Abstract
Predictive coding has been proposed as a framework to understand neural processes in neuropsychiatric disorders. We used this approach to describe mechanisms responsible for attentional abnormalities in autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). We monitored brain dynamics of 59 children (8–15 yr old) who had ASD or ADHD or who were control participants via high-density electroencephalography. We performed analysis at the scalp and source-space levels while participants listened to standard and deviant tone sequences. Through task instructions, we manipulated top-down expectation by presenting expected and unexpected deviant sequences. Children with ASD showed reduced superior frontal cortex (FC) responses to unexpected events but increased dorsolateral prefrontal cortex (PFC) activation to expected events. In contrast, children with ADHD exhibited reduced cortical responses in superior FC to expected events but strong PFC activation to unexpected events. Moreover, neural abnormalities were associated with specific control mechanisms, namely, inhibitory control in ASD and set-shifting in ADHD. Based on the predictive coding account, top-down expectation abnormalities could be attributed to a disproportionate reliance (precision) allocated to prior beliefs in ASD and to sensory input in ADHD.
Keywords: ADHD, ASD, predictive coding, MMN, P300
predictive coding has emerged as a framework to disentangle the neural processes underlying cognitive impairments in neuropsychiatric disorders (Fogelson et al. 2014; Friston 2012; Limongi et al. 2014). Although expectation biases favor anticipated task-relevant stimuli in neurotypical subjects (Chennu et al. 2013), this process could be affected in individuals with top-down processing abnormalities, such as autism spectrum disorders (ASD) or attention deficit hyperactivity disorder (ADHD). In this study, we assessed the influences of top-down expectation alongside bottom-up stimuli predictability in ASD and ADHD children by using high-density electroencephalography (hdEEG) markers of predictive coding as measured with an event-related potential (ERP) paradigm.
According to the predictive coding framework (Friston 2009), bottom-up prediction errors flowing upward allow adaptive inferences about sensory signals, in turn producing top-down predictions that propagate downward (Chennu et al. 2013). Recently, Pellicano and Burr (2012) and Lawson et al. (2014) proposed that ASD individuals generate impaired top-down predictions, resulting in failures to contextualize sensory information. Alternatively, Van de Cruys et al. (2014) have suggested that these individuals fail in the flexible adjustment of precision. Thus, in unambiguous or structured contexts, they generate overfitted predictions that cannot be generalized in unexpected or unpredictable contexts. Therefore, ASD subjects would exhibit normal or even enhanced neural responses to expected stimuli, but they would have difficulties with unexpected events. Furthermore, abnormal prefrontal cortex (PFC) activation associated with executive dysfunction (Dichter et al. 2009) could be related to these aberrant top-down expectations. Although two recent studies (Robic et al. 2014; Skewes et al. 2015) have indirectly supported the predictive coding model in ASD at the behavioral level, no evidence has been tested at the neural level.
In ADHD, although no empirical or theoretical arguments based on predictive coding have been stated, deficits in top-down expectation could explain the observed symptoms of inattention and distractibility. Specifically, difficulties in generating predictions would increase reliance on novel sensory evidence. Accordingly, ADHD individuals (and contrary to ASD subjects) exhibit higher or even exaggerated neural responses to novel/unexpected stimuli (Gumenyuk et al. 2005) and lower responses to expected cues (Marzinzik et al. 2012). Additionally, abnormal PFC activation and executive dysfunction (Hart et al. 2013) could be related to difficulties in top-down expectation. However, these predictions have not yet been tested.
In the current study, we unified these ideas to explore the neural underpinnings of top-down expectation in ASD and ADHD children. We used a modified auditory task previously used to test the predictive coding model (Bekinschtein et al. 2009; Chennu et al. 2013). This task included simple tones that were contextually grouped into sequences to create and then deviate from stimulus patterns. Participants were instructed to attend to stimuli deviating on frequency (expected) while stimuli deviating on laterality (unexpected) were also presented. We first investigated the ERP markers of predictive coding: bottom-up prediction error indexed by the mismatch negativity (MMN), followed by top-down expectation responsible for the P300 (Chennu et al. 2013). On the basis of task manipulation, we predicted that group differences would manifest in P300 responses (Bekinschtein et al. 2009; Chennu et al. 2013). Children with ASD would show reduced P300 responses to unexpected deviants but enhanced responses to expected stimuli. In contrast, children with ADHD would exhibit reduced P300s to expected deviants and stronger responses to unexpected deviants. Second, to explore the frontal mechanisms underlying this pattern of disassociation, we reconstructed cortical sources of P300s. Finally, we investigated the control mechanisms associated with group differences in top-down processing by exploring the associations between P300 markers and performance in executive function (EF) tasks. We expected that individual variability in these neural markers would be associated with abnormal EF.
MATERIALS AND METHODS
Participants
Fifty-nine participants were assessed, comprising 24 children diagnosed with ASD, 16 children with ADHD, and 19 typically developing participants. Individuals in the ASD and ADHD groups were selected from a 50-outpatient population of the Institute of Cognitive Neurology (INECO) and their related institutions using the following inclusion criteria: 1) age range between 8 and 15 yr, similar to that used in previous studies (Brumback et al. 2012; Gumenyuk et al. 2004) and 2) ADHD or ASD diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5; American Psychiatric Association 2013). Children in both ASD and ADHD groups were evaluated during admission interviews to the specialized clinic of developmental disorders, where they underwent a detailed examination that included neuropsychiatric assessment, neurological examination, and neuropsychological evaluation. To measure ASD symptoms, we used the Developmental, Diagnostic and Dimensional Interview (3Di; Santosh et al. 2009). This diagnostic instrument is similar to the Autism Diagnostic Interview (ADI-R; Lord et al. 1994), with which it correlates strongly (Skuse et al. 2004), and emulates its algorithms for measures of social communication impairments as well as restricted and repetitive behaviors (Mandy et al. 2012). To quantify ADHD symptom presentations, we used the Conners' Parent Rating Scale Revised: Short form (CPRS-R:S; Conners 1997), which assesses both inattentive and hyperactive-impulsive symptoms (see Table 1). Most ADHD subjects (11/16) were currently taking methylphenidate, and some ASD subjects were taking risperidone (5/24). To control the potential effects of these medications on ERP responses (Iwanami et al. 2001; Nieman et al. 2002; Paul-Jordanov et al. 2010; Sawada et al. 2010), these individuals stopped their medication 48 h before testing. Regarding long-term effects of these medications, given that they either improve or have no effects on ERP modulations (Adler et al. 2004; Iwanami et al. 2001; Paul-Jordanov et al. 2010; Seifert et al. 2003), any abnormal ERP responses would not be explained by the medication.
Table 1.
Means (SD) and group differences in demographics, diagnosis symptoms, and executive functions
| ASD | ADHD | Control | P Values* | |
|---|---|---|---|---|
| n | 24 | 15 | 19 | |
| Matching measures | ||||
| Age | 10.38 (1.97) | 11.73 (2.43) | 11.63 (2.43) | 0.104 |
| Sex, males:females | 23:1 | 11:4 | 15:4 | 0.121 |
| Fluid intelligence | 39.63 (9.83) | 39.70 (8.93) | 40.16 (8.20) | 0.970 |
| ASD symptoms (3di) | ||||
| Social communication deficits (cut-off: 10) | 13.23 (4.18) | 3.46 (3.82) | 0.000 | |
| Restricted and repetitive behaviors (cut-off: 3) | 6.09 (2.57) | 1.32 (2.82) | 0.000 | |
| ADHD symptoms (CPRS-R:S) | ||||
| Inattention (cut-off: 9) | 9.09 (4.46) | 11.10 (4.43) | 0.067 | |
| Hyperactivity (cut-off: 7) | 5.50 (3.53) | 9 (5.85) | 0.274 | |
| ADHD index (cut-off: 20) | 17.88 (6.87) | 23 (7.91) | 0.093 | |
| Executive functions | ||||
| Working memory | 12.91 (2.43) | 14.93 (4.20) | 15.37 (2.75) | 0.026 |
| Inhibitory control | 5.97 (6.41) | 4.47 (3.38) | 4.84 (3.30) | 0.601 |
| Set-shifting | 61.33 (29.40) | 47.20 (24.68) | 45.63 (23.63) | 0.101 |
Values are means (SD); n = no. of participants.
P values are from ANOVA test for age, fluid intelligence, and executive functions; χ2 for sex; and 2-tailed Student's test for autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) symptoms. 3di, Developmental, Diagnostic and Dimensional Interview; CPRS-R:S, Conner's Parent Rating Scale Revised: Short form.
Twenty-two control participants were recruited from neighboring schools. Exclusion criteria for this group were the following: 1) age outside the range of 8–15 yr and (2) history of intellectual disability, neurological, or psychiatric diseases. By using group-wise matching criteria, 19 of these participants were selected to form a control group, matched for age [F(2, 55) = 2.35, P = 0.104], sex [χ2(2, n = 58) = 4.22, P = 0.121], and fluid intelligence [Raven's progressive matrices test (Raven et al. 2008); F(2, 55) = 0.22, P = 0.970] to both the ASD and ADHD groups (see Table 1).
Parental informed consent was obtained in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of INECO.
EF Tasks
Participants completed a brief EF assessment of classical tasks that included 1) digit span subtests from the Wechsler Intelligence Scale for Children, fourth edition (WISC IV; Wechsler 2003) to assess working memory. This test consisted of two parts: first, children were required to repeat numbers verbatim as they were stated by the examiner; second, the numbers were repeated in reverse order. The score was calculated from the sum of both parts (maximum 32 points). 2) The children's version of the Hayling test was performed to evaluate inhibitory control (Shallice et al. 2002). Participants had to complete sentences with a word that made coherent sense (first part) and later with words that did not fit in the context of the sentence (second part). We measured the number of errors committed in the second part; therefore, higher scores represented worse performance. 3) The Trail Making Test (TMT) was performed to assess set-shifting (Spreen and Gaddes 1969). In this task children were instructed to draw lines connecting numbers in ascending order (TMT-A) and later to alternate between numbers and letters (TMT-B). The performance was calculated by the subtraction of time required in TMT-B minus TMT-A; therefore, longer times represented worse performance. Table 1 shows the mean, SD, and statistical comparisons of these tasks for all groups.
Experimental Task
We adapted a well-established ERP paradigm (Bekinschtein et al. 2009; Chennu et al. 2013) to design an auditory task suitable for children (e.g., abridged version) and focused on expectancy manipulation. Auditory stimuli were presented via earphones using Psychtoolbox (version 3) running in MATLAB on a desktop computer. Stimuli consisted of sequences of five complex 50-ms-duration sounds spaced 150 ms apart. Each complex sound was composed of three sinusoidal tones, either type A (500, 1,000, and 2,000 Hz) or type B (350, 700, and 1,400 Hz), identical to those used previously (Bekinschtein et al. 2009; Chennu et al. 2013).
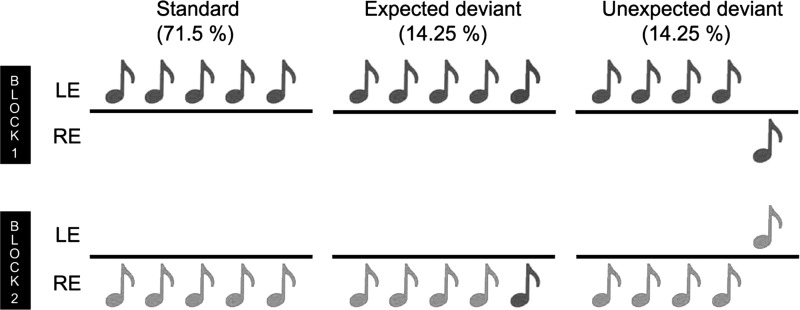
Three sequences of complex sounds were included (see Fig. 1): 1) standard sequences that contained five identical tones (AAAAA or BBBBB) presented monaurally to either the right or left ear; 2) expected deviant sequences (monaural) that included four identical sounds and a tone of the other type (AAAAB or BBBBA; these 5 tones were also presented monaurally to either the right or left ear); and 3) unexpected deviant sequences (interaural) in which all tones were identical, but the first four were presented in one ear and the fifth tone in the opposite ear (AAAAA or BBBBB). The task included two blocks of stimuli. In each block, 71.5% of the sequences were standard, 14.25% were expected deviants, and the remaining 14.25% were unexpected deviants. In block 1, the standard and unexpected deviant condition consisted of type A tones and expected deviant sequences comprised type B tones. The standard and deviant sequences were presented in the left ear, and the unexpected deviants were presented in the right ear. In block 2, type B sounds were used for standard and unexpected deviant sequences, whereas type A tones were used for expected deviant stimuli. In the second block, the standard and expected deviant stimuli were presented in the right ear, whereas unexpected deviants were presented in the left ear.
Fig. 1.
Experimental task. Auditory stimuli consisted of sequences of 5 tones of type A (black) or B (gray) presented in 2 blocks. Each block included standard sequences (71.5%), with 5 repetitions of the same tone (left); expected deviant sequences (14.25%), in which the fifth tone differed from the previous tone in type (middle); and unexpected deviant sequences (14.25%), in which all the tones were identical except the fifth tone was presented in the opposite ear (right). In block 1, tones of type A were used for standard and unexpected deviant sequences and type B for expected deviants. In contrast, in block 2, tones of type B were used for standard and unexpected sequences and type A for expected deviant stimuli. LE, left ear; RE, right ear.
The task included ∼220 trials (sequences), equally divided into the two blocks. The interval between consecutive sequences was randomly sampled from a uniform distribution between 700 and 1,000 ms. Each block began with a habituation phase consisting of a 3-s pause followed by 12 presentations of the standard sequence that would occur throughout the rest of the block. This phase was followed by the test phase, consisting of a pseudorandomly ordered mix of the three conditions in which both deviant conditions were interspersed among the standard condition. The duration of each sequence was ∼3–4 s.
Participants were asked to listen to the auditory stimulation and count the monaural deviant sequences (rare or uncommon sequences presented in the same ear as the standard or common sequences). At the end of each block, they were asked to report this count. Through these instructions, expectation was manipulated using two types of deviant stimuli: 1) expected deviants, which were the monaural deviant sequences that participants were instructed to attend to, and 2) unexpected deviants, namely, interaural deviant sequences that were novel stimuli not explicitly specified in the instructions.
High-Density EEG Data Collection and Preprocessing
During the experiment, 128-channel hdEEG signal was recorded using a Biosemi amplifier, sampled at 1,024 Hz and referenced to linked mastoids. Data were downsampled to 256 Hz and bandpass filtered at 0.5 and 20 Hz. The epochs were extracted between −200 and 1,300 ms relative to the start of the presentation of each sequence. Given that we analyzed ERP responses after the fifth tone, these epochs were baseline-corrected relative to the mean activity during the −200- to 0-ms window preceding the onset of the fifth tone (Bekinschtein et al. 2009; Chennu et al. 2013). Data containing excessive eye movement or muscular artifacts were rejected by a quasi-automated procedure; noisy channels and epochs were identified by calculating their normalized variance and then manually rejected or retained by visual confirmation. Rejected channels were interpolated using spherical spline interpolation. There were no significant differences in the number of channels interpolated or epochs rejected in the three conditions and groups. The retained data were jointly re-referenced to the linked mastoid electrodes. These processing steps were implemented using custom MATLAB scripts that used EEGLAB functionality (Delorme and Makeig 2004).
Data Analysis
ERP markers of predictive coding.
The MMN and P300 components were compared within groups in pairs of conditions (standard vs. deviant) and between groups for deviant conditions (expected and unexpected) as previously reported (Chennu et al. 2013). Time windows were selected between 100 and 200 ms for the MMN marker and within 200 and 600 ms for the P300 marker. The epochs in the habituation phase were excluded from this analysis. In both within- and between-group comparisons, epochs in each condition were averaged subject-wise. The number of epochs contributing to each participant's ERPs was equalized before averaging by rejecting a random subset of epochs in the condition with exceeding epochs. These averages were passed to the FieldTrip analysis (Maris and Oostenveld 2007), a procedure that compared each spatiotemporal point in the subject-wise averages using one-tailed dependent (for within-group comparisons) or independent (for between-group comparisons) sample t-tests. A nonparametric clustering method was introduced to address the resulting multiple comparisons problem (Bullmore et al. 1999). The t values of adjacent spatiotemporal points with P values of <0.05 were clustered together by summating their t values, and the largest cluster was retained. A minimum of two neighboring electrodes (within a 4-cm radius) was required to pass this threshold to form a cluster (Chennu et al. 2013). The cluster-level t value was calculated as the sum of the individual t values at the points within the cluster. To assess the significance of a spatiotemporal cluster identified as above, this procedure was repeated 1,000 times, with recombination and randomized resampling of the subject-wise averages before each repetition using a Monte Carlo method (Manly 2007). After each repetition, the t value of the largest cluster identified was retained. The proportion of these 1,000 randomized t values greater than the originally identified cluster-level t value was used to calculate a nonparametric P value for the originally identified cluster.
Source space reconstruction.
Cortical sources of both P300 markers of deviant conditions (expected and unexpected) were reconstructed with Brainstorm (Tadel et al. 2011). Following previous studies in children and adolescents (Escobar et al. 2014; Liu et al. 2014), we first calculated a forward model using the OpenMEEG Boundary Element Method (Gramfort et al. 2010) on the cortical surface of a template MNI brain (colin27) with a 1-mm resolution. In the next step, an inverse model was constrained using weighted minimum norm estimation (Baillet et al. 2011) to estimate source activation in picoampere-meters. To plot cortical maps, grand-averaged activation values were normalized by calculating z scores at each time point relative to baseline activity within the −200- to 0-ms window and then spatially smoothed with a 5-mm kernel. Subject-wise activation time courses were extracted by averaging activity within regions of interest (ROIs) visually identified in the grand-average cortical maps. Finally, time courses for each ROI were compared between groups using a FieldTrip-based analysis similar to that used for ERPs but restricted to clustering in the temporal dimension (Chennu et al. 2013). For group comparisons, time windows were selected between 200 and 400 ms for early P300 components and within 400 and 600 ms for late P300 markers.
Associations between cortical markers and EF tasks.
Spearman rank correlation was used to explore associations between cortical markers of top-down expectation and EF tasks. To obtain cortical markers, we first performed single-trial ERP cluster analyses with individual participants in which standard and deviant sequences were compared. We then used the t values of significant ERP spatiotemporal clusters between both conditions in each participant as a global score for cortical measures. These scores were associated with the three outcomes of each EF task (working memory, inhibitory control, and set-shifting). We first used the Mahalanobis distance method (Tabachnick and Fidell 2001) to exclude multivariate outliers and reported the associations (after excluding any outliers) that were statistically significant as indicated by an α value <0.05.
RESULTS
We explored the successive levels of ERP-markers for predictive coding: the bottom-up prediction error indexed by the early MMN and influences of top-down expectation indexed by the late P300 (Chennu et al. 2013). According to the predictive coding framework (Feldman and Friston 2010; Friston 2009), prediction error corresponds to the mismatch between sensory input and top-down predictions about that input. These predictions are learned and updated on the basis of preceding stimuli (prior experience). In our paradigm, predictability is generated by the repetition of standard stimuli in the habituation phase. Prediction error could then be greater for deviant sequences compared with standard sequences and could be interpreted as a failure to predict deviant stimuli. Neural mechanism underlying this process may correspond to a gain control that reflects the bottom-up learning of predictability. Prediction errors can also be enhanced by gain top-down mechanisms representing the predictive precision of a stimulus before it is presented (Chennu et al. 2013). This can be changed a priori by task instructions that manipulate conditional expectation of an attentional set (expected deviants).
In both MMN and P300 indexes and following previous studies (Bekinschtein et al. 2009; Chennu et al. 2013), we compared deviant and standard conditions in each group by using within-subjects analyses. Deviant conditions were then compared between groups. Additionally, we further explored the influences of manipulation of expectation by contrasting the effects of expected and unexpected deviant conditions in top-down processing.
As stated in the Introduction, we expected to find group differences in top-down expectation (P300). To examine this hypothesis with both temporal and neuroanatomical constraints, we analyzed both the ERP and the source space of frontal generators. Accordingly, we reconstructed cortical sources of P300 responses associated with both deviant conditions.
Finally, we examined the involved cognitive control mechanisms related to abnormalities in top-down expectation by performing correlations between P300 markers and performance in EF tasks.
Bottom-Up Processing Indexed by the MMN
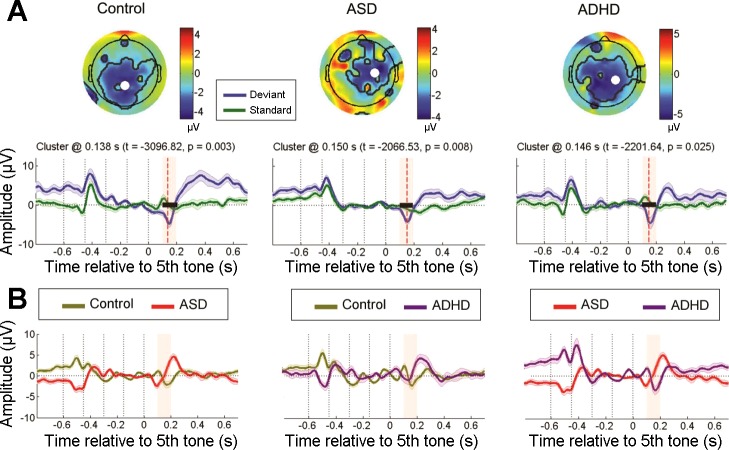
Figure 2A compares the early MMN responses generated by standard and expected monaural deviant sequences for each group. The spatiotemporal cluster analysis identified significantly larger early MMN activation generated by deviants in the three groups: controls (cluster t = −3,096.82, P = 0.003), ASD individuals (cluster t = −2,066.53, P = 0.008), and ADHD children (cluster t = −2,201.64, P = 0.025). Likewise, no significant differences were found between groups in this deviant condition; i.e., not for control vs. ASD groups, control vs. ADHD groups, or ASD vs. ADHD groups (Fig. 2B). These results suggest that prediction error generated in the early stages of auditory processing is normally developed in control participants and both ASD and ADHD groups.
Fig. 2.
Bottom-up processing indexed by the mismatch negativity (MMN). A: within-group differences between monaural deviant and monaural standard conditions for each group. Top plots show the spatial topography of the cluster at the time point of maximal difference between the pair of conditions. Thick black lines outline the spatial extent of the cluster, and the white dot therein indicates the electrode at which the maximum difference was observed. Bottom plots show grand-average event-related potential (ERP) time courses at this electrode in microvolts. Shaded bars around the ERPs indicate SE. The thick black horizontal line indicates the temporal extent of the significant cluster, and the red dashed vertical line depicts the time point at which the topography above is plotted (only when significant clusters were found). This time point is also specified above the plot, in addition to the Monte Carlo t and P values of the cluster. The pink-shaded rectangular box indicates the time window within which the conditions/groups were compared. Vertical black dotted lines indicate the onset of each of the 5 tones in the sequence. B: between-group differences in monaural deviant condition in the MMN temporal window. The plots depict time courses of the cluster at the electrode in which maximal differences between groups were observed for this condition. No significant differences were observed in these group comparisons. ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder.
Top-Down Expectation Indexed by the P300
In this section, we separately analyze the P300 responses for expected and unexpected deviant stimuli in both electrode and source spaces.
Expected deviant sequences.
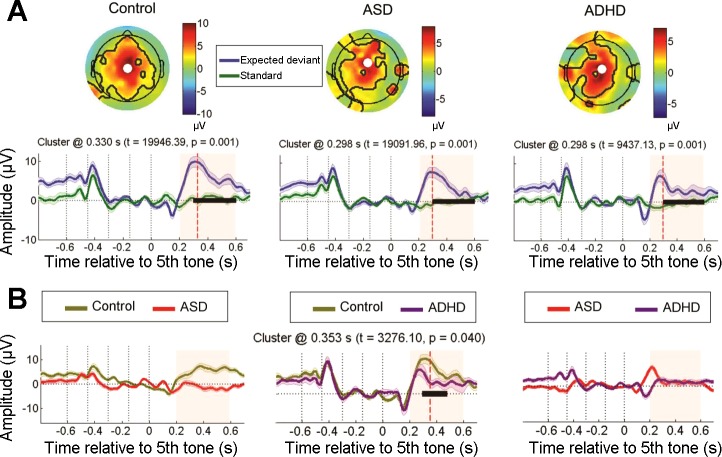
ELECTRODE SPACE.
P300s evoked by expected deviant sequences were significantly larger than standard sequences in the control (cluster t = 19,946.39, P = 0.001), ASD (cluster t = 11,699.81, P = 0.006), and ADHD groups (cluster t = 9,437.13, P = 0.001; Fig. 3A). However, between-group comparisons of this deviant condition revealed that ADHD individuals showed lower P300 responses than controls (cluster t = 3,276.10, P = 0.040; Fig. 3B). No significant differences were observed in this condition for ADHD vs. ASD groups or ASD vs. control groups. Although all participants generated P300s to expected deviant sequences, between-group analyses showed that this ERP-based marker of top-down expectation was relatively reduced in ADHD compared with control participants.
Fig. 3.
Top-down expectation for expected deviant sequences indexed by the P300. A: within-group differences between expected deviant and standard conditions for each group in the P300 time window (see Fig. 2 for details). B: between-group differences in expected deviant condition at the P300 peak.
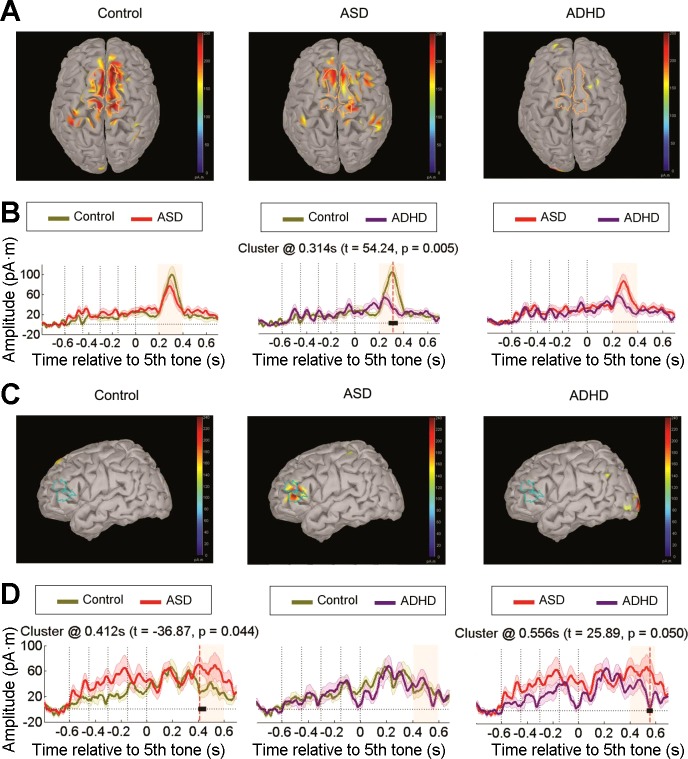
SOURCE SPACE.
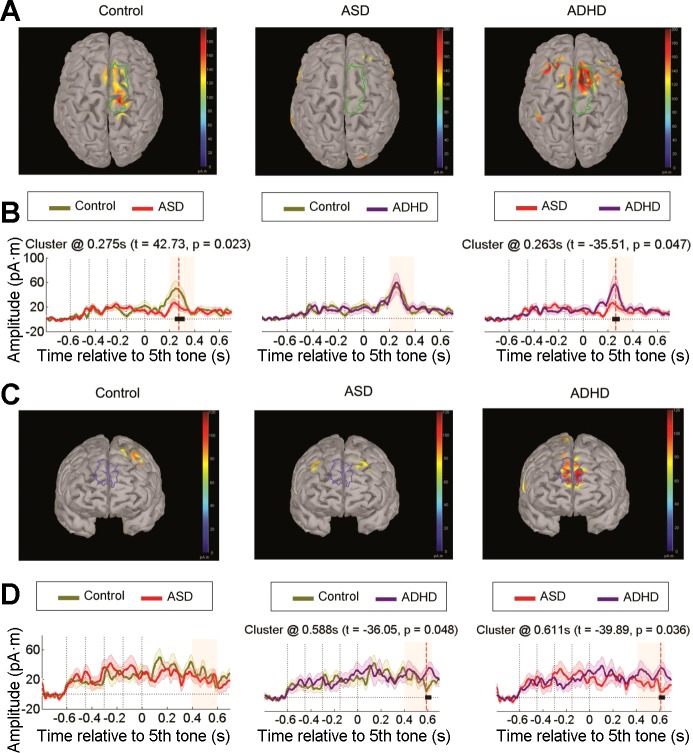
Figure 4A shows bilateral superior frontal cortex (FC) activation for expected deviants at the P300 peak in the control and ASD group. However, no such activation was observed in ADHD individuals. The temporal cluster between-group analyses (Fig. 4B) revealed significantly larger activation in this source for control compared with the ADHD group (cluster t = 54.24, P = 0.005). No significant differences were found between control vs. ASD groups and ASD vs. ADHD groups. Additionally, left dorsolateral prefrontal cortex (DLPFC) activation was observed in the late phase of the P300 component (≈500 ms) only in the ASD group (Fig. 4C). Importantly, ASD subjects presented greater activation than both control (cluster t = −36.87, P = 0.044) and ADHD participants (cluster t = 25.89, P = 0.050; Fig. 4D). No significant clusters were observed in the control vs. ADHD group.
Fig. 4.
Source reconstruction for expected deviant sequences. A: cortical activation maps at the P300 peak for each group highlighting stronger responses in the bilateral superior frontal cortex. B: activation time courses in picoampere-meters, comparing group differences for this region of interest (ROI) by cluster analyses (see Fig. 2 for details). C: cortical activation maps at the late phase of the P300 component exhibiting higher left dorsolateral prefrontal cortex activation in the ASD group. D: activation time courses of group differences in this ROI by cluster analyses.
Together, these results suggest a reduced cortical engagement in ADHD individuals for stimuli that require focused attention (expected sequences). Conversely, ASD individuals exhibited heightened frontal activation in a late time period, suggesting a stronger attentional updating in response to expected stimuli.
Unexpected deviant sequences.
ELECTRODE SPACE.
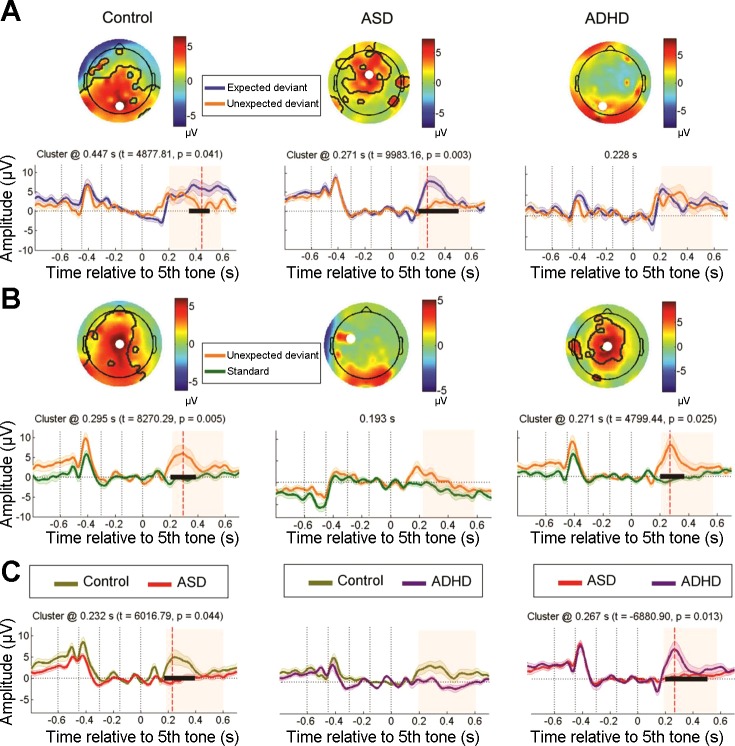
First, both expected and unexpected deviant stimuli were compared in each group (Fig. 5A). In the control (cluster t = 4,877.81, P = 0.041) and ASD groups (cluster t = 11,699.81, P = 0.006), significantly higher P300 responses were observed for expected compared with unexpected stimuli. However, no significant clusters between these conditions were found in the ADHD group. These findings are consistent with the results presented above and suggest that neural tuning of expectation to anticipated stimuli is reduced in children with ADHD, resulting in a lack of cortical distinction between expected and unexpected sequences.
Fig. 5.
Top-down expectation for unexpected deviant sequences indexed by the P300. A: within-group differences between expected and unexpected deviant conditions for each group (see Fig. 2 for details). B: within-group comparisons between standard and unexpected deviant sequences in the P300 time window. C: between-group differences in the unexpected deviant condition.
Therefore, we explored the attentional responses to unexpected deviant stimuli. Higher P300 markers for this deviant condition compared with standard sequences were observed in the control (cluster t = 5,597.99, P = 0.019) and ADHD groups (cluster t = 7,653.74, P = 0.008). However, no such effect was observed in ASD subjects (Fig. 5B). Similarly, between-group analyses for unexpected deviant condition revealed a reduced P300 response in the ASD group compared with control (cluster t = 6,016.79, P = 0.044) and ADHD groups (cluster t = −6,954.46, P = 0.041; Fig. 5C).
These results suggest that children with ASD generated minimal responses to unexpected deviant sequences (despite their novelty). Instead, they fixated on the expected sequences that were explicated in the task instructions.
SOURCE SPACE.
Figure 6A shows that regions in the right superior FC were activated for unexpected deviants at the P300 peak in the control and ADHD groups, whereas no such frontal activation was observed in ASD subjects. Therefore, this source was significantly less active in the ASD group compared with the control (cluster t = 42.73, P = 0.023) and ADHD groups (cluster t = −35.51, P = 0.047; Fig. 6B).
Fig. 6.
Source reconstruction for unexpected deviant sequences. A: cortical activation maps at the P300 peak for each group at the right superior frontal cortex. B: activation time courses in picoampere-meters, comparing group differences for the previous ROI by cluster analyses (see Fig. 2 for details). C: cortical activation maps at the late phase of the P300 showing stronger responses in the prefrontal cortex (bilaterally) in the ADHD group. D: activation time courses for group differences in this ROI by cluster analyses.
In this deviant condition, a high bilateral PFC activation was observed in the late phase of the P300 component, but only in ADHD subjects (Fig. 6C). The between-group comparisons of this source (Fig. 6D) confirmed that the ADHD group displayed a significantly greater activation compared with the control (cluster t = −36.05, P = 0.048) and ASD groups (cluster t = 39.89, P = 0.036). Altogether, these analyses indicate that although frontocortical activation to unexpected deviant sequences was reduced in children with ASD, children with ADHD were much more responsive to these novel stimuli and evidenced higher late cortical engagement.
Associations Between ERP Markers of Top-Down Expectation and EF Tasks
In the following section, we separately report the significant correlations between the P300 markers of each deviant condition and behavioral performance on EF tasks in each group (see details in Data Analysis).
Expected deviant condition.
Significant associations between the ERP marker of expected sequences and EF performance were found in the control and ASD groups. Specifically, increased cortical response, quantified by the t value of the statistically significant ERP spatiotemporal cluster, was associated with high working memory in the control group (rs = 0.47, P = 0.043) and greater inhibitory control in the ASD group (rs = 0.62, P = 0.002). No significant associations were found in the ADHD group.
Unexpected deviant condition.
For the ERP marker of unexpected sequences, significant associations were observed in the ADHD group only. Increased neural responses to unexpected sequences were associated with greater set-shifting abilities (rs = 0.61, P = 0.035). No significant associations were observed in the control and ASD groups.
In summary, neural signatures of top-down expectation were related to different executive skills across groups. In control children, higher working memory was associated with cortical markers of attention to expected or task-relevant stimuli, suggesting higher attentional updating. Regarding children with ASD, greater inhibitory control was related to increased neural responses to expected stimuli, indicating higher cognitive control closely adhering to explicit task instructions. Finally, in children with ADHD, higher set-shifting skills were associated with enhanced cortical responses to unexpected sequences, showing greater resource allocation in switching between attentional sets during the task.
DISCUSSION
The current study explored top-down expectation alongside bottom-up salience using ERP markers of predictive coding in ASD, ADHD, and control children. Our results showed that children with ASD and children with ADHD share abnormalities in neural markers of top-down expectation and also present a dissociated pattern of atypical top-down processing. Compared with other groups, children with ASD presented reduced P300 and frontocortical responses (localized to the superior FC) to unexpected events but exhibited increased late frontal activation to expected stimuli (in the right DLPFC). In contrast, children with ADHD showed reduced P300 attention-related cortical responses to expected deviants (in superior FC) but demonstrated increased late frontal activation (in bilateral PFC) to unexpected/novel stimuli (see summary of main results in Table 2).
Table 2.
Summary of ERP and source reconstruction results
| Control | ASD | ADHD | Control vs. ASD | Control vs. ADHD | ASD vs. ADHD | |
|---|---|---|---|---|---|---|
| MMN | ||||||
| Electrode space | + | + | + | n.s. | n.s. | n.s. |
| P300 | ||||||
| Expected deviants | ||||||
| Electrode space | + | + | + | n.s. | Control>ADHD† | n.s. |
| P3a source space | ↑ | ↑ | ↓ | n.s. | Control>ADHD† | n.s. |
| P3b source space | ↓ | ↑ | ↓ | Control<ASD* | n.s. | ASD>ADHD* |
| Unexpected deviants | ||||||
| Electrode space | + | n.s. | + | Control>ASD* | n.s. | ASD<ADHD* |
| P3a source space | ↑ | ↓ | ↑ | Control>ASD* | n.s. | ASD<ADHD* |
| P3b source space | ↓ | ↓ | ↑ | n.s. | Control<ADHD† | ASD<ADHD† |
| Expected vs. unexpected | + | + | n.s. | n.a. | n.a. | n.a. |
In the electrode space, plus signs (+) indicate a significant event-related potential (ERP) modulation between deviant and standard conditions (see Data Analysis for details). In the source space, upward arrows indicate an observed peak activation in the selected source, whereas downward arrows indicate an absence of this peak activation. Comparisons represent between-group differences determined by employing cluster analysis.
Main results for the ASD group.
Main results for the ADHD group. n.a., not available; n.s., not significant.
Based on the current accounts of attention as precision in the predictive coding framework (Friston 2009), abnormalities in top-down expectation could be attributed to a disproportionate reliance (precision) allocated to either prior beliefs in children with ASD or to novel sensory input in children with ADHD. Therefore, this framework allows us to posit a broader mechanistic explanation for how this misallocation of attentional precision could commonly underpin the atypical responses to unpredictable contexts in ASD and enhanced bottom-up attentional capture in ADHD.
Additionally, these atypical neural responses were associated with different behavioral correlates; in children with ASD, high inhibitory control was related to focused attention to task-related stimuli (expected), and in children with ADHD, higher set-shifting strategies correlated with excessive switching. According to our knowledge, this study is the first empirical report that draws upon predictive coding to delineate the neural mechanisms that could be responsible for attentional abnormalities in ASD and ADHD individuals.
Bottom-Up Processing in Children with ASD or ADHD
As expected, normal prediction error signals indexed by MMN in response to deviants were observed in both ASD and ADHD subjects. Larger MMN amplitude elicited by deviant compared with standard stimuli has been interpreted as intact bottom-up learning of predictability at the early stage of predictive coding (Garrido et al. 2009).
Previous research on the MMN in individuals with ASD has revealed inconsistencies (Gomot and Wicker 2012), probably due to differences in attentional task demands (Dunn et al. 2008). For instance, typical MMN amplitude was exhibited in individuals with ASD if stimuli were attended to (Dunn et al. 2008). The specific instructions included in the current study consistently guided attention to deviants; therefore, normal MMN responses were observed in children with ASD. Regarding children with ADHD, our results are consistent with most of the studies that have reported normal MMN modulation in these individuals (Barry et al. 2003).
From a predictive coding perspective, these results suggest that deficits in both neurodevelopmental disorders would not be due to deficient bottom-up prediction error generation. On the contrary, they are more plausibly explained by abnormalities in top-down expectations and attention.
Top-Down Expectation in Children with ASD
Our results show that children with ASD presented diminished P300 amplitude and FC activation in response to unexpected stimuli and enhanced DLPFC activation in the late phase of the P300 responses to expected deviants. These results are consistent with previous studies in individuals with ASD that have found reduced P300 amplitude to novel/unexpected stimuli (O'Connor 2012) and greater dorsomedial PFC activation to target/expected stimuli (Dichter et al. 2009).
Based on predictive coding, our results suggest that ASD individuals could be impaired in their ability to adjust precision if faced with uncertainty due to inflexible expectation (Van de Cruys et al. 2014). In other words, the tendency to inhibit bottom-up influences and the attentional bias toward expected stimuli may trigger difficulties in adjusting precision in changing real-world environments. This finding aligns with recent accounts of predictive coding in ASD (Lawson et al. 2014) suggesting that ASD individuals fail to contextualize sensory evidence in relation to prior beliefs and that these difficulties mostly emerge in uncertain environments (Gomot and Wicker 2012). More specifically, it has been proposed (Van de Cruys et al. 2013, 2014) that the flexible adjustment of precision in particular contexts is lacking in ASD, leading to overfitted and nongeneralizable predictions. Attenuated priors also have been proposed to explain hypersensitivity to sensory stimulation in individuals with ASD (Lawson et al. 2014; Pellicano and Burr 2012). Although this explanation would differ from our account, sensory hypersensitivity has been associated with more severe manifestations of ASD (Tavassoli et al. 2014). Because we assessed ASD subjects with low-to-mild severity level, these previous accounts may be true for ASD populations with more severe symptomatology. Future studies should further explore this issue to delineate differences in predictive coding with increasing severity of ASD. Moreover, both attenuated and exacerbated priors indicate failures in adjusting precision (Lawson et al. 2014), and therefore, both impairments could emerge as a function of context uncertainty.
Furthermore, highly structured contexts may favor strong expectation and inhibition of distraction by unexpected events. Accordingly, individuals with ASD have shown intact performance if explicit information about task rules is available, but they fail in open-ended tasks in which rules must be inferred (Baez and Ibanez 2014; Baez et al. 2012; Senju et al. 2009; White et al. 2009). Possibly, these individuals employ higher and disproportionate cognitive control strategies to follow task instructions, attenuating the processing of unexpected stimuli. The observed association between top-down abnormalities and high inhibitory control performance in ASD supports this hypothesis.
Conversely, unstructured contexts could increase the volume of sensory channels, leading to overload. Whereas in the current study we have employed an explicit or structured task, future studies should explore top-down expectation using unstructured paradigms (i.e., without task instructions) to test this notion.
Top-Down Expectation in Children with ADHD
Children with ADHD exhibited reduced P300 amplitude and superior FC source activity for expected deviants but increased PFC activation in response to unexpected deviants in the late P300 window. These results parallel ADHD reports of diminished attentional-related neural response to target (expected) stimuli (Barry et al. 2003; Kemner et al. 1996; Marzinzik et al. 2012; Senderecka et al. 2012), as well as more pronounced attention switching to novel (unexpected) events (Gumenyuk et al. 2005; Keage et al. 2006; Kemner et al. 1996).
In our study, those abnormalities were related to higher set-shifting performance in children with ADHD. In typically developing individuals, this ability allows flexible switching between attentional sets. Nevertheless, inappropriate set-shifting to irrelevant/novel stimuli may reduce the attentional focus to expected stimuli in children with ADHD. This interpretation is consistent with previous studies that have reported abnormal attention-switching in these individuals (Cepeda et al. 2000; Nigg 2005).
Based on the predictive coding framework, our results suggest that difficulties in top-down expectation in children with ADHD are due to high precision ascribed to novel sensory evidence relative to task instructions. Although children with ADHD responded to failures of accurate prediction (unexpected deviants), they generated diminished prediction errors for expectations generated by task instructions. A disorder-specific failure to correctly place confidence in expectations could trigger difficulties to attenuate sensory salience and, consequently, increased distractibility.
Limitations and Further Assessment
Difficulties in responding to expected stimuli by children with ADHD were not identified in within-group analyses, which is possibly explained by the short task duration. Reduced P300 amplitude to targets in tasks with slower event rates, and therefore longer duration (Johnstone and Galletta 2013; Wiersema et al. 2006), has been observed in ADHD reports. Future studies should manipulate task duration in the assessment of attentional abnormalities in these individuals.
Because of the reduced sample size and well-known high variability reported in both neurodevelopmental disorders (Geurts et al. 2008; Gonzalez-Gadea et al. 2013; Gonzalez-Gadea et al. 2014), we used nonparametric analyses that lack interaction effects between groups and conditions, as well as the inclusion of covariables such as age, symptomatology, or comorbidity. Future studies with larger samples would further explore the influence of these variables by employing multivariate methods.
Conclusion
We have drawn on the current neuroscientific understanding of predictive coding in cortical information processing to provide a collective account of atypical attention responses in both ASD and ADHD. Children with neurodevelopmental disorders exhibited a double-dissociated neural pattern; children with ASD were strongly influenced by explicit task instructions and less affected by novel and unexpected stimuli, whereas children with ADHD were more influenced by task-irrelevant stimuli than by explicitly expected stimuli. These findings could help us understand the various symptoms in each disorder. In individuals with ASD, strong expectations may account for restricted interest and hyporeactivity to novel input, whereas in individuals with ADHD, attenuated expectations could explain distractibility symptoms.
From a theoretical perspective, predictive coding could help to unravel the neural underpinnings of abnormal information processing in neurodevelopmental disorders. Traditionally, these difficulties have been explained by deficits in high-level processes, such as theory of mind or EF in ASD (Baron-Cohen et al. 1985; Hill 2004) and EF in ADHD (Barkley 1997; Pennington and Ozonoff 1996), or by failures in low-level processes, including atypical perceptual functioning in ASD (Frith 1989; Mottron et al. 2006) and abnormalities in arousal levels in ADHD (Sergeant 2005). As predictive coding theories are further developed to provide broad-based explanations of cortical information processing, they could provide a valuable, theoretically motivated method to reconcile seemingly conflicting low -vs. high-level interpretations of ASD and ADHD.
GRANTS
This research was partially supported by grants from the National Scientific and Technical Research Council (CONICET), Fondo Nacional de Desarrollo Científico y Tecnológica (CONICYT/FONDECYT) Regular Grants 1130920 and 1140114, FONCyT-PICT Grants 2012-0412 and 2012-1309, and the INECO Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.G.-G., S.C., T.A.B., A.R., A.B., P.T., B.M., Y.S., L.S., F.A., M.S., J.M., F.M., and A.I. conception and design of research; M.L.G.-G. performed experiments; M.L.G.-G., S.C., and A.I. analyzed data; M.L.G.-G., S.C., T.A.B., A.R., F.A., and A.I. interpreted results of experiments; M.L.G.-G. and S.C. prepared figures; M.L.G.-G., S.C., F.A., M.S., and A.I. drafted manuscript; M.L.G.-G., S.C., T.A.B., A.R., A.B., P.T., B.M., Y.S., L.S., F.A., M.S., J.M., F.M., and A.I. edited and revised manuscript; M.L.G.-G., S.C., T.A.B., A.R., A.B., P.T., B.M., Y.S., L.S., F.A., M.S., J.M., F.M., and A.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Programa Argentino para Niños, Adolescentes y Adultos con Condiciones del Espectro Autista (PANAACEA), the Institute of Neurosciences (University of Favaloro, Buenos Aires, Argentina), and Centro Interdisciplinario de Tourette, TOC, TDAH, y Trastornos Asociados (CITTTA) for helping with the patient recruitment process.
REFERENCES
- Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry 161: 1822–1828, 2004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- Baez S, Ibanez A. The effects of context processing on social cognition impairments in adults with Asperger's syndrome. Front Neurosci 8: 270, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S, Rattazzi A, Gonzalez-Gadea M, Torralva T, Vigliecca N, Decety J, Manes F, Ibanez A. Integrating intention and context: assessing social cognition in adults with Asperger syndrome. Front Hum Neurosci 6: 302, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet S, Friston K, Oostenveld R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput Intell Neurosci 2011: 972050, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121: 65–94, 1997. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic-child have a theory of mind. Cognition 21: 37–46, 1985. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 114: 184–198, 2003. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA 106: 1672–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback TY, Arbel Y, Donchin E, Goldman MS. Efficiency of responding to unexpected information varies with sex, age, and pubertal development in early adolescence. Psychophysiology 49: 1330–1339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42, 1999. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Cepeda ML, Kramer AF. Task switching and attention deficit hyperactivity disorder. J Abnorm Child Psychol 28: 213–226, 2000. [DOI] [PubMed] [Google Scholar]
- Chennu S, Noreika V, Gueorguiev D, Blenkmann A, Kochen S, Ibanez A, Owen AM, Bekinschtein TA. Expectation and attention in hierarchical auditory prediction. J Neurosci 33: 11194–11205, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners K. Conners' Rating Scales–Revised: Technical Manual. New York: Multi-Health Systems, 1997. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci 4: 215–226, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. J Autism Dev Disord 38: 52–71, 2008. [DOI] [PubMed] [Google Scholar]
- Escobar MJ, Huepe D, Decety J, Sedeño L, Messow MK, Baez S, Rivera-Rei Á, Canales-Johnson A, Morales JP, Gómez DM, Schröeder J, Manes F, López V, Ibánez A. Brain signatures of moral sensitivity in adolescents with early social deprivation. Sci Rep 4: 5354, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H, Friston KJ. Attention, uncertainty, and free-energy. Front Hum Neurosci 4: 215, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Litvak V, Peled A, Fernandez-Del-Olmo M, Friston K. The functional anatomy of schizophrenia: A dynamic causal modeling study of predictive coding. Schizophr Res 158: 204–212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a rough guide to the brain? Trends Cogn Sci 13: 293–301, 2009. [DOI] [PubMed] [Google Scholar]
- Friston K. Prediction, perception and agency. Int J Psychophysiol 83: 248–252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the Enigma. Oxford: Blackwell, 1989. [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol 120: 453–463, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia 46: 3030–3041, 2008. [DOI] [PubMed] [Google Scholar]
- Gomot M, Wicker B. A challenging, unpredictable world for people with autism spectrum disorder. Int J Psychophysiol 83: 240–247, 2012. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gadea ML, Baez S, Torralva T, Castellanos FX, Rattazzi A, Bein V, Rogg K, Manes F, Ibanez A. Cognitive variability in adults with ADHD and AS: disentangling the roles of executive functions and social cognition. Res Dev Disabil 34: 817–830, 2013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gadea ML, Tripicchio P, Rattazzi A, Baez S, Marino J, Roca M, Manes F, Ibanez A. Inter-individual cognitive variability in children with Asperger's syndrome. Front Hum Neurosci 8: 575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Papadopoulo T, Olivi E, Clerc M. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed Eng Online 9: 45, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Alho K, Escera C, Naatanen R. Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8–13 years. Psychophysiology 41: 30–36, 2004. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Escera C, Hamalainen M, Huotilainen M, Hayrinen T, Oksanen H, Naatanen R, von Wendt L, Alho K. Electrophysiological evidence of enhanced distractibility in ADHD children. Neurosci Lett 374: 212–217, 2005. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70: 185–198, 2013. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci 8: 26–32, 2004. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Okajima Y, Isono H, Shinoda J, Kasai K, Hata A, Fukuda M, Nakagome K, Kamijima K. Effects of risperidone on event-related potentials in schizophrenic patients. Pharmacopsychiatry 34: 73–79, 2001. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Galletta D. Event-rate effects in the flanker task: ERPs and task performance in children with and without AD/HD. Int J Psychophysiol 87: 340–348, 2013. [DOI] [PubMed] [Google Scholar]
- Keage HA, Clark CR, Hermens DF, Kohn MR, Clarke S, Williams LM, Crewther D, Lamb C, Gordon E. Distractibility in AD/HD predominantly inattentive and combined subtypes: the P3a ERP component, heart rate and performance. J Integr Neurosci 5: 139–158, 2006. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag RJ, Camfferman G, van Engeland H. Event-related brain potentials in children with attention-deficit and hyperactivity disorder: effects of stimulus deviancy and task relevance in the visual and auditory modality. Biol Psychiatry 40: 522–534, 1996. [DOI] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci 8: 302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limongi R, Tomio A, Ibanez A. Dynamical predictions of insular hubs for social cognition and their application to stroke. Front Behav Neurosci 8: 380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Woltering S, Lewis MD. Developmental change in EEG theta activity in the medial prefrontal cortex during response control. Neuroimage 85: 873–887, 2014. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659–685, 1994. [DOI] [PubMed] [Google Scholar]
- Mandy WP, Charman T, Skuse DH. Testing the construct validity of proposed criteria for DSM-5 autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 51: 41–50, 2012. [DOI] [PubMed] [Google Scholar]
- Manly B. Randomization, Bootstrap, and Monte Carlo Methods in Biology. Boca Raton, FL: Chapman & Hall, 2007. [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. [DOI] [PubMed] [Google Scholar]
- Marzinzik F, Wahl M, Kruger D, Gentschow L, Colla M, Klostermann F. Abnormal distracter processing in adults with attention-deficit-hyperactivity disorder. PLoS One 7: e33691, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord 36: 27–43, 2006. [DOI] [PubMed] [Google Scholar]
- Nieman DH, Koelman JH, Linszen DH, Bour LJ, Dingemans PM, Ongerboer de Visser BW. Clinical and neuropsychological correlates of the P300 in schizophrenia. Schizophr Res 55: 105–113, 2002. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry 57: 1424–1435, 2005. [DOI] [PubMed] [Google Scholar]
- O'Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev 36: 836–854, 2012. [DOI] [PubMed] [Google Scholar]
- Paul-Jordanov I, Bechtold M, Gawrilow C. Methylphenidate and if-then plans are comparable in modulating the P300 and increasing response inhibition in children with ADHD. Attent Defic Hyperact Disord 2: 115–126, 2010. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn Sci 16: 504–510, 2012. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry 37: 51–87, 1996. [DOI] [PubMed] [Google Scholar]
- Raven J, Court J, andRaven J. (editors). Test de matrices progresivas. Manual. Escalas coloreadas, general y avanzada. Buenos Aires: Paidos, 2008. [Google Scholar]
- Robic S, Sonie S, Fonlupt P, Henaff MA, Touil N, Coricelli G, Mattout J, Schmitz C. Decision-Making in a Changing World: A Study in Autism Spectrum Disorders. J Autism Dev Disord 2014. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Mandy WP, Puura K, Kaartinen M, Warrington R, Skuse DH. The construction and validation of a short form of the developmental, diagnostic and dimensional interview. Eur Child Adolesc Psychiatry 18: 521–524, 2009. [DOI] [PubMed] [Google Scholar]
- Sawada M, Iida J, Ota T, Negoro H, Tanaka S, Sadamatsu M, Kishimoto T. Effects of osmotic-release methylphenidate in attention-deficit/hyperactivity disorder as measured by event-related potentials. Psychiatry Clin Neurosci 64: 491–498, 2010. [DOI] [PubMed] [Google Scholar]
- Seifert J, Scheuerpflug P, Zillessen KE, Fallgatter A, Warnke A. Electrophysiological investigation of the effectiveness of methylphenidate in children with and without ADHD. J Neural Transm 110: 821–829, 2003. [DOI] [PubMed] [Google Scholar]
- Senderecka M, Grabowska A, Gerc K, Szewczyk J, Chmylak R. Event-related potentials in children with attention deficit hyperactivity disorder: an investigation using an auditory oddball task. Int J Psychophysiol 85: 106–115, 2012. [DOI] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frith U. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 325: 883–885, 2009. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry 57: 1248–1255, 2005. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol 21: 43–71, 2002. [DOI] [PubMed] [Google Scholar]
- Skewes JC, Jegindo EM, Gebauer L. Perceptual inference and autistic traits. Autism 19: 301–3017, 2015. [DOI] [PubMed] [Google Scholar]
- Skuse D, Warrington R, Bishop D, Chowdhury U, Lau J, Mandy W, Place M. The developmental, dimensional and diagnostic interview (3di): a novel computerized assessment for autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 43: 548–558, 2004. [DOI] [PubMed] [Google Scholar]
- Spreen O, Gaddes WH. Developmental norms for 15 neuropsychological tests age 6 to 15. Cortex 5: 170–191, 1969. [DOI] [PubMed] [Google Scholar]
- Tabachnick B, Fidell L. Using Multivariate Statistics. Boston, MA: Allyn and Bacon, 2001. [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, Baron-Cohen S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 18: 428–432, 2014. [DOI] [PubMed] [Google Scholar]
- Van de Cruys S, de-Wit L, Evers K, Boets B, Wagemans J. Weak priors versus overfitting of predictions in autism: reply to Pellicano and Burr (TICS, 2012). Iperception 4: 95–97, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev 121: 649–675, 2014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children (4th ed). San Antonio, TX: Psychological Corporation, 2003. [Google Scholar]
- White SJ, Burgess PW, Hill EL. Impairments on “open-ended” executive function tests in autism. Autism Res 2: 138–147, 2009. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Roeyers H, Van Coster R, Baeyens D. Event rate and event-related potentials in ADHD. J Child Psychol Psychiatry 47: 560–567, 2006. [DOI] [PubMed] [Google Scholar]