Abstract
Previous studies have shown that the transcriptional regulator PsaR regulates the expression of the PsaR regulon consisting of genes encoding choline binding protein (PcpA), the extracellular serine protease (PrtA), and the Mn2+-uptake system (PsaBCA), in the presence of manganese (Mn2+), zinc (Zn2+), and cobalt (Co2+). In this study, we explore the Ni2+-dependent regulation of the PsaR regulon. We have demonstrated by qRT-PCR analysis, metal accumulation assays, β-galactosidase assays, and electrophoretic mobility shift assays that an elevated concentration of Ni2+ leads to strong induction of the PsaR regulon. Our ICP-MS data show that the Ni2+-dependent expression of the PsaR regulon is directly linked to high, cell-associated, concentration of Ni2+, which reduces the cell-associated concentration of Mn2+. In vitro studies with the purified PsaR protein showed that Ni2+ diminishes the Mn2+-dependent interaction of PsaR to the promoter regions of its target genes, confirming an opposite effect of Mn2+ and Ni2+ in the regulation of the PsaR regulon. Additionally, the Ni2+-dependent role of PsaR in the regulation of the PsaR regulon was studied by transcriptome analysis.
Introduction
Streptococcus pneumoniae, an encapsulated bacterium is a common cause of otitis media, bacterial meningitis, bacteremia, and pneumoniae, leading to millions of death every year, particularly in developing countries [1–3]. Although, S. pneumoniae has an asymptomatic association within the human nasopharyngeal cavity [4], it has also the ability to spread to other sites in the human body to cause severe infections [5–7]. The survival of S. pneumoniae in different niches inside the human body might depend on the availability of macro- and micro- nutrients on the respective infection sites. Metal ions are an integral part of nutrients, and play a vital role in the regulation of many cellular processes in S. pneumoniae [8–10]. The deprivation or excess of metal ions may result in impaired growth of bacterial cells [11]. Therefore, proper regulation of metal homeostasis is important for the survival of S. pneumoniae. For this purpose, S. pneumoniae possesses metal uptake and -efflux systems that are specific to different metal ions, including manganese (Mn2+), zinc (Zn2+), copper (Cu2+), and iron (Fe2+) [12–16]. These systems are tightly regulated by different transcriptional regulators in the presence of specific metal ions [9,10,16–18]. For example, the expression of the adc operon encoding Zn2+ transporters is repressed by transcriptional regulator AdcR in the presence of Zn2+ [10,19]. The cop operon, encoding proteins for Cu2+ homeostasis is activated by transcriptional regulator CopY in the presence of Cu2+ [16]. The expression of Mn2+ uptake system psaBCA is regulated by transcriptional regulator PsaR and is dependent on the balance between Mn2+, Zn2+, and cobalt (Co2+) [20,21].
The interference or competition of metal ions for metal-sensory proteins has been reported for many bacteria, including S. pneumoniae [9,16,21–25]. The interplay of metal ions on specific protein depends on the concentration of metal ions, the nature of the coordinating ligands [26–28], and the effect of the Irving-William stability series (where the order is Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+) on protein metal ion affinity [29]. In S. pneumoniae, the CopY-mediated expression of the Cu2+-efflux system depends on the availability of Cu2+ and Zn2+, where the Cu2+ induced expression of Cu2+-efflux system is nullified by the addition of high Zn2+ concentrations [16]. Similarly, the expression of the PsaR regulon (pcpA, psaBCA, and prtA) is repressed by Mn2+ and derepressed by Zn2+ [9]. In our previous study, we have demonstrated the opposite effect of Mn2+ and Co2+ in the regulation of the PsaR regulon [21]. Moreover, metal ions can also compete to bind with extracellular proteins, which ultimately results in the impaired homeostasis of other metal ions. In S. pneumoniae, Zn2+ and Cd2+ have been shown to cause intracellular Mn2+ deficiency [30,31]. Several proteins with ligase activity have been reported to bind with nickel (Ni2+) [32]. However, the role of Ni2+ in pneumococcal metabolism and virulence has not been determined.
Here, we used qRT-PCR, β-galactosidase assays, EMSAs, and ICP-MS analyses to investigate the role of Ni2+ in the regulation of the PsaR regulon in S. pneumoniae D39. Our results demonstrate that the expression of the PsaR regulon is highly derepressed in the presence of Ni2+ and that a high concentration of Ni2+ causes cell-associated Mn2+ deficiency in S. pneumoniae. Furthermore, an opposite effect of Mn2+ and Ni2+ on the PsaR-mediated expression of the PsaR regulon is found.
Material and Methods
Bacterial strains, growth conditions and DNA manipulation
All the bacterial strains and plasmids used in this study are listed in Table 1. S. pneumoniae D39 was grown in 1% Chelex 100 resin (Bio-rad)-treated Chemically Defined Medium (CDMchelex). Salts of metal ions, i.e. MnSO4 and NiSO4 were added separately as specified in the Results section. Escherichia coli strain EC1000 was cultured at 37°C. The following concentrations of antibiotics were used in the media for the selection of strains where necessary: tetracycline: 2.5 μg ml-1 for S. pneumoniae; chloramphenicol: 4 μg.ml-1 for Lactococcus lactis; and ampicillin; 100 μg.ml-1 for E. coli. Chromosomal DNA of S. pneumoniae D39 was used as a template for PCR amplification [33,34]. All bacterial strains used in this study were stored at -80°C in 10% (v/v) glycerol stock. Primers used in this study are based on the genome sequence of S. pneumoniae D39 and listed in Table 2.
Table 1. List of strains and plasmids used in this study.
| Strain/plasmid | Description | Source |
|---|---|---|
| S. pneumoniae | ||
| D39 | Serotype 2 strain, cps 2 | Laboratory of P. Hermans |
| RW100 | D39 ΔpsaR | [9] |
| RW104 | D39nisRK ΔbgaA::PprtA-lacZ; ErmR | [9] |
| RW109 | D39nisRK ΔpsaR ΔbgaA::PprtA-lacZ; ErmR | [9] |
| IM402 | D39 ΔbgaA::PpsaB-lacZ; TetR | [21] |
| IM403 | D39 ΔbgaA::PpcpA-lacZ; TetR | [21] |
| IM451 | RW100 ΔbgaA::PpsaB-lacZ; TetR | [21] |
| IM452 | RW100 ΔbgaA::PpcpA-lacZ; TetR | [21] |
| E. coli | ||
| EC1000 | KmR; MC1000 derivative carrying a single copy of the pWV1 repA gene in glgB | Laboratory collection |
| L. lactis | ||
| NZ9000 | MG1363 ΔpepN::nisRK | [39] |
Table 2. List of primers used in this study.
| Name | Nucleotide Sequence (5’→3’) |
|---|---|
| Primers for qRT-PCR | |
| prtA-F | GCAGCCTATGCCCCTAATG |
| prtA-R | GTTTTAGTGTCTATTACAGG |
| pcpA-F | CCAATCCTAGCAGATACTCC |
| pcpA-R | GTAGGAATCGTGAATGG |
| psaB-F | CCTCAGTGTCTCCTACAAAG |
| psaB-R | GGCAATTCGGTGTAAGG |
| psaC-F | CCATTTCCTACAAAATGCCTT |
| psaC-R | TCCAAAGACAATGGCTCC |
| psaA-F | CTCGTTCTCTTTCTTTCTG |
| psaA-R | CTTAACGTCTTCAGGAA |
| gyrA-F | CGAGGCACGTATGAGCAAGA |
| gyrA-R | GACCAAGGGTTCCCGTTCAT |
β-galactosidase assays
The β-galactosidase assays were performed as described before [35] by using derivatives of S. pneumoniae D39 grown till mid-exponential phase of growth (OD600 = 0.25) in triplicate in CDMchelex at 37°C supplemented with different metal ion concentrations (w/v) as mentioned in the Results section. Standard deviation was calculated from three independent replicates of each sample.
Quantitative real time (qRT)-PCR experiments
For qRT-PCR, S. pneumoniae D39 wild-type was grown in CDM with and without the addition of 0.3 mM Ni2+ and harvested at mid-exponential growth phase. RNA was isolated as described before [16]. Additionally, RNA was treated with DNase I (RNase-free) (Thermo Fisher Scientific, St. Leon-Rot, Germany) for 60 min at 37°C to remove any DNA contamination. qRT-PCR was performed in triplicates as described before [16]. The transcription level of the target genes was normalized to gyrA transcription using the relative expression software tool [36].
Inductively coupled plasma-mass spectrometry (ICP-MS) analysis
To measure the intracellular concentrations of metal ions, S. pneumoniae D39 was grown till OD600 = 0.2–0.25 in 20 ml CDMchelex supplemented with either 0.02 mM MnSO4, 0.02 mM MnSO4 + 0.1 mM NiSO4, 0.02 mM MnSO4 + 0.3 mM NiSO4, or 0.02 mM MnSO4 + 0.5 mM NiSO4. Cell cultures were washed twice with CDMchelex medium and twice with overnight Chelex (Sigma) treated phosphate-buffered saline (PBS) with 1 mM nitrilotriacetic acid. The cell pellets were dried overnight in a Speedvac at room temperature and lysed in 2.5% nitric acid (Ultrapure, Sigma Aldrich) for 10 min at 95°C by vigorous vortexing. ICP-MS analysis on the lysed cell samples were performed as described before [31]. Amounts of metal ions are expressed in the Result section as μg g-1 dry weight of cells.
DNA Microarray Analysis
To observe the impact of the psaR deletion on the transcriptome of S. pneumoniae in the presence of Ni2+, S. pneumoniae D39 wild-type and its isogenic psaR mutant (RW100) [9] were grown in two biological replicates in CDMchelex with 0.3 mM of NiSO4. (H2O)6. Cells were harvested at the mid-exponential growth phase. Further experiments were performed essentially as described before [37]. DNA microarray data were analyzed by using the MicroPrep software package as described before [38]. To identify differentially expressed genes a Bayesian p-value <0.001 and a fold-change cut-off of ≥ 2 were applied. The DNA microarray data have been deposited to Gene Expression Omnibus (GEO) with accession number GSE73818.
Purification of Strep-tagged PsaR and Electrophoretic mobility shift assays
The overexpression and purification of C-terminally Strep-tagged PsaR was achieved in L. lactis NZ9000 essentially as described before [9,39]. Electrophoretic mobility shift assays (EMSAs) were performed essentially as described previously [10]. In short, PCR products of PpcpA, PpsaB, PprtA, and PadcR were labeled with [γ-33P] ATP. EMSAs were carried out in buffer containing 20 mM Tris-HCL (pH 8.0), 5mM MgCl2, 8.7% (w/v) glycerol, 62.5 mM KCl. 25 μg/ml bovine serum albumin, 25 μg/ml poly (dI-dC), and 5000 cpm of [γ-33P] ATP-labeled PCR product. Reactions were incubated at 30°C for 30 min before loading on gels. Gels were run in 1 M Tris-borate buffer (PH 8.3) at 95 V for 90 min.
Results
Ni2+-dependent expression of the PsaR regulon in S. pneumoniae
In a previous study, we have shown that, like Zn2+, Co2+ also induces the expression of the PsaR regulon, while addition of Mn2+ causes repression of the PsaR regulon [21]. The PsaR regulon comprises the psa operon (psaBCA), encoding Mn2+-dependent ABC transporters, pcpA, encoding a choline binding protein and prtA, encoding a serine protease. In this study, we decided to explore the impact of Ni2+ on the expression of the PsaR regulon. To investigate the impact of Ni2+ on the expression of the PsaR regulon, cells were grown in CDM with either 0 or 0.3 mM Ni2+, and qRT-PCR was performed. qRT-PCR data revealed that the expression of pcpA, psaBCA, and prtA was highly upregulated in the presence of 0.3 mM Ni2+ compared to 0 mM Ni2+ (Table 3), suggesting the putative role of Ni2+ in the regulation of the PsaR regulon.
Table 3. The relative expression of prtA, psaB, psaC, psaA, and pcpA genes was normalized with the housekeeping gene gyrA.
The log2 fold increase is relative to the expression in the D39 wild-type grown in CDMchelex with 0.3 mM Ni2+ to that with 0 mM Ni2+. Standard deviation of three independent replications is given in parentheses.
| Gene tag a | Function b | Fold Raito |
|---|---|---|
| spd_0558 | Cell wall-associated serine protease PrtA | 4.53 (1.22) |
| spd_1461 | Manganese ABC transporter, ATP-binding protein, PsaB | 2.83 (0.24) |
| spd_1462 | Manganese ABC transporter, permease protein, PsaC | 3.15 (0.30) |
| spd_1463 | Manganese ABC transporter, ATP-binding protein, PsaA | 5.88 (1.55) |
| spd_1965 | Choline binding protein PcpA | 10.53 (3.09) |
To further verify the role of Ni2+ in the regulation of the PsaR regulon in S. pneumoniae, the D39 wild-type strain containing either PpcpA-lacZ, PpsaB-lacZ, or PprtA-lacZ was grown in CDMchelex and CDMchelex-Mn2+ (CDMchelex without Mn2+) with the addition of 0, 0.1, 0.3 or 0.5 mM Ni2+, and β-galactosidase assays were performed. Our β-galactosidase data (Miller Units) revealed that the expression of the PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ increased significantly with increasing concentrations of Ni2+ in CDMchelex and CDMchelex-Mn2+ (Table 4). However, the expression of these transcriptional lacZ-fusions was much higher in CDMchelex-Mn2+ compared to CDMchelex due to the unavailability of Mn2+ in CDMchelex-Mn2+. This data indicates that the expression of pcpA, psaBCA, and prtA is regulated by Ni2+ and in agreement with our qRT-PCR analysis data mentioned above.
Table 4. β-galactosidase activity (miller units) of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ in S. pneumoniae D39 wild-type and ΔpsaR (RW100)grown in CDMchelex and CDMchelex-Mn2+ supplemented with various concentrations of Ni2+ (mM).
Standard deviation of three independent replications is given in parentheses, whereas ND stands for not determined. Noteworthy, lacZ was fused to the 3’ end of prtA* on the native chromosomal location, using plasmid pOR113. This might explain the lower Miller Units of PprtA compared to PpcpA and PpsaB.
| β-galactosidase Activity (Miller Units) | |||||||
|---|---|---|---|---|---|---|---|
| Medium | D39 (wt) | D39 ΔpsaR | |||||
| PpcpA | PpsaB | PprtA* | PpcpA | PpsaB | PprtA* | ||
| CDMchelex | |||||||
| Ni2+ [0.0] | 29 (7) | 68 (8) | 0.57 (0.06) | 1363 (35) | 1290 (30) | 2.1 (0.2) | |
| Ni2+ [0.1] | 48 (4) | 118 (11) | 0.92 (0.06) | 1226 (42) | 1230 (40) | 2.1 (0.3) | |
| Ni2+ [0.3] | 73 (6) | 218 (20) | 1.38 (0.1) | 1195 (52) | 1220 (24) | 2.2 (0.4) | |
| Ni2+ [0.5] | 101 (8) | 419 (15) | 1.57 (0.1) | 1190 (23) | 1202 (55) | 2.0 (0.2) | |
| CDMchelex-Mn 2+ | |||||||
| Ni2+ [0.0] | 84 (10) | 565 (40) | 0.74 (0.2) | ND | ND | ND | |
| Ni2+ [0.1] | 160 (12) | 628 (43) | 1.24 (0.2) | ND | ND | ND | |
| Ni2+ [0.3] | 360 (36) | 873 (50) | 1.62 (0.1) | ND | ND | ND | |
| Ni2+ [0.5] | 571 (30) | 1072 (102) | 1.87 (0.1) | ND | ND | ND | |
PsaR mediates expression of the PsaR regulon in the presence of Ni2+
To check, whether the observed Ni2+-dependent high expression of the PsaR regulon is mediated by the Mn2+/ Zn2+/ Co2+-responsive transcriptional regulator PsaR, the psaR mutant strain (RW100) containing PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ were grown in CDMchelex with 0, 0.1, 0.3 or 0.5 mM Ni2+. The expression of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ was highly derepressed in the psaR mutant. We did not observe significant difference in the expression of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ in the psaR mutant strain at different concentrations of Ni2+ (Table 4), indicating that PsaR mediates the Ni2+-dependent expression of the PsaR regulon.
To analyze the impact of psaR deletion on the global gene expression of S. pneumoniae and find more targets of PsaR in the presence of Ni2+, transcriptome of psaR mutant strain was compared with S. pneumoniae D39 wild-type strain grown in CDMchelex with 0.3 mM Ni2+. The expression of psaR was significantly downregulated, confirming the inactivation of psaR in the psaR deletion strain. The expression of pcpA, psaBCA, and prtA was highly upregulated in the psaR mutant (Table 5). This data further confirms our β-galactosidase data mentioned above indicating Ni2+-dependent derepression of the PsaR regulon. We did not find any new target of PsaR in the presence of Ni2+. Notably, an operon (spd_0616-spd_618) encoding amino acid ABC transporter proteins was downregulated in our transcriptomic analysis, but in our β-galactosidase assay we did not observe any activity of the respective promotor of this operon in the psaR mutant (Data not shown here).
Table 5. Summary of transcriptome comparison of S. pneumoniae D39 wild-type strain with ΔpsaR grown in CDM with 0.3 mM Ni2+.
| Gene tag a | Function b | Ratio c | P-value |
|---|---|---|---|
| spd_0616 | Amino acid ABC transporter, ATP-binding protein | -4.40 | 1.32E-05 |
| spd_0617 | Amino acid ABC transporter, permease protein | -5.99 | 7.46E-07 |
| spd_0618 | Amino acid ABC transporter, permease protein | -6.19 | 4.27E-07 |
| spd_0558 | Cell wall-associated serine protease PrtA | 3.02 | 6.65E-05 |
| spd_1461 | Manganese ABC transporter, ATP-binding protein | 2.39 | 7.47E-05 |
| spd_1462 | Manganese ABC transporter, permease protein, putative | 2.52 | 1.46E-04 |
| spd_1450 | Iron-dependent transcriptional regulator (PsaR) | -4.43 | 7.45E-07 |
| spd_1632 | Hypothetical protein | -2.22 | 9.00E-04 |
| spd_1965 | Choline binding protein PcpA | 14.42 | 2.65E-09 |
Opposite effect of Ni2+ and Mn2+ in the regulation of the PsaR regulon
Previous studies showed that the PsaR-mediated expression of the PsaR regulon depends on the balance between Mn2+, Co2+ and/ or Zn2+ [9,21,31]. In this study, we observed that the expression of the PsaR regulon was highly derepressed in response to various Ni2+ concentrations. Therefore, we decided to explore the influence of Ni2+ and Mn2+ together on the expression of the PsaR regulon. The expression of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ in S. pneumoniae D39 wild-type was measured at different concentrations of Ni2+ and Mn2+ in CDMchelex and CDMchelex-Mn2+ (Table 6). β-galactosidase data (Miller units) showed that high expression of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ at 0.1 or 0.3 mM of Ni2+ was nullified by the addition of 0.02 or 0.05 mM Mn2+ (Table 6). However, Mn2+ repression was higher in CDMchelex compared to CDMchelex-Mn2+. This might be due to the fact that CDMchelex contains 5–7 μM of Mn2+ which is enough to cause the repression of the PsaR regulon [21]. These results suggest that the Mn2+-dependent repression of the PsaR regulon is derepressed by the addition of Ni2+.
Table 6. Expression level (in Miller units) of PpcpA-lacZ, PpsaB-lacZ, and PprtA-lacZ in D39 wild-type in CDMchelex and CDMchelex-Mn2+ supplemented with different concentrations of Ni2+ and Mn2+ (mM).
Standard deviation of three independent replicates is indicated in bars.
| β-galactosidase Activity (Miller Units) | |||
|---|---|---|---|
| Medium | D39 (wt) | ||
| PpcpA | PpsaB | PprtA | |
| CDMchelex | 29 (3) | 72 (9) | 0.50 (0.07) |
| Ni2+ [0.1] | 32 (4) | 99 (10) | 0.91 (0.08) |
| Ni2+ [0.3] | 66 (6) | 200 (28) | 1.20 (0.2) |
| Ni2+ [0.1] + Mn2+[0.02] | 20 (5) | 79 (7) | 0.55 (0.05) |
| Ni2+ [0.3] + Mn2+[0.02] | 36 (27) | 142 (12) | 0.69 (0.1) |
| Ni2+ [0.1] + Mn2+[0.05] | 20 (5) | 79 (7) | 0.30 (0.05) |
| Ni2+ [0.3] + Mn2+[0.05] | 36 (27) | 142 (12) | 0.35 (0.1) |
| CDMchelex-Mn2+ | 90 (15) | 550 (50) | 0.70 (0.2) |
| Ni2+ [0.1] | 180 (18) | 640 (48) | 1.10 (0.2) |
| Ni2+ [0.3] | 390 (60) | 890 (106) | 1.40 (0.1) |
| Ni2+ [0.1] + Mn2+[0.02] | 100 (10) | 450 (70) | 0.80 (0.05) |
| Ni2+ [0.3] + Mn2+[0.02] | 280 (47) | 565 (120) | 0.90 (0.1) |
| Ni2+ [0.1] + Mn2+[0.05] | 50 (10) | 210 (70) | 0.30 (0.05) |
| Ni2+ [0.3] + Mn2+[0.05] | 120 (47) | 335 (120) | 0.60 (0.1) |
Ni2+ counteracts the Mn2+-PsaR interaction with PpcpA, PpsaBCA, and PprtA
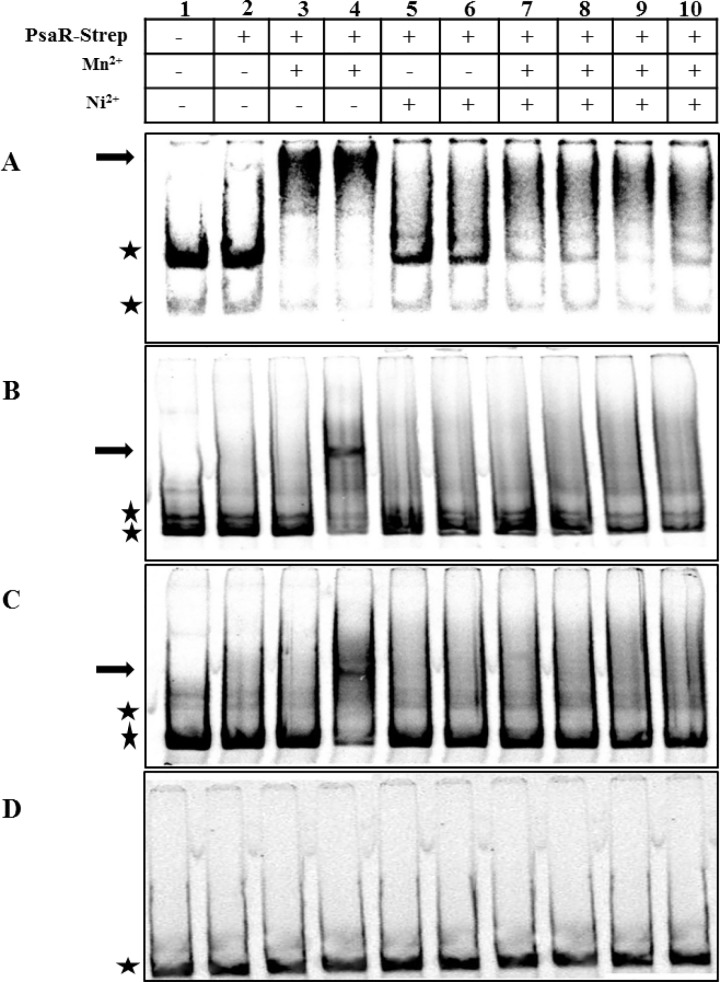
To find out whether the observed opposite effects of Ni2+ and Mn2+on the expression of pcpA, psaBCA, and prtA are mediated by the direct DNA binding activity of the PsaR protein, the effects of these metal ions on the binding of PsaR-Strep tag to 33P-labeled promoters of pcpA, psaB, and prtA were studied in vitro. The promotor region of phtB was used as a negative control. Due to the metal-ion chelating ability of EDTA, we decided to exclude it from all buffers used to perform EMSAs. PsaR-Strep tag was not able to bind with the promoter regions of pcpA, psaB, and prtA without the addition of any metal ion (Fig 1A, 1B and 1C. Lane 2) which is in agreement with the previous study [9]. First of all, we checked the DNA binding activity of PsaR-Strep to the promoter regions of pcpA, psaB, and prtA with different concentrations of Mn2+. We observed that 0.05 and 0.1 mM Mn2+ were able to stimulate the binding of PsaR-Strep tag to the promoter region of pcpA. However, only 0.1 mM Mn2+ was able to stimulate the binding of PsaR-Strep to the promoter regions of psaB and prtA (Fig 1A, 1B and 1C. Lane 4). No binding of PsaR-Strep to the promoter regions of psaB, and prtA was observed at 0.05 mM Mn2+ (Fig 1A, 1B and 1C. Lane 3). Interestingly, no shift in the promoter regions of pcpA, psaB, and prtA was observed with 0.2 or 0.4 mM Ni2+ (Fig 1A, 1B and 1C. Lane 5 and 6), suggesting that Ni2+ does not stimulate the binding of PsaR with pcpA, psaB, and prtA promoters. Previously, it has been shown that Zn2+ binds to the PsaR in such a way which leads to the inactivation of Mn2+-PsaR interaction with the promoter regions of pcpA, psaB, and prtA [9]. We hypothesized that like Zn2+, Ni2+ also interferes in the Mn2+-dependent binding of PsaR-Strep to the promoter regions of pcpA, psaB, and prtA. Therefore, we decided to explore the influence of Ni2+ on the in vitro Mn2+-PsaR-Strep tag interaction. Interestingly, the binding of PsaR to all three promoters in the presence of Mn2+ was impaired with the addition of Ni2+ (Fig 1 Lanes 7–10). This data suggests that the Mn2+-PsaR interaction with pcpA, psaB, and prtA promoters is competed away in the presence of Ni2+, indicating a direct role of Ni2+ in the regulation of the PsaR regulon through PsaR.
Fig 1. In vitro interaction of PsaR-Strep tag with the promoter regions of pcpA (A), psaB (B), prtA (C), and phtB (D).
PsaR-Strep was added at concentration of 30 nM as indicated above panel, while lane 1 is without added protein. Arrows indicate the position of shifted probe and asterisks indicate the position of free probe. Mn2+ was added with concentrations of 0.05 mM in lanes 3, 7, and 9, and 0.1 mM in lane 4, 8, and 10. Ni2+ was added with concentrations of 0.2 mM in lanes 5, 7, and 9, and 0.4 mM in lanes 6, 8, and 10.
A high concentration of Ni2+ in the medium leads to Mn2+ deficiency in the cells
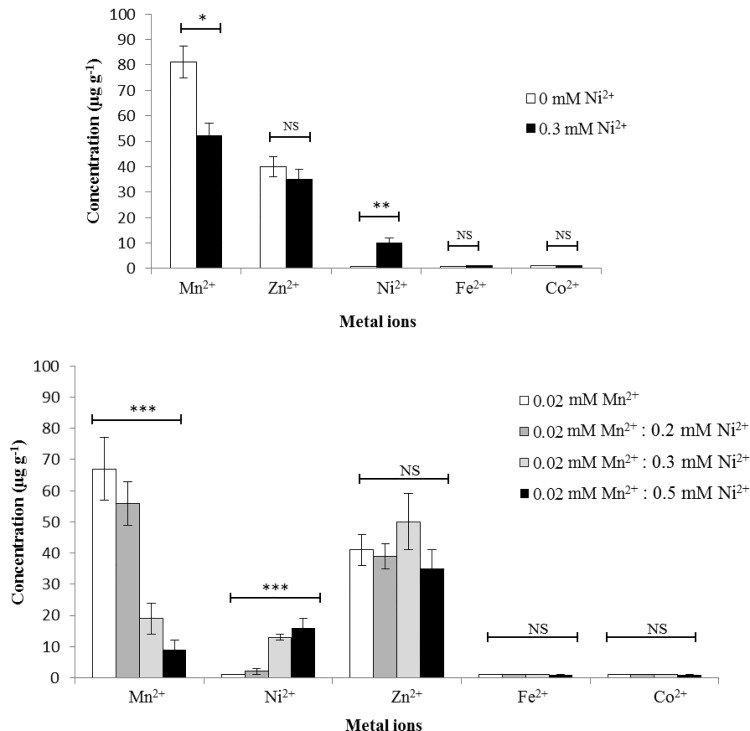
To determine the cell-associated concentrations of metal ions, we performed an ICP-MS analysis on the cells grown in CDMchelex either with 0 or 0.3 mM of Ni2+. ICP-MS data revealed that the cells grown in the presence of 0.3 mM Ni2+ accumulate 10-fold (P<0.01, One way ANOVA) more Ni2+ (Fig 2A) compared to cells grown in the absence of Ni2+. No significant difference in the concentrations of other metal ions was observed in our ICP-MS analysis except for Mn2+. The concentration of Mn2+ was reduced by 1.5-fold (P<0.01, One way ANOVA) in the presence of Ni2+ (Fig 2A). This data indicates that high concentration of Ni2+ leads to Mn2+ deficiency in the cell. To study this in more details, we have checked the impact of various concentrations of Ni2+ on the cell-associated Mn2+. Cells were grown in CDMchelex with the addition of 0.02 mM Mn2+, and 0, 0.1, 0.3 or 0.5 mM Ni2+. As expected, addition of Ni2+ in medium leads to an increased cell-associated Ni2+ concentration. The cell-associated Ni2+ concentration was increased by 2-fold (P<0.01, One way ANOVA) at 0.1 mM Ni2+, 13-fold at 0.3 mM Ni2+, and 16-fold at 0.5 mM Ni2+ when compared to 0 mM Ni2+ (Fig 2B). ICP-MS analyses data further revealed that an increasing concentration of Ni2+ leads to a decrease in the concentrations of Mn2+. The cell-associated concentration of Mn2+ was decreased by 1.25-fold (P<0.01, One way ANOVA) at 0.1 mM Ni2+, 3.52-fold (P<0.01, One way ANOVA) at 0.3 mM Ni2+, and 7.4-fold (P<0.01, One way ANOVA) at 0.5 mM Ni2+ (Fig 2B) when compared to the Mn2+ concentration at 0 mM Ni2+. Notably, the cell-associated concentration of other metal ions (Zn2+, Fe2+, and Co2+) was not affected (Fig 2). This data demonstrate that Ni2+ has ability to cause Mn2+ starvation which ultimately leads to the high expression of the PsaR regulon in the presence of Ni2+.
Fig 2. (A) Cell-associated metal ion concentrations (expressed ug g-1) of S. pneumoniae D39 wild type when grown in CDMchelex with either 0 mM or 0.3 mM Ni2+. (B) Metal ions contents of S. pneumoniae D39 wild-type when grown in CDMchelex containing 0.02 mM Mn2+ with addition of 0, 0.1, 0.3 or 0.5 mM Ni2+. The statistical significance of the differences in the mean metal concentrations was determined by one-way ANOVA (NS not significant, *P<0.01, and ***P<0.0001).
Discussion
Adherence to epithelial cells of human nasopharynx is the primary step of S. pneumoniae towards the pathogenesis [40]. The pneumococcal surface adhesion protein, PsaA and choline binding protein, PcpA are among those proteins that promote pneumococcal adherence in nasopharyngeal epithelial cells and colonization in mice [14,41,42]. Similarly, PrtA, a serine protease containing an LPXTG-anchor motif, is expressed on the surface of nearly all virulent pneumococcal strains and is required for full virulence in animal models [43,44]. The pcpA, psaBCA, and prtA genes comprise the PsaR regulon and their expression is regulated by transcriptional regulator PsaR [9]. The role of Mn2+, Zn2+, and Co2+ in the regulation of pcpA, psaBCA, and prtA (PsaR regulon) has already been established [9,21,45]. In this study, we investigated the role of Ni2+ on the expression of the PsaR regulon. The expression of the PsaR regulon was increased with the increasing concentrations of Ni2+ and this increased expression of the PsaR regulon is directly linked with cell-associated Mn2+ deficiency caused by a high concentration of Ni2+. Moreover, Mn2+ and Ni2+ have opposite regulatory effects on the expression of the PsaR regulon in S. pneumoniae. Where, Mn2+-binding represses the expression of the PsaR regulon, Ni2+ derepresses the repression caused by Mn2+.
Mn2+ is an important transition metal ion that is a cofactor for many pneumococcal proteins which are involved in the colonization, virulence, and resistance to oxidative stress in S. pneumoniae [15]. Mn2+ accumulation shows significant flexibility and cells can survive even at a 3% concentration of the normal accumulation level [45,46]. S. pneumoniae has a dedicated system for Mn2+ transport (PsaBCA) that consists of two ABC transporters (PsaBC) and a cell surface salute binding protein (PsaA) [47–49]. Previous studies have shown that PsaA is not only important for virulence [14,41], but also has a direct role in the accumulation of cell associated Mn2+ [48,49]. PsaA has the ability to bind Zn2+ and Mn2+ [46,48]. The binding affinity of PsaA to Zn2+ is much higher compared to that of Mn2+, and PsaA-Zn2+ interaction led to the ~40% decrease in cell associated Mn2+ accumulation [31,46]. Structural studies of PsaA have revealed that Cd2+ can also bind to PsaA and ultimately results in the reduction of cell-associated Mn2+ [30]. Recently, it was shown that PsaA can also bind to other d-block elements including Ni2+ [50]. This unique property of PsaA to bind with different metal ions makes its role very important in the life style of S. pneumoniae. In our ICP-MS analysis, we observed a cell-associated Mn2+ deficiency in the presence of relatively high concentrations of Ni2+. Therefore, based on our ICP-MS data, we can speculate that most likely Ni2+ interacts with PsaA, which leads to Mn2+ deficiency.
Biochemical studies of transcriptional regulator PsaR of S. pneumoniae showed that PsaR harbors two pairs of metal binding sites where Mn2+ or Zn2+ can bind [51]. Similarly, Mn2+-responsive regulators DtxR from Corynebacterium diphtheria and MntR from Bacillus subtilis, which are homologous of PsaR, also have two metal binding sites [52,53]. The binding of DtxR to the tox operon in C. diphtheria not only depends on the availability of Mn2+ but also on Co2+, Fe2+, and Ni2+ [54]. Similarly, The Mn2+-dependent DNA binding activity of MntR in B. subtilis is diminished in the presence of Ni2+, Zn2+, and Fe2+ [55–57]. The metal responsive transcriptional regulators, ScaR of Streptococcus gordonii and SloR of Streptococcus mutants also belongs to DtxR family, and are homologous to PsaR [58–60]. Interestingly, the PsaR binding site is similar to the operator sequences of ScaR and SloR [61]. This might suggest that PsaR uses a similar mechanism of metal ion competition for regulatory metal ion homeostasis as other member of DxtR family regulators adopt.
It has been previously demonstrated that PsaR represses the expression of the PsaR regulon in the presence of Mn2+ whereas Zn2+ and Co2+ relieved this repression [21,61]. Moreover, the in vitro studies of the interaction of PsaR to its target promotors showed that both Zn2+ and Co2+ could bind to PsaR in a different way [21]. When Zn2+ interacts with PsaR, it relieves the PsaR interaction with the promoter regions of the PsaR regulon, whereas Co2+, just like Mn2+, stimulates the interaction of PsaR with the promoter regions of the PsaR regulon [9,21]. Here, we demonstrated that Mn2+-PsaR interaction leads to the binding of PsaR to the promoter regions of pcpA, psaBCA, and prtA which is an agreement with previous studies [9]. However, the Mn2+-PsaR interaction with pcpA, psaB, and prtA promoters was alleviated by the addition of Ni2+ which suggests that the observed transcriptional response of the PsaR regulon is directly linked to the interaction of Ni2+ and Mn2+ on the PsaR-promoter interactions. In conclusion, we have shown that the interaction of PsaR to Ni2+ plays a similar role as Zn2+, to induce derepression by PsaR in competition with Mn2+.
Data Availability
The DNA microarray data have been deposited to Gene Expression Omnibus (GEO) under the accession number GSE73818.
Funding Statement
The authors received no specific funding for this work.
References
- 1. McGeer A, Low DE. Is resistance futile? Nat Med. 2003;9: 390–392. 10.1038/nm0403-390 [DOI] [PubMed] [Google Scholar]
- 2. Saha SK, Naheed A, Arifeen S El, Islam M, Al-Emran H, Amin R, et al. Surveillance for invasive Streptococcus pneumoniae disease among hospitalized children in Bangladesh: antimicrobial susceptibility and serotype distribution. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48 Suppl 2: S75–81. 10.1086/596544 [DOI] [PubMed] [Google Scholar]
- 3. Zaidi AKM, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J. 2009;28: S10–18. 10.1097/INF.0b013e3181958769 [DOI] [PubMed] [Google Scholar]
- 4. Mitchell TJ. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol. 2003;1: 219–230. 10.1038/nrmicro771 [DOI] [PubMed] [Google Scholar]
- 5. Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4: 144–154. 10.1016/S1473-3099(04)00938-7 [DOI] [PubMed] [Google Scholar]
- 6. Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30: 189–209. 10.1055/s-0029-1202938 [DOI] [PubMed] [Google Scholar]
- 7. Obaro S, Adegbola R. The pneumococcus: carriage, disease and conjugate vaccines. J Med Microbiol. 2002;51: 98–104. [DOI] [PubMed] [Google Scholar]
- 8. Gupta R, Shah P, Swiatlo E. Differential gene expression in Streptococcus pneumoniae in response to various iron sources. Microb Pathog. 2009;47: 101–109. 10.1016/j.micpath.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 9. Kloosterman TG, Witwicki RM, van der Kooi-Pol MM, Bijlsma JJE, Kuipers OP. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J Bacteriol. 2008;190: 5382–5393. 10.1128/JB.00307-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shafeeq S, Kloosterman TG, Kuipers OP. Transcriptional response of Streptococcus pneumoniae to Zn2+) limitation and the repressor/activator function of AdcR. Met Integr Biometal Sci. 2011;3: 609–618. 10.1039/c1mt00030f [DOI] [PubMed] [Google Scholar]
- 11. Wakeman CA, Skaar EP. Metalloregulation of Gram-positive pathogen physiology. Curr Opin Microbiol. 2012;15: 169–174. 10.1016/j.mib.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 2011;82: 904–916. 10.1111/j.1365-2958.2011.07862.x [DOI] [PubMed] [Google Scholar]
- 13. Jomaa M, Terry S, Hale C, Jones C, Dougan G, Brown J. Immunization with the iron uptake ABC transporter proteins PiaA and PiuA prevents respiratory infection with Streptococcus pneumoniae. Vaccine. 2006;24: 5133–5139. 10.1016/j.vaccine.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 14. Rajam G, Anderton JM, Carlone GM, Sampson JS, Ades EW. Pneumococcal surface adhesin A (PsaA): a review. Crit Rev Microbiol. 2008;34: 163–173. 10.1080/10408410802383610 [DOI] [PubMed] [Google Scholar]
- 15. Rosch JW, Gao G, Ridout G, Wang Y- D, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol. 2009;72: 12–25. 10.1111/j.1365-2958.2009.06638.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, et al. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol. 2011;81: 1255–1270. 10.1111/j.1365-2958.2011.07758.x [DOI] [PubMed] [Google Scholar]
- 17. Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJE, Kuipers OP. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol Microbiol. 2007;65: 1049–1063. 10.1111/j.1365-2958.2007.05849.x [DOI] [PubMed] [Google Scholar]
- 18. Ulijasz AT, Andes DR, Glasner JD, Weisblum B. Regulation of iron transport in Streptococcus pneumoniae by RitR, an orphan response regulator. J Bacteriol. 2004;186: 8123–8136. 10.1128/JB.186.23.8123-8136.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plumptre CD, Hughes CE, Harvey RM, Eijkelkamp BA, McDevitt CA, Paton JC. Overlapping Functionality of the Pht Proteins in Zinc Homeostasis of Streptococcus pneumoniae. Infect Immun. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun. 2006;74: 1171–1180. 10.1128/IAI.74.2.1171-1180.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manzoor I, Shafeeq S, Kloosterman TG, Kuipers OP. Co2+-dependent gene expression in Streptococcus pneumoniae: Opposite effect of Mn2+ and Co2+ on the expression of the virulence genes psaBCA, pcpA and prtA. Microb Physiol Metab. 2015;6: 748 10.3389/fmicb.2015.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287: 15544–15556. 10.1074/jbc.M111.330365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72: 844–858. 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvie DR, Andreini C, Cavallaro G, Meng W, Connolly BA, Yoshida K, et al. Predicting metals sensed by ArsR-SmtB repressors: allosteric interference by a non-effector metal. Mol Microbiol. 2006;59: 1341–1356. 10.1111/j.1365-2958.2006.05029.x [DOI] [PubMed] [Google Scholar]
- 25. Ong C- LY, Potter AJ, Trappetti C, Walker MJ, Jennings MP, Paton JC, et al. Interplay between manganese and iron in pneumococcal pathogenesis: role of the orphan response regulator RitR. Infect Immun. 2013;81: 421–429. 10.1128/IAI.00805-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Couñago RM, McDevitt CA, Ween MP, Kobe B. Prokaryotic substrate-binding proteins as targets for antimicrobial therapies. Curr Drug Targets. 2012;13: 1400–1410. [DOI] [PubMed] [Google Scholar]
- 27. Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109: 4644–4681. 10.1021/cr900077w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460: 823–830. 10.1038/nature08300 [DOI] [PubMed] [Google Scholar]
- 29. Irving H, Williams RJP. 637. The stability of transition-metal complexes. J Chem Soc Resumed. 1953; 3192–3210. 10.1039/JR9530003192 [DOI] [Google Scholar]
- 30. Begg SL, Eijkelkamp BA, Luo Z, Couñago RM, Morey JR, Maher MJ, et al. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae. Nat Commun. 2015;6: 6418 10.1038/ncomms7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobsen FE, Kazmierczak KM, Lisher JP, Winkler ME, Giedroc DP. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Met Integr Biometal Sci. 2011;3: 38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun X, Yu G, Xu Q, Li N, Xiao C, Yin X, et al. Putative cobalt- and nickel-binding proteins and motifs in Streptococcus pneumoniae. Met Integr Biometal Sci. 2013;5: 928–935. 10.1039/c3mt00126a [DOI] [PubMed] [Google Scholar]
- 33. Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. 1944. Mol Med Camb Mass. 1995;1: 344–365. [PMC free article] [PubMed] [Google Scholar]
- 34. Lanie JA, Ng W-L, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, et al. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 2007;189: 38–51. 10.1128/JB.01148-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Afzal M, Shafeeq S, Kuipers OP. LacR Is a Repressor of lacABCD and LacT Is an Activator of lacTFEG, Constituting the lac Gene Cluster in Streptococcus pneumoniae. Appl Environ Microbiol. 2014;80: 5349–5358. 10.1128/AEM.01370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Afzal M, Manzoor I, Kuipers OP. A Fast and Reliable Pipeline for Bacterial Transcriptome Analysis Case study: Serine-dependent Gene Regulation in Streptococcus pneumoniae. J Vis Exp. 2015; 10.3791/52649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shafeeq S, Afzal M, Henriques-Normark B, Kuipers OP. Transcriptional profiling of UlaR-regulated genes in Streptococcus pneumoniae. Genomics Data. 2015;4: 57–59. 10.1016/j.gdata.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuipers OP, de Ruyter PGG., Kleerebezem M, de Vos WM. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64: 15–21. 10.1016/S0168-1656(98)00100-X [DOI] [Google Scholar]
- 40. Rosch JW, Mann B, Thornton J, Sublett J, Tuomanen E. Convergence of Regulatory Networks on the Pilus Locus of Streptococcus pneumoniae. Infect Immun. 2008;76: 3187–3196. 10.1128/IAI.00054-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frolet C, Beniazza M, Roux L, Gallet B, Noirclerc-Savoye M, Vernet T, et al. New adhesin functions of surface-exposed pneumococcal proteins. BMC Microbiol. 2010;10: 190 10.1186/1471-2180-10-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sánchez-Beato AR, López R, García JL. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol Lett. 1998;164: 207–214. [DOI] [PubMed] [Google Scholar]
- 43. Mirza S, Wilson L, Benjamin WH, J., Novak J, Barnes S, Hollingshead SK, et al. Serine protease PrtA from Streptococcus pneumoniae plays a role in the killing of S. pneumoniae by apolactoferrin. Infect Immun. 2011;79: 2440–2450. 10.1128/IAI.00489-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bethe G, Nau R, Wellmer A, Hakenbeck R, Reinert RR, Heinz HP, et al. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol Lett. 2001;205: 99–104. [DOI] [PubMed] [Google Scholar]
- 45. Eijkelkamp BA, Morey JR, Ween MP, Ong CY, McEwan AG, Paton JC, et al. Extracellular Zinc Competitively Inhibits Manganese Uptake and Compromises Oxidative Stress Management in Streptococcus pneumoniae. PLoS ONE. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, et al. A Molecular Mechanism for Bacterial Susceptibility to Zinc. PLoS Pathog. 2011;7: e1002357 10.1371/journal.ppat.1002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dintilhac A, Alloing G, Granadel C, Claverys JP. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25: 727–739. [DOI] [PubMed] [Google Scholar]
- 48. Lawrence MC, Pilling PA, Epa VC, Berry AM, Ogunniyi AD, Paton JC. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Struct Lond Engl 1993. 1998;6: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 49. McAllister LJ, Tseng H-J, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53: 889–901. 10.1111/j.1365-2958.2004.04164.x [DOI] [PubMed] [Google Scholar]
- 50. Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O’Mara ML, et al. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10: 35–41. 10.1038/nchembio.1382 [DOI] [PubMed] [Google Scholar]
- 51. Lisher JP, Higgins KA, Maroney MJ, Giedroc DP. Physical Characterization of the Manganese-Sensing Pneumococcal Surface Antigen Repressor from Streptococcus pneumoniae. Biochemistry (Mosc). 2013;52: 7689–7701. 10.1021/bi401132w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D’Aquino JA, Tetenbaum-Novatt J, White A, Berkovitch F, Ringe D. Mechanism of metal ion activation of the diphtheria toxin repressor DtxR. Proc Natl Acad Sci U S A. 2005;102: 18408–18413. 10.1073/pnas.0500908102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Golynskiy MV, Gunderson WA, Hendrich MP, Cohen SM. Metal binding studies and EPR spectroscopy of the manganese transport regulator MntR. Biochemistry (Mosc). 2006;45: 15359–15372. 10.1021/bi0607406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tao X, Murphy JR. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem. 1992;267: 21761–21764. [PubMed] [Google Scholar]
- 55. Golynskiy MV, Davis TC, Helmann JD, Cohen SM. Metal-induced structural organization and stabilization of the metalloregulatory protein MntR. Biochemistry (Mosc). 2005;44: 3380–3389. 10.1021/bi0480741 [DOI] [PubMed] [Google Scholar]
- 56. Lieser SA, Davis TC, Helmann JD, Cohen SM. DNA-binding and oligomerization studies of the manganese(II) metalloregulatory protein MntR from Bacillus subtilis. Biochemistry (Mosc). 2003;42: 12634–12642. 10.1021/bi0350248 [DOI] [PubMed] [Google Scholar]
- 57. Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35: 1454–1468. [DOI] [PubMed] [Google Scholar]
- 58. Jakubovics NS, Smith AW, Jenkinson HF. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol Microbiol. 2000;38: 140–153. [DOI] [PubMed] [Google Scholar]
- 59. Kitten T, Munro CL, Michalek SM, Macrina FL. Genetic Characterization of a Streptococcus mutans LraI Family Operon and Role in Virulence. Infect Immun. 2000;68: 4441–4451. 10.1128/IAI.68.8.4441-4451.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oram DM, Avdalovic A, Holmes RK. Analysis of Genes That Encode DtxR-Like Transcriptional Regulators in Pathogenic and Saprophytic Corynebacterial Species. Infect Immun. 2004;72: 1885–1895. 10.1128/IAI.72.4.1885-1895.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kloosterman TG, Witwicki RM, van der Kooi-Pol MM, Bijlsma JJ, Kuipers OP. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J Bacteriol. 2008;190: 5382–5393. 10.1128/JB.00307-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoskins J, Alborn WE Jr, Arnold J, Blaszczak LC, Burgett S, DeHoff BS, et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol. 2001;183: 5709–5717. 10.1128/JB.183.19.5709-5717.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DNA microarray data have been deposited to Gene Expression Omnibus (GEO) under the accession number GSE73818.