Abstract
The cercal system of crickets detects low-frequency air currents produced by approaching predators and self-generated air currents during singing, which may provide sensory feedback to the singing motor network. We analyzed the effect of cercal stimulation on singing motor pattern generation to reveal the response of a singing interneuron to predator-like signals and to elucidate the possible role of self-generated air currents during singing. In fictive singing males, we recorded an interneuron of the singing network while applying air currents to the cerci; additionally, we analyzed the effect of abolishing the cercal system in freely singing males. In fictively singing crickets, the effect of short air stimuli is either to terminate prematurely or to lengthen the interchirp interval, depending on their phase in the chirp cycle. Within our stimulation paradigm, air stimuli of different velocities and durations always elicited an inhibitory postsynaptic potential in the singing interneuron. Current injection in the singing interneuron elicited singing motor activity, even during the air current-evoked inhibitory input from the cercal pathway. The disruptive effects of air stimuli on the fictive singing pattern and the inhibitory response of the singing interneuron point toward the cercal system being involved in initiating avoidance responses in singing crickets, according to the established role of cerci in a predator escape pathway. After abolishing the activity of the cercal system, the timing of natural singing activity was not significantly altered. Our study provides no evidence that self-generated cercal sensory activity has a feedback function for singing motor pattern generation.
Keywords: cercal sensory system, air stimulus, singing central pattern generator interneuron, escape response
terrestrial animals are constantly subjected to air currents, which, among others, can be of atmospheric origin, produced by approaching predators and conspecifics, or self-generated.
Crickets, cockroaches, and locusts detect air currents through the cerci, two appendages at the rear of the abdomen (Edwards and Palka 1974) that carry up to 2,000 mechanosensitive filiform hairs (Chiba et al. 1992). Each filiform hair is innervated by a single sensory neuron, which makes synaptic connections with ascending interneurons, like the giant interneurons (GIs), in the terminal abdominal ganglion (TAG; Edwards and Palka 1974). Stimulation of the cercal pathway with salient air currents as generated by predators triggers escape responses and aggressive and freezing reactions and alters the singing behavior in male crickets (Baba and Shimozawa 1997; Dambach et al. 1983; Kohstall-Schnell and Gras 1994; Lewkiewicz and Zuk 2004; Matsuura et al. 2002). Air currents directed to the cerci evoke silencing responses in singing crickets during which the animals transiently lower their wings and stop singing (Dambach and Rausche 1985; Hedwig 2000a). In addition, singing crickets also generate low-velocity air currents, which are coupled to the wing movements underlying the production of sound pulses (Kämper and Dambach 1981). These are detected by the cercal system and were thought to provide sensory feedback for singing motor pattern generation (Dambach et al. 1983; Dambach and Rausche 1985).
As cercal activity is implicated to detect both predator-generated and self-generated air currents, it may require stimulus- and context-specific processing by the central nervous system (CNS). During singing at least two cercal GIs are inhibited by a corollary discharge mechanism (Schöneich and Hedwig 2015), but we do not yet understand whether and how the singing motor network responds to cercal air current stimulation.
In the two-spotted field cricket Gryllus bimaculatus DeGeer, males sing by rhythmically opening and closing their front wings at a rate of 20–30 Hz, with each closing movement producing a short 20-ms sound pulse. Current evidence shows that the cricket singing activity is generated by a central pattern generator (CPG) network, which extends from the metathoracic to the first unfused abdominal ganglion A3 (Hennig and Otto 1996; Schöneich and Hedwig 2011, 2012). One core element of the singing CPG, the ascending opener interneuron in A3 (A3-AO), is rhythmically active in phase with the singing rhythm, i.e., opener and closer activity underlying wing movements. The A3-AO depolarizes in phase with the opener wing motoneuron activity and hyperpolarizes in phase with the closer wing motoneuron activity. This interneuron elicits singing motor activity and resets the chirp pattern when stimulated by intracellular current injection (Schöneich and Hedwig 2012). The recent identification of the A3-AO interneuron now offers the possibility to analyze how the effect of cercal air current stimuli is integrated at the level of the singing CPG and to shed some light on how air stimuli related to noxious and innocuous signals are processed at the level of the singing network in order to produce adaptive responses. To analyze the processing of cercal activity we studied the effect of cercal air stimulation in fictively and freely singing crickets.
MATERIALS AND METHODS
Animals.
Experiments were performed on adult male crickets (G. bimaculatus DeGeer) from 7 to 21 days after the final ecdysis. The animals were kept individually in plastic containers at 28°C with a 12:12-h light-dark cycle and were provided with ad libitum food, a mixture of muesli, fish food and cat food, and water. All experiments were carried out at 23–24°C and complied with the principles of laboratory animal care (ASAB Ethics Committee 2006).
Dissection and pharmacological brain stimulation.
Crickets were cooled in a fridge for ≤30 min and were subsequently fixed dorsal side up on a Plasticine block by restraining all legs with metal clamps. The head was waxed to a metal holder, the anterior head capsule was opened, and the brain was exposed. After the wings and the pronotum were removed, the abdomen and thorax were opened via a dorsal midline incision and the ventral CNS was exposed. All peripheral nerves to the thoracic and abdominal ganglia were cut, except for mesothoracic wing nerve 3A (Meso Nv3A), cercal nerves, and nerves of the head ganglia.
Exposed nervous tissue was continuously perfused in insect saline (in mmol/l: 140 NaCl, 10 KCl, 7 CaCl2, 8 NaHCO3, 1 MgCl2, 5 N-trismethyl-2-aminoethanesulfonic acid, 4 d-trehalose dehydrate) adjusted to pH 7.4. To elicit fictive singing, glass capillaries filled with eserine salicylate (10−2 mol/l; Sigma-Aldrich, St Louis, MO) in saline were inserted into the ventral protocerebrum and the eserine was then pressure injected (Pneumatic PicoPump PV820; WPI, Sarasota, FL; details are given in Schöneich and Hedwig 2012; Wenzel and Hedwig 1999).
The singing motor pattern was monitored by recording from Meso Nv3A, which was lifted with a double-hook electrode made from 100-μm platinum wire (Fig. 1) and insulated from body fluids with Vaseline. The sound pulse pattern, which constitutes the chirps, is reflected in the rhythmic alternation of opener and closer motoneuron bursts in the Meso Nv3A recording (Fig. 1, inset). The signal was amplified with a differential AC amplifier (model 1700; A-M Systems, Sequim, WA).
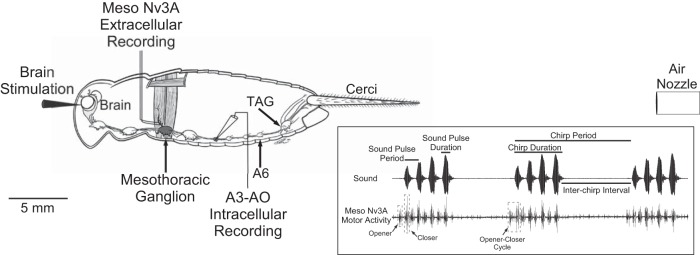
Fig. 1.
Schematic diagram of the experimental design with the cricket central nervous system (CNS). Indicated is the site of the eserine injection into the brain, the mesothoracic wing-nerve recording (Meso Nv3A), the intracellular recording of neuron A3-AO in abdominal ganglion A3, the air current stimulation with a nozzle placed 20 mm behind the cerci, producing an air current from the back to the front of the animal, the A6 ganglion, and the terminal ganglion (TAG). Inset: schematic representation of a cricket calling song with 3 chirps composed of 4 sound pulses each and its putative corresponding wing-opener and wing-closer motoneuron activity in Nv3A. The opener-closer cycle refers to 1 sequence of opener-closer activity. The song parameters analyzed (sound pulse period, sound pulse duration, chirp period, chirp duration) are indicated.
Intracellular recordings of singing CPG neurons.
The abdominal opener interneuron (A3-AO) of the cricket singing CPG was recorded in abdominal ganglion A3 (Fig. 1). The ganglion was stabilized between a tungsten ring and a stainless steel platform; an optic fiber was used for illumination. Microcapillaries were pulled (DMZ-Universal Puller; Zeitz-Instruments, Martinsried, Germany) from thick-walled (ID 0.58 mm) or middle-walled (ID 0.7 mm) borosilicate glass tubes (OD 1 mm; Hilgenberg, Malsfeld, Germany). They were filled with 2 M potassium acetate, giving resistances of 40–60 MΩ. Intracellular recordings were made in bridge mode (SEC10-05LX amplifier; NPI, Tamm, Germany), sampled at 40 kHz per channel (Micro1401 mk II; CED, Cambridge, UK) and stored on a PC hard drive.
Sensory stimulation.
Air stimuli were delivered to the cerci from a metal tube with 1.5-mm inner diameter positioned at a distance of 20 mm behind the animal, producing an airflow from the back to the front of the animal (Fig. 1). Air current velocities, as measured at 20 mm in front of the tube's nozzle, could be adjusted between 0.014 and 0.56 m/s with a minishaker unit or a Pneumatic PicoPump (for details see Hedwig 2000a; Meyer and Hedwig 1995). Stimuli durations (20–1,000 ms) were controlled with CED Spike2 software (CED). All stimuli deflected the filiform hairs on both cerci, as verified by observation with a dissecting microscope. Air velocity calibrations for the PicoPump and minishaker system were performed with an opto-electronic system described by Hedwig (2000b). This system has a time constant of 60 μs and detects movement amplitudes from 20 mm to 10 μm, so it is ideal to record both fast and slow movements. A nylon thread (200-mm length, 0.15-mm diameter, weight of 4.3 mg) was coated at its tip with 0.3 mg of Scotchlite reflective paint 7120 (3M Laboratories, Neuss, Germany), giving a final weight of 4.6 mg (0.023 mg/mm). The nylon thread was attached to a micromanipulator, and the reflective tip was positioned at 20 mm in front of the PicoPump/minishaker nozzle at the center of the opto-electronic diode. The optical measuring system was calibrated by moving the reflective tip in steps of 5 mm over 40 mm. The distance was plotted against the voltage, giving a linear relationship between distance and voltage (Hedwig 2000b). To each air stimulus, the reflective tip was displaced and a corresponding voltage change detected by diode was recorded. The voltage difference was taken over a 10-ms time period during the initial linear phase of the movement. This voltage difference was used to calculate the distance the reflective tip had moved over the 10-ms period in response to the air stimulus and allowed to calculate the air current velocity.
The transmission delay from the air stimulus to an interneuronal response recorded extracellularly from the connectives between A6 and TAG was determined and taken into account in the data analysis of the inhibitory postsynaptic potential (IPSP) properties to stimuli of different velocities and durations. This latency was 30.7 ± 7.8 ms (mean ± SD) for stimuli with lower velocity (0.014–0.05 m/s) and 13.7 ± 1.3 ms for the higher-velocity stimuli (0.53–0.56 m/s).
Our operational definition of the effects of cercal stimulation on singing follows Dambach et al. (1983) and Faure and Hoy (2000), as “song pausing” or “interchirp interval extension” and “song cessation” or “silencing.” During pausing/interval extension singing is briefly disturbed for <1,000 ms, whereas during song cessation/silencing singing is interrupted and stops for >1,000 ms.
Abolishing activity of cercal sensory system.
From day 8 after final ecdysis, we performed two types of experiments to evaluate the stability of singing natural activity after the cercal sensory system was blocked. First, in a microdissection the connectives between abdominal ganglion A6 and the TAG were cut (Fig. 1). Male crickets were mounted ventral side up in a Plasticine block on a Peltier element (Peltier-Technik, Fürth, Germany) and cooled to 6°C. The intersegmental soft membrane between abdominal segment A6 and the terminal segment was incised to expose the nerve cord between the A6 and TAG. The target connectives were cut with a fine pair of scissors. The ventral cuticle was folded back and the wound sealed by drying hemolymph. Second, in a manipulation to inactivate the wind-sensitive hairs, a drop of glue (UHU solvent-free multipurpose adhesive; UHU, Bühl, Germany) was spread along the cerci to cover all sensory hairs (Fig. 1).
After both procedures, all crickets were touched at the cerci with a paintbrush to ensure that there was no behavioral response. For 2 nights before and over 15 nights after either manipulation singing was recorded at 23–24°C, with a standard PC microphone and Cool Edit 2000 software (Syntrillium Software, Phoenix, AZ) with a sampling rate of 48 kHz. These long-term recordings provided a substantial data set covering any intraindividual variability in singing activity. After the animals had died, the nervous system was examined under a dissecting microscope to confirm the site of the applied lesion to the connectives.
Data analysis.
All individual IPSP measurements (duration, amplitude, and onset) were performed for A3-AO recordings in periods when the animal was not singing. The average membrane potential over 100 ms prior to the onset of the IPSP was calculated, establishing a baseline value. To determine the IPSP duration, the timing when the membrane potential reached the baseline value, after the air stimulation, was considered as the end of the IPSP if the average membrane potentials for the next 50 ms were not statistically different (P > 0.05) from the baseline value.
Electrophysiological data were analyzed with CED Spike2 software and with NEUROLAB (Knepper and Hedwig 1997). For data that did not follow a Gaussian distribution (D'Agostino and Pearson omnibus normality test) or did not have equal variances (F-test), the median value and interquartile range (IQR: 25th percentile/75th percentile) is given (N = number of animals; n = total number of analyzed events). Song recordings were analyzed with NEUROLAB (Knepper and Hedwig 1997), for which means ± SD (N = number of animals) of sound pulse and chirp period and duration were calculated (Fig. 1, inset). Statistical analysis of the song parameters was carried out with a two-way ANOVA with animal and microdissection/manipulation as between-subject factors. When appropriate, post hoc planned comparisons were performed contrasting the song parameter before and after the microdissection/manipulation in individual animals. Multiple comparisons were corrected with the Holm-Šídák test. For statistical analysis, we used GraphPad Prism 6 (La Jolla, CA).
RESULTS
Cercal stimulation causes inhibition of the A3-AO CPG interneuron.
To investigate the effect of cercal air stimulation on the singing motor pattern generator network, we intracellularly recorded the A3-AO ascending opener interneuron during fictive singing. The fictive singing pattern mirrors the natural calling song and is generated after all peripheral nerves are cut (Poulet and Hedwig 2006; Schöneich and Hedwig 2011; see Fig. 1, inset, for terminology of singing pattern). Since no self-generated sensory feedback is present, it is possible to study directly the effect of cercal stimulation on singing motor pattern generation.
Air stimuli were delivered with a range of velocities encompassing intensities induced by wing movements during singing (0.014–0.05 m/s) and intensities that would be detected as a disturbance (0.53–0.56 m/s). Higher air current velocities that evoke evasive responses like kicking (Dumpert and Gnatzy 1977) or running (Oe and Ogawa 2013) were not used, as they interfered with the intracellular recording.
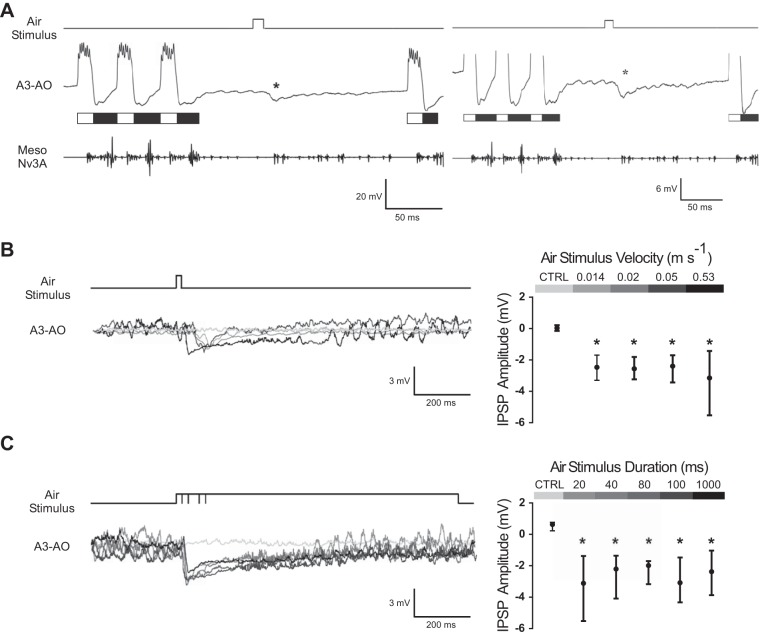
During fictive singing, the recordings of the A3-AO revealed its rhythmic membrane potential oscillations coupled to the motor pattern cycle with excitation in the opener phase and inhibition in the closer phase. When an air stimulus was applied to the cerci, an IPSP was elicited (Fig. 2A). Stimuli with different velocities elicited an IPSP in the A3-AO with different latencies, with the response to higher velocities occurring earlier because of its faster arrival at the cerci (Fig. 2B). These different times of arrival were considered to calculate a corrected latency for high- and low-velocity stimuli (see materials and methods for details). Application of a 20-ms stimulus elicited an IPSP in A3-AO independently of the air stimulus velocity used (0.014, 0.02, 0.05, and 0.53 m/s; Fig. 2B). Neither IPSP latency nor amplitude differed between velocities (Kruskal-Wallis: P = 0.0982 for IPSP latency and Kruskal-Wallis: P = 0.86 for IPSP amplitude; N = 3 and n = 90 for low-velocity stimuli and N = 5 and n = 50 for high-velocity stimuli; Fig. 2B). Since no differences were found between velocities, data was pooled across them. Taking into account the transmission delay, for all velocities the median IPSP onset was 17.3 ms (IQR: 13.9 ms/19 ms; N = 8, pooled data; n = 140). The maximum IPSP amplitude, from all pooled velocities, reached a median value of −2.53 mV (IQR: −3.29 mV/−1.74 mV), which was significantly different from the average membrane potential 100 ms before the air stimulus for all stimulations (Mann-Whitney U-test: P < 0.05; N = 8, pooled data; n = 140; Fig. 2B). The membrane potential of A3-AO showed a longer IPSP after a 20-ms stimulus of 0.53 m/s (median: 92 ms; IQR: 56 ms/147 ms) compared with stimuli velocities between 0.014 and 0.05 m/s (median: 29 ms; IQR: 23 ms/39 ms). The IPSP duration was significantly different between the different velocity ranges (0.014–0.05 vs. 0.53 m/s; Mann-Whitney U-test: P < 0.0001; pooled data for each velocity range, N = 3 and n = 90 for low-velocity stimuli and N = 5 and n = 50 for high-velocity stimuli).
Fig. 2.
Rhythmic motor activity and synaptic response of the A3-AO interneuron to air currents delivered to the cerci during fictive singing. White and black bars indicate the opener and closer phases of the A3-AO activity, respectively. A: example of the inhibitory postsynaptic potential (IPSP; indicated by asterisks) in the A3-AO to a 20-ms air stimulus presented in the interchirp interval. The air-evoked IPSP is smaller than the inhibition occurring during the closer phase (black bars). Vertical scale bars refer only to the intracellular recording of A3-AO. B, left: applying air stimuli of different velocities leads to similar IPSP amplitudes, although with longer IPSPs for faster stimuli; light gray line represents the membrane potential of the A3-AO where no stimulus was applied. Right: median and interquartile range (IQR; 25th and 75th percentiles) of the inhibitory response to the control (CTRL; average membrane potential 100 ms prior to all stimuli) and different stimuli velocities (N = 3 and n = 90 for low-velocity stimuli and N = 5 and n = 50 for high-velocity stimuli; *P < 0.05) are shown. C, left: applying air stimuli of different durations induces similar IPSPs; light gray line represents the membrane potential of the A3-AO where no stimulus was applied. Right: median and IQR (25th and 75th percentiles) of the inhibitory response to the control (average membrane potential 100 ms prior to all stimuli) and different stimuli durations (N = 5 and n = 50 for 20- and 80-ms stimuli durations and N = 7 and n = 70 for 40-, 100-, and 1,000-ms stimuli durations; *P < 0.05) are shown. In B and C, the inhibition amplitude was calculated from the difference between the average membrane potential 100 ms prior to the stimulus and the maximum inhibition. In the averaged membrane potential, oscillations of the signal are due to rhythmic A3-AO activity, when the crickets resumed singing after the air stimulus.
In addition to air current velocities, we also used different stimuli durations (20, 40, 80, 100, 1,000 ms; Fig. 2C), with velocities ranging from 0.53 to 0.56 m/s. In the A3-AO, an IPSP occurred independently of the stimulus duration (Fig. 2C). Neither IPSP latency nor amplitude differed between the different durations (Kruskal-Wallis: P = 0.4 for IPSP latency and Kruskal-Wallis: P = 0.93 for IPSP amplitude; N = 5 and n = 50 for 20- and 80-ms stimuli durations and N = 7 and n = 70 for 40-, 100-, and 1,000-ms stimuli durations). Since no differences were found between durations, data were pooled across them. Considering the transmission delay, the neuron responded after a median of 14.2 ms (IQR: 13.2 ms/16.9 ms; N = 11, pooled data; n = 310). The maximum IPSP amplitude, for all stimuli durations, reached a median value of −2.44 mV (IQR: −3.82 mV/−1.55 mV), which was significantly different from the average membrane potential 100 ms before the air stimulus for all stimulations (Mann-Whitney U-test: P < 0.05; N = 11, pooled data; n = 310). The median IPSP duration elicited by the 1,000-ms air stimulus was 340 ms (IQR: 158 ms/576 ms), which was significantly different from the 92-ms IPSP duration elicited by the 20-ms air stimulus (Mann-Whitney U-test: P < 0.001; N = 5 and n = 50 for 20-ms stimulus duration and N = 7 and n = 70 for 1,000-ms stimulus duration).
The median inhibition induced by the air stimulation was −2.5 mV (IQR: −3.34 mV/−1.59 mV; N = 14, pooled data; n = 400). This inhibition is smaller than the hyperpolarization observed during the closer phase of A3-AO activity, which ranged between −6 and −12 mV (Fig. 2A, right).
Effect of short air currents on singing motor pattern generation.
To test the impact of cercal stimulation on motor pattern generation in fictively singing males, we applied single 20-ms air stimuli of 0.53 m/s spaced by at least 2-s intervals. We considered the moment of the IPSP onset and its maximum amplitude as indicating the main effects of the air current, disregarding the duration of the gradual waning IPSP. The averaged IPSP latency elicited by a 20-ms (0.53 m/s) air stimulus was 29.1 ± 1 ms (N = 5, n = 50); this value includes the stimulus transmission delay. The 29-ms latency was used as a temporal indicator of the stimulus effect. When the effect of the air stimulus occurred within a chirp (Fig. 3A, top), the chirp was truncated but the subsequent interchirp interval did not change significantly (before air stimuli: median of 258 ms, IQR: 212 ms/307 ms; after air stimuli: median of 283 ms, IQR: 217 ms/352 ms; Mann-Whitney U-test: P = 0.72; N = 9; n = 55). When the effect of the air stimulus fell within an interchirp interval (Fig. 3A, middle and bottom), an inhibition of A3-AO occurred and the interval was extended (N = 9; n = 48). A phase response diagram demonstrates the effect of air stimuli presented at different phases of the chirp period in Fig. 3B. Values below 1 indicate a shortening of the relative chirp period, and values above indicate an extension (Fig. 3B). Stimulation with an air stimulus during a chirp, i.e., from phase 0.0 to 0.43 of the chirp cycle, truncated the chirp and thereby shortened the chirp period (median = 0.92, Wilcoxon signed-rank test: W = −650, P = 0.0059; N = 9, n = 55; Fig. 3B), especially when the effect of the stimulus occurred in the first half of a chirp. When the stimulus fell within the interchirp interval, i.e., from phase 0.43 to 1.0 of the chirp cycle, the interval was extended and the generation of the subsequent chirp was delayed by up to 3 cycles (median = 1.77; Wilcoxon signed-rank test: W = 1,176, P < 0.0001; N = 9, n = 48; Fig. 3B). This delay to the onset of the next chirp was not compensated in the following chirps. An effect similar to the delay reported here was previously described as a reset of the chirp pattern by the air stimulus (Dambach et al. 1983). The data presented here are not consistent with a reset, since we always observed a delay of the next chirp and never observed that the air stimulus shortened the interchirp interval, although the premature termination of chirps caused a shortening of the chirp period.
Fig. 3.

A3-AO activity during fictive singing and responding to 20-ms air current stimuli delivered to the cerci. A: examples of stimuli occurring during an ongoing chirp (top), during the beginning of the interchirp interval (middle), and at the time when a chirp is expected to occur (bottom). Inhibition of the A3-AO is indicated by *. B: phase response diagram of the chirp pattern in relation to the effect of the air stimuli (N = 9, n = 103). Tn−1, chirp period before air stimulation; t, time from beginning of the chirp to time of the stimulation effect on the A3-AO (i.e., 29 ms after onset of air stimulus); Tn, chirp period after air stimuli. Filled circles represent the effect of stimuli falling within the chirp; the end of the chirp is indicated by vertical dashed line. Open circles represent the effect of air stimuli during the interchirp interval; vertical solid line represents beginning of the next chirp. A value of 1 indicates that the chirp period was not altered by the air pulse (horizontal dashed line). Gray circles in the diagram correspond to the examples in A.
Analyzing the timing of the air stimulus effect relative to the opener and closer phases of the opener interneuron provided more detail on how cercal activity affects the CPG activity underlying the generation of opener-closer activity. Again, the moment when the IPSP was elicited in the singing interneuron was considered as the time of the stimulus effect, i.e., 29 ms after the onset of air stimulus. The analysis (Fig. 4; N = 4, n = 118) revealed a different response depending on whether the stimulus had an effect within the opener phase (depolarization) or the closer phase (hyperpolarization) of the A3-AO. For example, if the effect of the air stimuli occurred within the first opener phase (Fig. 4A, top, C, and D) 100% of the chirps (n = 24) were truncated immediately. When the effect of the air stimuli occurred in the first closer phase (Fig. 4, B–D) 71% of the chirps immediately stopped (n = 30), but in 29% of the cases one more opener-closer cycle was generated and chirps were composed of two opener-closer cycles (n = 6). Whenever the effect of an air stimulus occurred within the opener phase (N = 4; n = 53) all chirps were truncated; in these cases the effect of the air stimuli arrived between 0.01 and 17.7 ms (mean ± SD: 8 ± 5 ms; Fig. 4C, i and ii) after the first spike of the ongoing A3-AO burst (Fig. 4D). When the stimulus effect occurred in the closer phase (Fig. 4D; N = 4, n = 65) its precise timing was crucial: if the effect occurred between 16.2 and 31.3 ms (mean ± SD: 23 ± 5 ms) after the first spike of the preceding A3-AO burst the chirp was truncated (N = 4; n = 45); however, if the stimuli effect occurred at a later stage, between 30.7 and 46.8 ms (mean ± SD: 40 ± 5 ms; Fig. 4Ciii) after the first spike of the preceding A3-AO burst, one more opener-closer cycle occurred before the chirp stopped (Fig. 4, B and C; N = 4, n = 20). The duration of the opener period in fictive singing animals was 42 ± 3 ms. Then, it appears that when the stimulus effect arrived 40 ms after the A3-AO burst the next opener-closer cycle of the CPG was already initiated, explaining why one more cycle was generated.
Fig. 4.
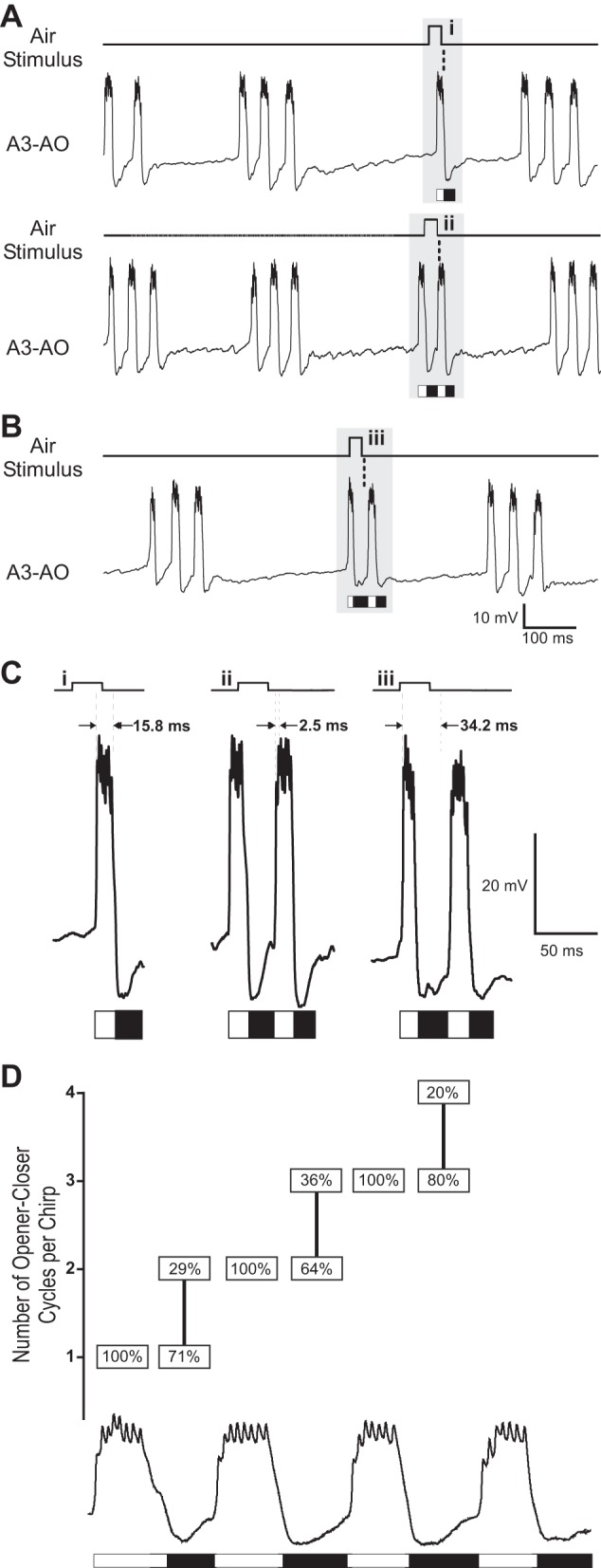
Effect of 20-ms air stimuli within a chirp. A: if the effect of the stimulus falls within the opener phase (white bars), the chirp is always prematurely terminated. B: if the effect of the stimulus occurs within the closer phase (black bars), the next opener-closer cycle is still produced. In A and B, stippled line indicates time of stimulus effect in A3-AO after onset of air stimulus (i.e., 29 ms). C: higher temporal and amplitude resolution of the stimulus effect in A3-AO (i–iii; gray areas in A and B). The time between the first spike of the preceding opener burst and the stimulus effect (i.e., 29 ms) is indicated by 2 vertical dashed lines with the corresponding interval in milliseconds. D: y-axis represents number of opener-closer cycles produced while an air stimulus was delivered during the chirp. Boxes indicate % of opener-closer cycles per chirp generated depending on the timing of the air-evoked inhibition within the opener (N = 4, n = 53) or closer (N = 4, n = 65) phase as indicated by white (opener) and black (closer) bars. Note that the calling song analyzed did not have more than 4 opener-closer cycles; stimuli occurring during the 4th cycle were not considered.
Previous data indicated that the chirp pattern in singing crickets couples to the timing of periodically delivered air stimuli only if the difference between chirp and air stimulation period does not exceed 10% (Dambach et al. 1983; Dambach and Rausche 1985). To test whether singing activity would be entrained by the air stimuli, we applied sets of nine 20-ms air stimuli at either 2.75 (Fig. 5A) or 2.9 (Fig. 5B) Hz while recording the A3-AO activity (N = 6, at least 3 sequences of 9 pulses at either frequency). In most cases (N = 5 of 6 animals), the singing activity ceased after the first two air stimuli and a rhythmic inhibition was evident for the remaining seven air stimuli. In one animal, singing continued during the rhythmic stimulation and the air stimuli always delayed the onset of the subsequent chirp (Fig. 5, A and B). For the existence of entrainment between air stimulation and singing activity, we would expect a temporal coupling. Analyzing the timing of the effect of the air stimulation (i.e., 29 ms after the onset of air stimulus) and the start of the chirps with a peristimulus time histogram revealed no coupling between the stimuli pattern and the A3-AO activity. All chirps started before the air stimulus (Fig. 5C), and no chirps were initiated after the stimulus effect.
Fig. 5.
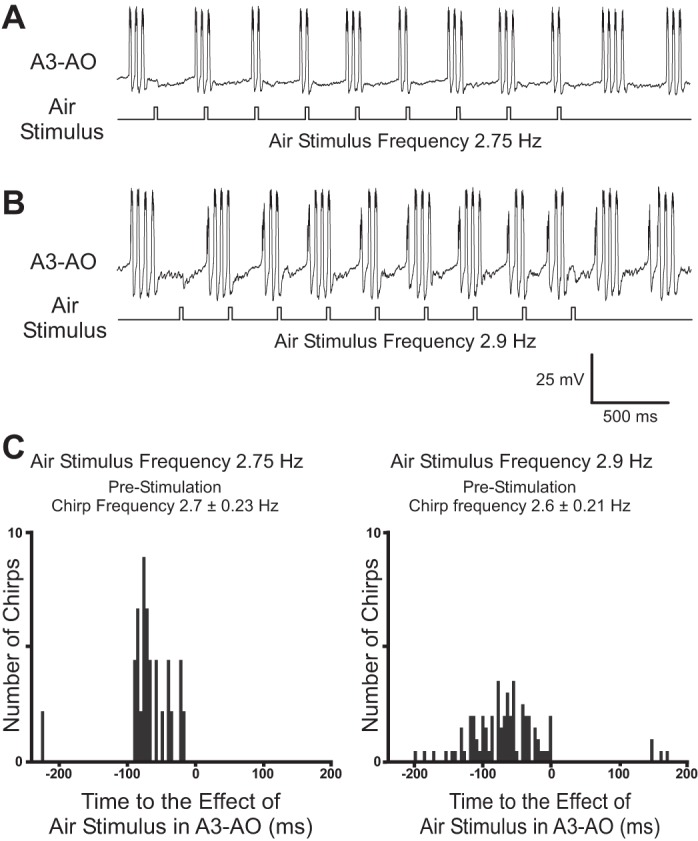
A and B: intracellular recording of A3-AO in a fictively singing cricket while periodic air stimuli were applied at a frequency of 2.75 Hz (A) or 2.9 Hz (B). C: peristimulus time histograms for the 2 stimulation frequencies. Diagrams are aligned to the onset (0 ms) of the air-evoked IPSP; for 2.75 Hz: 26 chirps and 45 stimuli (N = 1); for 2.9 Hz: 93 chirps and 198 stimuli (N = 1).
Intracellular current injection in A3-AO overrides cercal inhibition.
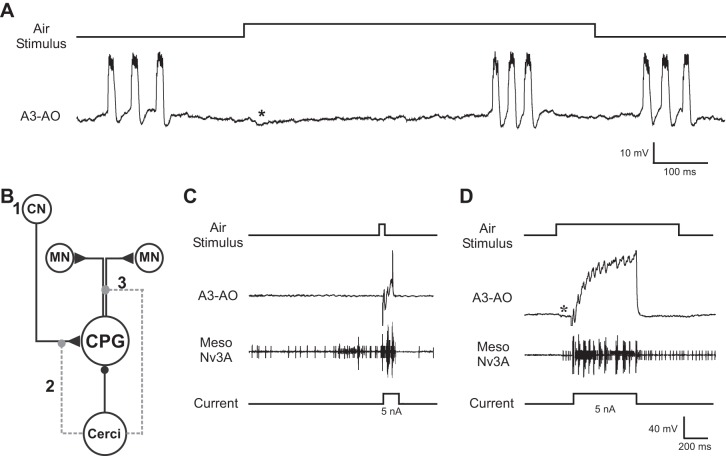
During fictive singing a pausing effect was elicited by stimulating the cerci with a long air stimulus (1,000 ms, 0.56 ± 0.11 m/s), which caused a significant lengthening of the interchirp interval (median: 946 ms, IQR: 821 ms/998 ms; Mann-Whitney U-test: P < 0.0001; N = 7, n = 79; Fig. 6A). Therefore, in some animals singing resumed while the air current was still present (Fig. 6A; see also Fig. 2C). This pausing effect may indicate either that the input to the singing CPG by the brain command neuron is inhibited or that the coupling between the CPG and the motor network is transiently blocked (Fig. 6B). We therefore tested whether current injection in A3-AO would elicit singing motor activity even during the cercal-evoked inhibition. We combined air stimulus and current injection in A3-AO and timed the current injection to fall into the inhibition triggered by the air stimulation, either at its start or after 100 ms.
Fig. 6.
Depolarizing current injection into A3-AO during inhibition elicited by air current stimulation. A: a 1,000-ms stimulus delivered to the cerci during the interchirp interval elicits an IPSP (*) and extends the interval. B: diagram of the putative cricket singing network. When singing, the central pattern generator (CPG) is excited (triangle) by the calling song brain command neuron (CN) and drives the motor neuron network (MN). Air stimulation of the cerci forwards an inhibition (circle) to the CPG. The cercal inhibition does not modulate the activity of the CN (1), but it may alter either the connection between the CN and CPG (2) or the connection between the CPG and MN network (3). C: a 20-ms air stimulus elicits inhibition in the opener interneuron. Injection of 100-ms, +5-nA depolarizing current pulse during the inhibition evokes rhythmic singing motor activity in A3-AO and the mesothoracic motoneurons. Vertical scale bars refer only to intracellular recording of A3-AO. D: application of a 1,000-ms air stimulus and injection of a 500-ms depolarizing current (+5 nA) during the stimulus-evoked IPSP (*) elicits rhythmic singing activity in A3-AO and the motoneurons of the wing nerve. Note that the Meso Nv3A recording also represents breathing motor activity. Vertical scale bars refer only to intracellular recording of A3-AO.
Injecting +5-nA depolarizing current of 100-ms (Fig. 6C) or 500-ms (Fig. 6D) duration during the inhibition mediated by the cercal activity (N = 15) always elicited rhythmic opener-closer cycles in A3-AO and caused rhythmic motor activity in the wing nerve. This is consistent with the generation of singing activity as previously shown (Schöneich and Hedwig 2011, 2012). It demonstrates that during the inhibition the singing CPG is not decoupled from the motor output network and demonstrates that the cercal suppression of motor pattern generation can be overcome by an excitatory drive to this CPG interneuron.
Removing cercal sensory system does not affect stability of singing pattern.
If feedback via a sensory pathway modulates CPG activity, then ablation of the sensory structure should alter the timing of the motor network activity (Pearson and Ramirez 1990; Wolf and Pearson 1988).
Singing males were recorded before and after abdominal connectives were cut (N = 3) or the cerci were inactivated (N = 4), and the period and duration of pulses and chirps were analyzed (Fig. 7). Males with inactivated cerci and cut connectives resumed singing on the same day or 2–3 days after the operation, respectively. If the self-generated air currents contribute to stabilize the singing activity, then an increased variation in the timing of the song pattern (Fig. 1, inset) should occur after the cercal system is abolished (Fig. 7).
Fig. 7.
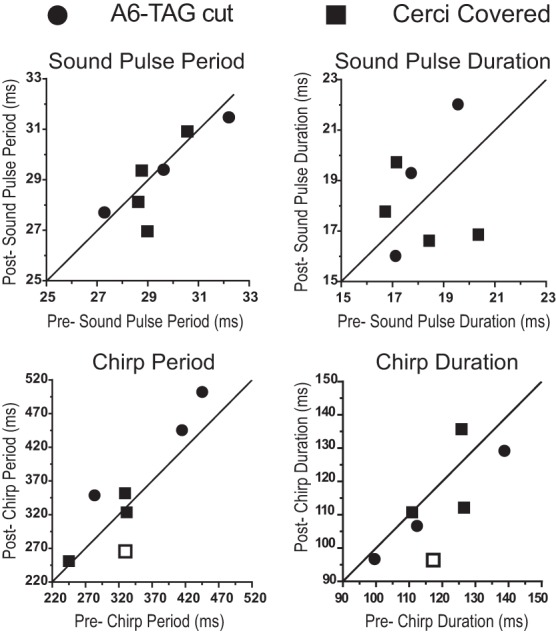
Effect of blocking cercal sensory activity on calling song parameters in freely singing male Gryllus bimaculatus (N = 7). Analysis of different song parameters, as indicated in Fig. 1, in freely singing crickets before (x-axis) and after (y-axis) the connectives between A6 and TAG were cut (N = 3, circles) and the cerci were covered (N = 4, squares). Each symbol represents the mean for 1 individual animal, before and after the block of cercal sensory activity, and the line through the origin represents the situation where the pre and post measures have the same mean parameter value. Open squares represent 1 animal with cerci covered, where the 2-way ANOVA revealed a significant interaction between manipulation and animal factors.
In the intact males, quantitative analysis of singing activity before removal of cercal sensory feedback (N = 7, ∼46,000 chirps per animal) demonstrated that pulse period and pulse duration were highly robust; the standard deviation of the mean for single specimens ranged from 0.2 to 2.4 ms for the sound pulse period and from 0.2 to 3 ms for the sound pulse duration.
Comparing the crickets before and after connectives were cut, a two-factor ANOVA (microdissection × animal; see details below) revealed no significant main effect of the microdissection in any of the parameters analyzed; it revealed a significant main effect of the animals in sound pulse period and chirp period and duration, but the interaction between microdissection and animal was not significant for any of the parameters (Fig. 7; ∼40,000 chirps per animal). As no main effect of the microdissection factor was found in any of the parameters analyzed, the data were pooled across animals for each parameter, for a before and after overall comparison. The pooled mean sound pulse period was 29.6 ± 2.3 ms (N = 3) before and 28.8 ± 2 ms (N = 3) after the microdissection. For the sound pulse period, the main effect for the microdissection factor was not significant [F(1,24) = 0.008, P = 0.93]; there was a significant main effect for the animal factor [F(2,24) = 4.666, P = 0.02] but no significant interaction between microdissection and animal [F(2,24) = 0.089, P = 0.91]. The pooled mean sound pulse duration was 18.1 ± 2.3 ms (N = 3) before and 19.1 ± 4.4 ms (N = 3) after the microdissection. For the sound pulse duration, no significant main effect for the microdissection factor [F(1,24) = 0.337, P = 0.57], the animal factor [F(2,24) = 1.945, P = 0.17], or the interaction between microdissection and animal [F(2,24) = 0.386, P = 0.68] was found. The pooled mean chirp period was 378 ± 94 ms (N = 3) before and 410 ± 73 ms (N = 3) after microdissection. For this parameter, the main effect for the microdissection factor was not significant [F(1,24) = 2.134, P = 0.16]; a significant main effect for the animal factor occurred [F(2,24) = 5.358, P = 0.014], but there was no significant interaction between microdissection and animal [F(2,24) = 0.122, P = 0.88]. The pooled mean chirp duration was 118 ± 21 ms (N = 3) before and 105 ± 11 ms (N = 3) after microdissection. For the chirp duration the main effect for the microdissection factor was not significant [F(1,24) = 1.494, P = 0.23]; there was a significant main effect for the animal factor [F(2,24) = 18.78, P < 0.0001] but no significant interaction between microdissection and animal [F(2,24) = 0.159, P = 0.85].
Also in animals with glue-covered cerci (Fig. 7), a two-factor ANOVA (manipulation × individual animal) revealed no significant main effect of the manipulation for any of the parameters analyzed; there was a significant main effect of the animals for sound pulse period and chirp period and duration. In three of the animals there was no significant interaction between manipulation and animal in any of the parameter analyses. In one particular animal an interaction occurred for the chirp period and duration (∼267,000 chirps per animal analyzed). As before, data were pooled across animals for each parameter, for a before and after overall comparison. The crickets had a pooled mean sound pulse period of 29.2 ± 1.4 ms before (N = 4) and 28.8 ± 1.8 ms (N = 4) after the cerci were covered. For sound pulse period, the main effect for the manipulation factor was not significant [F(1,32) = 0.529, P = 0.47]; a significant main effect for the animal factor occurred [F(3,32) = 6.993, P = 0.001], but there was no significant interaction between manipulation and animal [F(3,32) = 1.634, P = 0.2]. The pooled mean sound pulse duration was 18.1 ± 2.1 ms (N = 4) before and 17.6 ± 2.6 ms (N = 4) thereafter. For sound pulse duration, no significant main effect for the manipulation factor [F(1,33) = 0.057, P = 0.81], the animal factor [F(3,33) = 0.607, P = 0.62], or the interaction between manipulation and animal [F(3,32) = 2.218, P = 0.1045] was found. The pooled mean chirp period was 306 ± 45 ms (N = 4) before and 297 ± 45 ms (N = 4) after the cerci were covered. The main effect for the manipulation factor was not significant [F(1,32) = 0.583, P = 0.45]; there was a significant main effect for the animal factor [F(3,32) = 13.94, P < 0.0001] and for the interaction between manipulation and animal [F(3,32) = 2.906, P = 0.0497]. A pairwise comparison revealed in one of four animals a significant reduction of the chirp period after the manipulation (P = 0.02; chirp period before 326 ± 45 ms and after 265 ± 15 ms; Fig. 7). The pooled mean chirp duration was 120 ± 10 ms (N = 4) before and 114 ± 16 ms (N = 4) after the manipulation. For the chirp duration, the main effect for the manipulation factor was not significant [F(1,32) = 2.839, P = 0.1], but there was a significant main effect for the animal factor [F(3,32) = 8.814, P = 0.0002] and for the interaction between manipulation and animal [F(3,32) = 3.532, P = 0.02]. A pairwise comparison revealed, in the same animal as before, a significant reduction of the chirp duration after the manipulation (P = 0.03; chirp duration before 117 ± 12 ms and after 96 ± 8 ms; Fig. 7). Overall, both types of experiments never showed a main effect of the removal of cercal sensory activity on the singing motor pattern. The interactions between the manipulation and animal factors were found exclusively in one particular animal on the level of the chirp pattern, because of an overall reduction of the number of syllables per chirp after the manipulation. Before the manipulation, 68% of the chirps had four sound pulses and 30% of the chirps had five sound pulses; after the manipulation, the percentage of chirps with four sound pulses increased to 91% and only 4% of chirps had five sound pulses.
DISCUSSION
In crickets and other singing insects, air currents to the cerci initiate defensive mechanisms, such as silencing (Lewkiewicz and Zuk 2004; Spangler 1984), running, jumping, or kicking (Gnatzy and Heußlein 1986; Gras and Hörner 1992). Together, these secondary defensive mechanisms may represent an efficient antipredator strategy (Lewkiewicz and Zuk 2004; Müller and Robert 2002). On the other hand, singing crickets tolerate self-generated air currents when singing for many hours. This poses the question of how the singing motor system of crickets responds to salient predator-generated and low-amplitude self-generated air current stimuli.
Our data show that activation of the cercal system mediates an inhibitory effect on the singing motor network. The actual maintenance and stabilization of rhythmic singing motor activity appear to be independent of the self-generated cercal sensory activity.
Air current stimulation.
We used a wide range of air current velocities (0.014–0.56 m/s) and durations (20–1,000 ms), which cover the velocities and durations given for singing crickets (Kämper 1984) and for disturbance effects by approaching predators (Dupuy et al. 2012; Gnatzy and Heußlein 1986; Gnatzy and Kämper 1990; Triblehorn and Yager 2006). The stimuli used here were a uniform onset of bulk air movement toward the cerci, from the back to the front of the animal, which does not represent the natural situation. The precise spatial structure of the cricket's self-generated air currents during singing is unknown; an air current flowing from the front to the back can be expected because of the wing opening and closing movements. Since different dendritic branches of the GIs provide different receptive fields to directional air stimuli (Jacobs et al. 1986; Ogawa et al. 2004), the fact that the air stimuli might only represent approaching predators from the back of the animal cannot be excluded in the present study. Previous experimental conditions for stimulation of naturally singing crickets (Dambach et al. 1983; Dambach and Rausche 1985) could not be replicated, as the stimulation details, e.g., air current velocity and nozzle distance from the cerci, were not given. Despite the use of a highly restricted preparation in this study, and possible differences in experimental conditions compared with previous behavioral experiments (Dambach et al. 1983; Dambach and Rausche 1985), our air stimuli of ∼0.50 m/s reproduced essential data observed in unrestrained animals (see details below). For that reason, we argue that the inhibitory response of the A3-AO interneuron to the different air stimuli reliably represents the processing of air velocity stimuli in fictive and naturally singing crickets.
Cercal stimulation has inhibitory effect on singing motor pattern generation.
Stimuli of ∼0.50 m/s always had an inhibitory effect on the singing motor activity and caused either a premature termination of chirps or a lengthening of the interchirp interval that was not compensated in the subsequent chirps. Air stimulus of 1,000 ms even induced a pause of the singing activity. Dambach et al. (1983) also showed a lengthening of the interchirp interval upon cercal stimulation. Repetitive stimulation with a rate close to the chirp rate apparently led to a temporal coupling of the singing pattern to the cercal stimuli, although the singing activity was always delayed and never advanced by the cercal stimulus. In our experiments, we found no evidence that air stimulation triggered the generation of a chirp or entrained the singing CPG.
Cercal stimulation has inhibitory effect on A3-AO interneuron.
Low-velocity air currents, which might be related to self-generated stimuli, and high-velocity air currents, which could represent predator-like stimuli, may require adaptive differential processing at the level of the CNS. However, the A3-AO responded to all stimuli velocities (0.0014–0.56 m/s) and durations (20–1,000 ms) with an inhibition. At this level of the singing motor network the amplitude and latency of the neural response were similar for both low and high stimulus velocities and did not reveal a differential, stimulus-specific neural response.
When a singing male is stimulating its own cerci, this stimulus elicits responses in several classes of TAG interneurons. Recently, Schöneich and Hedwig (2015) described a rhythmic corollary discharge inhibition of two ventral GIs (8-1a and 8-1b), which would reduce the giant fiber response to self-generated air currents in singing crickets. The cricket singing system could use this corollary discharge inhibition of the GIs to prevent self-generated air currents from initiating silencing or escape responses (Schöneich and Hedwig 2015). Since crickets can detect extremely weak air currents (Dangles et al. 2007), the fact that the IPSP amplitude induced in the A3-AO is independent of stimulus velocity, in the range tested, is surprising, although the IPSP durations are significantly different. According to our data, even low-velocity air currents elicit an inhibitory effect on the singing motor network. If self-generated air currents in singing crickets elicit IPSPs in the A3-AO, four scenarios might explain the maintenance of singing despite an inhibition convened by the cercal stimulation. First, the measurements of the self-generated air currents (Kämper 1984) overestimated the velocity of these currents. Second, the corollary discharge mechanism (Schöneich and Hedwig 2015) is sufficient to inhibit the central response to the self-generated air currents even at the level of the cercal afferents via presynaptic inhibition. In the cercal afferents of the locust and crickets presynaptic inhibition has been demonstrated (Boyan 1988; Levine and Murphey 1980). However, when air current stimuli are applied in the interchirp interval the corollary discharge is not effective and the cercal pathway can have an impact on singing motor pattern. Third, cercal sensory system might adapt to repetitive air stimulation, by spike suppression (Hörner 1992) or by postsynaptic Ca2+-dependent depression (Ogawa et al. 2001; Ogawa and Oka 2015). This might allow the animal to cancel out reafferent inputs from the cerci caused by the animal's own movement (Hörner 1992) or to extract only differences in specific stimuli parameters, like acceleration (Camhi and Nolen 1981). Thus adaptation might prevent the cricket from responding to the self-generated stimuli. Finally, a role may be attributed to the different IPSP durations in the maintenance of singing. The cerci may produce a modulatory influence on the cricket escape pathway, referred to as tonic inhibition in the crayfish (Krasne and Wine 1975; Vu and Krasne 1992). Tonic IPSPs in the lateral giant neuron show a slow decay rate and are essential for the coordination of competing behaviors (Vu et al. 1993). Tonic inhibition reduces the likelihood that a feeding crayfish produces an escape response upon detection of modest threats (Bellman and Krasne 1983; Krasne and Lee 1988), although stronger stimuli can override this inhibition and elicit an appropriate escape response (Vu and Krasne 1992).
At which level does cercal stimulation modulate the CPG?
Cercal sensory input does not directly modulate the activity of the descending singing command neuron (Hedwig 2000a). The CPG opener interneuron A3-AO always received an inhibition after delivery of an air stimulus, even during rhythmic repetitive application. However, the amplitude of the IPSP elicited in A3-AO was not sufficient to prevent the generation of singing motor activity. Even during the inhibition triggered by an air current it was possible to drive spike activity in A3-AO by depolarizing current injection, which elicited rhythmic singing motor activity. This shows that the cercal-mediated inhibition does not block the pathway between the singing CPG and the motoneuron network. However, this cercal-mediated inhibition could act at the link between the brain command neurons and the A3-AO and/or at other yet unknown elements of the singing CPG network presynaptic to the opener interneuron (Fig. 6B).
Does the cercal system provide feedback for the generation of singing motor activity?
Previous studies suggested that in male crickets the singing CPG receives stabilizing sensory feedback from the cercal wind-sensitive pathway (Dambach et al. 1983; Dambach and Rausche 1985; Kämper and Dambach 1981). To analyze the impact of sensory feedback on CPG networks, three procedures were proposed (McClellan and Jang 1993; Pearson and Ramirez 1997): ablation of the sensory structure and testing for entrainment and reset effects by the sensory stimulus.
After removal of the cercal sensory system, there was no significant alteration in the temporal structure of the calling songs produced, except for one particular cricket at the level of chirp period and duration. Since crickets produce air currents with each wing movement, sensory feedback would be expected at the level of sound pulses and not chirps. Therefore, we argue that the reduction of chirp period and duration observed in this one animal might be related more to external conditions (e.g., temperature, humidity) than to the removal of the cercal system. Nonetheless, the fact that there was no alteration in the sound pulse parameters analyzed in the other specimen demonstrates the robustness of the central singing motor system and indicates its independence of any stabilizing self-generated sensory feedback from the cercal system. In a wide variety of rhythmic behaviors like walking (Andersson et al. 1981; Pearson 1972; Pearson and Iles 1970; Perret and Cabelguen 1980) or flying (Pearson and Ramirez 1990; Pearson and Wolf 1987; Wolf and Pearson 1988), in the sting defensive mechanism of the honeybee (Ogawa et al. 1995), or in the ovipositor system of crickets (Ogawa et al. 2011), the underlying CPG network does not provide all the timing cues for the generation of the intact pattern (Pearson 1987). In these rhythmic systems, sensory feedback is essential to the final motor output, with deafferentation or ablation of proprioceptors producing significant changes of the motor pattern. In the cricket singing system sensory feedback from wing structures is also known to alter the wing movements (Elliott et al. 1982; Elliott and Koch 1983; Schäffner and Koch 1987), but the data do not reveal whether this feedback has an impact on the singing CPG or if it acts directly on the rhythmic output of the motoneurons.
For a stabilization of singing activity by cercal input, one would expect an excitatory effect on the neurons of the singing CPG coupled to the air stimuli. This occurs in the cercal modulation of the cockroach flight system (Libersat 1992), the initiation of the onset of the elevator activity in locust flight (Pearson and Wolf 1988; Wolf and Pearson 1987), or the transition between stance and swing phase in walking (Andersson et al. 1981; Bässler 1986; Sillar et al. 1986). As the A3-AO responded to all air stimuli tested with an inhibition, at the level of this CPG interneuron there is no evidence for cercal sensory feedback stabilizing singing motor pattern generation.
The apparent entrainment of the chirp pattern by repetitive air current stimuli (Dambach et al. 1983; Dambach and Rausche 1985) may be explained by the fact that cercal stimulation can cause an inhibition in the singing CPG and extend chirp periods by a factor of 2–3 (Fig. 3), well beyond the period of the applied stimulus pattern. Therefore, the apparent triggering of an advanced chirp by a subsequent stimulus may occur during repetitive stimulation.
The lengthening of the interchirp interval by air current stimulation was previously regarded as a reset of the chirp rhythm (Dambach and Rausche 1985). An input that can cause a reset of a CPG network should also elicit or phase advance the motor activity (Duysens 1977; Lennard 1985; Pinsker 1977). We hesitate to interpret these effects mediated by cercal stimulation as a reset but rather judge them as an inhibition and a decrease of the excitatory drive to the CPG, which transiently slows down the whole CPG network; similar effects on the singing motor system occur by driving the command neuron for the calling song (Hedwig 2000a). In contrast to the tegula feedback in the flying locust (Pearson and Wolf 1988; Wolf and Pearson 1987), our data do not provide evidence that cercal feedback is incorporated into the activity of the singing CPG in a way to drive the singing motor pattern. Like Hedwig (2000a), we also observed a premature chirp truncation, demonstrating the inhibitory effects on the singing network. The chirp termination was not reported in early experiments with intact singing crickets and may be due to differences in experimental paradigms (Dambach et al. 1983; Dambach and Rausche 1985).
Conclusions/future approaches.
The data reported here demonstrate that activation of the cercal system mediates an inhibitory effect on the singing motor network. This shows a very efficient mechanism to terminate song generation and is closely linked to song-pausing responses or the preparation for an escape. The system seems to be an example of low-level neural decision-making to allow a faster response to an approaching predator. The decision between singing and silencing after cercal stimulation occurs at the level of the abdominal singing CPG, avoiding any loop via the brain (Hedwig 2000a). An interesting question that emerges is how the cercal pathway interferes so efficiently with the singing motor network to induce rapid termination of the chirp pattern. We speculate that it also inhibits some not yet identified neurons of the singing CPG.
GRANTS
P. F. Jacob was supported by the Fundação para a Ciência e a Tecnologia, Portugal (SFRH/BD/51901/2012).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.F.J. and B.H. conception and design of research; P.F.J. performed experiments; P.F.J. analyzed data; P.F.J. and B.H. interpreted results of experiments; P.F.J. and B.H. prepared figures; P.F.J. drafted manuscript; P.F.J. and B.H. edited and revised manuscript; P.F.J. and B.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Malcolm Burrows and Tim Bayley for constructive comments on the manuscript, Stefan Schöneich for advice and fruitful discussions, Charlotte Hartle for the cricket illustration, and Nigel Hall and Steve Ellis for technical support. Furthermore, we thank the two anonymous reviewers for their many insightful comments and suggestions on the manuscript.
REFERENCES
- Andersson O, Forssberg H, Grillner S, Wallén P. Peripheral feedback mechanisms acting on the central pattern generators for locomotion in fish and cat. Can J Physiol Pharmacol 59: 713–726, 1981. [DOI] [PubMed] [Google Scholar]
- ASAB Ethics Committee. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 71: 245–253, 2006. [DOI] [PubMed] [Google Scholar]
- Baba Y, Shimozawa T. Diversity of motor responses initiated by a wind stimulus in the freely moving cricket, Gryllus bimaculatus. Zoolog Sci 14: 587–594, 1997. [Google Scholar]
- Bässler U. Afferent control of walking movements in the stick insect Cuniculina impigra. J Comp Physiol A 158: 351–362, 1986. [Google Scholar]
- Bellman KL, Krasne FB. Adaptive complexity of interactions between feeding and escape in crayfish. Science 221: 779–781, 1983. [DOI] [PubMed] [Google Scholar]
- Boyan GS. Presynaptic inhibition of identified wind-sensitive afferents in the cercal system of the locust. J Neurosci 8: 2748–2757, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi JM, Nolen TG. Properties of the escape system of cockroaches during walking. J Comp Physiol A 142: 339–346, 1981. [Google Scholar]
- Chiba A, Kämper G, Murphey R. Response properties of interneurons of the cricket cercal sensory system are conserved in spite of changes in peripheral receptors during maturation. J Exp Biol 164: 205–226, 1992. [Google Scholar]
- Dambach M, Rausche G. Low-frequency airborne vibrations in crickets and feed-back control of calling song. In: Acoustic and Vibrational Communication in Insects, edited by Kalmring K, Elsner N. Hamburg, Germany: Parey, 1985, p. 177–182. [Google Scholar]
- Dambach M, Rausche HG, Wendler G. Proprioceptive feedback influences the calling song of the field cricket. Naturwissenschaften 70: 417–418, 1983. [Google Scholar]
- Dangles O, Pierre D, Christidès JP, Casas J. Escape performance decreases during ontogeny in wild crickets. J Exp Biol 210: 3165–3170, 2007. [DOI] [PubMed] [Google Scholar]
- Dumpert K, Gnatzy W. Cricket combined mechanoreceptors and kicking response. J Comp Physiol A 122: 9–25, 1977. [Google Scholar]
- Dupuy F, Steinmann T, Pierre D, Christidès JP, Cummins G, Lazzari C, Miller J, Casas J. Responses of cricket cercal interneurons to realistic naturalistic stimuli in the field. J Exp Biol 215: 2382–2389, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J. Fluctuations in sensitivity to rhythm resetting effects during the cat's step cycle. Brain Res 133: 190–195, 1977. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Palka J. The cerci and abdominal giant fibres of the house cricket, Acheta domesticus. I. Anatomy and physiology of normal adults. Proc Biol Sci 185: 83–103, 1974. [DOI] [PubMed] [Google Scholar]
- Elliott C, Koch U. Sensory feedback stabilizing reliable stridulation in the field cricket Gryllus campestris L. Anim Behav 31: 887–901, 1983. [Google Scholar]
- Elliott C, Koch U, Schäffner KH, Huber F. Wing movements during cricket stridulation are affected by mechanosensory input from wing hair plates. Naturwissenschaften 69: 288–289, 1982. [Google Scholar]
- Faure PA, Hoy RR. The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera; Tettigoniidae). J Comp Physiol A 186: 129–142, 2000. [DOI] [PubMed] [Google Scholar]
- Gnatzy W, Heußlein R. Digger wasp against crickets. Naturwissenschaften 73: 212–215, 1986. [Google Scholar]
- Gnatzy W, Kämper G. Digger wasp against crickets. II. An airborne signal produced by a running predator. J Comp Physiol A 167: 551–556, 1990. [Google Scholar]
- Gras H, Hörner M. Wind-evoked escape running of the cricket Gryllus bimaculatus. I. Behavioural analysis. J Exp Biol 171: 189–214, 1992. [Google Scholar]
- Hedwig B. Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83: 712–722, 2000a. [DOI] [PubMed] [Google Scholar]
- Hedwig B. A highly sensitive opto-electronic system for the measurement of movements. J Neurosci Methods 100: 165–171, 2000b. [DOI] [PubMed] [Google Scholar]
- Hennig RM, Otto D. Distributed control of song pattern generation in crickets revealed by lesions to the thoracic ganglia. Zoology 99: 268–276, 1996. [Google Scholar]
- Hörner M. Wind-evoked escape running of the cricket Gryllus bimaculatus. II. Neurophysiological analysis. J Exp Biol 171: 215–245, 1992. [Google Scholar]
- Jacobs GA, Miller JP, Murphey RK. Integrative mechanisms controlling directional sensitivity of an identified sensory neuron. J Neurosci 6: 2298–2311, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämper G. Abdominal ascending interneurons in crickets: responses to sound at the 30-Hz calling-song frequency. J Comp Physiol A 155: 507–520, 1984. [Google Scholar]
- Kämper G, Dambach M. Response of the cercus-to-giant interneuron system in crickets to species-specific song. J Comp Physiol A 141: 311–317, 1981. [Google Scholar]
- Knepper M, Hedwig B. NEUROLAB, a PC-program for the processing of neurobiological data. Comput Methods Programs Biomed 52: 75–77, 1997. [DOI] [PubMed] [Google Scholar]
- Kohstall-Schnell D, Gras H. Activity of giant interneurons and other wind sensitive elements of the terminal ganglion in the walking cricket. J Exp Biol 193: 157–181, 1994. [DOI] [PubMed] [Google Scholar]
- Krasne FB, Lee SC. Response-dedicated trigger neurons as control points for behavioral actions: selective inhibition of lateral giant command neurons during feeding in crayfish. J Neurosci 8: 3703–3712, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne FB, Wine JJ. Extrinsic modulation of crayfish escape behaviour. J Exp Biol 63: 433–450, 1975. [DOI] [PubMed] [Google Scholar]
- Lennard P. Afferent perturbations during “monopodal” swimming movements in the turtle: phase-dependent cutaneous modulation and proprioceptive resetting of the locomotor rhythm. J Neurosci 5: 1434–1445, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RB, Murphey RK. Pre- and postsynaptic inhibition of identified giant interneurons in the cricket (Acheta domesticus). J Comp Physiol A 135: 269–282, 1980. [Google Scholar]
- Lewkiewicz DA, Zuk M. Latency to resume calling after disturbance in the field cricket, Teleogryllus oceanicus, corresponds to population level differences in parasitism risk. Behav Ecol Sociobiol 55: 569–573, 2004. [Google Scholar]
- Libersat F. Modulation of flight by the giant interneurons of the cockroach. J Comp Physiol A 170: 379–392, 1992. [Google Scholar]
- Matsuura T, Kanou M, Yamaguchi T. Motor program initiation and selection in crickets, with special reference to swimming and flying behavior. J Comp Physiol A 187: 987–995, 2002. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Jang W. Mechanosensory inputs to the central pattern generators for locomotion in the lamprey spinal cord: resetting, entrainment, and computer modeling. J Neurophysiol 70: 2442–2454, 1993. [DOI] [PubMed] [Google Scholar]
- Meyer J, Hedwig B. The influence of tracheal pressure changes on the responses of the tympanal membrane and auditory receptors in the locust Locusta migratoria L. J Exp Biol 198: 1327–1339, 1995. [DOI] [PubMed] [Google Scholar]
- Müller P, Robert D. Death comes suddenly to the unprepared: singing crickets, call fragmentation, and parasitoid flies. Behav Ecol 13: 598–606, 2002. [Google Scholar]
- Oe M, Ogawa H. Neural basis of stimulus-angle-dependent motor control of wind-elicited walking behavior in the cricket Gryllus bimaculatus. PLoS One 8: e80184, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Baba Y, Oka K. Dendritic calcium accumulation regulates wind sensitivity via short-term depression at cercal sensory-to-giant interneuron synapses in the cricket. J Neurobiol 46: 301–313, 2001. [PubMed] [Google Scholar]
- Ogawa H, Baba Y, Oka K. Directional sensitivity of dendritic calcium responses to wind stimuli in the cricket giant interneuron. Neurosci Lett 358: 185–188, 2004. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kagaya K, Saito M, Yamaguchi T. Neural mechanism for generating and switching motor patterns of rhythmic movements of ovipositor valves in the cricket. J Insect Physiol 57: 326–338, 2011. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kawakami Z, Yamaguchi T. Motor patterns of the stinging response in the honeybee, Apis mellifera. J Exp Biol 189: 39–47, 1995. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Oka K. Direction-specific adaptation in neuronal and behavioral responses of an insect mechanosensory system. J Neurosci 35: 11644–11655, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Central programing and reflex control of walking in the cockroach. J Exp Biol 56: 321–330, 1972. [Google Scholar]
- Pearson KG. Central pattern generation: a concept under scrutiny. In: Advances in Physiological Research, edited by McLennan H, Ledsome JR, McIntosh CH, Jones DR. New York: Springer US, 1987, p. 167–185. [Google Scholar]
- Pearson K, Iles JF. Discharge patterns of coxal levator and depressor motoneurones in the cockroach, Periplaneta americana. J Exp Biol 52: 139–165, 1970. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Ramirez JM. Influence of input from the forewing stretch receptors on motoneurones in flying locusts. J Exp Biol 151: 317–340, 1990. [Google Scholar]
- Pearson KG, Ramirez JM. Sensory modulation of pattern-generating circuits. In: Neurons, Networks, and Motor Behavior, edited by Stein PS, Grillner S, Selverston AI, Stuart DG. Cambridge, MA: MIT Press, 1997, p. 225–236. [Google Scholar]
- Pearson KG, Wolf H. Comparison of motor patterns in the intact and deafferented flight system of the locust. J Comp Physiol A 160: 259–268, 1987. [Google Scholar]
- Pearson KG, Wolf H. Connections of hindwing tegulae with flight neurones in the locust, Locusta migratoria. J Exp Biol 135: 381–409, 1988. [Google Scholar]
- Perret C, Cabelguen JM. Central and reflex participation in the timing of locomotor activations of bifunctional muscle, the semitendinosus, in the cat. Brain Res 106: 390–395, 1976. [DOI] [PubMed] [Google Scholar]
- Pinsker HM. Aplysia bursting neurons as endogenous oscillators. I. Phase-response curves for pulsed inhibitory synaptic input. J Neurophysiol 40: 527–543, 1977. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science 311: 518–522, 2006. [DOI] [PubMed] [Google Scholar]
- Schäffner KH, Koch U. Effects of wing campaniform sensilla lesions on stridulation in crickets. J Exp Biol 129: 25–40, 1987. [Google Scholar]
- Schöneich S, Hedwig B. Neural basis of singing in crickets: central pattern generation in abdominal ganglia. Naturwissenschaften 98: 1069–1073, 2011. [DOI] [PubMed] [Google Scholar]
- Schöneich S, Hedwig B. Cellular basis for singing motor pattern generation in the field cricket (Gryllus bimaculatus DeGeer). Brain Behav 2: 707–725, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneich S, Hedwig B. Corollary discharge inhibition of wind-sensitive cercal giant interneurons in the singing field cricket. J Neurophysiol 113: 390–399, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, Skorupski P, Elson RC, Bush BM. Two identified afferent neurones entrain a central locomotor rhythm generator. Nature 323: 440–443, 1986. [Google Scholar]
- Spangler HG. Silence as a defense against predatory bats in two species of calling insects. Southwest Nat 29: 481–488, 1984. [Google Scholar]
- Triblehorn JD, Yager DD. Wind generated by an attacking bat: anemometric measurements and detection by the praying mantis cercal system. J Exp Biol 209: 1430–1440, 2006. [DOI] [PubMed] [Google Scholar]
- Vu ET, Krasne FB. Evidence for computational distinction between proximal and distal neuronal inhibition. Science 255: 1710–1712, 1992. [DOI] [PubMed] [Google Scholar]
- Vu ET, Lee SC, Krasne FB. The mechanism of tonic inhibition of crayfish escape behavior: distal inhibition and its functional significance. J Neurosci 13: 4379–4393, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel B, Hedwig B. Neurochemical control of cricket stridulation revealed by pharmacological microinjections into the brain. J Exp Biol 202: 2203–2216, 1999. [DOI] [PubMed] [Google Scholar]
- Wolf H, Pearson KG. Comparison of motor patterns in the intact and deafferented flight system of the locust. J Comp Physiol A 160: 269–279, 1987. [Google Scholar]
- Wolf H, Pearson KG. Proprioceptive input patterns elevator activity in the locust flight system. J Neurophysiol 59: 1831–1853, 1988. [DOI] [PubMed] [Google Scholar]