Abstract
Shock, sepsis, and multiorgan failure are associated with inflammation, morbidity, and high mortality. The underlying pathophysiological mechanism is unknown, but evidence suggests that pancreatic enzymes in the intestinal lumen autodigest the intestine and generate systemic inflammation. Blocking these enzymes in the intestine reduces inflammation and multiorgan dysfunction. We investigated whether enzymatic blockade also reduces mortality after shock. Three rat shock models were used here: hemorrhagic shock, peritonitis shock induced by placement of cecal material into the peritoneum, and endotoxin shock. One hour after initiation of hemorrhagic, peritonitis, or endotoxin shock, animals were administered one of three different pancreatic enzyme inhibitors—6-amidino-2-naphtyl p-guanidinobenzoate di-methanesulfate, tranexamic acid, or aprotinin—into the lumen of the small intestine. In all forms of shock, blockade of digestive proteases with protease inhibitor attenuated entry of digestive enzymes into the wall of the intestine and subsequent autodigestion and morphological damage to the intestine, lung, and heart. Animals treated with protease inhibitors also survived in larger numbers than untreated controls over a period of 12 weeks. Surviving animals recovered completely and returned to normal weight within 14 days after shock. The results suggest that the active and concentrated digestive enzymes in the lumen of the intestine play a central role in shock and multi-organ failure, which can be treated with protease inhibitors that are currently available for use in the clinic.
INTRODUCTION
Sepsis, shock, and multiorgan dysfunction are associated with strong markers for inflammation and severe cell dysfunction, and are the number one cause of mortality in intensive care units with hundreds of thousands of deaths every year in the United States alone (1–4). The mechanism is unknown, and no treatments exist other than alleviation of symptoms, such as “early goal-directed therapy,” to match oxygen delivery to its demand (5, 6). Antithrombotic therapy was the only treatment available (7), but despite protective effects in preclinical studies, it was withdrawn in 2011 owing to lack of effectiveness in septic patients.
The intestine has long been recognized to play a central role in shock. Initial evidence suggests that inflammation in several forms of shock may involve the digestive system (8). Digestive enzymes, synthesized in the pancreas and transported to the lumen of the intestine, may generate inflammatory and even cytotoxic mediators if they pass across the mucosal barrier from the lumen into the wall of the intestine (9–14). Breakdown of the mucosal barrier allows unrestricted access of digestive enzymes into the intestinal wall (15), which in turn leads to multiorgan failure (16, 17). Among these pancreatic enzymes, the serine proteases are quite effective in generating inflammation in acute shock (18).
Suppression of pancreatic protease activities in the lumen of the intestine substantially reduces systemic levels of inflammatory markers and attenuates microvascular inflammation in experimental shock caused by intestinal ischemia (19–23) or endotoxin administration (24). Enteral pancreatic enzyme blockade reduces markers for inflammation in the microcirculation of rat peripheral organs (16, 25) and enhances post-shock recovery rates in pigs (26). However, past experimental shock studies have been limited to relatively short observation periods of hours and have not investigated how direct inhibition of pancreatic digestive enzymes after shock affects long-term survival over months.
Human shock is complex, and an experimental model can only simulate selected aspects. We therefore used three separate rodent models of experimental shock for long-term survival studies, with consideration for the fact that there may be multiple pathways that lead to injury of the intestine and an involvement of the digestive enzymes. Each shock model was treated by blockade of the digestive pancreatic enzymes inside the small intestine, which resulted in significantly reduced organ injury and improved survival.
RESULTS
Experimental hemorrhagic, peritonitis, and endotoxic shock
We used three rat models of experimental shock: hemorrhagic, peritonitis, and endotoxin. We also applied three different pancreatic pro-tease inhibitors to reduce the possibility that the particular inhibitory profile of an inhibitor solely determined the outcome. The inhibitors were injected directly into the lumen of the small intestine 1 hour after initiation of shock to block the high [typically submillimolar (18)] concentrations of degrading serine proteases inside the digestive tract. In the peritonitis model, in which digestive enzymes are also present in the peritoneal cavity, protease inhibitors were also placed into the peritoneum. The primary endpoint for this study was survival over the course of 12 weeks, but we also assessed plasma protease activity; intestinal villus morphology; interstitial lesion formation in the intestine, lung, and heart; plasma cardiac troponin; lung edema; body weight; and animal activity levels.
In three hemorrhagic shock groups (n = 27 controls; n = 27 treated), femoral venous blood was withdrawn to reduce mean arterial blood pressure to a preselected value of 35 mmHg. After 1 hour of blood pressure reduction, digestive serine proteases were blocked by infusion of an inhibitor, 6-amidino-2-naphtyl p-guanidinobenzoate di-methanesulfate (ANGD; 0.45 mM), directly into about eight equally spaced spots in the lumen of the intestine. Enzyme blockade increased the 12-week survival rate from 25% in the untreated hemorrhagic controls (n = 12) to 83% in the treated animals (n = 12) (Table 1A). With an alternative protease inhibitor, tranexamic acid (127 mM), the survival rate increased to 100% in treated animals (n = 10), with 20% survival in the controls (n = 10), and with the serine protease inhibitor aprotinin (0.45 mM), survival increased from 0% in untreated controls (n = 5) to 80% in treated animals that received enteral blockade (Table 1A).
Table 1.
Three-month survival rates after experimental shock. Animals were divided into hemorrhagic (A), peritonitis (B), or endotoxic (C) shock and randomized between untreated control and treated animals. Data are numbers of surviving animals/total animals. P values were determined with Fisher’s two-tailed exact test, comparing percent survival of treatment group to the respective untreated control group. i.p., intraperitoneal.
| Inhibitor (route of administration) | Untreated control animals (% surviving) | Treated animals (% surviving) | P |
|---|---|---|---|
| A. Hemorrhagic shock | |||
| ANGD (enteral) | 3/12 (25) | 10/12 (83) | 0.0123 |
| Tranexamic acid (enteral) | 2/10 (20) | 10/10 (100) | 0.0001 |
| Aprotinin (enteral) | 0/5 (0) | 4/5 (80) | 0.0476 |
| B. Peritonitis shock | |||
| ANGD (enteral) and ANGD/ciprofloxacin/albumin (i.p.) | 1/10 (10) | 9/10 (90) | 0.0011 |
| Tranexamic acid (enteral) and tranexamic acid/ciprofloxacin/albumin (i.p.) | 0/5 (0) | 5/5 (100) | 0.0079 |
| Aprotinin (enteral) and aprotinin/ciprofloxacin/albumin (i.p.) | 0/5 (0) | 4/5 (80) | 0.0476 |
| C. Endotoxic shock | |||
| ANGD (enteral) | 4/13 (30) | 10/11 | 0.0045 |
| Tranexamic acid (enteral) | 2/12 (16) | 8/12 | 0.0360 |
In the case of hemorrhagic shock, we followed the ANGD- and the tranexamic acid–treated survivors (n = 10 in each group) over an extended period (up to 18 months) and saw no premature death. In all of the hemorrhagic shock groups, all nonsurvivors died from cardiac and respiratory arrest within 8 hours of initiation of blood pressure reduction. For this reason, survival curves cannot be resolved below 1 day.
Next, we tested pancreatic enzyme blockade as a means to increase the survival rate in a peritonitis model of shock, which was generated by infusion of cecal material into the peritoneum (n = 20 controls; n = 20 treated). Serine protease inhibitors were administered into the lumen of the intestine in the same manner as in hemorrhagic shock. In addition, the protease inhibitors were mixed with an antibiotic (ciprofloxacin) and a free fatty acid–binding protein (albumin) and then administered into the peritoneum 1 hour after placement of the fecal material. Only 1 of 20 untreated control animals survived peritonitis shock, for an overall survival rate of 5% (Table 1B). When using ANGD in this dual enteral/peritoneal treatment protocol, the 12-week survival rate of treated animals (n = 10) was 90%, compared to 10% (n = 10) in untreated controls. Tranexamic acid and aprotinin resulted in 100 and 80% survival rates, respectively, in treated animals (n = 5 each), compared to 0% survival in untreated controls (n = 5 each) (Table 1B). All nonsurvivors experienced cardiac and respiratory arrest within ~12 hours after placement of fecal material into the peritoneum.
In a third set of experiments, we blocked pancreatic enzymes in the lumen of the intestine in an endotoxic shock model at 1 hour after endotoxin (Gram-negative lipopolysaccharide derived from Escherichia coli) administration. In the ANGD group, 91% of animals (n = 11) survived, compared to 30% in the untreated control animals (n = 13) (Table 1C). When tranexamic acid was used, the survival rate was 75% (n = 12), with 30% (n = 13) in the untreated control animals. All nonsurvivors experienced cardiac and respiratory arrest within ~12 hours after endotoxin administration, with the exception of one tranexamic acid–treated animal that died on the third day after shock.
Viewed collectively across all models of shock and all protease inhibitors in Table 1, the enteral blockade of the digestive enzymes significantly reduced mortality in 70 treated animals compared with 72 untreated controls (P < 0.0001, Fisher’s two-tailed exact test).
Experimental peritonitis shock in aged animals
The experimental results shown in Table 1 were carried out in rats that were young but mature (15 to 20 weeks). To test the use of protease blockade at an advanced age (93 weeks), we induced peritonitis shock in 12 animals but with the amounts of cecal material delivered into the peritoneum lower than those given in the younger animals. Of these animals, we randomly treated six with tranexamic acid. Survival over an observation period of 10 weeks after shock was 17% in the untreated controls (one of six animals) and 83% in the treated animals (five of six) (P = 0.08, Fisher’s two-tailed exact test).
Reduced inhibitor concentrations in peritonitis shock
Tranexamic acid required higher concentrations in the enteral fluid than the other protease inhibitors. When tranexamic acid was administered at a lower dose (45 versus 127 mM), the survival rate still increased from 12.5 to 75% (n = 12 animals per group) (P = 0.012, Fisher’s two-tailed exact test) (table S1). However, at a lower concentration of tranexamic acid (16 mM), there was no trend toward enhancement of survival rates (zero of two animals survived).
Digestive protease activity
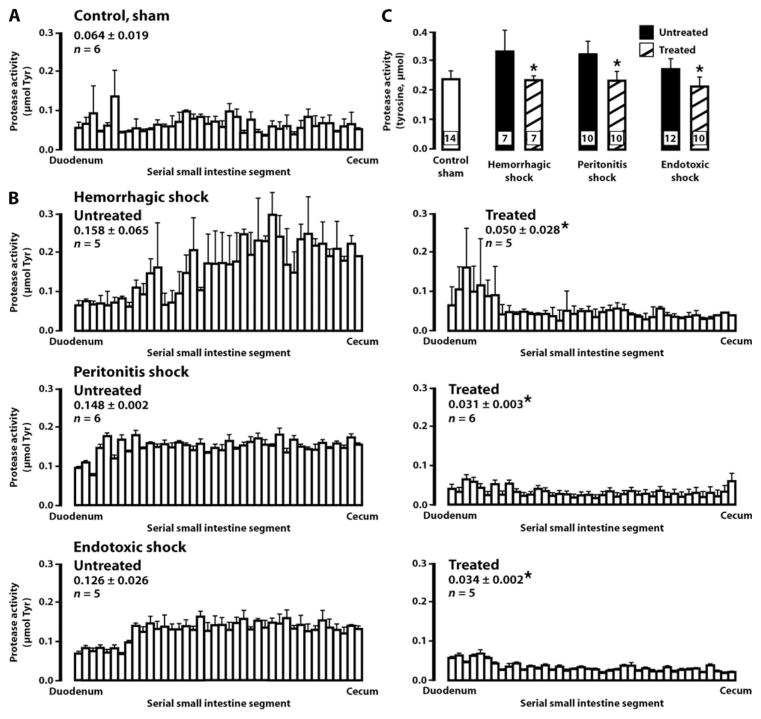
Leakage of pancreatic proteases from the lumen and elevation of protease activity in the wall of the intestine and in plasma is a common event in the different shock models of this study. Protease activity was tested with a casein substrate in the wall of the intestine and in the femoral plasma. Measurements in wall homogenates were made within hours after induction of shock. The average protease activity in wall specimen of normal, nonischemic control intestine was 0.064 μmol of liberated tyrosine (Fig. 1A). In all untreated shock models tested, the average levels were significantly raised in the intestinal wall during shock (Fig. 1B) (P < 0.05, untreated shock versus sham control by Student’s t test). Protease activity decreased significantly in ANGD-treated animals for each shock group (Fig. 1B). The average protease activity values were restored to below-normal levels upon treatment with ANGD, suggesting that even sham control rats (who were not treated with an inhibitor) have some protease activity in the intestinal wall that is blocked with a serine protease inhibitor. The protease activities in the plasma of each shock group at the time of tissue collection were also significantly elevated but decreased to normal levels upon treatment with ANGD (Fig. 1C). Tranexamic acid caused similar reduction of average protease activity of 0.037 ± 0.005 (SD) μmol of tyrosine in the intestinal wall and plasma in hemorrhagic shock (fig. S1).
Fig. 1.
Serine protease activity in the intestinal wall and in femoral plasma. Enzyme activity was measured as a function of liberated tyrosine (Tyr) at 40 individual segments of the wall of the rats’ small intestines along from the duodenum (left position on the abscissa) to the cecum (right position). (A) Protease activity in the intestinal lumen of nonischemic sham controls. Data are averages ± SD. (B) Protease activity in all shock models. The intestines in hemorrhagic shock were collected at 1 hour after the hypotensive period, in peritonitis shock at 4 hours after placement of cecal material, and in endotoxic shock at 4 hours after endotoxin administration. Data are averages ± SD with the number of rats (n) indicated. (C) Protease activity values in the plasma of nonischemic sham controls, and in hemorrhagic, peritonitis, and endotoxic shock rats without (black bars) and with (hashed bars) protease inhibitor ANGD in the lumen. Samples were collected at the same periods after shock as the intestine in (A). Data are averages ± SD, with the number of plasma samples in each group indicated in each column and one plasma sample per rat. In (B) and (C), *P < 0.05 versus respective untreated group by analysis of variance (ANOVA).
Animal physiology and organ damage after shock
Mean arterial blood pressures before and during hypotension in the hemorrhagic shock model were the same in the treated and untreated groups (table S2). However, the blood pressure after return of all shed blood was significantly higher in the treatment groups (averages ranged from 95.6 to 103.3 mmHg across all three treatment groups) compared with untreated controls (63.3 mmHg) (table S2). Blocking digestive proteases with any of the inhibitors restored the mean arterial pressure 30 min after return of shed blood volume to within about 15% of its value before hypotension. Similarly, femoral mean blood pressures at 2 hours and just before the femoral catheters were withdrawn were higher in the survivor groups exposed to endotoxic shock and treated with protease inhibitor ANGD (table S2).
Treated animals that survived returned to their weight gain (3 to 5 g/day) and to their pre-shock body weight within 14 days (fig. S2A). There was a delayed recovery in the few untreated animals that survived hemorrhagic (n = 2) and endotoxic (n = 2) shock in the tranexamic acid treatment group. There were no survivors in the peritonitis shock group. Furthermore, survivors with digestive enzyme blockade returned to normal activities (degree of movement, walking, and climbing) (fig. S2B), food consumption (drinking and chewing on food pellets) (fig. S2, C and D), and bowel movements (fig. S2E) within ~2 days after general anesthesia. Untreated surviving animals showed delayed post-anesthetic activity (fig. S2B). These results are in line with similar observations in a pig hemorrhagic shock model after enteral protease inhibition (26).
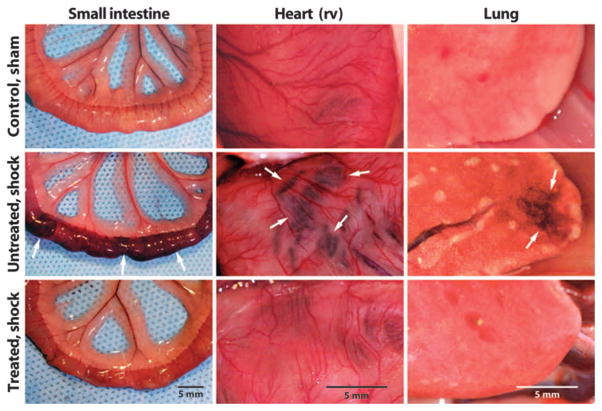
Gross inspection (Fig. 2) and histology (Fig. 3) of organ tissues at the time of death in animals without protease blockade, and in a separate group of animals with blockade of digestive enzymes, showed a morphology that looked similar among the shock models. All untreated hemorrhagic shock animals showed distended small intestines, swollen Peyer’s patches, and extensive diffuse microhemorrhages with blood cell accumulation in the lumen of the small intestine (Fig. 2). In contrast, all animals treated with ANGD along the intestine had an intact intestine with only minimal microhemorrhages and tissue swelling (Fig. 2). In peritonitis and endotoxic shock, untreated animals also exhibited tissue damage due to microhemorrhages in the intestine, heart, and lung. In contrast, treated animals had reduced levels of red cell diathesis (fig. S3). Treatment with tranexamic acid resulted in similar reduction of microhemorrhages in hemorrhagic shock, peritonitis, and endotoxic shock compared with ANGD (fig. S3).
Fig. 2.
Enteral protease blockade attenuates acute organ damage. Representative macroscale view of small intestine, heart [right ventricle (rv)], and lung in sham control (n = 5) and at 2 hours after the hypotensive period of hemorrhagic shock without (n = 3) and with the protease inhibitor ANGD (n = 3). Characteristic lesion sites due to escape of red blood cells from microvessels (Fig. 3) are marked by arrows.
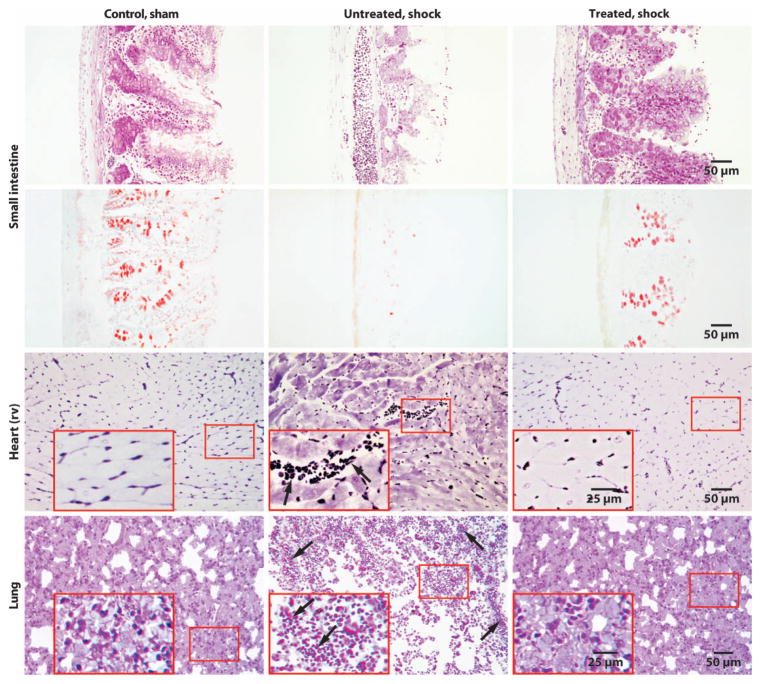
Fig. 3.
Enteral protease blockade prevents intestinal tissue damage and microhemorrhages in heart and lung. Representative cross sections of the small intestine, heart [right ventricle (rv)], and lung in nonischemic sham controls (n = 3) and in hemorrhagic shock without (n = 3) and with (n = 3) ANGD. Sections were labeled with toluidine blue, and the intestine was also labeled with mucin stain (second row, red). The tissues were collected at 1 hour after hypotension. Cleavage of intestinal villus tips (arrowheads) is prominent in untreated shock. Insets show extravasation of red blood cells into the cardiac interstitium (arrows) and into the alveolar space of the lung (arrows) in the untreated cases.
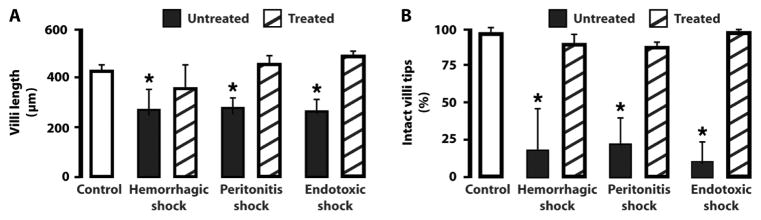
Histology of the intestinal wall 4 hours after induction of shock demonstrated reduced villus length in each model with autodigestion of intestinal wall tissue, including loss of the epithelium at the tips of a high fraction of villi, loss of morphological features in the villi, and reduced mucin layer and goblet cell staining (Figs. 3 and 4 and fig. S4). The destruction of the intestinal wall and villi was attenuated by protease inhibition in the lumen of the intestine (Figs. 3 and 4 and fig. S4), which is in line with previous observations in rats (16, 19). The heart and lung sections showed accumulation of red blood cells outside of blood vessels in the interstitial space (arrows in Fig. 3 and fig. S4), which was significantly attenuated in the treated groups. In heart muscle, the average number of red blood cells per tissue cross section (280 μm × 210 μm) located outside of blood vessels in the extravascular space was 0 ± 0 (average ± SD) in controls, 110 ± 61 in the shock group, and 8 ± 8 in the ANGD treatment group (Fig. 3) (P = 0.022 versus untreated, ANOVA).
Fig. 4.
Villus length and fraction of villi with intact epithelial lining after enteral tranexamic acid treatment. (A) Villus lengths. (B) Fraction of villi with intact epithelial lining. Data are averages ± SD; n = 3 rats in each group with 30 to 40 villi per rat derived from three sections at three equally spaced locations between duodenum and proximal ileum. Sham control is the same for all three shock models. *P < 0.05 compared to respective treated group, ANOVA.
The average plasma cardiac troponin levels and the lung wet/dry weight ratios at 4 hours after shock were significantly elevated in each shock group (Fig. 5) compared with sham controls. These levels were reduced by enteral treatment with tranexamic acid. We chose to use only one inhibitor here, because it is an approved inhibitor in several countries, including the United States.
Fig. 5.
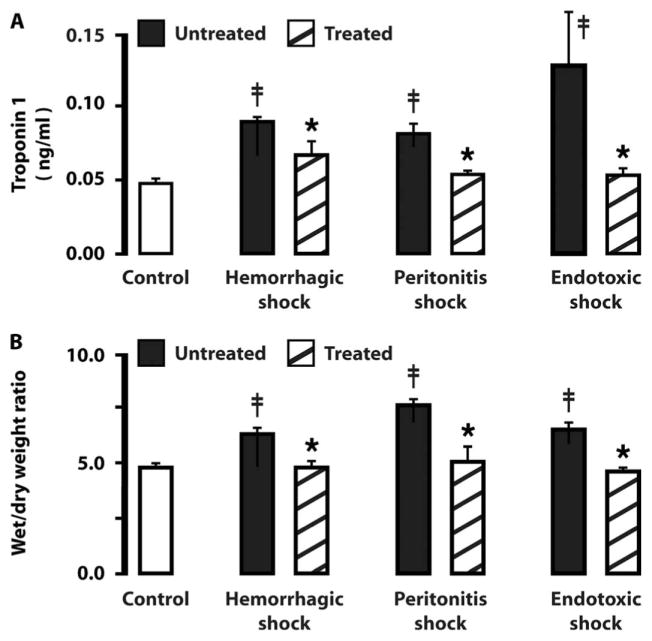
Plasma cardiac troponin levels and lung weight without and with enteral tranexamic acid treatment. (A) Plasma cardiac troponin I levels at 4 hours after induction of shock. (B) Lung wet/dry weight ratios at 4 hours after induction of shock. Data are averages ± SD (n = 3 rats per group). *P < 0.05 versus untreated, ANOVA; ‡P < 0.05 compared to control, ANOVA.
DISCUSSION
The high mortality in sepsis and shock may have its origin in the digestive system. Normally, pancreatic enzymes degrade most biomolecules within hours as part of digestion. The digestive enzymes are powerful because they are concentrated and active in the small intestine and are able to digest one’s own tissue in a relatively nonspecific manner. Auto-digestion of the intestinal wall is prevented normally by compartmentalization of the pancreatic enzymes within the lumen of the intestine (15). Insults, such as hypoperfusion of the intestine, endotoxin, or cecal material, can raise the permeability of the mucosal barrier, allowing these enzymes to leak into the intestinal wall. This results in autodigestion. To counter this autodigestive process, we hypothesized that blockade of the digestive enzymes via the lumen of the intestine would alleviate shock symptoms and prolong survival in rodent models. The current data show that this approach improves survival after experimental shock with various inhibitors, and may be feasible in more complex situations in patients. The key requirement is to reduce the activity of digestive enzymes once they have escaped from the lumen of the intestine and to minimize their leakage in the first place (13). Although it is known that systemic digestive enzyme levels are elevated in shock patients (27), the time frame over which the enzymes appear in the systemic circulation is yet unclear. This information will be needed to optimize intervention and block digestive enzymes as soon as possible.
To enhance survival rate in the three rodent shock models, several conditions had to be met. First, the digestive enzymes along the entire length of the lumen of the small intestine needed to be maximally blocked. Detailed postmortem autopsies of treated animals that did not survive the shock protocol showed that along the lumen of the ileum, there were sites where a bolus of partially digested food may have prevented the inhibitors from completely penetrating the lumen (fig. S5). At these sites, we saw diffuse microhemorrhages in the wall of the intestine and blood cells in the lumen, similar to features that were prominent in the intestine of untreated animals that did not survive (Fig. 2).
Second, the degrading activity of the digestive enzymes needed to be blocked as soon as possible after compromise of the mucosal barrier: after hypoperfusion in hemorrhagic shock or after administration of fecal material or endotoxin in the peritonitis or endotoxic shock model, respectively. Pilot studies in rats showed that the survival rate depends on the timing of the blockade of digestive enzymes. For example, in hemorrhagic shock with 2 hours of hypotension, if the blockade with ANGD is carried out at 4 hours after initial blood pressure reduction, there was no protection against a drop in central blood pressure and mortality (survival rate, zero of four). Animals treated “late” had extensive morphological damage in the intestine before the digestive enzymes were blocked, indicating that, in the rat, damage to the mucosal barrier by digestive enzymes can occur within minutes (28). We selected a 1-hour time point for treatment after hypotension as a compromise between the need to block digestive enzymes as early as possible and the delay imposed by clinical reality (transporting a patient to the clinic and detection of markers for shock). If the enzyme blockade were applied at earlier periods—for example, as pretreatment in elective surgery—lower inflammation and higher survival rates would be expected (16, 19, 20).
A third requirement to enhance survival rates with the current enteral treatment protocol was the use of low concentrations of heparin (0.5 U/ml of blood volume estimated as 6% body weight, intravenously) to minimize generation of diffuse bleeding in the intestine. An anticoagulant is a requirement for hemorrhagic shock protocols. A serine protease inhibitor like ANGD may partly block coagulation enzymes so that the combination of heparin and ANGD may lead to bleeding in the intestine in rat hemorrhagic shock. Elevation of the ANGD concentrations in the lumen of the intestine by 0.5 to 1 mM led to increased bleeding into the intestine. Because no fluid resuscitation was carried out in the current set of experiments, the loss of blood volume either into the intestinal lumen or into the peritoneal cavity reduced central blood pressure, thereby extending the ischemic state and causing further compromise of the intestinal barrier. If the current shock protocol were to be carried out with fluid resuscitation as an intervention, the fluid loss from bleeding might be compensated for with improved control of the central blood pressure.
Autopsies of nonsurviving, untreated, and treated animals showed, without exception, diffuse bleeding into the intestine (Fig. 2) and fluid loss into the abdominal cavity, regardless of the shock model. These observations suggest that one of the mechanisms for protection provided by the blockade of digestive enzymes is preservation of the mucosal barrier (28). Maximal preservation of the mucosal barrier is critical for optimal attenuation of multiorgan dysfunction and failure. Ischemia or the presence of inflammatory mediators enhances permeability and opens tight junctions between epithelial cells (29–31) with entry of high–molecular weight species into the intercellular gap between mucosal epithelial cells. Once digestive enzymes enter into the wall of the intestine, they initiate an autodigestion process with destruction of the villus structures (28, 32) and activation of proenzymes, which in turn amplifies the action of the pancreatic enzymes (25). The current study shows that digestive enzymatic blockade minimizes damage to the mucin mucosal layer and to the cells in the intestine, preserves the morphology of the villi and the epithelium, and reduces tissue lesion formation in the form of escape of red blood cells into the wall of the intestine. Preservation of the mucosal barrier by blockade of digestive enzymes may be especially relevant in patients scheduled for elective surgery with high risk for postsurgical shock.
The enzyme inhibitors in hemorrhagic and endotoxic shock were administered into the lumen of the intestine without intravenous injections. As small–molecular weight competitive protease inhibitors, however, these molecules may be transported into the portal venous circulation or the lymphatics and enter the central circulation. Intravenous administration only of protease inhibitors in shock associated with intestinal ischemia has been ineffective in preventing inflammation and reducing multiorgan failure in animals (19, 33). In the case of peritonitis, where intraluminal material is transferred into the peritoneal cavity (for example, in ruptured appendix or intestinal punctures), a combination of digestive enzymes, intestinal bacteria, and cytotoxic breakdown products (unbound free fatty acids) may leak into the peritoneum. As shown by the current results, in addition to enteral digestive protease inhibition, minimizing peritonitis also requires protection of the peritoneum from the cytotoxic effects of digestive enzymes (by protease inhibitor), intestinal bacteria (by antibiotic), and cytotoxic products generated by digestive enzymes, such as unbound free fatty acids (by albumin) (14).
The relatively high concentrations selected for the inhibitors in these experiments were guided by the requirement to block the high concentrations of the digestive proteases inside the lumen of the intestine (18). ANGD and aprotinin are effective pancreatic protease inhibitors (34, 35). Tranexamic acid is predominantly a trypsin and plasminogen activator inhibitor (28, 36, 37) and is less effective against chymotrypsin or elastase. It was therefore given at higher enteral concentrations, but it did not generate bleeding in the rat intestine in any of the current shock models. Blockade at only a single time point was necessary, indicating that escape of digestive enzymes in the experimental models, including the aged ones, occurs predominantly during early periods of shock. Intravenous administration of the inhibitors is not effective because the high concentrations required for digestive enzyme, especially using competitive inhibitors that need to match the protease concentrations in the intestine, cannot be achieved without side effects unless delivered directly into the lumen of the intestine.
Maintenance of the systemic blood pressure is central to the effectiveness of any intervention against sepsis and multiorgan failure. Enteral blockade of digestive enzymes serves to preserve the arterial blood pressure already in the immediate post-shock period. We have seen this not only in hemorrhagic and endotoxic shock with multiple protease inhibitors (table S2) but also with ANGD (19), gabexate mesilate, and aprotinin (20, 38) in a shock model produced by superior mesentery artery occlusion and with gabexate mesilate in endotoxic shock (24). If fluid resuscitation by intravenous infusion is used to maintain the blood pressure after hemorrhagic shock, the enteral protease blockade serves to reduce the amount of fluid required to preserve the systemic blood pressure (23). The inhibitors administered into the lumen of the intestine in the different shock models have in common that each minimizes autodigestion and, at the same time, blood pressure reduction; as such, autodigestion and blood pressure reduction may be closely linked events in shock.
A major limitation of the experimental models of shock used in this study is that they do not simulate chronic preexisting conditions that may be present in patients. The intestines in the experimental animals were intact before injury during the acute shock protocol. This situation may apply to patients scheduled for elective surgery, in which the intestine is initially intact but may be damaged during surgery. However, it is different in patients with chronic preexisting conditions, prolonged injury to the intestinal mucosal barrier, and a reduced ability to repair the barrier. In such patients, longer periods of enteral protease blockade may be required to minimize intervention by digestive protease activity with healing of the mucosal barrier. Preexisting conditions may be associated with a permeable intestine and leakage of enzymes. If they reach the peritoneal fluid, lavage of the peritoneum with pancreatic enzyme inhibitors needs to be considered. If digestive enzymes have already spread into tissues outside the peritoneum, additional protease inhibition should be considered and its effectiveness determined.
Translation of the current approach should be guided by measurements of digestive enzyme activity and intestinal permeability and/or by detection of autodigestion breakdown products. Optimal delivery methods for the inhibitors into the lumen of the intestine need to be developed because the inhibitor administration in the current study design is invasive. In this regard, it is noteworthy that digestive enzyme blockade reversed severe septic shock in one immunosuppressed patient with Fournier’s gangrene at 4 years after heart transplantation, who was treated by consent with continuous enteral pancreatic protease inhibition delivered via nasogastric tube over several days (39). Previous intravenous administration of the inhibitor did not reduce septic indicators.
In conclusion, the collective evidence points to a cascade in septic shock and multiorgan failure that starts after breakdown of the mucosal barrier with escape of the powerful digestive enzymes from the lumen into the wall of the intestine. The digestive enzymes are further transported into the systemic circulation via the portal venous system, via the intestinal lymphatics (thus bypassing the liver), and possibly also via leakage across the intestinal serosa into the peritoneum. Because digestive enzymes readily degrade autologous tissue or various food constituents, smaller–molecular weight proinflammatory and cytotoxic mediators are produced, which can also be carried from the intestine into the systemic circulation. The combination of digestive enzymes and their degradation products causes frank tissue destruction and autodigestion of the intestine, and may cause receptor cleavage even in more peripheral organs, loss of associated cell functions, and systemic inflammation. Depending on the extent to which the intestine is subject to autodigestion, multiorgan morbidity and mortality may result. Effective future interventions against organ failure in sepsis and shock need to stop the tissue destruction by blocking digestive enzymes in addition to symptomatic support of organ functions.
MATERIALS AND METHODS
Animals
The protocol was reviewed and approved by the University of California San Diego Animal Subjects Committee. Adult male Wistar rats (Harlan Sprague-Dawley) were used (age 15 to 20 weeks, 350 to 450 g) unless indicated otherwise. Throughout the study, the animals were maintained on a regular diet (Harlan Teklad Rodent diet 8604, 0.29% sodium by weight) without any restriction and water ad libitum. Surgical procedures were carried out under sterile conditions.
General anesthesia was administered (pentobarbital sodium, 50 mg/kg, Abbott Laboratories, intramuscularly), followed by placement of a femoral artery side branch catheter (polyethylene 50 tubing) for continuous measurement of blood pressure, and a femoral venous catheter for blood exchanges. The animals were heparinized with minimal concentrations of sodium heparin (0.5 USP units/ml of blood volume estimated as 6% body weight). Higher concentrations of heparin induce bleeding during the course of the shock protocol and were avoided. The body temperature was carefully maintained at 37°C by keeping the animals on a water-heated support and by use of a heat blanket.
The femoral blood pressure was monitored continuously (MacLab 8e, AD Instruments Inc.) until the animals showed initial signs of recovery from the primary anesthesia. No supplemental anesthesia was administered; instead, at this time (at a minimum of 30 min after return of all shed blood volume), the femoral catheters were removed. The skin over the incision sites was closed by sutures and superficially covered with antibiotic ointment.
After the shock protocol, the animals were kept under observation in water-heated cages (37°C) with free access to water and solid food pellets for about 6 hours and then transferred into the vivarium. In a subgroup of surviving animals, the body weight, water intake (ml/day), food consumption (g/day), and fecal output (g/day) were measured daily over a period of 2 weeks after shock.
Activity index
After shock, the animal’s activity was daily observed over a period of 2 weeks and quantitated in terms of an activity index (0 = death; 1 = alive but no movement; 2 = slow movement; 3 = walking; 4 = normal mobility with walking and climbing), and at the same time, body weight, daily food pellet, and water consumption as well as fecal output were measured.
Digestive enzyme blockade
To block digestive enzymes in the lumen of the intestine, we made a mid-line incision (2.5 cm) to temporarily expose the small intestine. In the case of hemorrhagic shock, GoLYTELY [polyethylene glycol 3350 (60 g/liter) and electrolytes for oral solution; Braintree Laboratories Inc.] with and without the pancreatic protease inhibitor (ANGD, 0.45 mM, nafamostat mesilate, Torii Pharmaceutical; dissolved in 5% glucose in saline) were used in the treatment and untreated control groups. Tranexamic acid (127 mM, Pfizer Inc.) and aprotinin (0.45 mM, Bayer Health Care Pharmaceuticals) were dissolved in GoLYTELY.
In the case of peritonitis shock, each protease inhibitor was also dissolved in saline and mixed with an antibiotic [ciprofloxacin (10 mg/ml), Hospira Inc.] and with albumin (40 mg/ml, Sigma-Aldrich Co.) and administered 1 hour after placement of cecal material as lavage into the peritoneum (2 ml). The untreated peritonitis shock group received the same volume of saline into the peritoneum and the same volume of GoLYTELY into the lumen of the intestine but without additives.
The inhibitor solutions were injected with a needle (BD Sub-Q 26G 5/8 PrecisionGlide Needle, Becton Dickinson & Co.) into the lumen of the intestine over its entire length from the duodenum to the cecum. The injections were spaced in intervals of about 5 cm for a total of about eight injections. A total fluid volume of 15 ml (at 39°C) was injected into the lumen of the small intestine and 2 ml was injected into the cecum in a 350-g rat.
Care was taken that all lumen segments of the small intestine were filled with the enzyme inhibitor to achieve uniform blockade of the digestive enzymes. The injections resulted in a small distension of the small intestine but did not impair its return into the peritoneal cavity. After enzyme blockade, the intestine was returned gently into the peritoneal cavity, and the incision was closed by absorbable suture in the abdominal muscle and silk suture in the skin. All animals received a narcotic [Buprenex, Reckitt Benckiser Healthcare (UK) Ltd.; 0.1 mg/kg, intramuscularly] for 3 days after surgery for pain management, and incision sites were covered with antibiotic ointment (Neosporin + Pain Relief, Pfizer). No skin infections developed at any incision site during the study. The intestinal injection to block the digestive enzymes required less than 10 min, so that with opening and closure of the skin, the entire procedure required less than 25 min.
In general, handling of the intestine was minimized and overinflation was avoided. Care was taken to avoid puncture of arterioles or venules because they tend to bleed during and after the hypotensive period. Exposure of intestine to ambient temperature was also minimized. If the intestine was exposed for more than 5 to 10 min, the core temperature dropped to 34 to 35°C. The core temperature could not be returned to 37.5°C during anesthesia if it had cooled.
Experimental shock protocols
Hemorrhagic shock was initiated by reduction of blood volume (~40% of whole blood volume based on 6% body weight) to achieve a blood pressure of 35 mmHg for a period of 2 hours, at which time the entire shed blood was returned. Blood withdrawal was carried out over a period of about 5 min. The blood was stored at 4°C and warmed to 37°C just minutes before reinjection via the femoral venous catheter.
During the hypotensive period, one observes a compensatory period during which time small blood volumes need to be withdrawn to maintain the blood pressure at 35 mmHg. This period is followed by a decompensatory period during which blood needs to be returned to maintain the preselected blood pressure. The time point at which the transition from compensatory to decompensatory period occurred was different from animal to animal. It started between 40 and 60 min and, in some animals, occurred even later (four control animals and three treated animals had decompensation at a time less than 60 min).
In the peritonitis model, cecal material (900 mg/kg derived from multiple donor rats, diluted by addition of 1 ml of 5% glucose saline solution to 1 g of cecal material) was injected into the peritoneal cavity and gently spread by mild skin compression over the abdomen. In old rats, a lower amount of cecal material was injected (500 mg/kg). In the endotoxic shock model, Gram-negative endotoxin (lipopolysaccharide derived from E. coli; serotype 0111:B4; L2630, 5 mg/kg, nonpurified, Sigma-Aldrich) in normal saline was administered (intravenously) over a period of about 5 min.
The digestive enzymes were blocked at 60 min of hypotension in hemorrhagic shock and at 60 min after cecal material administration and endotoxin in the peritonitis and endotoxin shock models, respectively. Untreated animals received an identical treatment without the enzyme blocker. In the case of protease inhibition with tranexamic acid, we also studied a shock sham group (control) that received the protease inhibitor but was not subjected to any form of shock (n = 10 rats).
Protease activity in the small intestine
To determine the level of protease activity, we isolated the entire length of the small intestine by separating it from the mesentery. Fresh specimens were cut into ~2.5-cm-long segments, opened longitudinally, and rinsed to eliminate the luminal content. Thereafter, each specimen was individually homogenized (0.5-g tissue in 2.0-cm3 buffered saline). The intestinal homogenates were centrifuged to eliminate solid tissue residue, and 25 μl per sample of the supernatant was used for protease activity measurements (Protease Colorimetric Detection Kit, product code PC0100, Sigma-Aldrich). The kit detects a spectrum of proteases (serine, cysteine, metallo, and aspartic). Plasma protease activity was detected in the same way with 25 μl per sample.
Tissue histology
Selected tissues were excised, fixed (10% neutral-buffered formalin), embedded (Poly/Bed 812 Resin Test Kit, product no. 18107, Ted Pella Inc.), sectioned (1-μm thickness), and stained with toluidine blue for tissue histology and mucin distribution on intestinal sections (Rapid Mucin Stain Kit, catalog number 24208, Polysciences Inc.). The lumen of the intestine was rinsed with saline before fixation to display sites in which digestive enzymes had degraded the intestinal tissue by auto-digestion. Heart and lung sections were prepared in the same fashion and stained with toluidine blue.
In heart muscle, the number of blood cells located outside of blood vessels in the interstitial space was counted on the sections under a 100× objective (numerical aperture, 1.4). At this magnification, the endothelium is clearly visible to allow distinction between cells inside and cells outside of microvessels. Ten random sections (280 μm × 210 μm) per rat heart were analyzed and averaged. Three rats per group were used for comparison of groups.
Measurements of villus length (measured from the tips to the junction between submucosa and the muscularis) and fraction of villi with intact epithelial lining (as detected morphologically on 1-μm-thin resin sections stained with toluidine blue) was collected at 4 hours after induction of shock (arterial blood pressure reduction in hemorrhagic shock, administration of cecal material, and endotoxin in peritonitis and endotoxic shock, respectively).
Troponin measurements
The plasma troponin levels were measured with an enzyme-linked immunosorbent assay kit to rat cardiac troponin I (catalog no. CSB-E08594r, Cusabio Biotech Co.).
Lung fluid accumulation
The wet weight/dry weight ratio for the entire lungs was measured before and after drying for 5 days at 60°C, and confirmation of constant dry weight between days 3 and 5.
Statistics
All measurements are shown as averages ± SD. Comparison between treated and untreated groups was carried out by Student’s t test. Fisher’s two-tailed exact test was used to determine the relationship between survival and the presence of the digestive enzyme blockade. Repeated-measures ANOVA followed by Bonferroni post hoc test was used to compare sham controls with untreated and treated shock groups. Selected comparisons (see figure legends) between treated and sham control groups were carried out by Student’s t test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank J. Chow and M. Santamaria-Aviles for their effective assistance with experiments.
Funding: Supported by an unrestricted gift from Leading BioSciences Inc. and by NIH grants HL 67825 and GM 85072.
Footnotes
Author contributions: F.A.D. designed the study and carried out the experiments and data analyses. D.B.H. designed the experiments. G.W.S.-S. designed the experiments, analyzed the data, and wrote the manuscript.
Competing interests: G.W.S.-S. is a scientific advisor to Leading BioSciences Inc. D.B.H., F.A.D., and G.W.S.-S. own equity in InflammaGen, a company by Leading BioSciences Inc., which develops therapy for shock patients.
www.sciencetranslationalmedicine.org/cgi/content/full/5/169/169ra11/DC1
Fig. S1. Protease activity after enteral tranexamic acid treatment.
Fig. S2. Body weight change, activity index, food consumption, and fecal output after enteral tranexamic acid treatment.
Fig. S3. Macroscale view of tissue lesions after enteral enzymatic blockade in hemorrhagic, peritonitis, and endotoxic shock.
Fig. S4. Interstitial microhemorrhages in peritonitis and endotoxic shock after enteral protease inhibition.
Fig. S5. Macroscale view of intestinal lesions in animals that did not survive after blockade of digestive enzymes.
Table S1. Three-month survival rates after peritonitis shock with combined enteral and intraperitoneal tranexamic acid treatment at two different concentrations.
Table S2. Mean femoral artery blood pressure under general anesthesia before induction of shock and before the time of catheter withdrawal.
REFERENCES AND NOTES
- 1.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: A common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 2.Waxman K. Shock: Ischemia, reperfusion, and inflammation. New Horiz. 1996;4:153–160. [PubMed] [Google Scholar]
- 3.Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 4.Barroso-Aranda J, Schmid-Schönbein GW. Transformation of neutrophils as indicator of irreversibility in hemorrhagic shock. Am J Physiol. 1989;257:H846–H852. doi: 10.1152/ajpheart.1989.257.3.H846. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, Bernard GR. Therapeutic intervention and targets for sepsis. Annu Rev Med. 2005;56:225–248. doi: 10.1146/annurev.med.56.082103.104356. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.Martí-Carvajal A, Salanti G, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev. 2008;1:CD004388. doi: 10.1002/14651858.CD004388.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Schönbein GW, Hugli TE. A new hypothesis for microvascular inflammation in shock and multiorgan failure: Self-digestion by pancreatic enzymes. Microcirculation. 2005;12:71–82. doi: 10.1080/10739680590896009. [DOI] [PubMed] [Google Scholar]
- 9.Lefer AM, Glenn TM. Role of the pancreas in the pathogenesis of circulatory shock. Adv Exp Med Biol. 1971;23:311–335. doi: 10.1007/978-1-4615-9014-9_31. [DOI] [PubMed] [Google Scholar]
- 10.Leffler JN, Litvin Y, Barenholz Y, Lefer AM. Proteolysis in formation of a myocardial depressant factor during shock. Am J Physiol. 1973;224:824–831. doi: 10.1152/ajplegacy.1973.224.4.824. [DOI] [PubMed] [Google Scholar]
- 11.Kistler EB, Hugli TE, Schmid-Schönbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183–192. [PubMed] [Google Scholar]
- 12.Kramp WJ, Waldo S, Schmid-Schönbein GW, Hoyt D, Coimbra R, Hugli TE. Characterization of two classes of pancreatic shock factors: Functional differences exhibited by hydrophilic and hydrophobic shock factors. Shock. 2003;20:356–362. doi: 10.1097/01.shk.0000082442.66379.90. [DOI] [PubMed] [Google Scholar]
- 13.Penn AH, Schmid-Schönbein GW. The intestine as source of cytotoxic mediators in shock: Free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294:H1779–H1792. doi: 10.1152/ajpheart.00902.2007. [DOI] [PubMed] [Google Scholar]
- 14.Penn AH, Hugli TE, Schmid-Schönbein GW. Pancreatic enzymes generate cytotoxic mediators in the intestine. Shock. 2007;27:296–304. doi: 10.1097/01.shk.0000235139.20775.7f. [DOI] [PubMed] [Google Scholar]
- 15.Chang M, Alsaigh T, Kistler EB, Schmid-Schönbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One. 2012;7:e40087. doi: 10.1371/journal.pone.0040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzal F, DeLano FA, Young C, Rosario HS, Schmid-Schönbein GW. Pancreatic protease inhibition during shock attenuates cell activation and peripheral inflammation. J Vasc Res. 2002;39:320–329. doi: 10.1159/000065544. [DOI] [PubMed] [Google Scholar]
- 17.Kistler EB, Alsaigh T, Chang M, Schmid-Schönbein GW. Impaired small-bowel barrier integrity in the presence of lumenal pancreatic digestive enzymes leads to circulatory shock. Shock. 2012;38:262–267. doi: 10.1097/SHK.0b013e31825b1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldo SW, Rosario HS, Penn AH, Schmid-Schönbein GW. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock. 2003;20:138–143. doi: 10.1097/01.shk.0000073866.47824.ae. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772–1777. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinal lumen: Attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Fitzal F, DeLano FA, Young C, Schmid-Schönbein GW. Improvement in early symptoms of shock by delayed intestinal protease inhibition. Arch Surg. 2004;139:1008–1016. doi: 10.1001/archsurg.139.9.1008. [DOI] [PubMed] [Google Scholar]
- 22.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 23.Doucet JJ, Hoyt DB, Coimbra R, Schmid-Schönbein GW, Junger WG, Paul LW, Loomis WH, Hugli TE. Inhibition of enteral enzymes by enteroclysis with nafamostat mesilate reduces neutrophil activation and transfusion requirements after hemorrhagic shock. J Trauma. 2004;56:501–510. doi: 10.1097/01.ta.0000114536.98447.f7. [DOI] [PubMed] [Google Scholar]
- 24.Fitzal F, Delano FA, Young C, Rosario HS, Junger WG, Schmid-Schönbein GW. Pancreatic enzymes sustain systemic inflammation after an initial endotoxin challenge. Surgery. 2003;134:446–456. doi: 10.1067/s0039-6060(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 25.Rosário HS, Waldo SW, Becker SA, Schmid-Schönbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164:1707–1716. doi: 10.1016/S0002-9440(10)63729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HD, Malinoski DJ, Borazjani B, Patel MS, Chen J, Slone J, Nguyen XM, Steward E, Schmid-Schönbein GW, Hoyt DB. Inhibition of intraluminal pancreatic enzymes with nafamostat mesilate improves clinical outcomes after hemorrhagic shock in swine. J Trauma. 2010;68:1078–1083. doi: 10.1097/TA.0b013e3181da78b1. [DOI] [PubMed] [Google Scholar]
- 27.Malinoski DJ, Hadjizacharia P, Salim A, Kim H, Dolich MO, Cinat M, Barrios C, Lekawa ME, Hoyt DB. Elevated serum pancreatic enzyme levels after hemorrhagic shock predict organ failure and death. J Trauma. 2009;67:445–449. doi: 10.1097/TA.0b013e3181b5dc11. [DOI] [PubMed] [Google Scholar]
- 28.Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297–305. doi: 10.1097/SHK.0b013e318240b59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan XD, Chang H, Qu XW, Caplan M, Gonzalez-Crussi F, Hsueh W. Platelet-activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br J Pharmacol. 2000;129:1522–1529. doi: 10.1038/sj.bjp.0702939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisham MB, Gaginella TS, von Ritter C, Tamai H, Be RM, Granger DN. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation. 1990;14:531–542. doi: 10.1007/BF00914274. [DOI] [PubMed] [Google Scholar]
- 31.Rollwagen FM, Li YY, Pacheco ND, Dick EJ, Kang YH. Microvascular effects of oral interleukin-6 on ischemia/reperfusion in the murine small intestine. Am J Pathol. 2000;156:1177–1182. doi: 10.1016/S0002-9440(10)64987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid-Schönbein GW. 2008 Landis Award lecture. Inflammation and the autodigestion hypothesis. Microcirculation. 2009;16:289–306. doi: 10.1080/10739680902801949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 34.Neligan PC. Plastic Surgery Educational Foundation DATA Committee, Aprotinin: Role in minimizing perioperative blood loss. Plast Reconstr Surg. 2005;116:324–327. doi: 10.1097/01.prs.0000173439.89107.7a. [DOI] [PubMed] [Google Scholar]
- 35.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981;661:342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol) 1980;14:41–47. [PMC free article] [PubMed] [Google Scholar]
- 37.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 38.Schimmeyer SM. thesis. University of California; San Diego: 2005. Intraintestinal enzyme inhibition in splanchnic arterial occlusion shock. [Google Scholar]
- 39.Lee YT, Wei J, Chuang YC, Chang CY, Chen IC, Weng CF, Schmid-Schönbein GW. Successful treatment with continuous enteral protease inhibitor in a patient with severe septic shock. Transplant Proc. 2012;44:817–819. doi: 10.1016/j.transproceed.2012.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.