Abstract
WRKY transcription factors constitute a very large family of proteins in plants and participate in modulating plant biological processes, such as growth, development and stress responses. However, the exact roles of WRKY proteins are unclear, particularly in non-model plants. In this study, Gossypium hirsutum WRKY41 (GhWRKY41) was isolated and transformed into Nicotiana benthamiana. Our results showed that overexpression of GhWRKY41 enhanced the drought and salt stress tolerance of transgenic Nicotiana benthamiana. The transgenic plants exhibited lower malondialdehyde content and higher antioxidant enzyme activity, and the expression of antioxidant genes was upregulated in transgenic plants exposed to osmotic stress. A β-glucuronidase (GUS) staining assay showed that GhWRKY41 was highly expressed in the stomata when plants were exposed to osmotic stress, and plants overexpressing GhWRKY41 exhibited enhanced stomatal closure when they were exposed to osmotic stress. Taken together, our findings demonstrate that GhWRKY41 may enhance plant tolerance to stress by functioning as a positive regulator of stoma closure and by regulating reactive oxygen species (ROS) scavenging and the expression of antioxidant genes.
Introduction
During their life span, plants are exposed to various biotic and abiotic stresses. Among these, environmental stresses, particularly drought and salt, which decrease the availability of water to the plant cell, are primary limiting factors for plant growth and development as well as crop yield and quality [1]. To cope with these environmental stresses, plants have evolved intricate acclimatization strategies to avoid or tolerate cellular dehydration.
Plant acclimatization responses usually involve changes to physiological and biochemical parameters. In terms of physiological changes, the stomata play a critical role in the control of water vapor flow [2]. Additionally, plants regulate the expression of genes involved in stress tolerance [3]. In both scenarios, the phytohormone abscisic acid (ABA) plays an important role [4, 5]. It has been well established that water-deficit stress causes significant accumulation of ABA. This increased endogenous ABA content then induces stomatal closure and the expression of various stress-related genes [6, 7]. Numerous transcription factors participate in the ABA response, including HD-ZIP [8], NAC [9], bHLH [10] and WRKY [11]. Among them, WRKY transcription factors, a large family of regulatory proteins, have received much attention in recent decades.
Much research has been performed on WRKY transcription factors since the first WRKY protein was characterized in sweet potato [12]. In Arabidopsis, there are 74 family members; rice has more than 100 family members. WRKY genes have also been identified in other species, such as soybean, barley, poplar, Pine spp. and Physcomitrella patens. All identified WRKY proteins contain either one or two DNA-binding domains consisting of a 60-amino acid region harboring a highly conserved WRKYGQK heptapeptide at its N-terminus with a zinc finger-like motif at its C-terminus. They can be classified on the basis of both the number of WRKY domains and the features of their zinc finger-like motif. Proteins with two WRKY domains belong to group I, whereas most proteins containing one WRKY domain belong to group II. Generally, members of groups I and II have the same zinc finger-like motif, with a pattern consisting of C-X4-5-C-X22-23-H-X1-H. A small subset including members with a C-X7-C-X23-H-X-C zinc finger-like motif and one WRKY domain is defined as group III [13].
According to previous reports, WRKY proteins are involved in multiple biotic stress responses as well as developmental and physiological processes, including trichome and seed coat development [14], regulation of seed size [15], modulation of leaf development [16] and regulation of leaf senescence [17]. Currently, WRKY proteins are also proposed to be involved in the regulation of plant responses to abiotic stresses. For example, GsWRKY20 was found to be induced by ABA, salt, drought and cold, and this protein enhanced the drought tolerance of transgenic Arabidopsis by regulating ABA signaling. This regulation was achieved due to the ability of GsWRKY20 to promote the expression of a negative regulator and repress the expression of a positive regulator of ABA signaling [18]. Li et al. reported that AtWRKY70 and AtWRKY54 cooperate to negatively regulate Arabidopsis stomatal closure and osmotic tolerance [19]. TaWKRY2 and TaWRKY19 were reported to be involved in drought resistance in wheat via direct binding to a downstream gene promoter or via an indirect mechanism [20]. As topics of extensive study, WRKY protein functions have been well examined in model plants, but their roles in crops are not yet clear. Cotton is planted all over the world; its role as a significant source of fiber, oil and biofuel products makes it an important cash crop worldwide [21, 22]. However, current information regarding cotton WRKY proteins is limited. In this report, we identified and characterized a functional WRKY group III gene named GhWRKY41. GhWRKY41 was induced by multiple environmental stresses, and overexpression of GhWRKY41 in Nicotiana benthamiana led to enhanced tolerance to drought and salt. The tissue-specific expression assessed by β-glucuronidase (GUS) staining suggested that GhWRKY41 was induced in the stoma under salt and drought stresses.
Materials and Methods
Plant material, growth conditions and treatments
Cotton (G. hirsutum L. cv. lumian 22) seeds were placed in wet cloths and germinated. Seedlings were then maintained in hydroponic culture for growth under greenhouse conditions at 25 ± 1°C with a 16-h light/8-h dark cycle (relative humidity of 60–75%). The seedlings were cultured until they were seven days old, at which point they were used for expression analysis. For tissue-specific expression analyses, roots, stems and cotyledons were harvested from the same plant, frozen in liquid nitrogen and stored at -80°C. The resulting uniform seedlings were sprayed or cultured with NaCl (200 mM), 15% polyethylene glycol 6000 (PEG 6000) (w/v), H2O2 (10 mM), ABA (100 μM), SA (2 mM) and ET released from ethephon (5 mM) or maintained under cold conditions (4°C) or hot conditions (37°C). The treated cotyledons were collected for RNA extraction. N. benthamiana seeds were surface-sterilized, planted in soil, and maintained under a 16-h light/8-h dark photoperiod at 25°C. Three- or four-leaf-stage N. benthamiana seedlings were transplanted separately into pots with soil and maintained under greenhouse conditions. The resulting uniform seedlings were used for further study. Each treatment was repeated at least twice.
GhWRKY41 cloning, vector construction and genetic transformation
The cDNA and genomic sequence of GhWRKY41 were isolated as previously described [23]. The primers that were used are shown in S1 Table. The GhWRKY41 cDNA sequence was cloned into the binary pBI121 vector at the XbaI and SalI sites; in this vector, the gene was under the control of the cauliflower mosaic virus 35S (CaMV35S) promoter. Thereafter, N. benthamiana was transformed with Agrobacterium tumefaciens (strain LBA4404) harboring the recombinant plasmid via the leaf disk method. Transgenic N. benthamiana seedlings were selected on MS agar medium containing 100 mg/L kanamycin and then transferred into soil in the greenhouse. The T3 progeny of transgenic seedlings were used for functional studies.
Subcellular localization of GhWRKY41
The open reading frame (ORF) of GhWRKY41 was inserted into the binary vector pBI121-GFP at the XbaI and XhoI sites, creating a fusion protein with green fluorescent protein (GFP) fused to the N-terminus of GhWRKY41. The fusion protein was under the control of the CaMV35S promoter. The recombinant plasmid was transformed into A. tumefaciens (strain GV3101). After overnight cell culture, A. tumefaciens was harvested by centrifugation and resuspended in infiltration media (for 100 mL: 1 mL of 1 M MES-KOH, pH 5.6, 333 μL of 3 M MgCl2, 100 μL of 150 mM acetosyringone). Five-week-old leaves of N. benthamiana were used for transformation [24]. The fluorescent signal of GhWRKY41-GFP was detected with a confocal microscope (LSM 510 META; Carl Zeiss) after 5 days of transformation. Leaves expressing the 35S-GFP construct were used as a control.
3, 3′-Diaminobenzidine (DAB) staining assay and measurement of malondialdehyde (MDA) and reactive oxygen species (ROS)-related enzyme activities
Accumulation of hydrogen peroxide (H2O2) was detected with DAB staining as previously described. In brief, N. benthamiana leaves were incubated in DAB solution (1 mg/mL, pH 3.8) for 24 h at 25°C in the dark. After staining, the leaves were soaked in 95% ethanol overnight to remove chlorophyll. MDA levels and catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX) activities were spectrophotometrically measured as previously described [25].
RNA isolation and quantitative real-time PCR (qPCR)
Total RNA was extracted from cotton and N. benthamiana using the cetyl-trimethyl-ammonium bromide (CTAB) method [26] and TRIzol reagent (Invitrogen, Carlsbad, CA, USA), respectively. First-strand cDNA was synthesized with EasyScript First-Strand cDNA Synthesis SuperMix (Transgen, Beijing, China). qPCR was subsequently performed to analyze the expression patterns of specific genes. G. hirsutum ubiquitin rRNA, G. hirsutum 18S rRNA and N. benthamiana β-actin genes were used separately as the standard controls. The following thermocycler program was used for amplification: 94°C for 5 min, followed by 26–32 cycles of 94°C for 40 s, 50–55°C for 40 s and 72°C for 40 s. Semi-quantitative RT-PCR was used to detect the expression levels of GhWRKY41 in the T1 progeny of transgenic plants and under Rhizoctonia solani treatment. G. hirsutum 18S rRNA and N. benthamiana β-actin genes were used separately as the standard controls. The primers used for qPCR are listed in S2 Table.
β-Glucuronidase (GUS) histochemical staining assay
Transgenic Arabidopsis plants harboring a ProGhWRKY41::GUS construct were generated via the floral dip method. T3 progeny were used to analyze promoter activity and were stained with GUS histochemical staining buffers as previously described [27].
Determination of stomatal aperture
Expanded leaves were floated in opening buffer (30 mM KCl and 10 mM MES-KOH, pH 6.15) for 2.5 h under a cool white light, and then the appropriate concentrations of ABA, PEG or NaCl solution were added to the opening buffer. After 2 h, the stomatal apertures were measured under a microscope. The aspect ratio was determined using the image processing software ImageJ.
Transcriptional activation analysis and yeast one-hybrid assay
To investigate its transcriptional activation, the ORF of GhWRKY41 was amplified using primers containing NcoI and BamHI sites. The fragment was then introduced into the bait plasmid pGBKT7 (Clontech) and fused to the GAL4 DNA-binding domain. pGBKT7-GhWRKY41 and the negative control pGBKT7 were transformed into the Y2H Gold yeast strain. The transformed cells were plated onto synthetic defined (SD) medium without tryptophan to identify positive transformants. Then, the positive transformants were grown on SD medium lacking tryptophan, histidine and adenine. The primers used in the transcriptional activation analysis are listed in S1 Table.
To investigate the DNA-binding activity of GhWRKY41, a yeast one-hybrid kit was used in accordance with the manufacturer’s protocol (Clontech, USA). The W-box and mW-box fragments were cloned into the pAbAi vector. The background AbAr expression of the strains was tested. The pGADT7 prey vector harboring the GhWRKY41 ORF (pGAD-GhWRKY41) was transformed into the aforementioned two strains. Yeast cells were also transformed with the empty pGADT7 vector as a control. The transformed yeast cells were first grown in leucine (Leu)- and uracil (Ura)-deficient SD medium (SD/-Leu/-Ura) to ensure the success of the transformation; they were then grown in leucine (Leu)-deficient SD medium (SD/-Leu) containing AbA (500 ng/mL). After culturing at 30°C for 4 days, the plates were examined.
Results
Sequence characterization of GhWRKY41
Previous studies have shown that group III WRKY family members play important roles in plant defense responses. To clarify the function of this family in cotton, we sought to isolate group III WRKY genes by using homology-based cloning methods. Based on the conserved WRKY domain sequence, a pair of degenerate primers, M1 and M2, was designed to isolate the internal conserved fragment of the cotton WRKY family. The primers 5P1/2, 5P4/4, 3P1/2 and Q1/2 were designed to perform 5′ rapid amplification of cDNA ends (RACE)-PCR, 3′ RACE-PCR and identification of the full-length cDNA sequence.
Full-length GhWRKY41 cDNA (GenBank accession number: 1693190) contains 1,231 nucleotides, including a 1,068-bp ORF, 36-bp 5′-untranslated region (5′-UTR) and a 127-bp 3′-UTR that was predicted to encode a 355-amino acid protein with a predicted molecular mass of 40 kDa and an isoelectric point of 5.42. A comparison of the protein sequences of GhWRKY41 and other plant WRKY proteins using DNAMAN demonstrated that GhWRKY41 shared high homology with other WRKY proteins; specifically, it was 66.11% homologous to PtWRKY12 (ACV92014.1) (Populus tomentosa), 63.09% to BgWRKY (BAG15875.1) (Bruguiera gymnorrhiza), 54.85% to GmWRKY41 (XP_003530379.1) (Glycine max) and 41.58% to AtWRKY41 (NP_192845.1) (Arabidopsis thaliana). The predicted GhWRKY41 protein contains a WRKY domain of approximately 60 amino acids that is composed of a conserved amino acid sequence (WRKYGQK) and a zinc-finger motif (C-C-H-C), indicating that GhWRKY41 belongs to group III of the WRKY family. Moreover, GhWRKY41 contains a nuclear localization signal, KKRK (S1A Fig).
To investigate the evolutionary relationships between WRKY proteins from different species, a phylogenetic analysis was performed based on their amino acid sequences using the software MEGA version 4.1 and the Neighbor-Joining method. The results demonstrated that GhWRKY41 was closely related to group III WRKY family members, including PtWRKY12, BgWRKY and CaWRKY30 (Capsicum annuum). These results further suggest that GhWRKY41 belongs to the group III WRKY family (S1B Fig).
GhWRKY41 is localized to the nucleus and demonstrates transcriptional activation and DNA binding activity
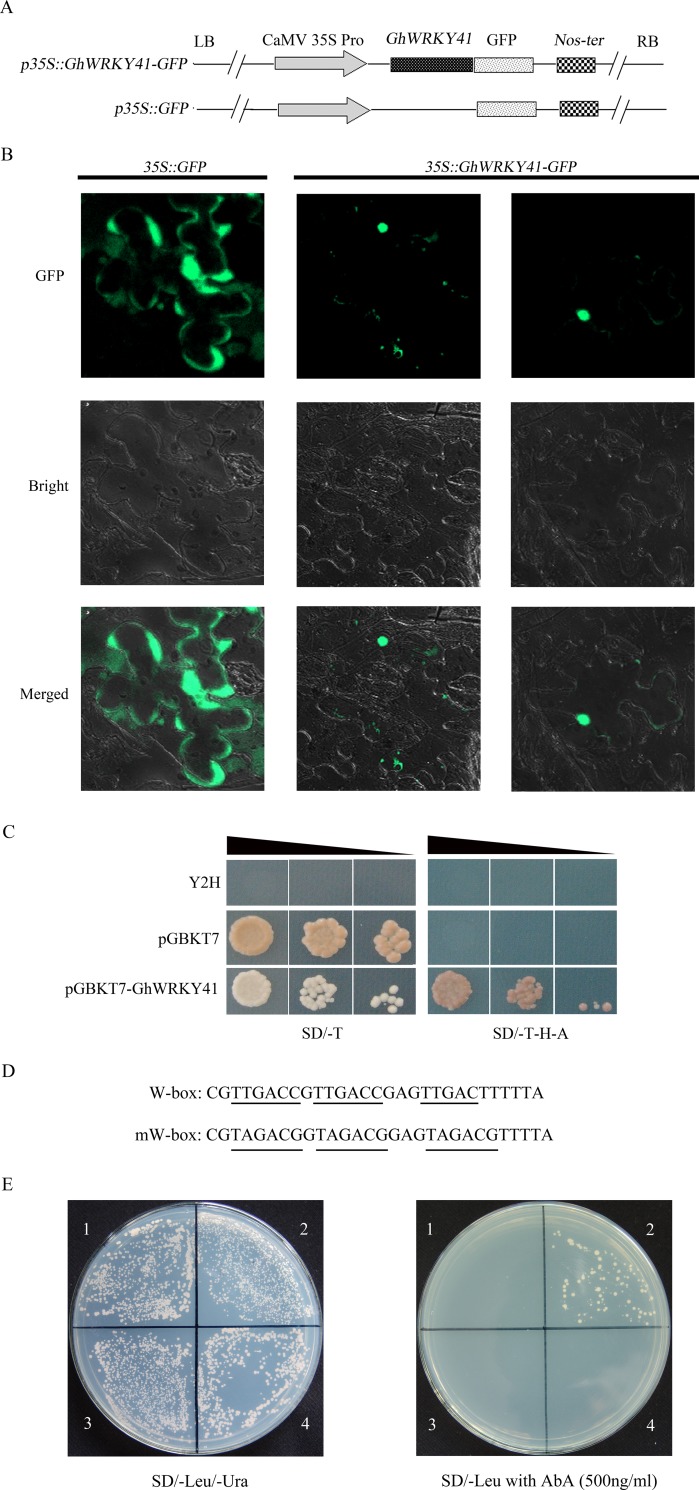
Results from the online protein subcellular location predictors Plant-mPloc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) and PSORT (http://psort.hgc.jp/form.html) suggested that GhWRKY41 was located in the nucleus. Additionally, a putative nuclear localization signal (KKRK) was identified in GhWRKY41. To confirm the nuclear localization of GhWRKY41, we constructed a vector that expressed a GhWRKY41-GFP fusion protein under the control of the CaMV35S promoter. A vector expressing p35S::GFP was also constructed to serve as the control. Plasmid vectors were individually introduced into N. benthamiana leaves, and fluorescence was observed with a confocal laser scanning microscope. The results showed that the GhWRKY41-GFP fusion protein emitted green fluorescence predominately in the nuclei, whereas GFP alone was found in multiple subcellular compartments, including the cytoplasm and nucleus. These results suggest that GhWRKY41 is a nuclear-localized protein (Fig 1A and 1B).
Fig 1. Characterization of GhWRKY41 as a transcriptional regulator.
(A) Schematic diagrams of the p35S::GhWRKY41-GFP fusion construct and the control p35S::GFP construct. (B) Transient expression of the p35S::GhWRKY41-GFP and p35S::GFP constructs in N. benthamiana. Green fluorescence was observed using a confocal microscope five days after transformation. (C) GhWRKY41 demonstrates transactivation activity. The full-length ORF of GhWRKY41 was subcloned into pGBKT7, and transformed yeast was selected on both SD-Trp and SD-Trp-His-Ade media. Positive transformants were further identified by spotting serial yeast dilutions (1/1, 1/10 and 1/100). The triangle indicates the dilutions from 1 to 100. (D) Sequences of three tandem W-boxes (TGAC) and mW-boxes. (E) Yeast one-hybrid assays. (1) pAbAi-Wbox + pGADT7. (2) pAbAi-Wbox + pGAD-GhWRKY41. (3) pAbAi-mWbox + pGADT7. (4) pAbAi-mWbox + pGAD-GhWRKY41.
To characterize the transcriptional activation activity of the GhWRKY41 protein, the plasmids pGBKT7-GhWRKY41 and pGBKT7 (negative control) were transformed into the yeast strain Y2H containing the upstream activating sequence (Clontech, UAS), which is specifically bound by the GAL4 binding domain. All transformants grew well on selective medium without tryptophan (SD-Trp). Moreover, transformants harboring pGBKT7-GhWRKY41 grew on selective medium lacking tryptophan, histidine and adenine (SD-Trp-His-Ade), but transformants harboring pGBKT7 did not grow on this media. These results indicated that GhWRKY41 could transactivate the expression of both HIS3 and ADE2 reporter genes in yeast, suggesting that GhWRKY41 may be a transcriptional activator (Fig 1C).
A yeast one-hybrid assay was performed to test the interaction between GhWRKY41 and the W-box cis-element. Yeast strains harboring the W-box and mW-box constructs did not grow in SD/-Ura containing AbA (500 ng/mL). Therefore, 500 ng/mL AbA can suppress the basal expression of the pAbAi vector. pGAD-GhWRKY41 was transformed into the two yeast strains. The empty vector (pGADT7) was also introduced into the two yeast strains to serve as a control. As shown in Fig 1E, all of the yeast cells grew in SD/-Leu/-Ura. However, only the yeast cells carrying the W-box construct and pGAD-GhWRKY41 grew in SD/-Leu containing AbA (500 ng/mL). These data indicated that GhWRKY41 specifically binds to the W-box in yeast.
Constitutive expression of GhWRKY41 in whole plants
A 1024-bp GhWRKY41 promoter fragment was isolated using the hiTAIL-PCR method and analyzed using PLACE and PlantCARE online software. Many abiotic stress-, biotic stress- and development-related cis-acting elements were identified (S3 Table).
To determine the expression pattern of GhWRKY41, the isolated promoter sequence of GhWRKY41 was cloned into the pBI121 vector to drive the expression of the GUS protein.
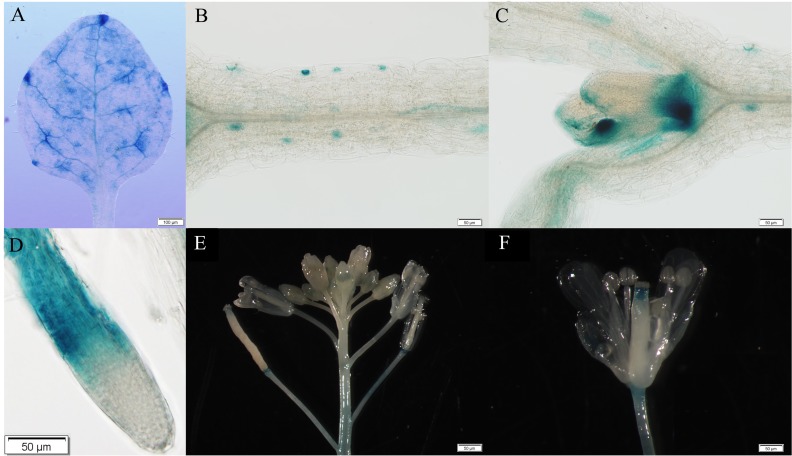
The staining assay showed that GhWRKY41 was expressed in most plant organs, including leaves, stems and roots (Fig 2A–2D). During the reproductive growth stage, staining was observed in the lower portion of the stigma, but no GUS signal was observed in pods (Fig 2E and 2F). Interestingly, a strong GUS signal was observed at the nodes, indicating probable roles for GhWRKY41 in plant development (Fig 2C).
Fig 2. Spatiotemporal expression patterns of ProGhWRKY41::GUS in transgenic Arabidopsis.
(A) Leaf, (B) stem, (C) bottom of bud, (D) root, (E) flower and (F) young silique flower. Bars are presented in the lower left corner.
Expression of GhWRKY41 is regulated by multiple abiotic stresses and signaling factors
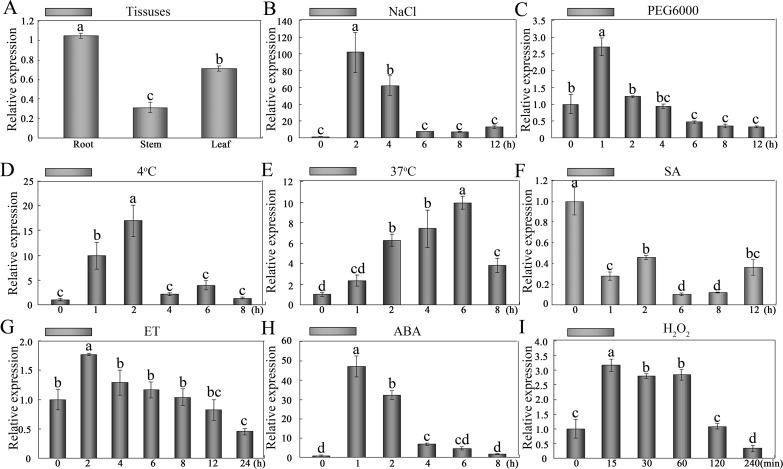
qPCR was performed to investigate the expression pattern of GhWRKY41. The expression of GhWRKY41 was higher in the leaf and root compared with the stem (Fig 3A). To further examine GhWRKY41, its transcript levels under treatment with various abiotic stresses, hormones and molecular signaling factors were analyzed by qPCR. As shown in Fig 3B–3E, abiotic stresses, including salt, drought, cold (4°C) and heat (37°C), markedly induced the expression of GhWRKY41. However, the induction patterns were different. NaCl treatment caused rapid, transient and powerful induction of GhWRKY41. Induction by PEG was also transient and rapid. mRNA began to accumulate immediately upon heat treatment and reached a maximum level at 6 h. Under cold treatment, transcript levels were increased at 1 h after the initiation of treatment and reached a maximum at 2 h.
Fig 3. Relative expression of GhWRKY41 in different tissues and in response to different stress factors.
Quantification of GhWRKY41 band intensity based on the expression of GhUbiquitin rRNA genes using Quantity One software. The roots, stems and cotyledons of 7-day-old cotton seedlings were used to assess the tissue-specific expression of GhWRKY41 (A). The seedlings were treated with 200 mM NaCl (B), with 15% PEG (C), at 4°C (D), at 37°C (E), with 2 mM salicylic acid (SA) (F), with 5 mM ethylene (ET) released from ethephon (G), with 10 μM abscisic acid (ABA) (H) or with 10 mM H2O2 (I).
Plant hormone and signaling factors are involved in multiple processes and regulate various signaling pathways. We also characterized the gene expression of GhWRKY41 under different hormone treatments. As shown in Fig 3F–3I, under ethylene (ET) treatment, the expression level of GhWRKY41 reached a peak at 2 h and then decreased back to its original level. ABA and H2O2 treatment also induced gene expression. However, salicylic acid (SA) treatment significantly repressed gene expression.
Overexpression of GhWRKY41 enhances drought tolerance of transgenic tobacco
To further investigate the function of GhWRKY41 in plants, we constructed transgenic N. benthamiana plants that overexpressed GhWRKY41. The transgenic seedlings were characterized by PCR. Seven independent T1 lines were selected on kanamycin, and the expression of GhWRKY41 was assessed. Based on the results, three representative lines (20, 24 and 28) that exhibited different expression levels were selected for further research. Lines 20, 24 and 28 were renamed OE1, OE2 and OE3, respectively, and T3 progeny of these lines were used for functional analysis (S2 Fig).
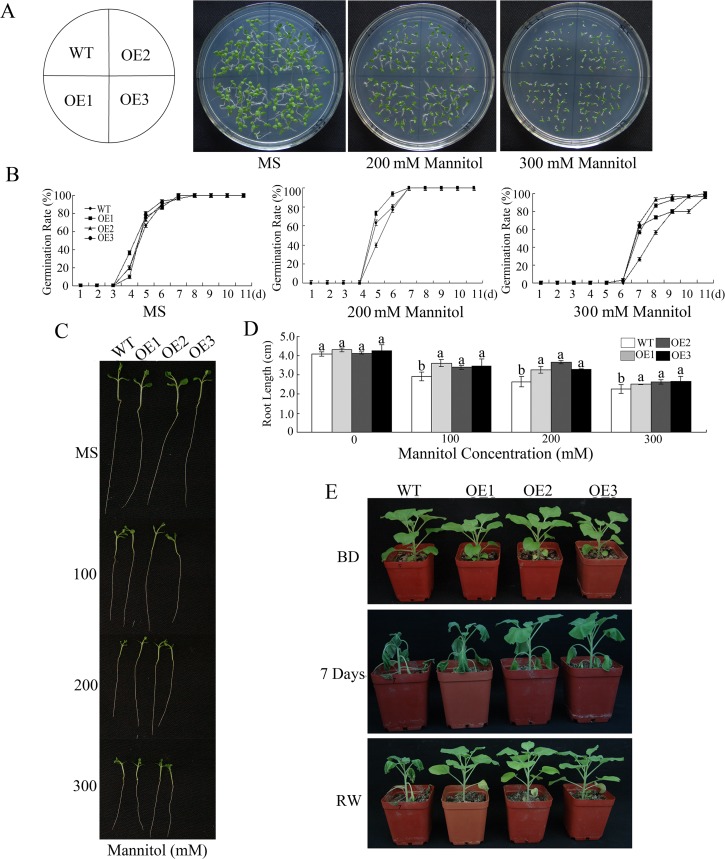
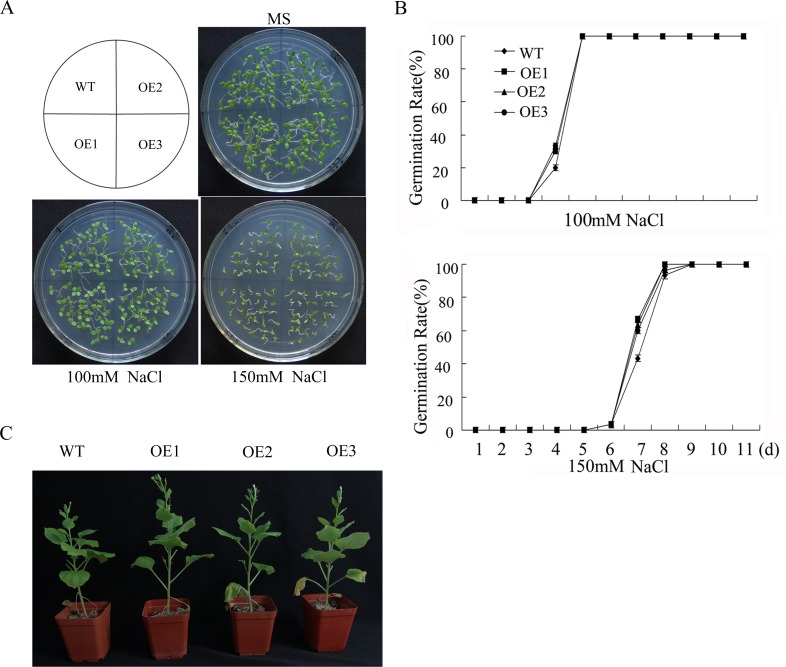
To evaluate the influence of GhWRKY41 on plant tolerance to drought, mannitol was used to mimic a water deficit. Transgenic tobacco seeds were surface-sterilized and germinated on MS agar medium containing 0, 200 or 300 mM mannitol. As shown in Fig 4A, there was no visible difference between the wild-type (WT) and transgenic (OE) lines on medium without mannitol. However, when the concentration of mannitol reached 200 mM, the transgenic lines began to exhibit earlier germination compare with the WT line, although all plants eventually showed a similar germination ratio (Fig 4B). Next, WT and OE seeds were germinated on MS agar medium and transferred to medium that contained mannitol to assay their growth after germination. The results showed that the root lengths of the WT and OE lines were similar on MS medium, but OE plants exhibited longer roots than WT plants on medium with mannitol (Fig 4C and 4D).
Fig 4. Drought tolerance test comparing wild-type and GhWRKY41-overexpressing N. benthamiana plants.
(A, B) Seed germination assay. (C) Post-germination seedling development of the WT and OE lines on MS supplemented with different concentrations of mannitol. (D) Primary root lengths of the seedlings 20 days after germination in the presence of different concentrations of mannitol. (E) Photograph of representative 8-week-old WT and OE plants grown in soil under drought conditions for 7 days and then watered for 3 days to allow them to recover.
To confirm GhWRKY41 function during vegetative growth, two-month-old plants grown in soil were deprived of water for drought treatment. As shown in Fig 4E, compared with WT plants, in which whole plants exhibited a severe wilt phenotype, transgenic plants suffered a slight wilt. Furthermore, transgenic plants recovered more rapidly than WT plants when they were rewatered 3 days following drought treatment.
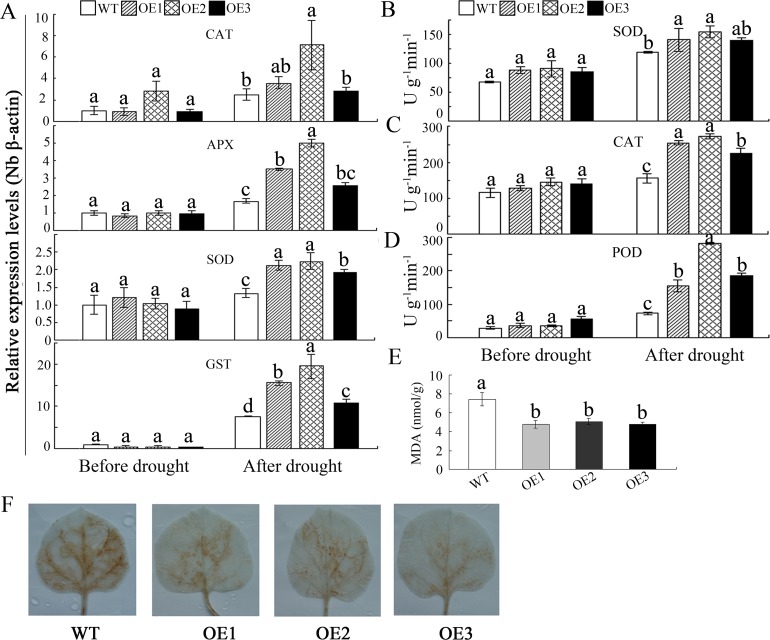
To investigate whether the tolerance of transgenic plants was related to oxidative stresses, MDA and H2O2 contents were analyzed. Under normal conditions, there was no significant difference between the WT and OE lines (data not shown). After treatment, there were marked increases in MDA and H2O2 in both WT and OE plants; furthermore, H2O2 and MDA accumulated to much lower levels in the transgenic lines than in the WT line in response to drought stress (Fig 5E and 5F).
Fig 5. Drought tolerance of WT and GhWRKY41-overexpressing N. benthamiana plants in the vegetative stage.
(A) Expression levels of antioxidant enzyme genes. (B-D) Analysis of antioxidant enzyme activity. (E) Drought-induced MDA accumulation in the WT and OE lines. (F) Drought-induced H2O2 accumulation detected via DAB staining.
To investigate the possible underlying cause of the decreased H2O2 accumulation in the transgenic lines in response to drought stress, the activities of major antioxidant enzymes were measured. Upon exposure to drought stress, there were marked increases in the activities of SOD, CAT and peroxidase (POD) in both the WT and transgenic lines. However, these increases were significantly greater in the transgenic lines than in the WT lines in response to drought stress (Fig 5B–5D).
To reveal the molecular mechanisms underlying enhanced drought stress tolerance in the transgenic lines, the expression of several antioxidant enzyme genes was assessed by qPCR. Under normal conditions, there was no significant difference in the expression of these genes. Under drought treatment, the expression levels of genes that encoded ROS-scavenging enzymes, such as SOD, CAT, APX and glutathione S-transferase (GST), were significantly increased in the transgenic lines compared to the WT lines (Fig 5A).
Overexpression of GhWRKY41 enhances the salt tolerance of transgenic tobacco
Osmotic stress can be caused by several environmental cues, such as drought, high salinity and low temperature. To elucidate whether the activation of WRKY41 led to drought tolerance alone or whether tolerance to other abiotic factors could be achieved, WT and transgenic plants were exposed to high-salt stress.
Transgenic tobacco seeds were surface-sterilized and germinated on MS agar medium containing 0, 150 or 200 mM NaCl. As shown in Fig 6A and 6B, there was no visible difference between the WT and transgenic lines on medium without NaCl. However, when the concentration of NaCl reached 150 mM, the transgenic lines exhibited earlier germination than the WT lines, although all plants eventually showed a similar germination ratio. Two-month-old plants watered with a 200 mM NaCl solution for 1 month were photographed. As shown in Fig 6C, WT plants were shorter in height and showed severe wilt.
Fig 6. Salt tolerance of WT and GhWRKY41-overexpressing N. benthamiana plants.
(A, B) Seed germination assay. (C) Photograph of representative 8-week-old WT and OE plants watered with 200 mM NaCl for 1 month.
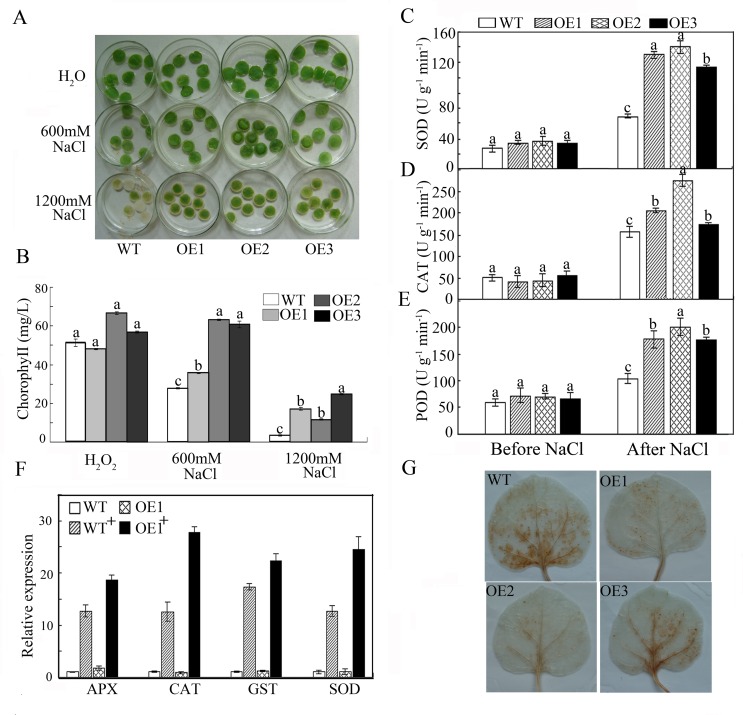
A leaf disk assay was performed to further study the function of GhWRKY41 at the vegetative stage. Leaf disks detached from two-month-old transgenic and WT plants were suspended in NaCl solutions ranging from 0 mM to 1,200 mM. Leaf disks from both transgenic and WT plants showed no significant changes in water with no added NaCl, although in the NaCl solutions, they all showed signs of bleaching. NaCl treatment caused severe damage in leaf disks from WT plants. This result was further confirmed by measuring leaf disk chlorophyll content before and after NaCl treatment (Fig 7A and 7B).
Fig 7. Salt tolerance of WT and GhWRKY41-overexpressing N. benthamiana plants in the vegetative stage.
(A) Leaf discs from WT and OE plants were incubated with NaCl at different concentrations (0, 600 or 1,200 mM) under greenhouse conditions. (B) Relative chlorophyll content was determined in the leaf discs of WT and OE plants following NaCl treatments. Disks floated in water were used as controls. The presented data are the means ± SE of three independent experiments (n = 6). (C-E) Analysis of stress-related enzyme activity. (F) Expression levels of stress-related genes. WT+ indicates the WT lines after NaCl treatment. OE1+ indicates the OE1 lines after NaCl treatment. (G) Salt-induced H2O2 accumulation detected via DAB staining.
To determine whether enhanced salt tolerance was related to oxidative stress, we measured the activities of major antioxidant enzymes. After salt treatment, there were marked increases in the activities of POD, CAT and SOD in both the WT and transgenic lines. However, these increases were significantly greater in the transgenic lines than in the WT lines in response to salt stress (Fig 7C–7E). The expression of several abiotic stress-response genes was assessed by qPCR, and the results were consistent with those of the enzyme activity assay (Fig 7F). To intuitively understand the oxidation states of the plants, leaves detached from transgenic and WT plants were stained with DAB. As shown in Fig 7G, WT plants stained darker than transgenic plants under salt stress.
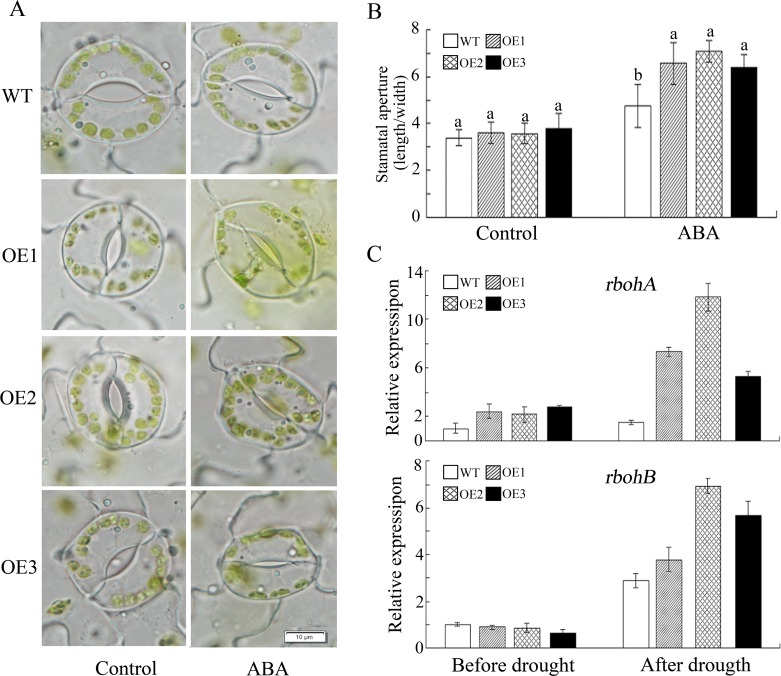
GhWRKY41 helps plants to cope with drought and salt stresses by enhancing stomata closure
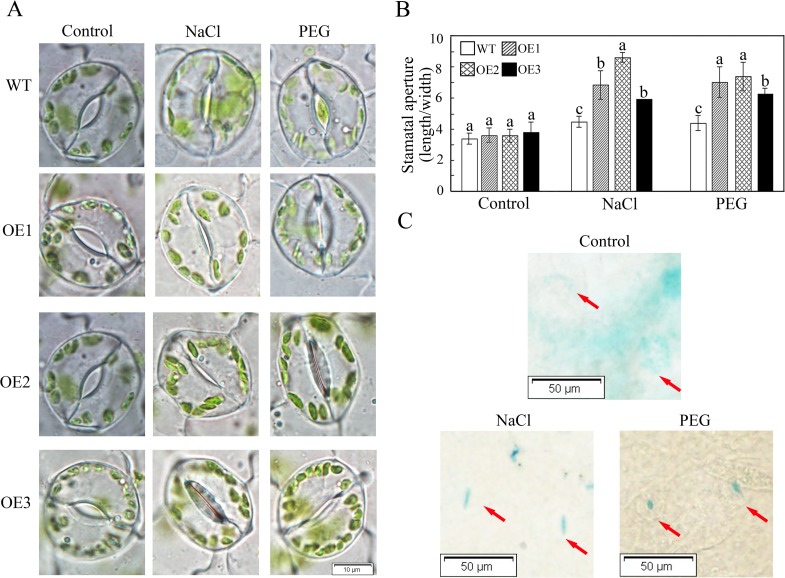
In response to drought or osmotic stress, plants are able to control their water content and reduce water loss. Thus, we explored the potential association of water balance regulation with the observed tolerance phenotype. To achieve this goal, we first compared the number of stomata per unit leaf area between WT and transgenic plants, but no significant differences were detected (data not shown). Then, we measured the stomatal aperture of the WT and transgenic lines under control and stress treatments. The transgenic lines exhibited a marked reduction in stomatal aperture (Fig 8A and 8B). And the stomatal conductance was tested by a leaf disk desiccation assay. The result showed that the stomatal conductance was significantly reduced in transgenic plants (S3 Fig). Moreover, in Arabidopsis transformed with proGhWRKY41::GUS and treated with salt and PEG, a GUS staining assay suggested that GhWRKY41 was highly expressed in stomata (Fig 8C). These results may indicate that GhWRKY41 enhances drought and salt stresses by regulating stomatal movement.
Fig 8. GhWRKY41 regulates stomatal movement.
(A) Comparison of stomatal aperture in response to salt and drought. (B) Stomatal aperture data were calculated from 50 stomata from the leaves of three different plants. Values are the mean ± SD. (C) GUS staining of leaves from transgenic Arabidopsis exposed to salt and drought treatments.
GhWRKY41 enhances osmotic tolerance in an ABA-dependent manner
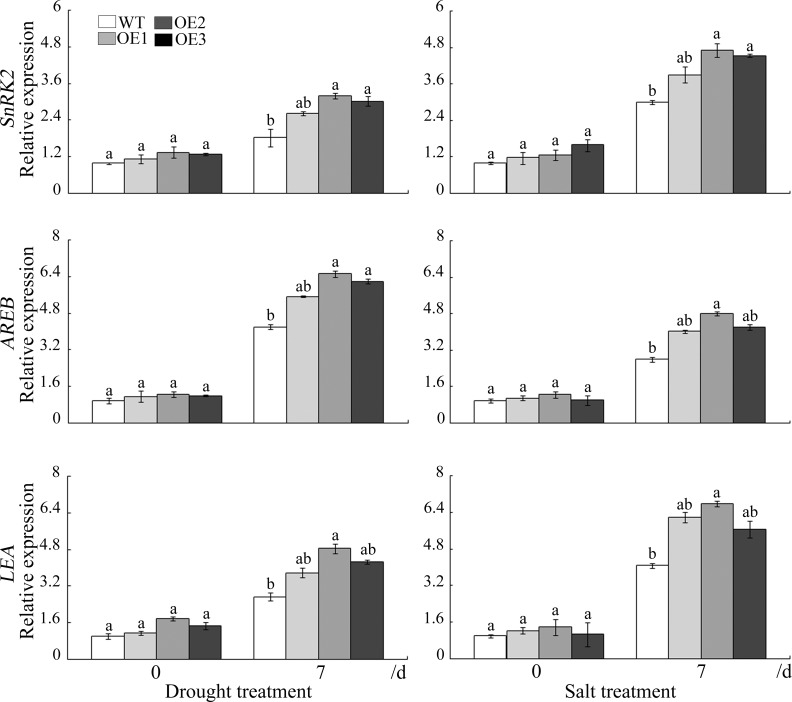
It is widely known that ABA participates in the regulation of stomatal movement. To explore whether GhWRKY41-dependent stomatal closure is related to ABA, we analyzed stomatal movement with or without ABA treatment. We found that ABA treatment for 2.5 h reduced the stomatal apertures of the WT and OE lines (Fig 9A and 9B). This reduction was more severe in the OE lines. In addition, the expression of rbohA and rbohB, which are reported to be involved in ABA signaling, was upregulated in the OE lines under drought stress (Fig 9C). The expression of genes associated with ABA signaling, including SnRK2 (SNF1-related protein kinase 2), AREB (ABA-responsive element binding protein) and LEA (late embryogenesis abundant protein), was also analyzed under drought and salt stress. As shown in Fig 10, the expression of these genes was significantly induced by drought or salt treatment. These results indicated that ABA participates in GhWRKY41-induced stomatal closure.
Fig 9. GhWRKY41 regulates stomatal movement in an ABA-dependent manner.
(A, B) Comparison of stomatal aperture in response to ABA treatment. (C) Expression pattern of rbohA and rbohB genes in WT and OE plants following drought stress. Data were calculated from 50 stomata from the leaves of three different plants. Values are the mean ± SD.
Fig 10. The expression of genes associated with ABA signaling in WT and OE plants following drought or salt stress.
Discussion
WRKY transcription factors constitute a large family in plants. Members of this family have been reported to be involved in various stress-related responses [28, 29]. However, the functions of WRKY proteins in non-model plants have remained unclear. In the present study, GhWRKY41 was isolated from cotton, a crop with critical economic importance. One WRKY domain and a typical group III C2HC zinc finger domain [30] were present in the GhWRKY41 sequence. Amino acid comparisons and phylogenetic analysis indicated that GhWRKY41 showed high similarity with PtWRKY12, BgWRKY, GmWRKY41 and AtWKRY41, all of which belong to the group III WRKY family. These results indicated that GhWRKY41 is a group III WRKY transcription factor.
The WRKY domain present in WRKY proteins can bind to W-box cis-acting elements that are mainly located in PR proteins. For this reason, WRKY proteins have been rigorously analyzed to determine their function in the plant defense response [31]. Moreover, Kalde et al. systematically analyzed the response of 13 Arabidopsis group III WRKY genes to SA and pathogens, and the results demonstrated that the majority of this subgroup could be upregulated by these treatments [32]. In our study, GhWRKY41 exhibited significant changes in response to osmotic stresses. This observation may indicate that GhWRKY41 has a novel role as a member of the group III family of proteins.
In addition to their roles in biotic resistance, the roles of WRKY proteins in abiotic tolerance have also been studied [33, 34]. The expression of GhWRKY41 was induced by osmotic stresses such as salt and drought. This induction is similar to that of BcWRKY46 and DgWRKY3 [35, 36]. Moreover, the in vivo role of GhWRKY41 in plant abiotic tolerance was clearly demonstrated in transgenic tobacco lines overexpression GhWRKY41. Under drought and salt stress conditions, WT plants were smaller, more withered and more chlorotic, while GhWRKY41-expressing tobacco plants were better able to adapt to these stresses. Similarly, constitutive expression of different WRKY genes, including VpWRKY2 and GsWRKY20, also resulted in greater tolerance to abiotic stresses in transgenic lines [18, 37]. Therefore, we conclude that GhWRKY41 is a functional WRKY transcription factor involved in the response to salt and drought stress.
ROS such as superoxide, hydrogen peroxide and hydroxyl radicals play a dual role in plants: they act as necessary signaling molecules, but they can cause damage to plant cells when overproduced under stress conditions [38]. Plants must maintain a ROS balance to minimize cellular damage caused by stress. Among the variety of ROS compounds, the present study investigated the effects of H2O2 in abiotic stress-resistant plants. Our study found that GhWRKY41-overexpressing plants exhibited lower H2O2 accumulation than WT plants under drought and salt stress conditions. MDA is widely recognized as a parameter that reflects lipid peroxidation [39]. GhWRKY41 might protect plants by promoting the degradation of MDA. Our results showed that overexpression of GhWRKY41 reduced the accumulation of H2O2 in transgenic seedlings. Moreover, the expression of GhWRKY41 was induced by treatment with H2O2. Consequently, we infer that overexpression of GhWRKY41 in tobacco confers drought and salt stress tolerance by promoting ROS elimination.
When plants exposed to stress overproduce ROS, they must develop tactics to scavenge ROS and protect cells. Enzymes such as SOD, CAT and POD act as ROS scavengers. An analysis of these enzymes showed that overexpression of GhWRKY41 markedly strengthened their activity under drought and salt stresses. At the molecular level, the expression of genes encoding these enzymes was also upregulated under stress. Thus, overexpression of GhWRKY41 may regulate these genes and induce the activity of a more efficient antioxidant system to counteract the oxidative stress evoked by salt and drought stress.
ABA acts as an important regulator and plays a complex role in abiotic stress signaling [40]. Various environmental stresses result in the rapid accumulation of ABA, leading to stomatal closure and the inhibition of stomatal opening, which reduces water loss by transpiration [41]. It has been reported that reduction of the water loss rate is a major factor contributing to drought tolerance, and transpirational water loss through the stomata is a key determinant of drought tolerance [19]. Li et al. reported that AtWRKY54 and AtWRKY70 enhanced tolerance to osmotic stress by regulating stomatal aperture. Salt stress is often accompanied by drought stress, and both stresses cause water deprivation through ABA-dependent and ABA-independent pathways [42]. A recent study showed that transient alkalinization, a remote effect of chloride stress, modulates the compartmental distribution of ABA between the leaf apoplast and the guard cells and is instrumental in inducing stomata closure during the initial stages of salt stress [43]. In our study, compared to the WT line, the transgenic lines showed enhanced stomatal closure under drought stress, salt stress and ABA treatment. ABA controls stomatal movement via a dual mechanism. Regulation can occur via the biochemical effects of ABA on guard cells, or it can occur via a decrease in water content in leaf vascular tissues [44]. Respiratory burst oxidase homolog proteins (rbohs) are NAD(P)H oxidase homologues that have a direct regulatory effect on Ca2+ and on the activity of the NADPH oxidase in plants [45]. Many rbohs can promote ABA-induced stomatal closing, ROS production, ABA-induced cytosolic Ca2+ increases and ABA activation of plasma membrane Ca2+-permeable channels in guard cells in Arabidopsis [46]. The GUS staining assay indicated that salt and drought stresses enhanced GhWRKY41 expression in guard cells. The upregulation of rbohA and rbohB expression in the OE lines under drought stress indicated that the overexpression of WRKY41 could enhance rbohA and rbohB activities to upregulate ROS production in the OE lines, thereby enhancing stomatal closure. However, darker DAB staining was observed in the WT lines. This result may be caused by antioxidant enzymes. These data suggest that GhWRKY41 acts as a positive regulator of stomatal closure in an ABA-dependent manner.
In summary, this study identified GhWRKY41 as a positive regulator of salt and drought stress responses. Overexpression of GhWRKY41 in tobacco improved plant tolerance to salt and drought stresses. This enhanced drought/salt tolerance in OE transgenic plants was associated with enhanced expression of several known stress-responsive genes and enhanced stomata closure, suggesting that overexpression of GhWRKY41 may lead to better osmotic adjustment, reduced membrane damage, and minimized oxidative stress. It should be noted that the function of GhWRKY41 in drought and salt tolerance must be directly addressed in cotton plants in the future. Additional work is also necessary to understand the molecular mechanisms of GhWRKY41 in salt and drought stress responses.
Supporting Information
(DOC)
(DOC)
(DOC)
(A) Alignment of the deduced GhWRKY41 protein sequence with other known WRKY homologs proteins. Identical amino acids are highlighted in blue. The WRKYGQK amino acids are boxed. The C and H residues in the zinc-finger motif are marked by a triangle. The nuclear localization signal (NLS), KKRK, is marked by an asterisk. (B) Phylogenetic analysis of the GhWRKY41 protein.
(TIF)
(A) Evaluation of GhWRKY41 expression in the T1 progeny of transgenic plants. (B) Evaluation of GhWRKY41 expression in the T3 progeny of three independent transgenic lines.
(TIF)
(TIF)
Acknowledgments
This work was financially supported by the National Science Foundation of China (grant Nos. 31171837 and 31471424).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the National Science Foundation of China (grant nos. 31171837 and 31471424).
References
- 1. Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, et al. (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dow GJ, Berry JA, Bergmann DC (2014) The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana . New Phytol 201:1205–1217. 10.1111/nph.12586 [DOI] [PubMed] [Google Scholar]
- 3. Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417. [DOI] [PubMed] [Google Scholar]
- 4. Jones RJ, Mansfield TA (1970) Suppression of Stomatal Opening in Leaves Treated with Abscisic Acid. J Exp Bot 21:714–719. [Google Scholar]
- 5. Zhu J- K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirayama T, Shinozaki K (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12:343–351. [DOI] [PubMed] [Google Scholar]
- 7. Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran L- SP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202:35–49. 10.1111/nph.12613 [DOI] [PubMed] [Google Scholar]
- 8. Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12:419–426. [DOI] [PubMed] [Google Scholar]
- 9. Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, et al. (2004) A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress signaling pathway. The Plant Journal 39:863–876. [DOI] [PubMed] [Google Scholar]
- 10. Li H, Sun J, Xu Y, Jiang H, Wu X, Li C (2007) The bHLH-type transcription factor AtAIB positively regulates ABA response in Arabidopsis . Plant Mol Biol 65:655–665. [DOI] [PubMed] [Google Scholar]
- 11. Jiang W, Yu D (2009) Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol 9:96 10.1186/1471-2229-9-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishiguro S, Nakamura K (1994) Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and β-amylase from sweet potato. Molecular and General Genetics MGG 244:563–571. [DOI] [PubMed] [Google Scholar]
- 13. Eulgem T, Rushton P, Robatzek S, Somssich I (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206. [DOI] [PubMed] [Google Scholar]
- 14. Johnson C, Kolevski B, Smyth D (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. The Plant cell 14:1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A (2005) MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis . Proc Natl Acad Sci U S A. 102(48):17531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren XJ, Huang WD, Li WZ, Yu DQ (2010) Tobacco transcription factor WRKY4 is a modulator of leaf development and disease resistance. Biologia Plantarum 54(4):684–690. [Google Scholar]
- 17. Besseau S, Li J, Palva E (2012) WRKY54 and WRKY70 cooperate as negative regulators of leaf senescence in Arabidopsis thaliana . J Exp Bot 63:2667–2679. 10.1093/jxb/err450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo X, Bai X, Sun X, Zhu D, Liu B, Ji W et al. (2013) Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J Exp Bot 64:2155–2169. 10.1093/jxb/ert073 [DOI] [PubMed] [Google Scholar]
- 19. Li J, Besseau S, Toronen P, Sipari N, Kollist H, Holm L, et al. (2013a) Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis . New Phytol 200:457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niu CF, Wei W, Zhou QY, Tian AG, Hao YJ, Zhang WK et al. (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant, Cell Environ 35:1156–1170. [DOI] [PubMed] [Google Scholar]
- 21. Gao X, Wheeler T, Li Z, Kenerley C, He P, Shan L (2011) Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt . The Plant journal 66:293–305. 10.1111/j.1365-313X.2011.04491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sunilkumar G, Campbell L, Puckhaber L, Stipanovic R, Rathore K (2006) Engineering cotton seed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc Natl Acad Sci U S A. 103:18054–18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi J, Zhang L, An H, Wu C, Guo X (2011) GhMPK16, a novel stress-responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol Biol 12:22 10.1186/1471-2199-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang W, Yang B, Yu BJ, Zhou Z, Li C, Jia M et al. (2013) Identification and analysis of MKK and MPK gene families in canola (Brassica napus L.). BMC Genomics 14:392 10.1186/1471-2164-14-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Zhang L, Wang X, Zhang W, Hao L, Chu X et al. (2013b) Cotton GhMPK6a negatively regulates osmotic tolerance and bacterial infection in transgenic Nicotiana benthamiana, and plays a pivotal role in development. The FEBS journal 280:5128–5144. [DOI] [PubMed] [Google Scholar]
- 26. Lu W, Chu X, Li Y, Wang C, Guo X (2013) Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana . PLoS ONE 8(7):e68503 10.1371/journal.pone.0068503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jefferson R (1987) Assaying chimeric genes in plants: The GUS gene fusion system Plant. Mol Biol Report 5:387–405. [Google Scholar]
- 28. Chen L, Song Y, Li S, Zhang L, Zou C, Yu D (2012) The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta 1819:120–128. 10.1016/j.bbagrm.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 29. Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15:247–258. 10.1016/j.tplants.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 30. Maeo K, Hayashi S, Kojima-Suzuki H, Morikami A, Nakamura K (2001) Role of conserved residues of the WRKY domain in the DNA-binding of tobacco WRKY family proteins. Biosci Biotechnol Biochem 65:2428–2436. [DOI] [PubMed] [Google Scholar]
- 31. Cheng Y, Zhou Y, Yang Y, Chi YJ, Zhou J, Chen JY et al. (2012) Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol 159:810–825. 10.1104/pp.112.196816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalde M, Barth M, Somssich IE, Lippok B (2003) Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Mol Plant-Microbe Interact 16:295–305. [DOI] [PubMed] [Google Scholar]
- 33. Jiang Y, Deyholos M (2009) Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69:91–105. 10.1007/s11103-008-9408-3 [DOI] [PubMed] [Google Scholar]
- 34. Ramamoorthy R, Jiang SY, Kumar N, Venkatesh PN, Ramachandran S (2008) A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol 49:865–879. 10.1093/pcp/pcn061 [DOI] [PubMed] [Google Scholar]
- 35. Liu QL, Zhong M, Li S, Pan YZ, Jiang BB, Jia Y et al. (2013) Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol Biochem 69:27–33. 10.1016/j.plaphy.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 36. Wang F, Hou X, Tang J, Wang Z, Wang S, Jiang F et al. (2012) A novel cold-inducible gene from Pak-choi (Brassica campestris ssp. chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol Biol Rep 39:4553–4564. 10.1007/s11033-011-1245-9 [DOI] [PubMed] [Google Scholar]
- 37. Li H, Xu Y, Xiao Y, Zhu Z, Xie X, Zhao H et al. (2010) Expression and functional analysis of two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild Vitis pseudoreticulata. Planta 232:1325–1337. 10.1007/s00425-010-1258-y [DOI] [PubMed] [Google Scholar]
- 38. Xiong L, Schumaker K, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. The Plant cell 14 Suppl:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. [DOI] [PubMed] [Google Scholar]
- 40. Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network Annual Reviews. Plant Biology 61:651–679. [DOI] [PubMed] [Google Scholar]
- 41. Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608. 10.1093/jxb/err460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geilfus CM, Mithöfer A, Ludwig-Müller J, Zörb C, Muehling KH (2015) Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytologist 10.1111/nph.13507 [DOI] [PubMed] [Google Scholar]
- 44. Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B et al. (2013) The dual effect of abscisic acid on stomata. New Phytol 197:65–72. 10.1111/nph.12013 [DOI] [PubMed] [Google Scholar]
- 45. Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). The Plant journal 14(3):365–70. [DOI] [PubMed] [Google Scholar]
- 46. Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL et al. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. The EMBO journal 22:2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(A) Alignment of the deduced GhWRKY41 protein sequence with other known WRKY homologs proteins. Identical amino acids are highlighted in blue. The WRKYGQK amino acids are boxed. The C and H residues in the zinc-finger motif are marked by a triangle. The nuclear localization signal (NLS), KKRK, is marked by an asterisk. (B) Phylogenetic analysis of the GhWRKY41 protein.
(TIF)
(A) Evaluation of GhWRKY41 expression in the T1 progeny of transgenic plants. (B) Evaluation of GhWRKY41 expression in the T3 progeny of three independent transgenic lines.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.