how the PFC regulates activity in downstream regions. Indeed, there is some evidence that hallucinogens excite efferent projections from the PFC. For example, DOI activates serotonergic neurons in the dorsal raphe nucleus indirectly by exciting the projection from mPFC (Martín-Ruiz et al., 2001; Puig et al., 2003). Similar findings have been reported for the projection to the ventral tegmental area (Puig et al., 2003). Additionally, a recent study by Mocci et al. (2014) assessed whether 5-HT2A receptors modulate the activity of the projection from mPFC to nucleus accumbens (NAc). Retrodialysis of DOI into the mPFC increased the extracellular level of Glu in the NAc by 174%, indicating that DOI activates NAc-projecting mPFC neurons. According to another report, 5-HT2A receptors excite cortico-cortical projections originating from mPFC (Avesar and Gulledge, 2012). In that study, microiontophretic application of 5-HT excited pyramidal neurons with commissural/callosal projections. Because 5-HT had no effect in the presence of the selective 5-HT2A antagonist MDL 11,939, the most reasonable interpretation is that the excitation is mediated by 5-HT2A receptors, but this needs to be confirmed using a selective agonist.
The PFC exerts top-down executive control over processing in temporal and parietal cortices (Tomita et al., 1999; Simons and Spiers, 2003; Merchant et al., 2011; Crowe et al., 2013). As shown by FDG-PET imaging, psilocybin increases absolute cerebral metabolic rates in the parietal and temporal cortices (Vollenweider, 1994; Volenweider et al., 1997). It is conceivable that hallucinogens could enhance the activity of neuronal ensembles in those regions by driving the firing of glutamatergic projections from the PFC. Moreover, 5-HT2A receptors are expressed at high to moderate densities in temporal and parietal cortical areas (Pazos et al., 1987; Lidow et al., 1989; Gross-Isseroff et al., 1990a,b; Hall et al., 2000), so the influence exerted by the PFC would act in concert with any local response induced by hallucinogens. Hallucinogenic drugs produce body image changes, derealization, and depersonalization (Guttmann and Maclay, 1936; Von Mering et al., 1957), effects that are specifically linked to altered activity in frontoparietal cortex and occipital cortex (Vollenweider and Geyer, 2001). This is not surprising because the posterior parietal cortex is part of the dorsal visual stream and generates multiple egocentric representations of space (Stein, 1992; Vallar et al., 1999; Schindler and Bartels, 2013). Likewise, hallucinogens enhance memory recall (Carhart-Harris et al., 2012b), sometimes producing extremely vivid recollections. Since the medial temporal lobe plays a crucial role in the storage and recall of autobiographical memories (Noulhiane et al., 2007), it has been proposed that hallucinogen effects on memory recall may be linked to activation of this region.
The amygdala, which is involved in generating fear responses and processing the emotional context of sensory input (LeDoux, 2000), is another subcortical structure potentially affected by changes in the activity of mPFC projections. An fMRI BOLD study in healthy volunteers revealed that psilocybin (0.16 mg/kg p.o.) reduces activation of the amygdala by negative and neutral pictures, and the BOLD signal change was inversely correlated with reports of increased positive mood (Kraehenmann et al., 2014). Likewise, an electrical neuroimaging study conducted by the same group found psilocybin impairs processing of facial expression valence in the amygdala and other limbic regions (Bernasconi et al., 2013). In healthy subjects, there is an inverse correlation between the density of mPFC 5-HT2A binding and the responsiveness of the amygdala to threatening stimuli (Fisher et al., 2009), suggesting processing in the amygdala is regulated by 5-HT2A receptors in mPFC. Hence, the ability of psilocybin to reduce emotional processing in the amygdala could potentially be a consequence of increased inhibitory top-down control from the PFC (Bernasconi et al., 2013).
The IL subregion of mPFC impairs fear conditioning by inhibiting central amygdaloid nucleus output neurons, which project to brainstem and hypothalamic sites responsible for expressing fear responses (Quirk et al., 2003). Although it was not initially clear how mPFC inhibits the amygdala because the projection is glutamatergic (Amaral and Insausti, 1992; Smith et al., 2000), the mechanism is now believed to involve excitation of GABAergic neurons in the intercalated nuclei of the amygdala (Berretta et al., 2005; Likhtik et al., 2008; Pinard et al., 2012). Psilocybin and TCB-2 have been shown to facilitate the extinction of fear conditioning in C57BL/6J mice (Catlow et al., 2013; Zhang et al., 2013), which could be a consequence of activating the projection from IL to the intercalated nuclei. However, it has not been ruled out that psilocybin and TCB-2 are acting directly in the amygdala; excitatory and inhibitory neurons in the amygdala express 5-HT2A receptors (McDonald and Mascagni, 2007; Bombardi, 2011), and DOI and other 5-HT2A agonists act locally to produce direct excitatory and indirect inhibitory effects in the amygdala (Rainnie, 1999; Stein et al., 2000; Sokal et al., 2005).
5.2.4. Interactions of the PFC with other structures: effects on cortico-striato-thalamo-cortical (CSTC) loops
It has been theorized that hallucinogen-induced altered states may arise in part through effects on cortico-striato-thalamo-cortical (CSTC) feedback loops (Vollenweider, 1994, 1998; Vollenweider and Geyer, 2001). CSTC loops are parallel, anatomically segregated circuits relaying information between the basal ganglia, thalamus, and cortex (Alexander et al., 1986, 1990). In each circuit, projections from multiple cortical regions converge in specific subregions of the striatum. The striatum, in turn, projects to the pallidum, which sends feedback to the cortex via the thalamus. In this regime, the thalamus serves as a filter that restricts or gates the flow of sensory and cognitive information to the cortex. There has been some debate about the exact number of CSTC loops (Middleton and Strick, 2001; Di Martino et al., 2008), but at least five have been putatively identified, each serving a different function. The limbic loop, for example, receives input from the temporal lobe, ACA, and medial orbitofrontal cortex, and links the ventral striatum (including NAc, lateral caudate, and ventromedial putamen), ventral pallidum (VP), and mediodorsal thalamus. Vollenweider and Geyer (2001) have proposed that psilocybin reduces thalamic filtering by activating 5-HT2A receptors in the limbic CSTC loop, resulting in excessive stimulation of frontal regions, hyperfrontality, and symptoms such as sensory overload and hallucinations.
Although involvement of CSTC loops in the effects of hallucinogens is admittedly speculative, it does receive some support from the fact that hallucinogens disrupt PPI in humans and in animal models (Sipes and Geyer, 1994; Padich et al., 1996; Vollenweider et al., 2007; Pálenícek et al., 2008; Halberstadt and Geyer, 2010; Quednow et al., 2012). Importantly, PPI is regulated by components of the limbic CSTC loop, including mPFC, NAc, and VP (Swerdlow et al., 2001). The VP appears to be responsible for the disruption of PPI by hallucinogens (Sipes and Geyer, 1997). DOI disrupts PPI when infused directly into the VP, but not when infused into the NAc. Likewise, infusion of M100907 into the VP prevents systemically administered DOI from disrupting PPI. It is important to note, however, that the PPI-disruptive effects of DOI are partially blocked when M100907 is infused into the dorsal striatum, so it is not entirely certain that the VP is the only site of action for DOI.
5.3. Visual cortex
Hallucinogens produce profound effects on visual perception. This includes visual distortions such as micropsia or macropsia, kinetopsia, pareidolias, hyperchromatopsia, dysmorphopsia, and polyopia-like trailing phenomena; elementary imagery composed of multicolored geometric patterns; and complex imagery with scenes, objects, and people (see Fig. 5). The visual imagery induced by hallucinogens is extremely vivid and can be observed with the eyes open or closed. When scientists began to experiment with mescaline at the end of the nineteenth century almost all of their work focused on the visual phenomenology (Prentiss and Morgan, 1885; Mitchell, 1896; Ellis, 1898, 1902; Knauer and Maloney, 1913; Klüver, 1926). Despite its highly subjective nature, the drug-induced imagery has been characterized in great detail (Klüver, 1928; Siegel and Jarvik, 1975). Heinrich Klüver (1928) was the first to recognize that all of the elementary geometric hallucinations induced by mescaline are elaborated variations of four basic forms, which he called form constants: (a) tunnels and funnels, (b) spirals, (c) lattices and checkerboards, and (d) cobwebs. The form constants are not unique to hallucinogens and can occur during a variety of hallucinatory states, including migraine aura (Sacks, 1995), epilepsy (Horowitz et al., 1967), sensory isolation (Heron et al., 1956), viewing flickering light (Becker and Elliott, 2006; Allefeld et al., 2011), and electrical cortical stimulation (Knoll et al., 1962; Horowitz et al., 1968).
Several theoretical explanations for geometric visual hallucinations have been proposed based on retinocortical mapping and the architecture of V1 (Ermentrout and Cowan, 1979; Tass, 1997; Bressloff et al., 2001, 2002; Baker and Cowan, 2009). According to these mathematical models, excitation of V1 neurons produces self-organizing patterns of activity that correspond to Klüver's form constants. The excitation of V1 is presumably driven by 5-HT2A receptors because ketanserin blocks the visual hallucinations induced by psilocybin (Vollenweider et al., 1998; Kometer et al., 2013). There are moderate to high densities of 5-HT2A receptors in V1 (Pazos et al., 1987; Lidow et al., 1989; Hall et al., 2000; López-Giménez et al., 2001), with the highest level occurring in geniculorecipient sublayer IVcβ (Lidow et al., 1989). Similar to other cortical regions, almost all glutamatergic pyramidal neurons and very few GABAergic interneurons in V1 express 5-HT2A mRNA (Watakabe et al., 2009; Moreau et al., 2010). A recent electrophysiology study conducted in anesthetized macaque monkeys revealed that DOI produces a combination of excitatory and inhibitory effects in V1, exciting neurons with low firing rates and inhibiting neurons with high firing rates (Watakabe et al., 2009). Since neuronal firing in V1 is driven by visual stimuli, one possible interpretation is that DOI reduces the response to visual input while enhancing spontaneous internally-driven activity. It is fairly well-established that hallucinogens reduce retinocortical transmission (Moore et al., 1976; Moore and Domino, 1978; Foote, 1982). Indeed, psilocybin inhibits N170 visually-evoked potentials in human subjects via 5-HT2A (Kometer et al., 2011, 2013). Visual input stabilizes network activity in V1 by driving inhibitory interneurons (Ozeki et al., 2009). Therefore, a reduction of visual input, coupled with an increase in the excitability of pyramidal neurons, could destabilize network activity in area V1, generating patterns of neuronal firing that are perceived as geometric form constants.
In contrast to the elementary visual hallucinations, which are linked to area V1, complex visual hallucinations probably arise from 5-HT2A activation in higher level visual areas. There is evidence that excitation of Brodmann area (BA) 19 and BA 37 can produce complex visual hallucinations (Ffytche et al., 1998; Holroyd and Wooten, 2006; Kazui et al., 2009). Among patients with Parkinson's disease, approximately 22% experience complex visual hallucinations (Fenelon et al., 2000). Their visual hallucinations are linked to elevated levels of 5-HT2A receptor binding in ventral visual pathway (Ballanger et al., 2010; Huot et al. 2010), and can be ameliorated by blocking 5-HT2A receptors. For example, a PET imaging study with [18F]setoperone found that visual hallucinations in Parkinson's patients are associated with unusually high levels of 5-HT2A binding in the inferooccipital gyrus (BA 19), fusiform gyrus (BA 20 and BA 37), and inferotemporal gyrus (BA 20) (Ballanger et al., 2010). According to another study conducted post-mortem, Parkinson's patients with visual hallucinations show elevated levels of 5-HT2A binding in the inferolateral temporal cortex (BA 21) (Huot et al., 2010). Two clinical trials have shown that the selective 5-HT2A inverse agonist pimavanserin reduces the severity of hallucinations in Parkinson's disease (Meltzer et al., 2010; Cummings et al., 2014). The atypical antipsychotics clozapine and risperidone, which block the 5-HT2A receptor, are also effective against the visual hallucinations (Kahn et al., 1991; Meco et al., 1997; Pollak et al., 2004).
6. Summary
Despite the complexity of hallucinogen effects, we are beginning to understand how these substances work in the brain. The 5-HT2A receptor was first identified about thirty years ago as a possible site of action of hallucinogens. It is now clear that most of the effects of hallucinogens are mediated by 5-HT2A activation. Although the vast majority of this evidence was derived from studies in animals, the resumption of human studies with hallucinogens has provided additional support.
Recent clinical trials have provided a highly detailed characterization of hallucinogen effects. However, most of this work has focused on one hallucinogen (psilocybin). By comparison, very little is known about the effects of other agents. This is especially true for ergoline and phenylalkylamine hallucinogens. One of the most characteristic properties of hallucinogens is how unpredictable their effects can be. The exact nature of the experience is highly variable and depends on the mood and expectations of the subject (the “set”) as well as the environment in which the drug is ingested (the “setting”) (Bercel et al., 1955; Osmond, 1957; Faillace and Szára, 1968; Freedman, 1968). Depending on the circumstances, the effects of hallucinogens may be perceived as being highly pleasurable or highly aversive (e.g., Aldous Huxley's description of mescaline as “heaven and hell”). Although hallucinogens act in a relatively unspecific manner (Grof, 1980), and hence a broad range of experiences are possible, previous clinical studies have confirmed that there is also a great deal of similarity between the effects of different hallucinogens. In other words, although it is impossible to predict exactly what type of experience will be produced by, for example, LSD or psilocybin, it appears that for the most part any experience produced by LSD can also occur with psilocybin. Thus, volunteers could not identify any clear differences between the subjective effects of those two compounds when administered by blind dosing (Hollister and Hartman, 1962; Wolbach et al., 1962a,b; Abramson and Rolo, 1967). However, those studies need to be repeated using modern psychometric assessment methods. Additionally, it is not clear to what extent those findings extend to other hallucinogens, or even to higher doses of LSD and psilocybin. One potentially unique aspect of the LSD experience is that it reportedly occurs in two distinct temporal phases (DeShon et al., 1952; Salvatore and Hyde, 1956; Freedman, 1968, 1984), but this needs to be confirmed by future investigations.
It appears that 5-HT2A activation is a common characteristic of serotonergic hallucinogens and is responsible for mediating their shared effects, but this does not eliminate the possibility that other receptors may play an ancillary role. There are pharmacological differences between the phenalkylamine, tryptamine, and ergoline classes, as well as between specific compounds within each class, and these differences could potentially influence the subjective effects (Halberstadt and Geyer, 2011). The receptors activated by hallucinogens may be analogous to individual musical notes that can be played in combination to generate chords associated with unique subjective impressions (Glennon, 1994), with 5-HT2A receptor activation being akin to the root note. Extramural investigations have attempted to categorize the existence of subtle subjective differences between the effects of different hallucinogens (e.g., Shulgin and Shulgin, 1991, 1997). However, it is not clear to what extent the apparent differences between individual compounds are influenced by expectation and by other factors. There are also dose-and route-dependent variations in the effects of hallucinogens, which can alter both the intensity and the qualitative nature of the response. Furthermore, even individual subjects may experience markedly different responses to the same drug on different occasions (Naranjo, 1973). The possibility exists that for hallucinogen effects, there may be just as much intra-drug variability as there is inter-drug variability. Only detailed, well-controlled clinical trials comparing multiple compounds over a wide range of doses will answer these questions. Nevertheless, it seems to be fairly well established that there are marked qualitative differences between the effects produced by serotonergic hallucinogens and by members of other drug classes. Although it was recently reported that subjects administered high doses of the NMDA antagonist dextromethorphan under double-blind conditions identified it as a classical hallucinogen when they were asked to classify it pharmacologically (Reissig et al., 2012), there are major confounds associated with this study. First, Reissig et al. (2012) acknowledged that most if not all of the study participants were expecting to receive psilocybin, and this may have influenced their response to dextromethorphan. Second, the subjects did not receive a hallucinogen as an active control, so the study did not actually quantify the similarity between the effects of dextromethorphan and hallucinogens. It is also surprising that none of the subjects classified dextromethorphan as a dissociative anesthetic, since dextromethorphan is abused for its dissociative-like effects (Boyer, 2004) and produces phencyclidine- and ketamine-like discriminative stimulus effects in rats (Nicholson et al., 1999; Narita et al., 2001).
Over the last decade, there has been renewed interest into the potential therapeutic uses for hallucinogens. Psilocybin can induce highly meaningful spiritual experiences (Griffiths et al., 2006), and some subjects have reported experiencing positive changes in mood and behavior that persist for many months (Griffiths et al., 2008). It may be possible to exploit these effects therapeutically. Recent clinical trials have investigated whether psilocybin has efficacy against anxiety in terminal cancer patients (Grob et al., 2011), and LSD has been tested as a potential adjunct for psychotherapy (Gasser et al., 2014). Several follow-up studies are currently in progress. It is anticipated that these and other studies will yield important insights into the psychopharmacology of hallucinogens, as well as showing whether there are potential medical uses for these drugs.
>Serotonergic hallucinogens are classified as phenylalkylamines and indoleamines.
>The two classes of hallucinogens produce similar subjective effects in humans and show cross-tolerance
>Hallucinogen effects are primarily mediated by the serotonin 5-HT2A receptor.
>Many effects of hallucinogens are mediated in the prefrontal cortex.
ACKNOWLEDGEMENTS
Supported by grants from NIMH (K01 MH100644), NIDA (R01 DA002925), and the Brain and Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abi-Saab WM, Bubser M, Roth RH, Deutch AY. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20:92–96. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Abramson HA, Jarvik ME, Gorin MH, Hirsch MW. Lysergic acid diethylamide (LSD-25): XVII. Tolerance development and its relationship to a theory of psychosis. J Psychol. 1956;41:81–105. [Google Scholar]
- Abramson HA, Rolo A. Comparison of LSD with Methysergide and Psilocybin on Test Subjects. In: Abramson H, editor. The Use of LSD in Psychotherapy and Alcoholism. Bobbs-Merrill; Indianapolis, IN: 1967. pp. 53–73. [Google Scholar]
- Abramson HA, Rolo A, Sklarofsky B, Stache J. Production of cross-tolerance to psychosis-producing doses of lysergic acid diethylamide and psilocybin. J Psychol. 1960;49:151–154. [Google Scholar]
- Abramson HA, Sklarofsky B, Baron MO, Fremont-Smith N. ysergic acid diethylamide (LSD-25) antagonists. II. Development of tolerance in man to LSD-25 by prior administration of MLD-41 (1-methyl-d-lysergic acid diethylamide). AMA Arch Neurol Psychiatry. 1958;79:201–207. [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD's effects on patterns of exploration in rats. Behav Neurosci. 1985b;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985c;23:461–468. doi: 10.1016/0091-3057(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Mescaline and LSD facilitate the activation of locs coeruleus neurons by peripheral stimuli. Brain Res. 1980;186:492–498. doi: 10.1016/0006-8993(80)90997-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Albertson DN, Grubbs LE. Subjective effects of Salvia divinorum: LSD- or marijuana-like? J Psychoactive Drugs. 2009;41:213–217. doi: 10.1080/02791072.2009.10400531. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits limking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allefeld C, Pütz P, Kastner K, Wackermann J. Flicker-light induced visual phenomena: frequency dependence and specificity of whole percepts and percept features. Conscious Cogn. 2011;20:1344–1362. doi: 10.1016/j.concog.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-asparate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Angrist B, Rotrosen J, Gershon S. Assessment of tolerance to the hallucinogenic effects of DOM. Psychopharmacologia. 1974;36:203–207. doi: 10.1007/BF00421802. [DOI] [PubMed] [Google Scholar]
- Appel JB, Callahan PM. Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. Eur J Pharmacol. 1989;159:41–46. doi: 10.1016/0014-2999(89)90041-1. [DOI] [PubMed] [Google Scholar]
- Appel JB, Cunningham KA. The use of drug discrimination procedures to characterize hallucinogenic drug actions. Psychopharmacol Bull. 1986;22:959–967. [PubMed] [Google Scholar]
- Appel JB, Freedman DX. Tolerance and cross-tolerance among psychotomimetic drugs. Psychopharmacologia. 1968;13:267–274. doi: 10.1007/BF00401404. [DOI] [PubMed] [Google Scholar]
- Appel JB, West WB, Buggy J. LSD, 5-HT (serotonin), and the evolution of a behavioral assay. Neurosci Biobehav Rev. 2004;27:693–701. doi: 10.1016/j.neubiorev.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Arnt J. Characterization of the discriminative stimulus properties induced by 5-HT1 and 5-HT2 agonists in rats. Pharmacol Toxicol. 1989;64:165–172. doi: 10.1111/j.1600-0773.1989.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Aronson H, Silverstein AB, Klee GD. Influence of lysergic acid diethylamide (LSD-25) on subjective time. AMA Arch Gen Psychiatry. 1959;1:469–472. doi: 10.1001/archpsyc.1959.03590050037003. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–2934. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Bak VK, Zhang ZQ, Rickard JF, Glennon JC, Fone KC, Bradshaw CM, Szabadi E. Effects of 5-HT2A receptor stimulation on the discrimination of durations by rats. Behav Pharmacol. 2006;17:51–59. doi: 10.1097/01.fbp.0000189810.69425.89. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Harkins K, Wang RY. Effects of (+/−)-DOI on medial prefrontal cortical cells: a microiontophoretic study. Brain Res. 1989;498:393–396. doi: 10.1016/0006-8993(89)91124-4. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits. 2012;6:12. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TI, Cowan JD. Spontaneous pattern formation and pinning in the primary visual cortex. J Physiol (Paris) 2009;103:52–68. doi: 10.1016/j.jphysparis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416–21. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- Balestrieri A. Studies on cross tolerance with LSD-25, UML-491 and JB-336. Psychopharmacologia. 1960;1:257–259. doi: 10.1007/BF00402747. [DOI] [PubMed] [Google Scholar]
- Balestrieri A, Fontanari D. Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148. Arch Gen Psychiatry. 1959;1:279–282. doi: 10.1001/archpsyc.1959.03590030063008. [DOI] [PubMed] [Google Scholar]
- Barclay Z, Dickson L, Robertson DN, Johnson MS, Holland PJ, Rosie R, Sun L, Fleetwood-Walker S, Lutz EM, Mitchell R. 5-HT2A receptor signalling through phospholipase D1 associated with its C-terminal tail. Biochem J. 2011;436:651–660. doi: 10.1042/BJ20101844. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bartoszyk GD, van Amsterdam C, Böttcher H, Seyfried CA. EMD 281014, a new selective serotonin 5-HT2A receptor antagonist. Eur J Pharmacol. 2003;473:229–230. doi: 10.1016/s0014-2999(03)01992-7. [DOI] [PubMed] [Google Scholar]
- Becker C, Elliott MA. Flicker-induced color and form: interdependencies and relation to stimulation frequency and phase. Conscious Cogn. 2006;15:175–196. doi: 10.1016/j.concog.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. Wet-dog shake behavior in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneš H, Deissler A, Rodenbeck A, Engfer A, Kohnen R. Lisuride treatment of Restless Legs Syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm. 2006;113:87–92. doi: 10.1007/s00702-005-0386-1. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Snyder SH. Stereospecific binding of D-lysergic acid diethylamide (LSD) to brain membranes: relationship to serotonin receptors. Brain Res. 1975;94:523–544. doi: 10.1016/0006-8993(75)90234-6. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Snyder SH. Serotonin and lysergic acid diethylamide binding in rat brain membranes: relationship to postsynaptic serotonin receptors. Mol Pharmacol. 1976;12:373–389. [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology (Berl) 2005;179:854–862. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Bercel NA, Travis LE, Olinger LB, Dreikurs E. Model psychoses induced by LSD-25 in normals: I. Psychophysiological investigations, with special reference to the mechanism of the paranoid reaction. Arch Gen Psychiatry. 1956;76:588–611. doi: 10.1001/archneurpsyc.1956.02330240026003. [DOI] [PubMed] [Google Scholar]
- Bernasconi F, Schmidt A, Pokorny T, Kometer M, Seifritz E, Vollenweider FX. Spatiotemporal brain dynamics of emotional face processing modulations induced by the serotonin 1A/2A receptor agonist psilocybin. Cereb Cortex. 2013 doi: 10.1093/cercor/bht178. in press. doi: 10.1093/cercor/bht178. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman WK, Goldstone S, Lhamon WT. Effects of lysergic acid diethylamide (LSD) on the time sense of normals; a preliminary report. AMA Arch Neurol Psychiatry. 1957;78:321–324. doi: 10.1001/archneurpsyc.1957.02330390103013. [DOI] [PubMed] [Google Scholar]
- Bobker DH. A slow excitatory postsynaptic potential mediated by 5-HT2 receptors in nucleus prepositus hypoglossi. J Neurosci. 1994;14:2428–2434. doi: 10.1523/JNEUROSCI.14-04-02428.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body S, Cheung TH, Bezzina G, Asgari K, Fone KC, Glennon JC, Bradshaw CM, Szabadi E. Effects of d-amphetamine and DOI (2,5-dimethoxy-4-iodoamphetamine) on timing behavior: interaction between D1 and 5-HT2A receptors. Psychopharmacology. 2006;189:331–343. doi: 10.1007/s00213-006-0575-0. [DOI] [PubMed] [Google Scholar]
- Body S, Chiang TJ, Mobini S, Ho MY, Bradshaw CM, Szabadi E. Effect of 8-OH-DPAT on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacol Biochem Behav. 2002;71:787–793. doi: 10.1016/s0091-3057(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda F, Bradshaw CM, Szabadi E. Effects of a 5-HT2 receptor agonist, DOI (2,5-dimethoxy-4-iodoamphetamine), and antagonist, ketanserin, on the performance of rats on a free-operant timing schedule. Behav Pharmacol. 2003;14:599–607. doi: 10.1097/00008877-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Bombardi C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with γ-aminobutyric acid. Brain Res. 2011;1370:112–128. doi: 10.1016/j.brainres.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Boyer EW. Dextromethophan abuse. Pediatric Emerg Care. 2004;20:858–863. doi: 10.1097/01.pec.0000148039.14588.d0. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PP, Middlemiss DN, Mylecharane EJ, Richardson BP, Saxena PR. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986;25:563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC. Geometric visual hallucinations, Euclidean symmetry and the functional architecture of striate cortex. Philos Trans R Soc Lond B Biol Sci. 2001;356:299–330. doi: 10.1098/rstb.2000.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC. What geometric visual hallucinations tell us about the visual cortex. Neural Comput. 2002;14:473–491. doi: 10.1162/089976602317250861. [DOI] [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Direct and indirect 5-HT receptor agonists produce gender-specific effects on locomotor and vertical activities in C57 BL/6J mice. Pharmacol Biochem Behav. 2009;94:194–203. doi: 10.1016/j.pbb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz NS, Zhou DF, Freedman DX. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1989;42:2439–2445. doi: 10.1016/0024-3205(88)90342-6. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Zhou DF, Freedman DX, Potter WZ. Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain. Neuropsychopharmacology. 1990;3:137–148. [PubMed] [Google Scholar]
- Buckner R, Andrews Hanna J, Schacter D. The brain's default network. Annals NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter S, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotnon-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Ann Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno E, Invernizzi RW. Strain-dependent serotonin neuron feedback control: role of serotonin 2C receptors. J Neurochem. 2010;114:1701–1710. doi: 10.1111/j.1471-4159.2010.06880.x. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Appel JB. Differentiation between the stimulus effects of (+)-lysergic acid diethylamide and lisuride using a three-choice, drug discrimination procedure. Psychopharmacology (Berl. 1990;100:13–18. doi: 10.1007/BF02245782. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology. 2013;70:112–121. doi: 10.1016/j.neuropharm.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl. 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012a;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Williams TM, Erritzoe D, Abbasi N, Bargiotas T, Hobden P, Sharp DJ, Evans J, Feilding A, Wise RG, Nutt DJ. Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry. 2012b;200:238–44. doi: 10.1192/bjp.bp.111.103309. [DOI] [PubMed] [Google Scholar]
- Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, Rung JP, Carlsson A. I. In vivo evidence for partial agonist effects of (-)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors. J Neural Transm. 2011;118:1511–1522. doi: 10.1007/s00702-011-0704-8. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology. 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. 2013;228:481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Díaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. A 5-hydroxytryptamine2 agonist augments γ-aminobutyric acid and excititory amino acid imputs into noradrenergic locus coeruleus neurons. Neuroscience. 1993;54:409–420. doi: 10.1016/0306-4522(93)90262-e. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Colombo G, Gessa G, Massi M. Autoradiographic analysis of 5-HT2A binding sites in the brain of Sardinian alcohol-preferring and nonpreferring rats. Eur J Pharmacol. 1999;373:13–19. doi: 10.1016/s0014-2999(99)00239-3. [DOI] [PubMed] [Google Scholar]
- Colasanti B, Khazan N. Electroencephalographic studies on the development of tolerance and cross tolerance to mescaline in the rat. Psychopharmacologia. 1975;43:201–205. doi: 10.1007/BF00429251. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers GJE, Janssen PAJ. A drug discrimination analysis of lysergic acid diethylamide (LSD): in vivo agonist and antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, a LSD-antagonist. J Pharmacol Exp Ther. 1982;221:206–214. [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warnet BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm. 2009;116:1591–1599. doi: 10.1007/s00702-009-0308-8. [DOI] [PubMed] [Google Scholar]
- Crowe DA, Goodwin SJ, Blackman RK, Sakellaridi S, Sponheim SR, MacDonald AW, 3rd, Chafee MV. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013;16:1484–1491. doi: 10.1038/nn.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Appel JB. Neuropharmacological reassessment of the discriminative stimulus properties of d-lysergic acid diethylamide (LSD). Psychopharmacology (Berl) 1987;91:67–73. doi: 10.1007/BF00690929. [DOI] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signaling at serotonin 5-HT2A, 5-HT2B and 5-HT2CVSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol Behav. 1996;60:1495–1500. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT2 receptors in prefrontal pyramidal neurons inhibits CaV 1.2 L-type Ca2+ currents via a PLCβ/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- De Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Delille HK, Mezler M, Marek GJ. The two faces of the pharmacological interaction of mGlu2 and 5-HT2A - relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology. 2013;70:296–305. doi: 10.1016/j.neuropharm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- DeShon HJ, Rinkel M, Solomon HC. Mental changes experimentally produced by LSD (d-lysergic acid diethylamide tartrate). Psychiatric Quart. 1952;26:33–53. doi: 10.1007/BF01568448. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dittrich A. Zusammenstellung eines Fragebogens (APZ) zur Erfassung abnormer psychischer Zustände [Construction of a questionnaire (APZ) for assessing abnormal mental states]. Zeitschrift für Klinische Psychologie und Psychotherapie. 1975;23:12–20. [Google Scholar]
- Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31:80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- Done J, Sharp T. Evidence that 5-HT2 receptor activation decreases noradrenaline release in rat hippocampus in vivo. Br J Pharmacol. 1992;107:240–245. doi: 10.1111/j.1476-5381.1992.tb14493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos RG, Valle M, Bouso JC, Nomdedéu JF, Rodríguez-Espinosa J, McIlhenny EH, Barker SA, Barbanoj MJ, Riba J. Autonomic, neuroendocrine, and immunological effects of Ayahuasca. A comparative study with D-amphetamine. J Clin Psychopharmacol. 2011;31:717–726. doi: 10.1097/JCP.0b013e31823607f6. [DOI] [PubMed] [Google Scholar]
- Dougherty JP, Aloyo VJ. Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology (Berl) 2011;215:581–593. doi: 10.1007/s00213-011-2207-6. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology (Berl) 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Rabin RA, Winter JC. Nefazodone in the rat: mimicry and antagonism of [-]-DOM-induced stimulus control. Pharmacol Biochem Behav. 2003;75:405–410. doi: 10.1016/s0091-3057(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol Biochem Behav. 2011;99:52–58. doi: 10.1016/j.pbb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Ellis H. Mescal: a new artificial paradise. Annual Rep Smithsonian Inst. 1898:537–548. [Google Scholar]
- Ellis H. Mescal: a study of a divine plant. Popular Sci Monthly. 1902;61:52–71. [Google Scholar]
- Ennis M, Aston-Jones G. A potent excitatory input to the nucleus locus coeruleus from the ventrolateral medulla. Neurosci Lett. 1986;71:299–305. doi: 10.1016/0304-3940(86)90637-3. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J Neurosci. 1989a;9:2973–2981. doi: 10.1523/JNEUROSCI.09-08-02973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Potent inhibitory input to locus coeruleus from the nucleus prepositus hypoglossi. Brain Res Bull. 1989b;22:793–803. doi: 10.1016/0361-9230(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Cowan JD. A mathematical theory of visual hallucination patterns. Biol Cybern. 1979;34:137–150. doi: 10.1007/BF00336965. [DOI] [PubMed] [Google Scholar]
- Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36. Mol Imaging Biol. 2013;15:376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- Faillace LA, Szára S. Hallucinogenic drugs: influence of mental set and setting. Dis Nerv System. 1968;29:124–126. [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335:728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: An fMRI study of visual hallucinations. Nat Neurosci. 1998;1:738–742. doi: 10.1038/3738. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 1995a;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: reassessment of LSD false positives. Psychopharmacology (Berl) 1995b;121:357–363. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote WE. Electrophysiological studies of d-lysergic acid diethylamide in the visual system. Neurosci Biobehav Rev. 1982;6:503–507. doi: 10.1016/0149-7634(82)90032-x. [DOI] [PubMed] [Google Scholar]
- Fox MA, French HT, Laporte JL, Blackler AR, Murphy DL. The serotonin 5-HT2A receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berlin) 2009;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- Freedman DX. On the use and abuse of LSD. Arch Gen Psychiatry. 1968;18:330–347. doi: 10.1001/archpsyc.1968.01740030074008. [DOI] [PubMed] [Google Scholar]
- Freedman DX. LSD: the bridge from human to animal. In: Jacobs BL, editor. Hallucinogens: neurochemical, behavioral, and clinical perspectives. Raven Press; New York: 1984. pp. 203–226. [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptor. Br J Pharmacol. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, Brenneisen R. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014 doi: 10.1097/NMD.0000000000000113. in press. doi: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav. 2002;73:317–326. doi: 10.1016/s0091-3057(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Gordon J, Adams LM. Behavioral effects of xylamine-induced depletions of brain norepinephrine: interaction with LSD. Pharmacol Biochem Behav. 1985;23:619–625. doi: 10.1016/0091-3057(85)90427-7. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–399. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Kaplan J, Stillman R, Wyatt RJ. The psychedelic model of schizophrenia: The case of N,N-dimethyltryptamine. Am J Psychiatry. 1976;133:203–208. doi: 10.1176/ajp.133.2.203. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of the serotonergic agent 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Life Sci. 1986;39:825–830. doi: 10.1016/0024-3205(86)90461-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res Monogr. 1991;116:25–44. [PubMed] [Google Scholar]
- Glennon RA. Clasical hallucinogens: an introductory overview. NIDA Res Monogr. 1994;146:4–32. [PubMed] [Google Scholar]
- Glennon RA. Arylalkylamine drugs of abuse: an overview of drug discrimination studies. Pharmacol Biochem Behav. 1999;64:251–256. doi: 10.1016/s0091-3057(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Hauck AE. Mechanistic studies on DOM as a discriminative stimulus. Pharmacol Biochem Behav. 1985;23:937–941. doi: 10.1016/0091-3057(85)90096-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, McKenney JD. Site-selective 5-HT agonists as discriminative stimuli. Pharmacologist. 1985;27:194. [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. The use of the drug discrimination paradigm for studying hallucinogenic agents. A review. In: Colpaert FC, Slangen JL, editors. Drug Discrimination: Applications in CNS Pharmacology. Elsevier Biomedical Press; Amsterdam: 1982. pp. 69–96. [Google Scholar]
- Glennon RA, Rosecrans JA, Young R, Gaines J. Hallucinogens as a discriminative stimuli: generalization of DOM to a 5-methoxy-N, N-dimethyltryptamine stimulus. Life Sci. 1979;24:993–997. doi: 10.1016/0024-3205(79)90317-5. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA, Sluster RM. N,N-Di-n-propylserotonin: binding at serotonin binding sites and a comparision with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem. 1988;31:867–870. doi: 10.1021/jm00399a031. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Seggel MR, Lyon RA. N-methyl derivatives of the 5-HT2 agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane. J Med Chem. 1987;30:930–932. doi: 10.1021/jm00388a032. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Young R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol Bull. 1986;22:953–958. [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983;91:189–196. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gorea E, Adrien J. Serotonergic regulation of noradrenergic coerulean neurons: electrophysiological evidence for the involvement of 5-HT2 receptors. Eur J Pharmacol. 1988;154:285–291. doi: 10.1016/0014-2999(88)90203-8. [DOI] [PubMed] [Google Scholar]
- Gorea E, Davenne D, Lanfumey L, Chastanei M, Adrien J. Regulation of noradrenergic coerulean neuronal firing mediated by 5-HT2 receptor: involvement of the prepositus hypoglossil nucleus. Neuropharmacology. 1991;30:1309–1318. doi: 10.1016/0028-3908(91)90028-a. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Sun JC. Functional role of the endocannabinoid system and AMPA/kainate receptors in 5-HT2A receptor-mediated wet dog shakes. Eur J Pharmacol. 2005;516:28–33. doi: 10.1016/j.ejphar.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Büll U, Sass H. Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology. 1999a;20:565–581. doi: 10.1016/S0893-133X(98)00089-X. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999b;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Smith RL, Barrett RJ, Sanders-Bush E. Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin-2A receptor signaling in rat cortex. Neuropsychopharmacology. 2005;30:1693–1702. doi: 10.1038/sj.npp.1300711. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218:649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22:621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar J. Psychedelic Drugs Reconsidered. New York: Basic Books. 91979. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Grof S. Realms of the human unconscious: Observations from LSD research. In: Walsh RN, Vaughan F, editors. Beyond Ego: Transpersonal Dimensions in Psychology. Jeremy P. Tarcher, Inc.; Los Angeles, CA: 1980. pp. 87–99. [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of [3H]ketanserin binding in the human brain postmortem: effect of suicide. Brain Res. 1990a;507:208–215. doi: 10.1016/0006-8993(90)90274-f. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res. 1990b;519:223–227. doi: 10.1016/0006-8993(90)90081-l. [DOI] [PubMed] [Google Scholar]
- Guttmann E, Maclay WS. Mescalin and depersonalization: therapeutic experiments. J Neurol Psychopathol. 1936;16:193–212. doi: 10.1136/jnnp.s1-16.63.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT2C receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hill HE, Belleville RE. Development of the Addiction Research Center Inventory (ARCI): selection of items that are sensitive to the effects of various drugs . Psychopharmacologia. 1963;4:155–166. doi: 10.1007/BF02584088. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT2A receptor. Psychopharmacology. 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology. 2013;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology. 2014;77:200–207. doi: 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Powell SB, Geyer MA. Role of the 5-HT2A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology. 2013;70:218–227. doi: 10.1016/j.neuropharm.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT2A receptors in the human brain using [3H]M100907 and [11C]M100907. Synapse. 2000;38:421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hampson CL, Body S, Den Boon FS, Cheung THC, Bezzina G, Langley RW, Fone KCF, Bradshaw CM, Szabadi E. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and D-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behv Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, Waberski TD, Gouzoulis-Mayfrank E. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermle L, Fünfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Heron W, Doane BK, Scott TH. Visual disturbances after prolonged perceptual isolation. Can J Psychol. 1956;10:13–18. doi: 10.1037/h0083650. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cognitive Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Herrmann WM, Horowski R, Dannehl K, Kramer U, Lurati K. Clinical effectiveness of lisuride hydrogen maleate: a double-blind trial versus methysergide. Headache. 1977;17:54–60. doi: 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- Hirschhorn ID, Winter JC. Mescaline and lysergic acid diethylamide (LSD) as discriminative stimuli. Psychopharmacologia. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Hoch P, Cattell JP, Pennes HH. Effects of mescaline and lysergic acid diethylamide (d. LSD-25). Am J Psychiatry. 1952;108:579–584. doi: 10.1176/ajp.108.8.579. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Clinical, biochemical and psychologic effects of psilocybin. Arch Int Pharmacodyn. 1961;130:42–52. [PubMed] [Google Scholar]
- Hollister LE. Chemical Psychoses: LAD and Related Drugs. Charles C. Thomas; Springfiled, IL: 1968. [Google Scholar]
- Hollister LE, Hartman AM. Mescaline, lysergic acid diethylamide and psilocybin: comparison of clinical syndromes, effects on color perception and biochemical measures. Comprehens Psychiat. 1962;3:235–241. doi: 10.1016/s0010-440x(62)80024-8. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Macnicol MF, Gillespie HK. An hallucinogenic amphetamine analog (DOM) in man. Psychopharmacologia. 1969;14:62–73. doi: 10.1007/BF00401535. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Prusmack JJ, Paulsen JA, Rosenquist N. Comparison of three psychotropic drugs (psilocybin, JB-329, and IT-290) in volunteer subjects. J Nerv Ment Dis. 1960;131:428–434. doi: 10.1097/00005053-196011000-00007. [DOI] [PubMed] [Google Scholar]
- Holohean AM, White FJ, Appel JB. Dopaminergic and serotonergic mediation of the discriminable effects of ergot alkaloids. Eur J Pharmacol. 1982;81:595–602. doi: 10.1016/0014-2999(82)90349-1. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Wooten GF. Preliminary fMRI evidence of visual system dysfunction in Parkinson's disease patients with visual hallucinations. J Neuropsychiatry Clin Neurosci. 2006;18:402–404. doi: 10.1176/jnp.2006.18.3.402. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ, Adams JE, Rutkin BB. Visual imagery on brain stimulation. Arch Gen Psychiat. 1968;19:469–486. doi: 10.1001/archpsyc.1968.01740100085013. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hughes RN. Chlordiazepoxide modified exploration in rats. Psychopharmacologia. 1972;24:462–469. doi: 10.1007/BF00423436. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, Pires D, Brotchie JM, Fox SH. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord. 2010;25:1399–1408. doi: 10.1002/mds.23083. [DOI] [PubMed] [Google Scholar]
- Isbell H. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia. 1959;1:29–38. doi: 10.1007/BF00408109. [DOI] [PubMed] [Google Scholar]
- Isbell H, Belleville RE, Fraser HF, Wikler A, Logan CR. Studies on lysergic acid diethylamide (LSD-25). I. Effects in former morphine addicts and development of tolerance during chronic intoxication. Arch Neurol Psychiatry. 1956;76:468–478. [PubMed] [Google Scholar]
- Isbell H, Jasinski DR. A comparison of LSD-25 with (–)-Δ9-trans-tetrahydrocannabinol (THC) and attempted cross tolerance between LSD and THC. Psychopharmacologia. 1969;14:115–123. doi: 10.1007/BF00403684. [DOI] [PubMed] [Google Scholar]
- Isbell H, Wolbach AB, Wikler A, Miner EJ. Cross tolerance between LSD and psilocybin. Psychopharmacologia. 1961;2:147–159. doi: 10.1007/BF00407974. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997;768:327–332. doi: 10.1016/s0006-8993(97)00703-8. [DOI] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Juncosa JI, Jr, Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE. Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci. 2013;4:96–109. doi: 10.1021/cn3000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn N, Freeman A, Juncos JL, Manning D, Watts RL. Clozapine is beneficial for psychosis in Parkinson's disease. Neurology. 1991;41:1699–1700. doi: 10.1212/wnl.41.10.1699. [DOI] [PubMed] [Google Scholar]
- Kazui H, Ishii R, Yoshida T, Ikezawa K, Takaya M, Tokunaga H, Tanaka T, Takeda M. Neuroimaging studies in patients with Charles Bonnet Syndrome. Psychogeriatrics. 2009;9:77–84. doi: 10.1111/j.1479-8301.2009.00288.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna JC, Sedman G. The subjective experience of time during lysergic acid diethylamide (LSD-25) intoxication. Psychopharmacologia. 1964;5:280–288. doi: 10.1007/BF02341260. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Glen A, Grewal S, Forbes I, Gadre A, Blackburn TP. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br J Pharmacol. 1994;111:797–802. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled ascess to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Klüver H. Mescal visions and edetic vision. Am J Psychol. 1926;37:502–515. [Google Scholar]
- Klüver H. Mescal—The Divine Plant and Its Psychological Effects. Kegan Paul; London: 1928. [Google Scholar]
- Knauer A, Maloney WJMA. A preliminary note on the psychic action of mescalin, with special reference to the mechanism of visual hallucinations. J Nerv Ment Dis. 1913;40:425–436. [Google Scholar]
- Knoll M, Krugler J. Subjective light pattern spectroscopy in the encephalographic frequency range. Nature. 1959;184:1823–1824. doi: 10.1038/1841823a0. [DOI] [PubMed] [Google Scholar]
- Knoll M, Kugler J, Eichmeier J, Höffer O. Note on the spectroscopy of subjective light patterns. Journal of Analytical Psychology. 1962;7:55–69. [Google Scholar]
- Kometer M, Cahn BR, Andel D, Carter OL, Vollenweider FX. The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol Psychiatry. 2011;69:399–406. doi: 10.1016/j.biopsych.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jäncke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33:10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, Vollenweider FX. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.010. in press. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. The role of 5-HT1A receptors in the locomotor suppressant effects of LSD: WAY-100635 studies of 8-OH-DPAT, DOI and LSD in rats. Behav. Pharmacol. 1996;7:551–559. [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003a;86:980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharrmacol Exp Ther. 2003b;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology. 2006;31:1682–1689. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007;145:900–910. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovits BZ, Visotsky HM, Ostfeld AM. LSD and JB318: a comparison of two hallucinogens. An exploratory study. Arch Gen Psychiatry. 1960;2:390–407. doi: 10.1001/archpsyc.1960.03590100030004. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lehmann-Masten VD, Geyer MA. Spatial and temporal patterning distinguishes the locomotor activating effects of dizocilpine and phencyclidine in rats. Neuropharmacology. 1991;30:629–636. doi: 10.1016/0028-3908(91)90083-n. [DOI] [PubMed] [Google Scholar]
- Leysen JE. Use of 5-HT receptor agonists and antagonists for the characterization of their respective receptor sites. In: Boulton AA, Baker GB, Butterworth R, editors. Drugs as tools in neurotransmitter research. Neuromethods. Vol. 12. Springer; Berlin: 1989. pp. 299–350. [Google Scholar]
- Leysen JE, Janssen PF, Niemegeers CJ. Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol. 1989;163:145–149. doi: 10.1016/0014-2999(89)90409-3. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther. 2008;324:827–833. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Quantitative autoradiographic mapping of serotonin 5-HT1 and 5-HT2 receptors and uptake sites in the neocortex of the rhesus monkey. J Comp Neurol. 1989;280:27–42. doi: 10.1002/cne.902800104. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall Michelle R., Griffiths Roland R., Mintzer Miriam Z. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Experimental and Clinical Psychopharmacology. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Vilaró MT, Palacios JM, Mengod G. Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol. 2001;429:571–589. doi: 10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lyon RA, Titeler M, Seggel MR, Glennon RA. Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens. Eur J Pharmacol. 1988;145:291–297. doi: 10.1016/0014-2999(88)90432-3. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Meester M. Illicit use of LSD or psilocybin, but not MDMA or nonpsychedelic drugs, is associated with mystical experiences in a dose-dependent manner. J Psychoactive Drugs. 2012;44:410–417. doi: 10.1080/02791072.2012.736842. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl. 2013;226:381–392. doi: 10.1007/s00213-012-2912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ. Behavioral evidence for mu-opioid and 5-HT2A receptor interactions. Eur J Pharmacol. 2003;474:77–83. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Activation of adenosine1 (A1) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56:1082–1087. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by mu-opiate receptor activation. Neuroscience. 1998;86:485–497. doi: 10.1016/s0306-4522(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or α1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 2009;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE. Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology. 2002;164:93–107. doi: 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Further evidence that the delayed temporal dopaminergic effects of LSD are mediated by a mechanism different than the first temporal phase of action. Pharmacol Biochem Behav. 2007;87:453–461. doi: 10.1016/j.pbb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl. 2005;180:427–435. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Martín-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews WD, Smith CD. Pharmacological profile of a model for central serotonin receptor activation. Life Sci. 1980;26:1397–1403. doi: 10.1016/0024-3205(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity in spikes in rat cerebellar cortex. J Physiol (Lond) 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JA, Sharif NA, Chen HH, Liao JC, Kelly CR, Glennon RA, Young R, Li JX, Rice KC, France CP. Pharmacological properties and discriminative stimulus effects of a novel and selective 5-HT2 receptor agonist AL-38022A [(S)-2-(8,9-dihydro-7H-pyrano[2,3-g]indazol-1-yl)-1-methylethylamine]. Pharmacol Biochem Behav. 2009;91:307–314. doi: 10.1016/j.pbb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146:306–320. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers G, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and β-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J. Med. Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- Meco G, Alessandri A, Giustini P, Bonifati V. Risperidone in levodopa-induced psychosis in advanced Parkinson's disease: an open-label, long-term study. Mov Disord. 1997;12:610–612. doi: 10.1002/mds.870120423. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of parkinson's disease psychosis. Neuropsychopharmacology. 2010;35:881–892. doi: 10.1038/npp.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Martínez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Merchant H, Crowe DA, Robertson MS, Fortes AF, Georgopoulos AP. Top-down spatial categorization signal from prefrontal to posterior parietal cortex in the primate. Front Syst Neurosci. 2011;5:69. doi: 10.3389/fnsys.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. A revised neuroanatomy of frontal-subcortical circuits. In: Lichter DG, Cumming JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. Guilford Press: New York, NY: 2001. pp. 44–58. [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding properties of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SW. Remarks on the effects of Anhelonium lewinii (the mescal button). Br Med J. 1896;2:1625–1629. doi: 10.1136/bmj.2.1875.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Effects of 5HT-1A agonists on locomotor and investigatory behaviors in rats differ from those of hallucinogens. Psychopharmacology (Berlin) 1989;98:321–329. doi: 10.1007/BF00451682. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Disassociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology (Berlin) 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Ann Rev Neurosci. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Mocci G, Jiménez-Sánchez L, Adell A, Cortés R, Artigas F. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology. 2014;79:49–58. doi: 10.1016/j.neuropharm.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuéllar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, López-Giménez JF, Meana JJ, Benson DL, González-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocci G, Jiménez-Sánchez L, Adell A, Cortés R, Artigas F. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology. 2014;79:49–58. doi: 10.1016/j.neuropharm.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Moore RH, Domino EF. Modification of the effects of LSD-25, d-amphetamine and tryptamine on electrically evoked responses in the visual system by methiothepin and octoclothepin. Arch Int Pharmacodyn Ther. 1978;236:66–73. [PubMed] [Google Scholar]
- Moore RH, Hatada K, Domino EF. Effects of N,N-dimethyltryptamine on electrically evoked responses in the cat visual system and modification by neuroleptic agents. Neuropharmacology. 1976;15:535–539. doi: 10.1016/0028-3908(76)90104-0. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Mueggler T, Razoux F, Russig H, Buehler A, Franklin TB, Baltes C, Mansuy IM, Rudin M. Mapping of CBV changes in 5-HT1A terminal fields by functional MRI in the mouse brain. Eur Neuropsychopharmacol. 2011;21:344–353. doi: 10.1016/j.euroneuro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD, Feilding A, Friston KJ, Nutt DJ. Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci. 2013;18:15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex . Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Naranjo C. The Healing Journey. Pantheon Books; New York: 1973. [Google Scholar]
- Narita M, Yoshizawa K, Nomura M, Aoki K, Suzuki T. Role of the NMDA receptor subunit in the expression of the discriminative stimulus effect induced by ketamine. Eur J Pharmacol. 2001;423:41–46. doi: 10.1016/s0014-2999(01)01089-5. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Structure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr Transp Signal. 2012;1:559–579. [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2, 4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N, N-diethyllysergamide (LSD). J Med Chem. 2002;45:4344–4349. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Hayes BA, Balster RL. Evaluation of the reinforcing properties and phencyclidine-like discriminative stimulus effects of dextromethorphan and dextrorphan in rats and rhesus monkeys. Psychopharmacology (Berl) 1999;146:49–59. doi: 10.1007/s002130051087. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Noulhiane M, Piolino P, Hasboun D, Clemenceau S, Baulac M, Samson S. Autobiographical memory after temporal lobe resection: neuropsychological and MRI volumetric findings. Brain. 2007;130(Pt 12):3184–3199. doi: 10.1093/brain/awm258. [DOI] [PubMed] [Google Scholar]
- Osmond H. A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci. 1957;66:418–434. doi: 10.1111/j.1749-6632.1957.tb40738.x. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Finn IM, Schaffer ES, Miller KD, Ferster D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron. 2009;62:578–592. doi: 10.1016/j.neuron.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahnke WN. Psychedelic drugs and mystical experience. Int Psychiatry Clin. 1969;5:149–162. [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Hoyer D, Cortes R. α1-Adrenoceptors in the mammalian brain: similar pharmacology but different distribution in rodents and primates. Brain Res. 1987;419:65–75. doi: 10.1016/0006-8993(87)90569-5. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Páleníček T, Fujáková M, Brunovský M, Horáček J, Gorman I, Balíková M, Rambousek L, Syslová K, Kačer P, Zach P, Bubeníková-Valešová V, Tylš F, Kubešová A, Puskarčíková J, Höschl C. Behavioral, neurochemical and pharmaco-EEG profiles of the psychedelic drug 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in rats. Psychopharmacology (Berl) 2013;225:75–93. doi: 10.1007/s00213-012-2797-7. [DOI] [PubMed] [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE. A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J. Med. Chem. 1998;41:5148–5149. doi: 10.1021/jm9803525. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Braden MR, Gundy E, Nichols DE. Differential phospholipase C activation by phenylalkylamine serotonin 5-HT2A receptor agonists. J Neurochem. 2005;95:1575–1584. doi: 10.1111/j.1471-4159.2005.03477.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain — IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–339. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979;16:687–699. [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- Piercey MF, Hoffmann WE, Smith MW, Hyslop DK. Inhibition of dopamine neuron firing by pramipexole, a dopamine D3 receptor-preferring agonist: comparison to other dopamine receptor agonists. Eur J Pharmacol. 1996;312:35–44. doi: 10.1016/0014-2999(96)00454-2. [DOI] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2012;205:112–124. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak P, Tison F, Rascol O, Destée A, Péré JJ, Senard JM, Durif F, Bourdeix I. Clozapine in drug induced psychosis in Parkinson's disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75:689–695. doi: 10.1136/jnnp.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentiss DW, Morgan FP. Anhalonium lewinii (mescal buttons): a study of the drug, with especial reference to its physiological action upon man, with report of experiments. Therap Gaz. 1895;19:577–585. [Google Scholar]
- Puig MV, Celada P, Díaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Geyer MA, Halberstadt AL. Serotonin and schizophrenia. In: Müller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; London: 2010. pp. 585–620. [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Raffaelli E, Jr, Martins OJ, dos Santos P, Dãgua Filho A. Lisuride in cluster headache. Headache. 1983;23:117–121. doi: 10.1111/j.1526-4610.1983.hed2303117.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotoninergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res. 1986;385:395–400. doi: 10.1016/0006-8993(86)91090-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Failure to block responses of locus coeruleus neurons to somatosensory stimuli by destruction of two major afferent nuclei. Synapse. 1989;4:162–164. doi: 10.1002/syn.890040210. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl) 2012;223:1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology (Berlin) 2005;182:197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jane F, Saletu B, Barbanoi MJ. Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology. 2004;50:89–101. doi: 10.1159/000077946. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Morte A, Urbano G, Jané F, Saletu B, Barbanoj MJ. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol. 2002;53:613–628. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Romero S, Grasa E, Mena E, Carrió I, Barbanoj MJ. Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology (Berl) 2006;186:93–98. doi: 10.1007/s00213-006-0358-7. [DOI] [PubMed] [Google Scholar]
- Riga M, Soria G, Tudela R, Artigas F. The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats. Reversal by antipsychotic drugs. Int J Neuropsychopharmacology. 2014 doi: 10.1017/S1461145714000261. in press. DOI: http://dx.doi.org/10.1017/S1461145714000261. [DOI] [PubMed]
- Rojas-Corrales MO, Gibert-Rahola J, Mico JA. Role of atypical opiates in OCD. Experimental approach through the study of 5-HT(2A/C) receptor-mediated behavior. Psychopharmacology (Berl) 2007;190:221–231. doi: 10.1007/s00213-006-0619-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DE, Isbell H, Miner EJ, Logan CR. The effect of N,N-dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide. Psychopharmacologia. 1964;5:217–227. doi: 10.1007/BF00413244. [DOI] [PubMed] [Google Scholar]
- Rosenberg DE, Wolbach AB, Miner EJ, Isbell H. Observations on direct and cross tolerance with LSD and D-amphetamine in man. Psychopharmacologia. 1963;5:1–15. doi: 10.1007/BF00405570. [DOI] [PubMed] [Google Scholar]
- Roth BL, Palvimaki EP, Berry S, Khan N, Sachs N, Uluer A, Choudhary MS. 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha–gamma versus theta–gamma codes dor distinct WM information. Trends Cognitive Sci. 2014;18:16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Sacks O. Migraine. Picador; London: 1995. [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology (Berl) 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Salvatore S, Hyde RW. Progression of effects of lysergic acid diethylamide (LSD). AMA Arch Neurol Psychiat. 1956;2:50–59. doi: 10.1001/archneurpsyc.1956.02330250052007. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Schindler A, Bartels A. Parietal cortex codes for egocentric space beyond the field of view. Curr Biol. 2013;23:177–182. doi: 10.1016/j.cub.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Schlemmer RF, Jr, Davis JM. A primate model for the study of hallucinogens. Pharmacol Biochem Behav. 1986;24:381–92. doi: 10.1016/0091-3057(86)90368-0. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. 1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT)2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the 'atypical' antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. The gamma oscillation: master and slave? Brain Topogr. 2009;22:24–26. doi: 10.1007/s10548-009-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J. Neurosci. 2006;26:1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serko A. Im Mescalinrausch. Jahrbücher fur Psychiatrie Neurologie. 1913;31:355–366. [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur J Pharmacol. 1984;102:23–29. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. PIHKAL: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Shulgin AT, Shulgin A. Transform Press; Berkeley, CA: 1997. TIHKAL: The Continuation. [Google Scholar]
- Siegel RK, Jarvik ME. Drug-induced hallucinations in animals and man. In: Siegel RK, West LS, editors. Hallucinations: Behavior, Experience, and Theory. John Wiley and Sons; New York: 1975. pp. 81–161. [Google Scholar]
- Silva MT, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–171. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. (1999) [DOI] [PubMed] [Google Scholar]
- Singewald N, Philippu A. Release of transmitters in the locus coeruleus. Prog. Neurobiol. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Singleton C, Marsden CA. Circadian variation in the head twitch response produced by 5-methoxy-N1,N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology (Berl) 1981;74:173–176. doi: 10.1007/BF00432688. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. J Pharmacol Exp Ther. 1995;275:1050–1057. [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 1999;144:248–254. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/-)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Sokal DM, Giarola AS, Large CH. Effects of GABAB, 5-HT1A, and 5-HT2 receptor stimulation on activation and inhibition of the rat lateral amygdala following medial geniculate nucleus stimulation in vivo. Brain Res. 2005;1031:141–150. doi: 10.1016/j.brainres.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT1A receptor-mediated inhibition and 5-HT2 as well as 5-HT3 receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stein JF. The representation of egocentric space in the posterior parietal cortex. Behav Brain Sci. 1992;15:691–700. doi: 10.1017/S0140525X00072605. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993a;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39:784–795. doi: 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Stubbs DA. Temporal differentiation and a free-operant psychophysical procedure. J Exp Anal Behav. 1980;33:167–185. doi: 10.1901/jeab.1980.33-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the Altered States of Consciousness rating scale (OAV). PLoS ONE. 2010;5:e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Marek GJ, Aghajanian GK. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience. 2001;105:55–69. doi: 10.1016/s0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tass P. Oscillatory cortical activity during visual hallucinations. J Biol Phys. 1997;23:21–66. doi: 10.1023/A:1004990707739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresa M, Silva A, Carlini EA, Claussen U, Korte F. Lack of cross-tolerance in rats among (-)Δ9-trans-tetrahydrocannabinol(Δ9-THC), cannabis extract, mescaline and lysergic acid diethylamide (LSD-25). Psychopharmacologia. 1968;13:332–340. doi: 10.1007/BF00414344. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon LA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropyl amine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex to executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vallar G, Lobel E, Galati G, Berthoz A, Pizzamiglio L, Le Bihan D. A fronto-parietal system for computing the egocentric spatial frame of reference in humans. Exp Brain Res. 1999;124:281–286. doi: 10.1007/s002210050624. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology (Berl) 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Verde G, Chiodini PG, Liuzzi A, Cozzi R, Favales F, Botalla L, Spelta B, Dalla Bonzana D, Rainer E, Horowski R. Effectiveness of the dopamine agonist lisuride in the treatment of acromegaly and pathological hyperprolactinemic states. J Endocrinol Invest. 1980;3:405–414. doi: 10.1007/BF03349379. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behaviour in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci. 2005;22:1120–1126. doi: 10.1111/j.1460-9568.2005.04307.x. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Foehring RC, Lee JC, Andrade R. Essential role for phosphatidylinositol 4,5-bisphosphate in the expression, regulation, and gating of the slow afterhyperpolarization current in the cerebral cortex. J Neurosci. 2011;31:18303–18312. doi: 10.1523/JNEUROSCI.3203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX. Evidence for a cortical-subcortical imbalance of sensory information processing during altered states of consciousness using positron emission tomography and [18F]fluorodeoxyglucose. In: Pletscher A, Ladewig D, editors. 50 Years of LSD: Current Status and Perspectives of Hallucinogens. Parthenon; Pearl River, NY: 1994. pp. 67–86. [Google Scholar]
- Vollenweider FX. Advances and pathophysiological models of hallucinogenic drug actions in humans: a preamble to schizophrenia research. Pharmacopsychiat. 1998;31(Suppl):92–103. doi: 10.1055/s-2007-979353. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Von Mering O, Morimoto K, Hyde RW, Rinkel M. Experimentally induced depersonalization. In: Hoch PH, Zubin J, editors. Experimental Psychopathology. Grune and Stratton; New York: 1957. pp. 66–77. [Google Scholar]
- Wallach MB, Hine B, Gershon S. Cross tolerance or tachyphylaxis among various psychotomimetic agents on cats. Eur J Pharmacol. 1974;29:89–92. doi: 10.1016/0014-2999(74)90174-5. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang QJ, Liu J, Ali U, Wu ZH, Chen L, Gui ZH, Wang Y, Hui YP. In vivo effects of activation and blockade of 5-HT2A/2C receptors in the firing activity of pyramidal neurons of medial prefrontal cortex in a rodent model of Parkinson's disease. Exp Neurol. 2009;219:239–248. doi: 10.1016/j.expneurol.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Watakabe A, Komatsu Y, Sadakane O, Shimegi S, Takahata T, Higo N, Tochitani S, Hashikawa T, Naito T, Osaki H, Sakamoto H, Okamoto M, Ishikawa A, Hara S, Akasaki T, Sato H, Yamamori T. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb Cortex. 2009;19:1915–1928. doi: 10.1093/cercor/bhn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci. 2010;4(pii):36. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI). Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB. Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. Science. 1982;216:535–537. doi: 10.1126/science.7071600. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC. Tolerance to a behavioral effect of lysergic acid diethylamide and cross-tolerance to mescaline in the rat: absence of a metabolic component. J Pharmacol Exp Ther. 1971;178:625–630. [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol. Biochem. Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol Biochem Behav. 1988;30:617–624. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–480. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol. 2011;22:805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M. Pre-treatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology (Berl) 2012;219:387–400. doi: 10.1007/s00213-011-2441-y. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, Hell D, Flohr H, Vollenweider FX. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Isbell H, Miner EJ. Cross tolerance between mescaline and LSD-25. With a comparison of the mescaline and LSD reactions. Psychopharmacologia. 1962a;3:1–14. doi: 10.1007/BF00413101. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Miner EJ, Isbell H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia. 1962b;3:219–223. doi: 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin2A (5HT2A) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nabeshima T, Ishikawa K, Yoshida S, Kameyama T. Phencyclidine-induced head-weaving and head-twitch through interaction with 5-HT1 and 5-HT2 receptors in reserpinized rats. Neuropharmacology. 1987;26:1489–1497. doi: 10.1016/0028-3908(87)90168-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res. 2006;82:251–260. doi: 10.1016/j.schres.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA, Rosecrans JA. The hallucinogen DOM as a discriminative stimulus. Commun Psychopharmacol. 1981;4:501–504. [PubMed] [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology. 2003;28:45–52. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Arsenault D. Gain modulation by serotonin in pyramidal neurones of the rat prefrontal cortex. J Physiol. 2005;566(Pt 2):379–394. doi: 10.1113/jphysiol.2005.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Marek GJ. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog. Neuropsychopharmacol. Biol Psychiatry. 2008;32:62–71. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ásgeirsdóttir HN, Cohen SJ, Munchow AH, Barrera MP, Stackman RW., Jr Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology. 2013;64:403–413. doi: 10.1016/j.neuropharm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Wang S, Liu J, Ali U, Gui ZH, Wu ZH, Hui YP, Wang Y, Chen L. Unilateral lesion of the nigrostriatal pathway decreases the response of interneurons in medial prefrontal cortex to 5-HT 2A/2C receptor stimulation in the rat. Brain Res. 2010;1312:127–137. doi: 10.1016/j.brainres.2009.11.052. [DOI] [PubMed] [Google Scholar]