Abstract
BACKGROUND
Epidemiologic and preclinical data suggest that higher intake and serum levels of vitamin D and higher intake of calcium reduce the risk of colorectal neoplasia. To further study the chemopreventive potential of these nutrients, we conducted a randomized, double-blind, placebo-controlled trial of supplementation with vitamin D, calcium, or both for the prevention of colorectal adenomas.
METHODS
We recruited patients with recently diagnosed adenomas and no known colorectal polyps remaining after complete colonoscopy. We randomly assigned 2259 participants to receive daily vitamin D3 (1000 IU), calcium as carbonate (1200 mg), both, or neither in a partial 2×2 factorial design. Women could elect to receive calcium plus random assignment to vitamin D or placebo. Follow-up colonoscopy was anticipated to be performed 3 or 5 years after the baseline examinations, according to the endoscopist’s recommendation. The primary end point was adenomas diagnosed in the interval from randomization through the anticipated surveillance colonoscopy.
RESULTS
Participants who were randomly assigned to receive vitamin D had a mean net increase in serum 25-hydroxyvitamin D levels of 7.83 ng per milliliter, relative to participants given placebo. Overall, 43% of participants had one or more adenomas diagnosed during follow-up. The adjusted risk ratios for recurrent adenomas were 0.99 (95% confidence interval [CI], 0.89 to 1.09) with vitamin D versus no vitamin D, 0.95 (95% CI, 0.85 to 1.06) with calcium versus no calcium, and 0.93 (95% CI, 0.80 to 1.08) with both agents versus neither agent. The findings for advanced adenomas were similar. There were few serious adverse events.
CONCLUSIONS
Daily supplementation with vitamin D3 (1000 IU), calcium (1200 mg), or both after removal of colorectal adenomas did not significantly reduce the risk of recurrent colorectal adenomas over a period of 3 to 5 years. (Funded by the National Cancer Institute; ClinicalTrials.gov number, NCT00153816.)
Vitamin D, an essential nutrient that is important for bone mineralization and calcium homeostasis,1 also has effects beyond bone and calcium. Many studies have shown it to be antineoplastic, particularly in the colorectum. In in vitro studies, vitamin D and its analogues have been shown to inhibit proliferation, induce differentiation, inhibit angiogenesis, and promote apoptosis in epithelial tissues.2,3 High vitamin D intake inhibits experimental carcinogenesis,2,3 even in animals that are vitamin D–replete.4 Observational studies of vitamin D intake5–7 and serum levels of 25-hydroxyvitamin D8–10 have shown inverse associations between these measures and the risk of colorectal cancer or adenoma.8–10 Trials of vitamin D supplementation have not shown a decrease in the incidence of colorectal cancer in association with supplementation,11–14 but these studies were limited by small numbers of events,11–13 low vitamin D doses,14 and relatively short follow-up periods for invasive cancer end points.11–14
High calcium intake is also associated with lower risks of colorectal neoplasia. Increases in dietary calcium have been shown to inhibit large-bowel carcinogenesis in animal models,15 and epidemiologic studies have shown lower risks of colorectal cancer and adenomas in association with higher calcium intake.16 Trials of calcium supplementation for adenoma prevention have shown reduced risks.17–19 Moreover, calcium and vitamin D may have synergistic chemopreventive effects against colorectal neoplasia.20,21
To further investigate the chemopreventive potential of vitamin D and calcium, we conducted a randomized trial of supplementation with calcium, vitamin D3, or both for the prevention of new colorectal adenomas in persons with a recent history of adenomas. We hypothesized that supplementation would reduce the risk of adenoma and that both agents together would reduce the risk more than calcium alone. Our secondary hypotheses addressed the relationship between vitamin D supplementation and the risk of advanced adenoma, as well as the effects of vitamin D supplementation among persons with baseline 25-hydroxyvitamin D levels in blood that were below the study median, as compared with the effects in persons with levels above the median.
METHODS
Participants
We conducted this randomized, multicenter, double-blind, placebo-controlled trial at 11 academic medical centers and associated medical practices in the United States. Enrollment of patients took place from July 2004 through July 2008. Staff members at each center enrolled patients 45 to 75 years of age who had at least one colorectal adenoma removed within 120 days before enrollment, had no remaining polyps after a complete colonoscopy, and were anticipated to undergo a 3-year or 5-year colonoscopic follow-up examination recommended by the treating endoscopist. Eligible patients were in good general health and did not have familial colorectal cancer syndromes or serious intestinal disease. We did not include patients who had conditions that indicated that the study agents would pose a health risk (e.g., a history of kidney stones or hyperparathyroidism) or who had conditions that would indicate a need for either agent (e.g., osteoporosis). We also did not include patients who had a serum calcium level that was outside the normal range, a creatinine level that was more than 20% above the upper limit of the normal range, or a 25-hydroxyvitamin D level that was lower than 12 ng per milliliter or higher than 90 ng per milliliter.
Study Design and Oversight
In a partial factorial design, we evaluated four regimens, all of which involved two identical tablets taken daily: 1000 IU of vitamin D3, 1200 mg of calcium as carbonate, both agents, or placebo. Women could elect to be randomly assigned to receive either calcium or calcium plus vitamin D (two-group randomization); all other patients were randomly assigned to receive one of the four regimens (full factorial randomization). The doses of study agents were chosen to increase the total intake substantially, with a margin of safety below the highest mean daily intake level believed unlikely to cause adverse effects in most people at the time that the trial began (2000 IU of vitamin D and 2.5 g of calcium).22 In accordance with the protocol, study treatment was to continue until the anticipated 3-year or 5-year colonoscopic examination.
At enrollment, participants provided information regarding demographic data, medical history, medications, nutritional supplements, behavioral factors, and diet (using the Block Brief 2000 food frequency questionnaire [Nutritionquest]). Enrollment was followed by a placebo run-in period of 56 to 84 days to identify and exclude participants who were considered unlikely to follow study procedures. Subsequent randomization by the coordinating center was performed with the use of computer-generated random numbers with permuted blocks and stratification according to clinical center, sex, anticipated colonoscopic examination at 3 years or 5 years, and full factorial or two-group randomization. All study staff were unaware of the treatment assignments, with the exception of the data analyst and statistician, some of the programmers, and pharmacy personnel.
Participants agreed to avoid taking study agents outside the trial. However, because of increasing publicity regarding the possible benefits of these supplements (especially vitamin D), daily personal use of up to 1000 IU of vitamin D, 400 mg of elemental calcium, or both were permitted, although discouraged, from April 2008 onward.
Participants were contacted by telephone every 6 months and queried regarding adherence to study agents, illnesses, medication and supplement use, dietary calcium intake (see the Supplementary Appendix, available with the full text of this article at NEJM.org), and colorectal procedures. Records were collected that included data on major medical events, colorectal surgical procedures, and endoscopic examinations. Two physicians who were unaware of the study group assignments adjudicated the diagnosis of adverse events. Bottles of study tablets were mailed to participants every 4 months. Patients who wanted to take a multivitamin were offered a special preparation that did not include calcium and vitamin D. The study intervention ended on August 31, 2013; the treatment-phase follow-up continued until November 30, 2013, to accommodate the final 5-year participants.
Blood levels of 25-hydroxyvitamin D, calcium, and creatinine were measured at baseline and at year 1, as well as at year 3 for participants with 5-year surveillance cycles. The level of 25-hydroxy- vitamin D was also measured shortly before the end-of-treatment examination. The laboratory methods are described in the Supplementary Appendix. Levels of 25-hydroxyvitamin D were seasonally adjusted according to the month in which the blood was drawn (see the Supplementary Appendix). The net change in 25-hydroxyvitamin D levels was defined as the posttreatment level minus the pretreatment level in participants who received vitamin D, minus that difference in participants who were given no vitamin D.
The study end points included all adenomas that were diagnosed in any colorectal endoscopic or surgical procedure at least 1 year after randomization and up to 6 months after the anticipated 3-year or 5-year colonoscopic examination. A single study pathologist who was unaware of the treatment assignments reviewed the slides for all excised colorectal lesions. We distinguished between lesions that were proximal to the splenic flexure and lesions that were more distal. Advanced adenomas were defined as those with cancer, high-grade dysplasia, more than 25% villous features, or an estimated diameter of at least 1 cm. Study diagnoses were compared with the diagnoses made by the pathologists at the clinical centers. Discrepancies were resolved by means of a detailed adjudication procedure (see the Supplementary Appendix).
The study was conducted and reported in accordance with the study protocol, which is available at NEJM.org. The authors designed the study, analyzed the data, wrote the manuscript, and vouch for the completeness and accuracy of the data and analysis. Pfizer Consumer Healthcare provided the study agents. No institution or company affected the analysis or the decision to submit the manuscript for publication.
All participants provided written informed consent; the research was approved by the institutional review board at each center. An independent data and safety monitoring committee oversaw the study.
Statistical Analysis
In our primary analysis, we compared the risk of one or more adenomas after randomization to vitamin D versus no vitamin D, calcium versus no calcium, and calcium plus vitamin D versus calcium alone. Participants who did not undergo the anticipated colonoscopic examination at 3 years or 5 years were included in the analysis if they had had a colonoscopic examination performed at least 1 year after randomization. The sample size and statistical power considerations are described in the Supplementary Appendix.
In the prespecified primary analysis, contingency tables and standard chi-square tests were used for the comparison of adenoma occurrence among randomized groups. The calcium analyses included only the participants who underwent full-factorial randomization. Subsequent multivariable generalized linear models for binary data were used to estimate adjusted risk ratios and confidence intervals. The covariates were age, sex, clinical center, number of baseline adenomas (one, two, or three or more), anticipated 3-year versus 5-year surveillance interval, and two-group versus full-factorial randomization. Clinical centers were grouped geographically when necessary because of sparse data. One subgroup analysis was prespecified: the effects of vitamin D in participants with baseline 25-hydroxyvitamin D levels below the overall median level were compared with the effects in those with levels above the median level. Eight additional post hoc subgroup analyses were conducted, as described in Figure 1. Interactions were assessed with the use of Wald tests. For interactions with variables that had more than two levels, we used a one-degree-of-freedom test for trend over medians within strata. In all analyses of randomly assigned treatments, participants were evaluated according to their assigned treatment group, regardless of their adherence to the study treatment and procedures. Sensitivity analyses were conducted with imputation of missing end points as either adenomas or no adenomas.
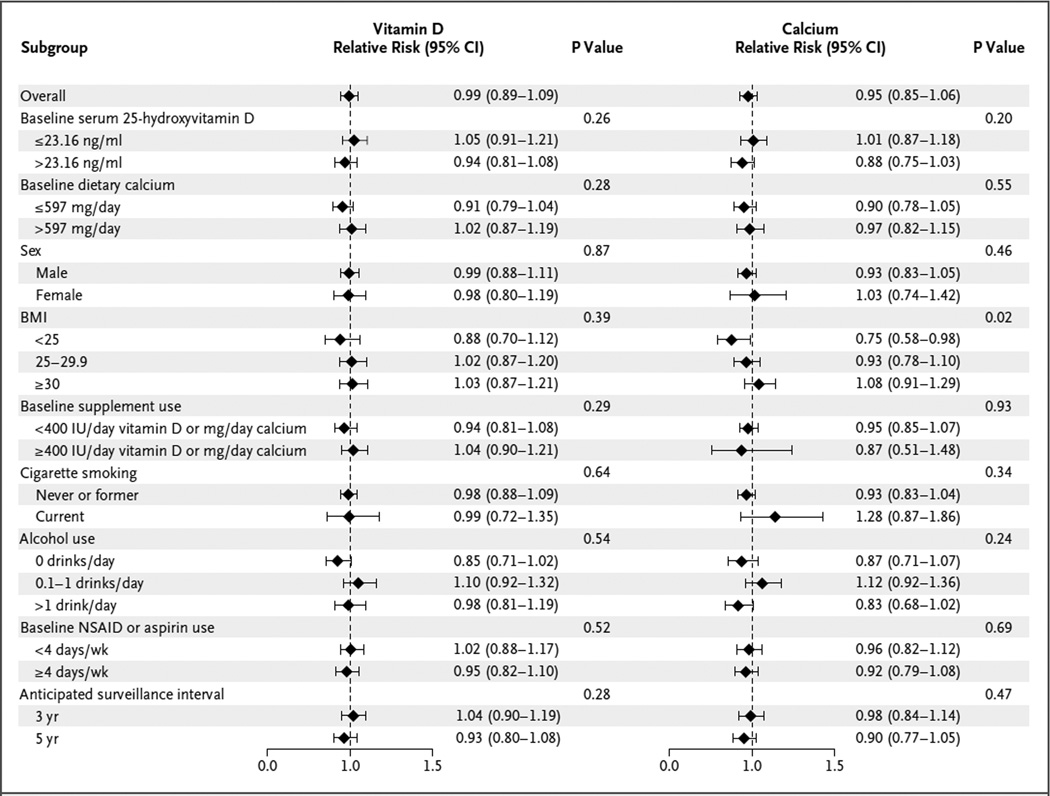
Figure 1. Subgroup Analysis of the Effects of Supplementation with Calcium or Vitamin D on the Development of One or More Adenomas.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. NSAID denotes nonsteroidal anti-inflammatory drug.
In a post hoc observational analysis, we similarly assessed associations between adenoma risk and baseline serum 25-hydroxyvitamin D levels among participants who were not randomly assigned to take vitamin D, as well as the association between adenoma risk and baseline calcium intake among participants who were not randomly assigned to receive calcium. The analyses were adjusted for a number of covariates, including (but not limited to) age, clinical center, surveillance interval (3 or 5 years), and number of baseline adenomas (one, two, or three or more).
Two-sided P values of less than 0.05 were considered to indicate statistical significance. Statistical analyses were conducted with the use of SAS software, version 9.4 (SAS Institute), and STATA software, version 12 (StataCorp).
RESULTS
Participants
During the period from July 2004 through July 2008, the study staff screened colonoscopy and pathology reports and found 19,083 apparently eligible patients (Fig. S1 in the Supplementary Appendix). Ultimately, 2813 participants entered the run-in period, and 2259 underwent randomization. The study population was middle-aged or older; most patients were non-Hispanic men, and more than 35% of the patients were obese (Table 1). A total of 11% of the participants had three or more adenomas at baseline, and 18% had one or more advanced adenomas. There were no material differences between treatment groups with regard to personal characteristics (Table 1). Fifteen patients who underwent randomization and were included in the analysis were later found not to have met all eligibility criteria (see the Supplementary Appendix).
Table 1.
Selected Baseline Characteristics According to Treatment Assignment.*
| Characteristic | Full Factorial Randomization |
Two-Group Randomization |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N=415) |
Calcium (N=419) |
Vitamin D (N = 420) |
Vitamin D plus Calcium (N = 421) |
Calcium plus Placebo (N = 295) |
Calcium plus Vitamin D (N=289) |
|||
| Sex— no. (%) | ||||||||
| Female | 60 (14.5) | 63 (15.0) | 62 (14.8) | 67 (15.9) | 295 (100) | 289 (100) | ||
| Male | 355 (85.5) | 356 (85.0) | 358 (85.2) | 354 (84.1) | 0 | 0 | ||
| Age — yr | 58.2±7.0 | 58.7±7.0 | 58.3±7.0 | 58.7±6.9 | 56.7±6.0 | 57.0±6.6 | ||
| Race — no./total no. (%)† | ||||||||
| White | 357/402 (88.8) | 354/404 (87.6) | 364/404 (90.1) | 350/404 (86.6) | 238/275 (86.5) | 237/271 (87.5) | ||
| Black | 27/402 (6.7) | 33/404 (8.2) | 25/404 (6.2) | 46/404(11.4) | 28/275 (10.2) | 25/271 (9.2) | ||
| Asian or Pacific Islander | 10/402 (2.5) | 10/404 (2.5) | 12/404 (3.0) | 6/404 (1.5) | 8/275 (2.9) | 7/271 (2.6) | ||
| Other | 8/402 (2.0) | 7/404 (1.7) | 3/404 (0.7) | 2/404 (0.5) | 1/275 (0.4) | 2/271 (0.7) | ||
| Non-Hispanic ethnic background — no./total no. (%)† | 395/414 (95.4) | 395/418 (94.5) | 396/420 (94.3) | 394/420 (93.8) | 267/295 (90.5) | 263/289 (91.0) | ||
| Family history of colorectal cancer— no./total no. (%) | 59/378 (15.6) | 77/395 (19.5) | 66/391 (16.9) | 75/393 (19.1) | 52/279 (18.6) | 43/270 (15.9) | ||
| BMI‡ | 29.0±4.9 | 29.5±5.1 | 29.1±4.6 | 29.0±5.1 | 28.8±6.0 | 28.3±5.4 | ||
| BMI ≥30— no./total no. (%)‡ | 147/414 (35.5) | 165/419 (39.4) | 163/420 (38.8) | 144/421 (34.2) | 112/294(38.1) | 92/288 (31.9) | ||
| Smoking status — no. (%) | ||||||||
| Never smoked | 187 (45.1) | 212 (50.6) | 217 (51.7) | 204 (48.5) | 205 (69.5) | 169 (58.5) | ||
| Former smoker | 193 (46.5) | 174 (41.5) | 164 (39.0) | 169 (40.1) | 62 (21.0) | 88 (30.4) | ||
| Current smoker | 35 (8.4) | 33 (7.9) | 39 (9.3) | 48 (11.4) | 28 (9.5) | 32(11.1) | ||
| At least one advanced adenoma at baseline — no./total no. (%) |
69/399 (17.3) | 79/399 (19.8) | 80/398 (20.1) | 76/404 (18.8) | 56/289 (19.4) | 47/281 (16.7) | ||
| Alcohol intake — drinks/day | 0.89±1.08 | 0.82±1.01 | 0.91±1.11 | 0.89±1.08 | 0.40±0.72 | 0.48±0.69 | ||
| Dietary calcium intake — mg/day | 672±313 | 718±326 | 643±286 | 652±303 | 604±295 | 656±313 | ||
| Baseline supplemental calcium intake — no./total no. (%)§ | ||||||||
| None | 201/387 (51.9) | 199/393 (50.6) | 207/396 (52.3) | 212/400 (53.0) | 64/257 (24.9) | 56/255 (22.0) | ||
| 1–499 elemental mg/day | 173/387 (44.7) | 172/393 (43.8) | 172/396 (43.4) | 169/400 (42.2) | 94/257 (36.6) | 86/255 (33.7) | ||
| ≥500 elemental mg/day | 13/387 (3.4) | 22/393 (5.6) | 17/396 (4.3) | 19/400 (4.8) | 99/257 (38.5) | 113/255 (44.3) | ||
| Baseline dietary vitamin D intake — lU/day | 138+97 | 146±96 | 131±102 | 130±92 | 124±96 | 134±98 | ||
| Baseline supplemental vitamin D intake — no./total no. (%)§ |
||||||||
| None | 204/391 (52.2) | 203/389 (52.2) | 209/397 (52.6) | 210/397 (52.9) | 84/246(34.1) | 71/233 (30.5) | ||
| 1–499 lU/day | 175/391 (44.8) | 170/389 (43.7) | 162/397 (40.8) | 175/397(44.1) | 120/246 (48.8) | 110/233 (47.2) | ||
| ≥500 lU/day | 12/391 (3.1) | 16/389(4.1) | 26/397 (6.5) | 12/397 (3.0) | 42/246(17.1) | 52/233 (22.3) | ||
| Baseline serum 25-hydroxyvitamin D level — ng/ml | 24.24±7.84 | 24.58±8.42 | 24.88±8.09 | 24.45±8.06 | 25.03±8.90 | 24.29±7.71 | ||
| Baseline aspirin use — no. (%) | ||||||||
| <4 days/wk | 255 (61.4) | 249 (59.4) | 255 (60.7) | 270 (64.1) | 227 (76.9) | 206 (71.3) | ||
| ≥4 days/wk | 160 (38.6) | 170 (40.6) | 165 (39.3) | 151 (35.9) | 68 (23.1) | 83 (28.7) | ||
| Baseline non-aspirin NSAID use — no. (%) | ||||||||
| <4 days/wk | 372 (89.6) | 367 (87.6) | 379 (90.2) | 388 (92.2) | 261 (88.5) | 253 (87.5) | ||
| ≥4 days/wk | 43 (10.4) | 52 (12.4) | 41 (9.8) | 33 (7.8) | 34(11.5) | 36 (12.5) | ||
Plus–minus values are means ±SD. The table includes all participants who underwent randomization. Women could elect to be randomly assigned to receive either calcium plus placebo or calcium plus vitamin D (two-group randomization); all other patients were randomly assigned to receive one of four regimens: vitamin D, calcium, both agents, or placebo (full factorial randomization). NSAID denotes nonsteroidal antiinflammatory drug.
Race and ethnic background were self-reported.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. A BMI of 30 or higher indicates obesity.
Supplemental values include those from separate supplements and multivitamins. Participants were asked to stop taking these personal supplements at enrollment as a condition of study entry. However, from April 2008 onward, daily personal use of up to 1000 IU of vitamin D, 400 mg of elemental calcium, or both were permitted, although discouraged. Patients who wanted to take a multivitamin were offered a special preparation that did not include calcium and vitamin D.
Adherence to the Study Procedures
The reported adherence to colonoscopy was excellent. Only 67 participants (3.0%) did not have a colonoscopic examination with associated histologic data at least 1 year after randomization, and 104 participants (4.6%) dropped out of the study, were lost to follow-up, or died; this left 2088 participants (92.4%) in the analysis (Fig. S1 in the Supplementary Appendix). Only approximately 1% of the colonoscopic examinations failed to reach the cecum; in 2.0% of procedures, the preparation of the colon was deemed “poor,” and in 6.8% it was deemed “fair.” The times to final colonoscopic examination are summarized in Table S2 in the Supplementary Appendix. A total of 1598 participants (77%) had a surveillance colonoscopic examination within 6 months before or after the anticipated 3-year or 5-year follow-up examination. A total of 60 participants (2.9%) had more than one colonoscopic examination during follow-up.
During the first year after randomization, 1974 of the 2259 participants who underwent randomization (87.4%) reported taking 80% or more of the study tablets; in the final year of treatment, 1663 participants (73.6%) reported this level of adherence. During the treatment period, 1719 participants (76.1%) reported taking at least 80% of the study tablets, and 1949 (86.3%) reported taking at least 50%. Only 98 of 2251 participants for whom data on personal vitamin supplementation were available (4.4%) reported on two or more semiannual interviews that they took personal vitamin D supplements of 1000 IU or more daily, 74 (3.3%) reported that they took 400 mg or more of calcium daily, and 78 (3.5%) reported that they took 500 to less than 1000 IU of vitamin D. The mean (±SD) net increase in serum 25-hydroxyvitamin D among participants randomly assigned to vitamin D was 7.83±13.4 ng per milliliter in a blood sample drawn shortly before the end of treatment.
Occurrence of Adenomas
In follow-up examinations, 3131 lesions that were potentially neoplastic were seen in 1301 participants. Pathological evaluations were available for 3012 lesions in 1280 participants. For 119 lesions, the tissue was lost, fulgurated without biopsy, or unsuitable for diagnosis; this left 2059 (99%) of the examined participants for whom we could determine adenoma status. Adenomas were diagnosed in 880 participants (43%).
Effects of Supplementation
The study interventions, alone or in combination, did not have a significant effect on the risk of adenoma (Table 2). The adjusted risk ratio for any adenoma among patients taking vitamin D as compared with patients who did not take vitamin D was 0.99 (95% confidence interval [CI], 0.89 to 1.09), and the adjusted risk ratio among patients taking calcium as compared with those who did not take calcium was 0.95 (95% CI, 0.85 to 1.06). Among patients taking vitamin D plus calcium versus those taking calcium alone, the adjusted risk ratio was 1.01 (95% CI, 0.88 to 1.15). The adjusted risk ratio among patients taking vitamin D plus calcium versus those taking neither agent was 0.93 (95% CI, 0.80 to 1.08). The findings for advanced adenomas also did not suggest meaningful effects. The results for proximal adenomas were similar to those for distal adenomas (Table S3 in the Supplementary Appendix).
Table 2.
Risk Ratios for Colorectal Adenoma Outcomes According to Treatment Assignment.*
| Treatment Assignment | One or More Adenomas† | One or More Advanced Adenomas‡ | ||
|---|---|---|---|---|
| No. of Patients/ Total No. (%) |
Risk Ratio (95% CI)§ |
No. of Patients/ Total No. (%) |
Risk Ratio (95% CI)§ |
|
| Vitamin D vs. no vitamin D | ||||
| No vitamin D | 442/1035 (42.7) | Reference | 98/1042 (9.4) | Reference |
| Vitamin D | 438/1024 (42.8) | 0.99 (0.89–1.09) | 98/1032 (9.5) | 0.99 (0.75–1.29) |
| Calcium vs. no calcium | ||||
| No calcium | 362/761 (47.6) | Reference | 77/764 (10.1) | Reference |
| Calcium | 345/762 (45.3) | 0.95 (0.85–1.06) | 81/773 (10.5) | 1.02 (0.76–1.38) |
| Calcium plus vitamin D vs. calcium alone |
||||
| Calcium | 259/655 (39.5) | Reference | 63/662 (9.5) | Reference |
| Calcium plus vitamin D | 259/643 (40.3) | 1.01 (0.88–1.15) | 56/648 (8.6) | 0.89 (0.63–1.26) |
| Calcium plus vitamin D vs. neither agent |
||||
| Neither calcium nor vitamin D | 183/380 (48.2) | Reference | 35/380 (9.2) | Reference |
| Calcium plus vitamin D | 174/381 (45.7) | 0.93 (0.80–1.08) | 37/387 (9.6) | 0.99 (0.63–1.56) |
The analyses of vitamin D versus no vitamin D included all participants who underwent randomization. The analyses of calcium versus no calcium and of vitamin D plus calcium versus neither agent were restricted to participants who underwent full factorial randomization. The analyses of vitamin D plus calcium versus calcium did not include participants who underwent full factorial randomization and were assigned to receive placebo or vitamin D alone.
P values, calculated with the use of a chi-square contingency-table test, are as follows: vitamin D versus no vitamin D, P = 0.98; calcium versus no calcium, P = 0.37; vitamin D plus calcium versus calcium, P = 0.79; and vitamin D plus calcium versus neither agent, P = 0.49.
Denominators differ between adenomas and advanced adenomas because of missing data for lesion size and an assumption that small lesions (<6 mm) with missing pathological data are not advanced adenomas (see the Supplementary Appendix).
Risk ratios were adjusted for age, clinical center, anticipated surveillance interval (3 or 5 years), a three-level variable for sex and type of randomization (male, female and two-group randomization, female and full factorial randomization), and number of baseline adenomas (1, 2, or ≥3).
In the subgroup analyses, we found almost no significant effects of supplementation (Fig. 1, and Fig. S2 in the Supplementary Appendix). The findings were similar among participants with baseline 25-hydroxyvitamin D levels below the study median of 23.2 ng per milliliter and those with levels above the study median. However, body-mass index (BMI) appeared to modify the effects of calcium on adenoma risk (P = 0.02): the lower the BMI, the greater the response to calcium supplementation. Findings with respect to advanced adenomas were broadly similar to those for all adenomas (Fig. 1, and Fig. S2 in the Supplementary Appendix). The findings did not differ significantly between participants who were using vitamin D or calcium supplements at baseline and those who were not or between those who had advanced adenomas at baseline and those who did not (Table S4, S5, and S6 in the Supplementary Appendix). There were no indications of effects in a per-protocol analysis, nor was there an association between adenoma risk and changes in 25-hydroxyvitamin D levels or total calcium intake from baseline to shortly before the end of treatment (Table S7 and S8 in the Supplementary Appendix). However, there was a suggestion that supplementation with vitamin D or calcium conferred lower risks among participants with longer surveillance (and treatment) intervals, although the differences were not significant (Fig. 1, and Table S9 in the Supplementary Appendix). The sensitivity analysis did not suggest that missing data distorted the primary analyses (Table S10 in the Supplementary Appendix).
Adverse events are shown in Table 3. Calcium supplementation was associated with a small, nonsignificantly greater risk of urolithiasis than no calcium supplementation, and vitamin D supplementation was associated with a nonsignificantly lower risk of urolithiasis than no vitamin D supplementation. Participants who were randomly assigned to take calcium had slightly higher serum creatinine levels than did participants who were not assigned to take calcium; the difference was of borderline significance. In addition, participants assigned to take calcium had significantly fewer myocardial infarctions than participants who were assigned to no calcium supplementation.
Table 3.
Adverse Events.*
| Adverse Event | Vitamin D versus No Vitamin D | Calcium versus No Calcium | ||||
|---|---|---|---|---|---|---|
| No Vitamin D (N = 1129) |
Vitamin D (N = 1130) |
P Value | No Calcium (N = 835) |
Calcium (N = 840) |
P Value | |
| number (percent) | number (percent) | |||||
| Death | 12 (1.1) | 15 (1.3) | 0.56 | 12 (1.4) | 13 (1.5) | 0.85 |
| Myocardial infarction with or with- Out revascularization |
7 (0.6) | 8 (0.7) | 0.80 | 9 (1.1) | 2 (0.2) | 0.03 |
| Revascularization without myocar- dial infarction |
11 (1.0) | 12 (1.1) | 0.84 | 8 (1.0) | 12 (1.4) | 0.38 |
| Stroke | 5 (0.4) | 9 (0.8) | 0.28 | 5 (0.6) | 3 (0.4) | 0.51 |
| Transient ischemic attack | 1 (0.1) | 3 (0.3) | 0.62 | 3 (0.4) | 0 | 0.12 |
| Cancer | ||||||
| Any | 61 (5.4) | 47 (4.2) | 0.17 | 46 (5.5) | 46 (5.5) | 0.98 |
| Colorectal | 2 (0.2) | 3 (0.3) | 1.00 | 0 | 2 (0.2) | 0.50 |
| Urolithiasis | 28 (2.5) | 19 (1.7) | 0.18 | 15 (1.8) | 20 (2.4) | 0.40 |
| Hypercreatininemia† | 67 (6.0) | 57 (5.1) | 0.35 | 40 (4.9) | 58 (7.0) | 0.06 |
| Hypercalcemia‡ | 28 (2.5) | 25 (2.2) | 0.67 | 5 (0.6) | 17 (2.0) | 0.01 |
| Hypercalcemia after albumin correction§ |
4 (0.4) | 3 (0.3) | 1.00 | 0 | 2 (0.2) | 0.50 |
| Fracture | 64 (5.7) | 55 (4.9) | 0.39 | 43 (5.1) | 37 (4.4) | 0.47 |
The table includes all confirmed events up to 30 days after the cessation of study treatment in all participants who underwent randomization. Data are the numbers of participants who had one or more occurrences of an adverse event and their percentage among all participants who were randomly assigned to the given group.
Hypercreatininemia was defined as a creatinine level in the blood sample drawn at year 1 (all participants) or year 3 (participants with a 5-year follow-up) that was above the normal range among participants who had creatinine levels in the normal range at baseline; 31 participants had baseline creatinine levels that were above the normal range. Data were available for 1113 participants in the group that received no vitamin D, 1115 participants in the group that received vitamin D, 822 participants in the group that received no calcium, and 825 participants in the group that received calcium.
Hypercalcemia was defined as a calcium level in the blood sample drawn at year 1 or year 3 that was above the normal range, before albumin correction.
Albumin correction was not available for 12 participants.
The observational associations between adenoma occurrence and baseline serum 25-hydroxyvi-tamin D level or baseline calcium intake roughly paralleled the findings for supplementation with vitamin D or calcium (Table 4). Among participants who were not given vitamin D, baseline 25-hydroxyvitamin D levels were not significantly associated with adenoma risk (risk ratio, quar-tile 4 vs. quartile 1, 0.98; 95% CI, 0.79 to 1.21). The results for baseline calcium intake among participants who were not given calcium were similar (risk ratio, quartile 4 vs. quartile 1, 0.95; 95% CI, 0.75 to 1.19). We also found no observational associations between the risk of advanced adenomas and baseline serum 25-hydrox-yvitamin D level or baseline calcium intake.
Table 4.
Observational Association of Baseline Serum 25-Hydroxyvitamin D Level and Dietary Calcium Intake with Risk of Colorectal Adenoma.
| Quartile of Level or Intake* | Baseline Serum 25-Hydroxyvitamin D Level† | Baseline Dietary Calcium Intake‡ | ||||
|---|---|---|---|---|---|---|
| No. of Patients/ Total No. (%) |
Adjusted Risk Ratio (95% CI)§ |
Adjusted Risk Ratio per 10 ng/ml (95% CI) |
No. of Patients/ Total No. (%) |
Adjusted Risk Ratio (95% CI)¶ |
Adjusted Risk Ratio per 200 mg (95% CI) |
|
|
Participants with one or more adenomas |
0.98 (0.90–1.07) |
0.99 (0.93–1.04) |
||||
| Quartile 1 | 108/256 (42.2) | Reference | 89/179 (49.7) | Reference | ||
| Quartile 2 | 115/271 (42.4) | 1.02 (0.83–1.25) |
94/170 (55.3) | 1.17 (0.95–1.46) |
||
| Quartile 3 | 117/257 (45.5) | 1.06 (0.86–1.30) |
69/170 (40.6) | 0.85 (0.65–1.08) |
||
| Quartile 4 | 102/251 (40.6) | 0.98 (0.79–1.21) |
84/184 (45.7) | 0.95 (0.75–1.19) |
||
|
Participants with one or more advanced adenomas║ |
0.91 (0.71–1.16) |
0.94 (0.80–1.10) |
||||
| Quartile 1 | 25/257 (9.7) | Reference | 21/176 (11.9) | Reference | ||
| Quartile 2 | 28/270 (10.4) | 1.11 (0.66–1.88) |
15/176 (8.5) | 0.72 (0.38–1.36) |
||
| Quartile 3 | 21/263 (8.0) | 0.83 (0.47–1.46) |
19/170 (11.2) | 0.96 (0.52–1.75) |
||
| Quartile 4 | 24/252 (9.5) | 1.06 (0.61–1.83) |
18/185 (9.7) | 0.82 (0.44–1.53) |
||
For 25-hydroxyvitamin D, quartiles of seasonally adjusted values were as follows: quartile 1, ≤18.411 IU; quartile 2, 18.412 to 23.178 IU; quartile 3, 23.179 to 29.326 IU; and quartile 4, ≥29.327 IU. For calcium intake, the quartiles were as follows: quartile 1, up to 435.7 mg; quartile 2, 437.8 to 596.7 mg; quartile 3, 596.8 to 831.2 mg; and quartile 4, ≥831.3 mg.
The analysis was restricted to participants who did not receive study vitamin D supplementation; risk ratios were adjusted for age, clinical center, anticipated surveillance interval (3 or 5 years), a three-level variable for sex and type of randomization (male, female and two-group randomization, female and full factorial randomization), number of baseline adenomas (one, two, or three or more), and calcium treatment assignment (participants who underwent two-group randomization are grouped with participants who underwent full factorial randomization and received calcium).
The analysis was restricted to participants who underwent full factorial randomization and did not receive study calcium supplementation; risk ratios were adjusted for age, clinical center, anticipated surveillance interval (3 or 5 years), sex, number of baseline adenomas (one, two, or three or more), and vitamin D treatment assignment; because of sparse data for advanced adenomas, clinical centers are grouped geographically into southeast (Georgia, North Carolina, South Carolina, and Puerto Rico), north (Ohio, New Hampshire, Iowa, and Minnesota), and west (Colorado, Texas, and California).
The P value for trend for adenomas was 0.86, and the P value for trend for advanced adenomas was 0.95.
The P value for trend for adenomas was 0.33, and the P value for trend for advanced adenomas was 0.99.
Denominators differ between adenomas and advanced adenomas because of missing data on lesion size and an assumption that small lesions (<6 mm) with missing pathological data are not advanced adenomas (see the Supplementary Appendix).
DISCUSSION
Contrary to our hypotheses, neither 1000 IU of vitamin D3 nor 1200 mg of calcium, taken daily alone or in combination, reduced the risk of colorectal adenomas. The findings were similar with regard to the risk of advanced adenomas, and vitamin D supplementation was ineffective even among participants who had lower baseline serum 25-hydroxyvitamin D levels. Unplanned subgroup analyses based on participant characteristics at baseline yielded similar results, with the exception of a lower risk of adenomas in association with calcium supplementation among participants with lower BMIs. Because of the number of subgroups examined, this finding may be due to chance. In observational analyses, we also found no association between either baseline 25-hydroxyvitamin D levels or dietary calcium intake and the risk of adenomas or advanced adenomas.
Our study dose of vitamin D (1000 IU per day) exceeded the currently recommended intake for adults up to 70 years of age (600 IU per day). However, a higher dose would have raised 25-hy-droxyvitamin D levels in serum more markedly and provided a more sensitive test of vitamin D chemoprevention. Meta-analyses have summarized the observational association between serum 25-hydroxyvitamin D level and colorectal cancer as relative risks of 0.74 pe r 10 ng per milliliter,6 0.85 per 10 ng per milliliter,23 and 0.96 per 100 IU per liter5 (0.85 per 10 ng per milliliter). These associations suggest that the net mean increase of 7.83 ng per milliliter in serum 25-hydroxyvitamin D levels in our study might yield a relative risk between 0.80 and 0.88, which is outside our confidence limits for all adenomas. In this sense, our findings provide evidence against the strong observational association reported in these meta-analyses.
A report of increasing colorectal cancer risk with increasing 25-hydroxyvitamin D levels24 was included in one meta-analysis9 that yielded a summary relative risk of 0.94 per 10 nmol per liter (0.90 per 10 ng per milliliter). This suggests that the increase of 7.83 ng per milliliter in 25-hydroxyvitamin D levels in our study might yield a relative risk of 0.92, which is within our confidence intervals and so statistically consistent with our data. Meta-analyses of the relationship between adenoma risk and serum 25-hydroxyvitamin D levels8,10 have shown summary relative risks between 0.82 and 0.93 per 20 ng per milliliter (0.93 to 0.97 per 7.75 ng per milliliter). Again, our data are consistent with this weaker association.8,10 A larger sample size, higher vitamin D dose, or perhaps a longer intervention might have been required to detect these associations. Also, the fact that vitamin D may have a weaker relationship with adenomas than with colorectal cancer might imply that vitamin D acts at a later stage of carcinogenesis than that at which adenomas develop.
In view of the strong data supporting a chemopreventive effect of calcium supplementation on colorectal carcinogenesis15,16,18,19 (including findings in our own previous trial),17 it is surprising that we found no effect of calcium. However, the lack of association between baseline dietary calcium intake and adenoma risk observed in our study population supports the negative findings for calcium in our trial. Whether the high prevalence of obesity in our study population explains the lack of a calcium effect requires further investigation.
Important adverse events in the trial were generally uncommon. However, calcium supplementation resulted in an unexpected, small increase in serum creatinine level,25 which was of uncertain clinical significance. There was a significantly lower risk of myocardial infarction among participants randomly assigned to receive calcium, a finding that contrasts with recent evidence.26 The lower cancer risk associated with vitamin D supplementation and the smaller number of participants receiving vitamin D or calcium in whom fractures occurred were all compatible with chance.
Our trial had several strengths. It was large enough to detect modest chemopreventive effects, adherence to study treatment was high, and participants largely avoided taking vitamin D and calcium in substantial amounts outside the study. We obtained findings from a follow-up colonoscopic examination in a high proportion of participants, and virtually all lesions underwent central pathological review. However, the vitamin D dose was lower than the dose many experts now recommend, and it was used for a limited time. The trial was conducted among patients with a recent history of colorectal adenomas, and the results might not apply to persons without such a history.
In summary, contrary to our expectation, supplementation with 1000 IU of vitamin D3, 1200 mg of calcium, or both did not significantly affect the risk of colorectal adenomas over a period of 3 to 5 years. We have no ready explanation for the finding with regard to calcium supplementation, but the lack of an observational association between the risk of adenomas and baseline dietary calcium intake in our population supports it. Our findings with regard to vitamin D are not inconsistent with a modest chemopreventive potential, but they do not support the more marked chemopreventive effect that has sometimes been posited.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institutes of Health, National Cancer Institute (CA098286, to Dr. Baron).
Appendix
The authors’ affiliations are as follows: the Departments of Medicine (J.A.B., D.J.R., R.R.) and Epidemiology (J.A.B., E.L.B., L.A.M., J.R.R.), Geisel School of Medicine at Dartmouth, Hanover, and Department of Medicine, Dartmouth–Hitchcock Medical Center, Lebanon (D.J.R., R.R.) — both in New Hampshire; the Departments of Medicine (J.A.B., R.S.S.) and Biostatistics (A.I.), University of North Carolina at Chapel Hill, Chapel Hill; the Department of Pathology, Fairview Southdale Hospital, Edina (D.C.S.), and the Division of Environmental Health Sciences, University of Minnesota School of Public Health (T.R.C.), Minnesota Gastroenterology (A.S.K.), Department of Medicine, University of Minnesota (A.S.), and Minneapolis Veterans Affairs (VA) Medical Center (A.S.), Minneapolis — all in Minnesota; the Department of Epidemiology, Rollins School of Public Health, Emory University and Winship Cancer Institute, Emory University, Atlanta (R.M.B., M.G.); the Department of Mathematics and Statistics, University of Vermont, Burlington (B.F.C.), and VA Outcomes Group, White River Junction (D.J.R.) — both in Vermont; the Department of Medicine, University of Colorado School of Medicine, Denver (D.J.A.); the Departments of Quantitative Health Sciences (G.J.B.) and Gastroenterology and Hepatology (C.A.B.), Cleveland Clinic, Cleveland; the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas M.D. Anderson Cancer Center, Houston (R.S.B.); Puerto Rico Cancer Center, Medical Sciences Campus, University of Puerto Rico, San Juan (M.C.-C.); the Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles (J.C.F.); Consultants in Gastroenterology, West Columbia, SC (M.E.S.); and the Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City (R.W.S.)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277:F157–F175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 2.Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20:R31–R47. doi: 10.1530/ERC-12-0381. [DOI] [PubMed] [Google Scholar]
- 3.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 4.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134(Suppl):3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 5.Touvier M, Chan DS, Lau R, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 7.Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:2958–2969. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE. Circulating levels of vitamin D, vitamin D receptor polymorphisms, and colorectal adenoma: a meta-analysis. Nutr Res Pract. 2011;5:464–470. doi: 10.4162/nrp.2011.5.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin Dwith or without calcium supplementation for prevention of cancer, fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 10.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53:10–16. doi: 10.1016/j.ypmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Avenell A, MacLennan GS, Jenkinson DJ, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial) J Clin Endocrinol Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 12.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecal-ciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorec-tal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 15.Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res. 1993;290:87–95. doi: 10.1016/0027-5107(93)90036-f. [DOI] [PubMed] [Google Scholar]
- 16.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 17.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 18.Bonithon-Kopp C, Kronborg O, Gia-cosa A, Räth U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. Lancet. 2000;356:1300–1306. doi: 10.1016/s0140-6736(00)02813-0. [DOI] [PubMed] [Google Scholar]
- 19.Chu DZ, Hussey MA, Alberts DS, et al. Colorectal Chemoprevention Pilot Study (SWOG-9041), randomized and placebo controlled: the importance of multiple luminal lesions. Clin Colorectal Cancer. 2011;10:310–316. doi: 10.1016/j.clcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 21.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 23.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein SJ, Yu K, Horst RL, Ashby J, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risks of colon and rectal cancer in Finnish men. Am J Epidemiol. 2011;173:499–508. doi: 10.1093/aje/kwq398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry EL, Mott LA, Melamed ML, et al. Calcium supplementation increases blood creatinine concentration in a randomized controlled trial. PLoS One. 2014;9(10):e108094. doi: 10.1371/journal.pone.0108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.