Abstract
We have studied how rewards modulate the occurrence of microsaccades by manipulating the size of an expected reward and the location of the cue that sets the expectations for future reward. We found an interaction between the size of the reward and the location of the cue. When monkeys fixated on a cue that signaled the size of future reward, the frequency of microsaccades was higher if the monkey expected a large vs. a small reward. When the cue was presented at a site in the visual field that was remote from the position of fixation, reward size had the opposite effect: the frequency of microsaccades was lower when the monkey was expecting a large reward. The strength of pursuit initiation also was affected by reward size and by the presence of microsaccades just before the onset of target motion. The gain of pursuit initiation increased with reward size and decreased when microsaccades occurred just before or after the onset of target motion. The effect of the reward size on pursuit initiation was much larger than any indirect effects reward might cause through modulation of the rate of microsaccades. We found only a weak relationship between microsaccade direction and the location of the exogenous cue relative to fixation position, even in experiments where the location of the cue indicated the direction of target motion. Our results indicate that the expectation of reward is a powerful modulator of the occurrence of microsaccades, perhaps through attentional mechanisms.
Keywords: eye movements, microsaccades, reward, target location
during fixation, our eyes are not completely still. The most prominent fixational eye movements are small rapid eye movements called microsaccades (Martinez-Conde et al. 2013). Microsaccades improve visual processing by aligning the fovea to the target (Ko et al. 2010), to sample selectively from information-rich regions (McCamy et al. 2014b; Otero-Millan et al. 2008), and by bringing faded or fading stimuli back to visibility (Martinez-Conde et al. 2006; McCamy et al. 2014a; Simons et al. 2006). Microsaccades potentiate visual-motor processing at least by enhancing the slow “ocular-following” eye movements that follow microsaccades (Chen and Hafed 2013). Yet, microsaccades (and large saccades) also can suppress visual processing (Hafed and Krauzlis 2010; Kagan et al. 2008).
The properties of the visual stimulus are an important factor in determining the rate of occurrence of microsaccades, but the exact parameters that control microsaccade rate remain unresolved. Some studies have highlighted the importance of stimuli that are close to the center of gaze (Ko et al. 2010). For these, the small amplitude of microsaccades makes them suitable for aligning the fovea relative to a small target. Nonvisual, contextual factors also modulate the rate and direction of microsaccades (Okada and Kobayashi 2014). Microsaccade direction is linked to attention (Engbert and Kliegl 2003; Hafed and Clark 2002; Laubrock et al. 2010) and the rate of microsaccades is modulated by rewards (Okada and Kobayashi 2014). Generally, changing the demands of the behavioral task modulates the rate of microsaccades in ways that do not depend solely on the properties of the visual stimulus (Gao et al. 2015; Hicheur et al. 2013; Siegenthaler et al. 2014). Finally, visual and nonvisual factors may interact in the control of microsaccades. For example, a link between covert attention and the direction of microsaccades appears for visual stimuli at eccentric locations (Hafed et al. 2011).
One of the most powerful contextual modulators of behavior is reward. Reward strongly modulates large eye movements such as saccades and smooth pursuit by changing the movement dynamics and by guiding decisions between multiple choices (Joshua and Lisberger 2012; Takikawa et al. 2002). A better understanding of whether and how reward modulates microsaccades might help us understand the factors that control microsaccades. Because the behavior can be characterized rigorously and the neural mechanisms for generating microsaccades are a topic of current research (Hafed et al. 2009; Hafed and Krauzlis 2012), the effect of reward on microsaccades might provide a model system for understanding how reward influences behavior at a neural level.
Our goal was to test how visual and nonvisual factors interact to modulate the rate of microsaccades. We designed tasks that control the size of the rewards and the location of a visual cue that signals the size of the future reward. We found large modulations of the rate of microsaccades that could be explained best by an interaction between reward and the location of the target. The nature of the interaction emphasizes the importance of the valence of the fixation target compared with its surround.
METHODS
Data were collected from four male rhesus macaque monkeys (Macaca mulatta). The monkeys had been prepared for eye movement recording using techniques described in detail previously (Joshua and Lisberger 2012). Some of the data were mined from experiments done for other purposes, and other data were obtained in new experiments designed for the purposes of this study. All procedures had been approved in advance by the Institutional Animal Care and Use Committees at University of California, San Francisco, and Duke University and were in strict compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, we implanted a head holder on the skull to allow us to restrain the monkeys' head movements. We also sutured a coil of wire to the sclera of one eye (Ramachandran and Lisberger 2005) to measure eye position using the magnetic search coil technique (Fuchs and Robinson 1966). After the monkeys had recovered from surgery, we trained them to track spots of light that moved across a video monitor placed in front of them.
Visual stimuli and experimental design.
Visual stimuli were displayed on a Barco monitor at a distance of 30 cm from the monkeys' eye. White and colored visual stimuli appeared as bright 0.5°-diameter circles on a dark background. All experiments were carried out in a dimly lit room. A computer performed all real-time operations and controlled sequences of target motion.
Stimuli were presented in discrete “trials.” At the start of a trial, a stationary white target appeared in the middle of the screen and monkeys were required to fixate within an invisible 2° × 2° window. After the monkey attained steady fixation on the white target, a colored circular spot appeared for a variable delay (500–700 ms for monkey P and 800-1,200 ms for monkeys Y, I, and R). The colored stimulus could either supplant the fixation point at the center of the screen to become the fixation target or appear eccentric on the screen while the fixation target remained present. At the end of the fixation period, the fixation target disappeared, and after a grace period of 150 ms, monkeys were required to keep their gaze within a 2° to 4° square window centered on the colored target as it moved at 30 deg/s for 600–750 ms. At the end of the trial, the target stopped and the monkey received a water or juice reward.
The colored spots cued the monkey about the size of the reward he would receive at the end of the trial. For monkey P, we associated a green target with a large reward (0.2–0.4 ml) and a yellow target with a small reward (0.05–0.1 ml). For monkeys I and Y, we reversed the association between color and reward size. For monkeys Y and R, we interleaved trials with central and eccentric cues. For monkeys I and P, the eccentric and central cues were not presented in the same behavioral session. Monkeys Y and R were used in additional experiments that associated reward size with blue and red cues. For monkey Y, we associated blue and red cues with large and small reward size; for monkey R, we reversed the association. After the monkey received the reward from one trial, a white fixation target reappeared in the center of the screen to start the next trial, and the monkey had 500 ms to achieve fixation. Because the rate of microsaccades is modulated dynamically following the saccade to the fixation target, we were not able to obtain a steady baseline estimate of microsaccade rate during fixation before the color cue appeared.
In trials with a central cue, the colored cue replaced the fixation spot at the center of the screen prior to movement (Fig. 1A). The monkey did not receive any cues about the possible directions of future target motion. At the end of the fixation period, the colored cue displaced to a location eccentric to the position of gaze (step) and immediately began moving toward the fixation point (ramp; Rashbass 1961). In many of our experiments, the colored cue appeared eccentric to the white fixation target (Fig. 1B) and cued both the direction of the future target motion (by its position) and the size of the future reward (by its color). At the end of the fixation period, the eccentric cue started to move from its eccentric location at 5° to 6° toward the middle of the screen. For monkey Y, in some sessions the eccentricity of the colored cue was 3°. In these sessions, at the end of the fixation period, the eccentric cue displaced again to a location that was 6° eccentric from fixation and began to move toward the position of fixation. We verified that the results did not depend on the initial eccentricity. In most experiments, the direction of target motion was chosen randomly from the four cardinal directions; in a minority of sessions, we tested movements in only the horizontal or vertical directions.
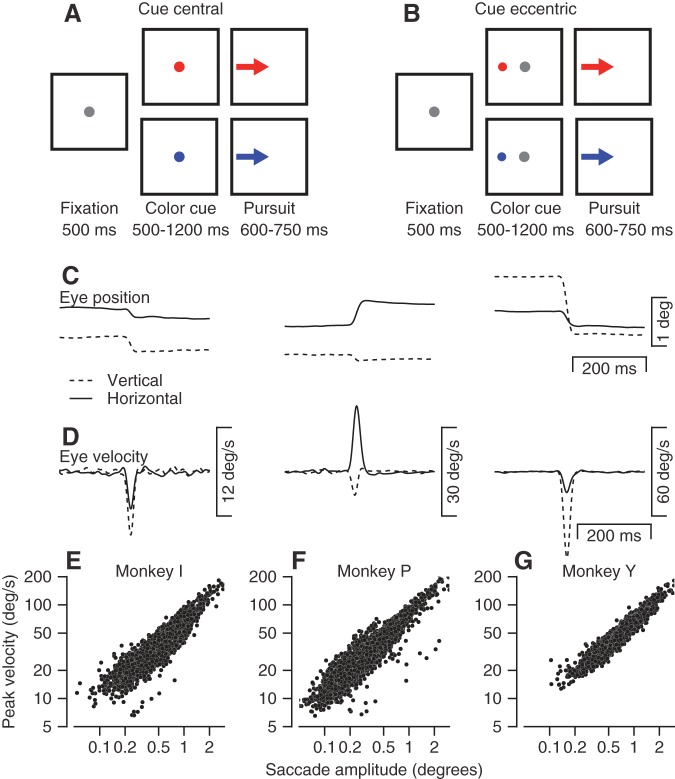
Fig. 1.
Behavioral tasks and properties of microsaccades. A and B: the sequences of snapshots illustrate the temporal structure of the behavioral task when the cue was presented at the center of the screen (A) or at an eccentric location (B). Dots represent stationary targets, and arrows represent targets moving through a step-ramp target motion. Targets are shown as gray, blue, and red spots for visibility even though during most of the experiments they were white, green, and yellow. C and D: examples of horizontal and vertical eye position (C) and eye velocity (D) traces for representative microsaccades. E–G: “main sequences” for microsaccades in 3 monkeys. Each symbol shows the peak velocity as a function of saccade amplitude for an individual saccade.
In an additional set of experiments, performed only on monkeys Y and R, we chose cue locations and directions of target motion so that the location of the eccentric color cue did not determine the direction of the subsequent target motion. After the color cue was presented at an eccentric location, it jumped with equal probability to a location along one of the four cardinal directions and started to move toward the center of the screen. To minimize generalization between experiments, we used new colors in monkey Y when the eccentric cue did not cue the direction of future motion (blue and red instead of green and yellow). Monkey R was used only for this experiment.
Finally, we performed a set of experiments on monkeys Y and R in which the target did not move. Monkeys fixated a stationary, central white target for 1 s, and then a color cue either replaced the central target or appeared eccentrically in addition to the fixation target. The monkeys were required to continue and fixate on the central target for an additional 1,550-1,950 ms and then received either large or small reward, depending on the color of the cue. We used the same colors as in the experiments that dissociated target motion from cue location. To try to eliminate any possible expectation of target motion, we repeated the experiment for 4–7 days (total of more than 13,000 trials per monkey). The results were stable across the data collection period. Note that monkey R was used only in the control experiments with the fixation task and for the experiments that dissociated cue position from the direction of subsequent target motion. Therefore, his data appear only in Fig. 5, which shows these controls.
Fig. 5.
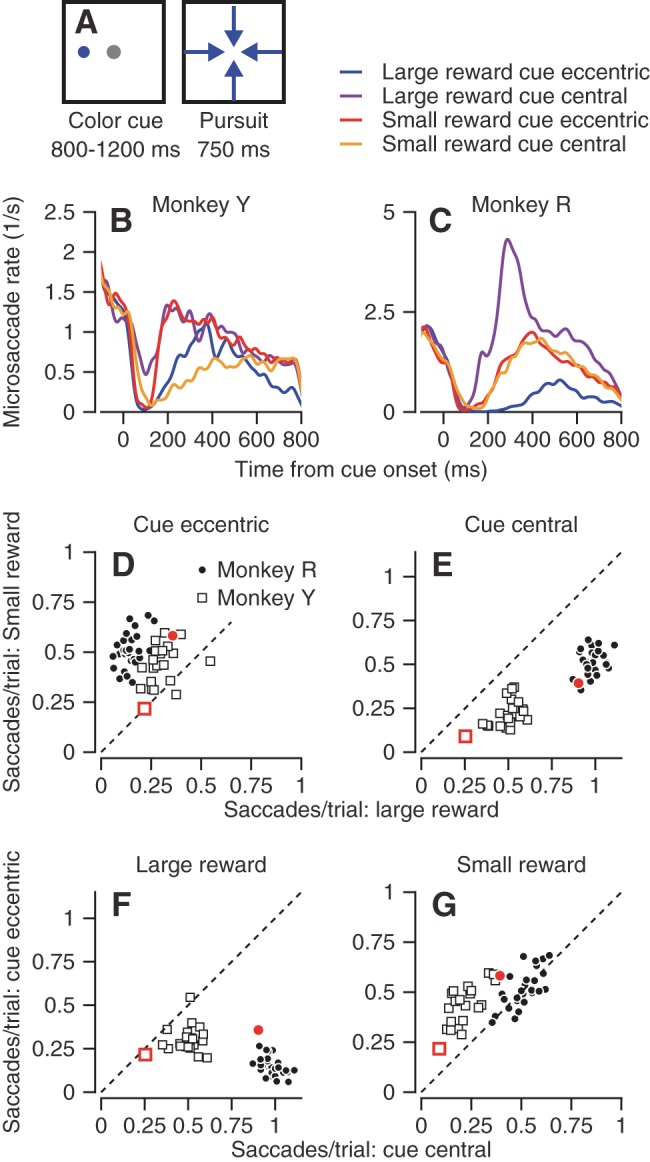
Modulation of the rate of microsaccades in experimental conditions where the location of an eccentric cue did not determine the future direction of target motion. A: schematics illustrating the task design. A cue was presented at an eccentric location and then jumped to a new eccentric location and moved toward the center of the screen, creating a modified step-ramp target motion. Dots represent stationary targets, and arrows represent moving targets. B and C: microsaccade rate as a function of time from the onset of the cue, across all sessions with interleaved conditions (the key for B and C appears above panel C). D–G: scatter plots show the number of microsaccades per trial when the monkeys were cued for a small vs. large reward with an eccentric vs. central cue (D vs. E) and when the cues were presented centrally vs. eccentrically with large and small rewards (F vs. G). Different symbols show data for different monkeys and represent data averaged for different days and different cue location conditions. Only data from conditions with more than 50 trials are included. Red symbols in D–G show the average results for a control experiment that used an eccentric or central colored cue for reward size on experimental days when the monkey never had to pursue a moving target.
Data analysis.
We used eye speed and acceleration thresholds to detect saccades automatically. During fixation, eye speeds >5 deg/s or eye accelerations >1,000 deg/s2 were marked as potential microsaccades. If the eye position displacement was smaller than 2°, we flagged the saccade for further analysis and then verified the automatic selection by visual inspection of the traces. We eliminated blinks by further excluding any small eye movements (<2°) that had durations longer than 80 ms. Changing the displacement ceiling for a microsaccade to 0.5° or 1°, or including eye movements with durations longer than 80 ms, did not alter any of our conclusions.
To obtain microsaccade frequencies, we averaged microsaccade occurrence across trials for each single condition on each experimental day. We used only conditions that provided more than 50 trials. For eccentric cues, we averaged the data for each cue direction separately. For central cues, we split the trials by future movement direction to allow a comparison between eccentric and central cue with similar number of trials. Grouping all the motion directions for the central cues did not alter any of our conclusions.
RESULTS
Reward size and cue location modulate the rate of microsaccades.
Microsaccades occur frequently during periods of fixation. We identified many microsaccades in the eye movements of our monkeys in the interval before the target motion that initiates pursuit eye movements (Fig. 1, C and D). As expected from prior publications (Zuber et al. 1965), the angular position displacement of microsaccades increased with peak eye velocity (Fig. 1, E–G). In the rest of this article, we analyze the frequency of microsaccades of amplitudes <2° as a function of time during the interval between the onset of the cue and the onset of target motion in the pursuit task. Our conclusions did not change if we restricted the definition of microsaccades to include only eye displacements smaller than 0.5° or 1°.
The rate of microsaccades was modulated dynamically during fixation of a stationary spot. Approximately 100 ms after the appearance of a colored cue for the size of the eventual reward, microsaccades always were suppressed for a short time (Hafed and Ignashchenkova 2013; Rolfs et al. 2008). When the cue appeared centrally (Fig. 2, A, C, and E), the suppression was followed by an increase in the rate of microsaccades if the cue predicted a large reward (blue traces). The rate of microsaccades remained low if the cue predicted a small reward (red traces) (Okada and Kobayashi 2014). The relationship between the rate of microsaccades and reward size reversed in all three monkeys when the color cue appeared eccentric to the fixation target (Fig. 2, B, D, and F). Now, after the characteristic suppression, microsaccades increased in rate more when the cue predicted a small vs. a large reward. The opposite relation between the blue and red traces in the right and left columns of Fig. 2 demonstrates the interaction between reward size and cue location.
Fig. 2.
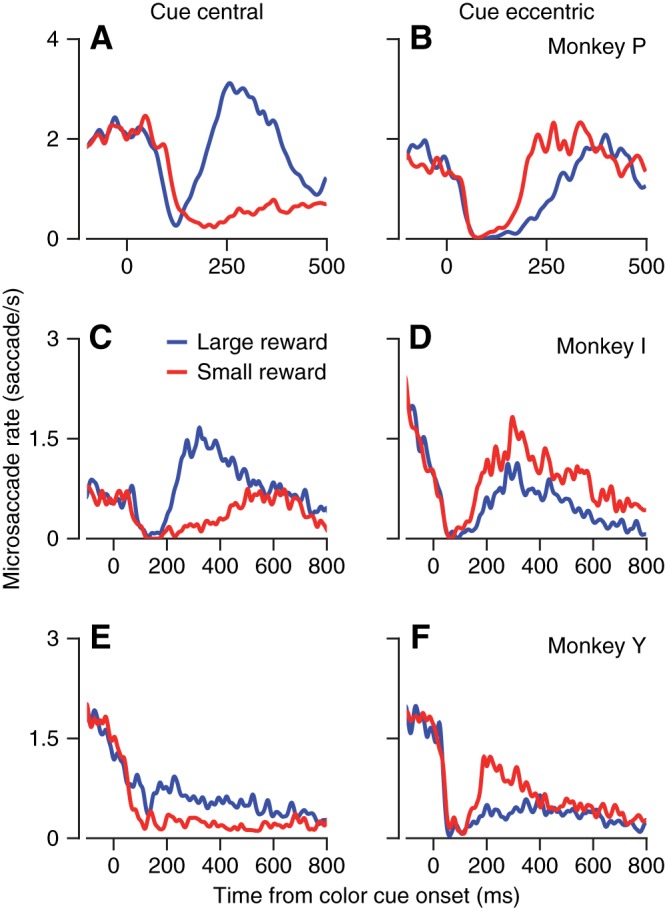
Modulation of microsaccade rate as a function of the time after appearance of a colored cue for future reward size. A–F: Traces show the average rate of microsaccades across all trials as a function of the time from the appearance of the colored cue. Blue and red traces show data from trials when the monkey was cued to expect a large or small reward, respectively. Left (A–C) and right (D–F) panels show data for a central vs. eccentric color cue.
The qualitative effects of expected reward size and cue location were consistent across monkeys, but details of the patterns of modulation varied. In monkeys Y and I, for example, the fixation duration was longer (800 ms) and the modulation of the rate of microsaccades diminished toward the end of the fixation interval. The duration of suppression of microsaccades after cue appearance was different for different monkeys in Fig. 2, as was the magnitude of the deviation of the rate of microsaccades relative to the rate at “time 0.”
To quantify the modulation of the microsaccade rate further, we calculated the number of microsaccades per trial during the first 500 ms after the color cue appeared. To allow statistical evaluation, we performed the analysis separately for each experimental session. In the sessions with a central cue, all three monkeys made more microsaccades when they were expecting a large vs. small reward in almost all sessions (Fig. 3A). In all three monkeys and in almost all sessions in which the color cue appeared eccentric to fixation (Fig. 3B), the monkeys made fewer microsaccades when they were expecting a large vs. small reward. The effects were statistically significant for all monkeys for both the central cue location (P < 10−8, paired t-test) and the eccentric cue location (P < 0.01, paired t-test).
Fig. 3.
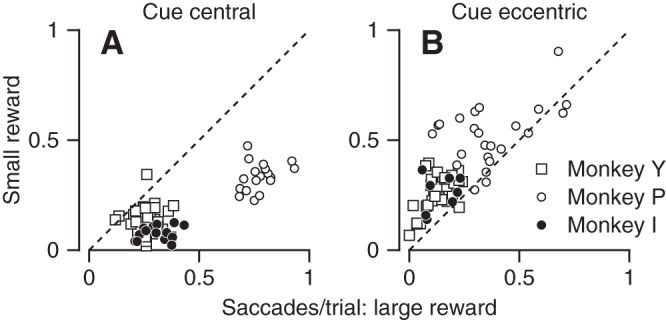
Quantitative summary of the effects of reward on the rate of microsaccades. Each symbol shows data from 1 experimental session. A and B: scatter plots of the number of saccades per trial when the monkey was cued for a small vs. large reward. Plots in A and B correspond to sessions when the cue appeared at a central or eccentric location, respectively. Different symbol shapes show data for different monkeys. Each symbol shows data from a single recording session and single motion direction. Only data from conditions with more than 50 trials are included.
All three monkeys exhibited an interaction between reward size and cue location. We show in Figs. 2 and 3 that reward size modulates the rate of microsaccades; the direction of the modulation depends on cue location. Next, we analyzed the data to test how reward size and cue location interact. We found a significant interaction between the reward size and cue location (Fig. 4, A–C; for all monkeys P < 0.01, 2-way ANOVA). Furthermore, the interception of the lines in Fig. 4, A–C, indicate that the location of the cue modulates the rate of microsaccades in both reward conditions. We compared the rate of microsaccades between sessions that presented the same color cue centrally vs. eccentric. All the monkeys made more microsaccades when the colored cue appeared centrally vs. eccentrically if the cue signaled a large reward (Fig. 4, A–C, left, open vs. filled symbols; P < 0.01, t-test). The effect reversed when the cue signaled a small reward (Fig. 4, A–C, right, open vs. filled symbols; P < 0.01, t-test): all three monkeys made fewer microsaccades when the color cue appeared centrally vs. eccentric.
Fig. 4.
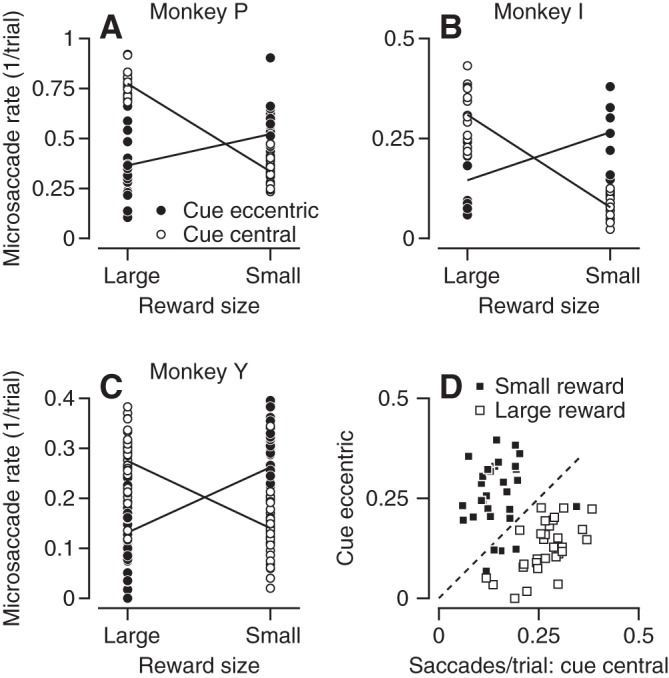
Quantitative summary of the interaction between cue position and reward size. A–C: filled and open symbols show data from different recording sessions that presented the color cue at eccentric (open) or central (filled) location. Lines with positive and negative slopes show the averages for conditions with central and eccentric cues. D: filled and open symbols show data obtained when monkey Y was cued for large vs. small rewards in sessions with randomly interleaved cue locations. Each symbol plots the saccades per trial for eccentric vs. central cues for different motion directions on different days. Only data from conditions with more than 50 trials are included.
The data presented in Figs. 2, 3, and 4, A–C, were collected in blocks that used either eccentric or central cue locations. To verify that presenting a cue location in a blocked design did not affect our results, we conducted experiments in monkey Y that cued large or small rewards in eccentric or central locations in interleaved trials. If the cue signaled a large reward, then the monkey still made more microsaccades when the color cue appeared centrally vs. eccentric (Fig. 4D, open squares). The effect reversed when the cue signaled a small reward (Fig. 4D, filled squares).
Dissociating cue location from upcoming movement direction.
In the experiments discussed in the previous section, eccentric colored cues signaled both the size the reward and the direction of the future target motion. To test whether the effect of cue location on the rate of microsaccades depends on the predictability of future target motion, we conducted experiments in which the location of the eccentric cue did not determine the direction future target motion (Fig. 5A). Monkeys fixated on a white target. An eccentric colored cue signaled the size of the future reward. After 800-1,200 ms of fixation, the color cue jumped to a position that was 6° eccentric in one of the four cardinal directions and started to move toward the center of the screen.
The interactions between cue location and reward size reported for the earlier experiments still were present when the color cue appeared eccentrically but did not determine future motion direction. First, the monkeys still made more microsaccades when an eccentric cue indicated a small reward (Fig. 5, B and C, red vs. blue traces, and Fig. 5D). The rate of microsaccades in the first 500 ms after the color cue appeared was larger for the small reward condition (Fig. 5D; P < 0.001 for both monkeys, paired t-test). Second, in interleaved trials with a central color cue, the monkeys made more microsaccades when they were cued for a large vs. small reward (Fig. 5, B and C, purple vs. yellow traces, and Fig. 5E). Third, the location of the cue modulated the rate of microsaccades when the reward was large. Both monkeys made more saccades when the colored cue appeared centrally vs. eccentrically (Fig. 5, B and C, purple vs. blue traces, and Fig. 5F). When the reward was small, monkey Y made more saccades when the cue was eccentric (Fig. 5B, yellow vs. red traces, and Fig. 5G, open boxes). The only difference from our prior results was that monkey R made similar numbers of microsaccades whether the colored cue signaled a small reward eccentrically or centrally (P = 0.2, paired t-test). We do not know whether the difference from our previous results (Fig. 3) is due to inter-subject variability or the difference in context. We also note that the effects documented in Fig. 5 evolved over different time courses during training in the different monkeys. Because there were some differences in training protocols, we do not have the data to isolate the parameters that affect the acquisition of the effects on microsaccade rate.
The requirement of accurate pursuit to receive a reward confirms that the monkeys are engaged in the task and provides a means to verify that they use the information about future reward (Joshua and Lisberger 2012). To test whether reward size and cue location modulate microsaccades even when monkeys are not preparing for a future movement, we trained monkeys Y and R in a task did not require pursuit. After a color cue appeared either centrally or eccentrically, the monkeys were require to fixate for another 1,550-1,950 ms and then received a small or large reward, depending on the color of the cue. The results followed the same broad pattern we found in the experiments with target motion, with some minor differences (red symbols in Fig. 5). Both monkeys made more microsaccades when signaled for a large reward vs. small reward centrally (Fig. 5E). The effect was reversed when the cue appeared eccentrically (Fig. 5D) but did not reach significance in monkey Y. The smaller effects in the fixation-only task for monkey Y could mean that he was less engaged in the fixation task or that preparation for a future pursuit movement has an effect on microsaccade rate.
Relation between reward size and saccade direction.
The direction of microsaccades during fixation might depend on the location of the cue relative to the fixation position (Hafed and Clark 2002; Hafed et al. 2011), the size of the reward signaled by the cue, and/or intrinsic directional anisotropies. To assess intrinsic anisotropies and reward size independent of the possible effect of cue location, we first analyzed only the trials that presented the cue at the fixation position. All three monkeys showed idiosyncratic directional anisotropies that depended weakly on the size of the reward signaled by the cue (Fig. 6, red vs. blue curves). Monkeys P and I showed strong biases toward vertical microsaccades (Fig. 6, A and B), and monkey Y showed a smaller bias with higher probabilities of upwards microsaccades (Fig. 6C). Statistical analysis verified that the size of the anisotropies exceeded the size of the effects of the reward. We assessed statistical significance by bootstrapping with samples from a uniform distribution matched to the number of microsaccades pooled across directions. The deviation from a uniform directional distribution was statistically larger (P < 0.05 for all monkeys) than the difference between distributions in the large and small reward conditions (blue vs. red curves in Fig. 6). Nevertheless, the small differences between the distributions for large and small rewards were statistically significant because of the large number of data points included in each distribution.
Fig. 6.
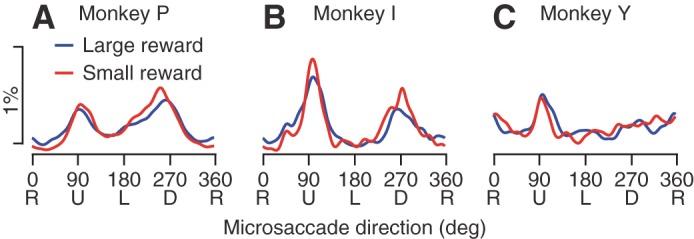
Intrinsic anisotropy in the direction of microsaccades. A–C: plots show the percentage of microsaccades in each direction in the first 500 ms after the appearance of the colored cue for reward size. Percentages were calculated in bins of 1° and smoothed circularly by a Gaussian kernel with an SD of 5°. Blue and red traces show results when the monkey was expecting a large vs. small reward, respectively. The colored cue always appeared at a central location to preclude biases in microsaccade direction. Different plots show data from different monkeys.
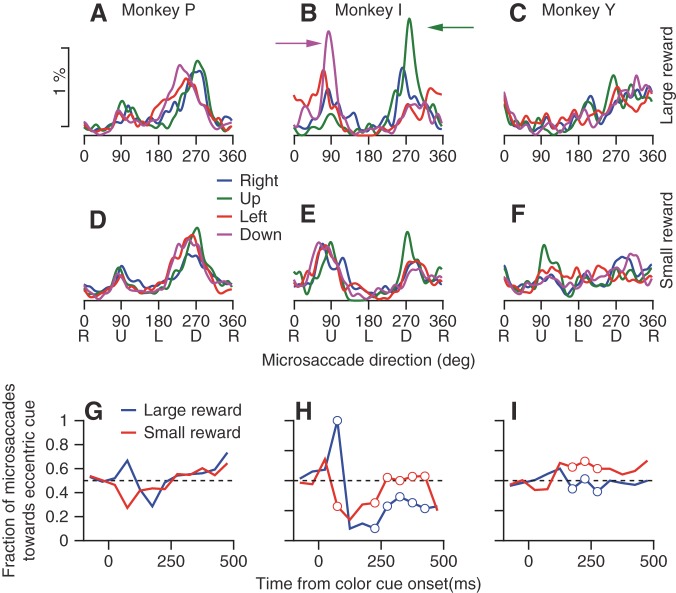
Next, we analyzed the trials with an eccentric cue to determine how the direction of microsaccades depended on the location of the color cue. Plots of the distribution of microsaccade direction (Fig. 7) mostly failed to show any effect of cue location. Each monkey's data were dominated by its intrinsic anisotropy, with some minor differences that depended on cue location. The only exception was monkey I, who made a high proportion of microsaccades away from the color cue when it appeared above or below the fixation target and signaled a large reward (Fig. 7B, green and magenta arrows). In monkey I only, the effect of cue location on microsaccade direction was clearly smaller when the cue signaled a small reward (Fig. 7E).
Fig. 7.
Absence of consistent biases in microsaccade direction related to the location of an eccentric cue. A–F: each graph shows the distribution of the directions of microsaccades in the first 500 ms after appearance of the colored cue. Different colored traces indicate the location of the colored cue relative to the position of fixation. Columns of panels at left, middle, and right show data for monkeys P, I, and Y. Rows of panels at top and bottom show the results when the monkey was cued to expect a large vs. a small reward. Percentages were calculated in bins of 1° and smoothed circularly by a Gaussian kernel with an SD of 5°. G–I: each graph shows the percentage of microsaccades that were directed toward the hemifield of the eccentric cue. Blue and red curves correspond to trials with large and small rewards, respectively. Open circles mark bins in which the fraction of microsaccades toward the hemifield of the cue were significantly different for large and small rewards (P < 0.05, χ2 test).
To test how the directions of the microsaccades are modulated dynamically across the interval after the appearance of the cue (Hafed and Ignashchenkova 2013), we calculated the fraction of microsaccades directed toward the hemifield of the eccentric color cue as a function of time in 50-ms bins. Just after cue appearance (50–100 ms), the direction of microsaccades was biased toward cues that signaled large rewards and away from cues that signaled small rewards (Fig. 7, G–I). The difference between microsaccade direction in trials with small and large reward reached significance only for one monkey (P < 0.05, χ2 test). In this time window, however, the rate of microsaccades was strongly suppressed (Fig. 2). The small number of microsaccades in the analysis window results in low power for the statistical test. The small number of microsaccades in the interval from 50 to 100 ms after the appearance of the color cue (fewer than 2% of the microsaccades occur in 10% of the first 500 ms) explains why we did not see this effect when we lumped the data across the whole 500 ms (Fig. 7, A–F); the overall distribution of microsaccade directions is dominated by the later microsaccades. Beyond this first interval, the time course of microsaccade directions varied across monkeys. For monkey I (Fig. 7H), microsaccades in the interval from 250 to 500 ms after cue appearance were directed away from the color cue more often when the cue signaled a large vs. a small reward. For monkey Y (Fig. 7I), there was a small but statistically significant bias (P < 0.05). For monkey P (Fig. 7G), we found no difference between the large and small reward conditions.
Relation between pursuit initiation and microsaccade timing.
We already know that monkeys initiate a faster pursuit eye movement when they are expecting a large vs. small reward (Joshua and Lisberger 2012). We also know that microsaccades enhance ocular following responses (Chen and Hafed 2013) but suppress other forms of visual processing (Hafed and Krauzlis 2010; Kagan et al. 2008). Therefore, we tested whether the occurrence of a microsaccade close to motion onset would enhance or suppress the initiation of pursuit in a way that was independent of the effect of reward size.
Microsaccades suppress the initiation of smooth pursuit in a way that superimposes on the effects of reward size. Microsaccades that occurred just before or after target motion onset were associated with slower eye movements in the initiation of pursuit. The effect of microsaccade timing is visible in averages of eye velocity as a function of time (Fig. 8A). Pursuit initiation was slower when a microsaccade ended between the onset of target motion and the initiation of the pursuit (blue and cyan traces) and was fastest when there was no microsaccade (black trace) or when the microsaccade occurred at least 50 ms before the onset of target motion (red and orange traces).
Fig. 8.
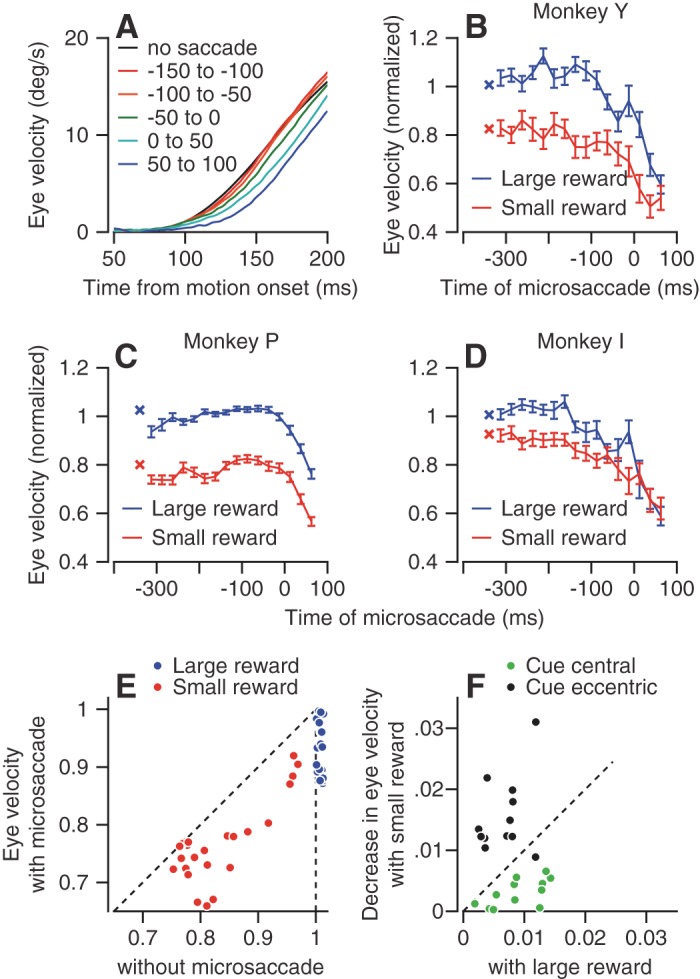
Effect of the time and occurrence of microsaccades on the eye velocity at the initiation of pursuit. A: each trace shows average eye velocity of monkey Y as a function of time from the onset of target motion. Data have been averaged across pursuit directions, reward expectations, and cue locations. Different colored traces show the initiation of pursuit when the last microsaccade occurred at different times relation to target motion onset. The black trace shows data from trials that lacked a microsaccade during the fixation period. B–D: the 2 connected sets of symbols in each graph show the normalized eye velocity at the initiation of pursuit as a function of the time of the last microsaccade relative to target motion onset. The target started to move at time 0. Blue and red symbols show data when the monkey had been cued to expect a large vs. small reward. Values were normalized by the average of the eye velocity in the large reward trials for that pursuit direction The × symbols that are not connected to the other traces show the normalized pursuit velocity in trials without microsaccades. Error bars show the SE of the means across directions and cue locations. E: scatter plots of the normalized pursuit eye velocity in trials with vs. without microsaccades in the last 200 ms before target motion. Blue and red symbols show data for trials with large vs. small rewards. Individual symbols show the normalized eye velocity of pursuit for different monkeys, movement directions, and cue locations. Only data from conditions that provided at least 10 microsaccades in the last 200 ms before the onset of target motion were included. F: symbols show the difference in the average normalized eye velocity in trials without vs. with microsaccades. Horizontal and vertical axes correspond to trials with large vs. small reward. Green and black dots correspond to trials with central vs. eccentric color cues.
To quantify how the timing of microsaccades modulates the impending initiation of pursuit, we binned the trials according to the time of the last microsaccade before pursuit initiation and averaged the eye velocity in the interval from 100 to 200 ms after the onset of target motion for the trials in each bin. In all three monkeys, pursuit eye velocity was lower in the analysis interval when microsaccades ended close to motion onset (Fig. 8, B–D). The smaller pursuit velocity was evident even for microsaccades that occurred before target motion onset in monkeys Y and I. The difference in eye velocity between trials without saccades and trials with a saccade in the last 100 ms before the onset of target motion was statistically significant (P < 0.01, t-test) for monkeys Y and I, but not for monkey P. In all three monkeys, the previously reported effect of reward size (Joshua and Lisberger 2012) persisted across most of the times of microsaccades (blue vs. red traces in Fig. 8, B–D).
For all monkeys, in all movement directions, for both reward sizes and for all cue locations, the overall pursuit velocity was lower when the monkey did vs. did not make a microsaccade in the interval from 200 ms before motion onset to 100 ms after target motion onset (Fig. 8E). The decrease in pursuit velocity did not depend whether microsaccades were directed toward or away from the direction of the pursuit movement (data not shown). In summary, at least for the initiation of pursuit to a bright target, we found that microsaccades suppress the visual-motor transformation that drives the initiation of pursuit.
Larger rewards lead to higher eye velocity in the initiation of pursuit, but reward size also modulates the rate of microsaccades. Extra microsaccades could decrease eye velocity if they occurred at strategic times (Fig. 8, A–E). Therefore, we conducted data analysis to ask how much of the effect of reward on pursuit velocity might be explained by the effects of reward and cue location on microsaccade occurrence. We subtracted the overall average pursuit velocity for all trials with a given cue location and reward size from the pursuit velocity in trials without microsaccades in the interval from 200 ms before to 100 ms after target motion onset. Excluding the microsaccades increased the eye velocity by around 0.5–3% (Fig. 8F). The effect of reward size on pursuit is an order of magnitude larger; eye velocity was 5–35% larger in trials with large vs. small reward (Fig. 8, A–D).
Figure 8E demonstrates the relatively small contribution of the microsaccade to overall velocity in trials with large reward by the slight deviation of the horizontal values of the blue symbols from 1 (y = 1, dashed vertical line). The much larger effect of the reward is demonstrated by the difference between the values of the blue and red symbols. As expected from the effect of cue location and reward size on the rate of microsaccades, the presence of microsaccades had tiny effects that did depend on the cue location and reward size. When monkeys made more microsaccades (i.e., small reward eccentric cue and large reward central cue), the decrease in the eye velocity due to microsaccades was larger (Fig. 8F).
DISCUSSION
We have found that the rate of microsaccades depends on the reward the monkey is expecting and on whether or not fixation is aimed at the visual stimulus that is a cue for future reward. The direction of microsaccades is determined mostly by anisotropies and only weakly by the location of a cue or the size of the reward. Microsaccades suppress the visual-motor transformation that drives the initiation of pursuit. Our results have implications for the role of microsaccades in vision and motor processing.
The function of microsaccades.
Microsaccades enhance visual processing by aligning the fovea at the target (Ko et al. 2010) and by bringing faded or fading stimuli back to visibility (Martinez-Conde et al. 2006; McCamy et al. 2014a; Simons et al. 2006). They also enhance the ocular following responses to a given stimulus motion (Chen and Hafed 2013), as do larger saccades (Kawano and Miles 1986). The modulation of the rate of microsaccades in our study supports the role of microsaccades in scanning foveal stimuli (Ko et al. 2010; Otero-Millan et al. 2013). We suggest that the monkey places a higher value on scanning a cue for a large reward than for a small reward and that microsaccades are biased toward scanning the fixation point vs. an eccentric target. Thus a foveal cue that predicts a large reward would evoke a large number of microsaccades, whereas a foveal cue that predicts a small reward would evoke fewer. An eccentric cue itself would not promote microsaccades because they do not assist with scanning an extrafoveal cue, but an eccentric cue that predicts a large reward might reduce the importance of the fixation point and thereby reduce the number of microsaccades made to scan it. When the eccentric cue is associated with small reward, the relative importance of the fixation cue might be increased and more microsaccades would be made to scan it.
We suggest that the pattern of microsaccades is a signature for the valuation of the eccentric and central targets. The lack of relation between the gain of pursuit and the occurrence of microsaccades shows that pursuit eye velocity is an independent measure of the valuation. We expect that our results will generalize also to other tasks in which eccentric and central target are valuated. Indeed, microsaccades increase with the size of the reward when the shape of a central fixation target signals the size of future reward (Okada and Kobayashi 2014). Engaging the monkeys in a motor task ensures the monkey will valuate the target, but we would expect to see the same trends in classical conditioning tasks that do not make reward contingent on a movement. For example, we observed effects of reward size on the rate of microsaccades even when the monkey did not have to prepare for a pursuit eye movement.
Our data do not support the prior conclusion that microsaccades enhance visual motion processing (Chen and Hafed 2013), and they show that the situation is different at least for the bright and high-contrast targets we have used. Consistent with studies that showed suppression of the responses of visual neurons by microsaccades (Hafed and Krauzlis 2010; Kagan et al. 2008), we found that microsaccades also suppress the visually driven eye velocity at the initiation of pursuit. In many cases the suppression of visual neural activity during microsaccades is followed by activity increases (Martinez-Conde et al. 2000, 2002; Martinez-Conde et al. 2013). The suppression of pursuit just after a microsaccade in our task indicates that these activity increases do not potentiate the visual motion processing for pursuit.
When considering an effect on processing of visual motion, we must consider whether activity is modulated not just at a single neuron but also at the level of the population. If the population responses in early motion processing are equally sensitive to saccades for both ocular following and pursuit, as seems likely, then the opposite effects of microsaccades on the behaviors would mitigate in favor of an alternative explanation. For example, the effect of microsaccades (and saccades) on ocular following might be mediated by control of the gain of visual-motor transmission for ocular following rather than by effects on visual motion processing. The different effect for pursuit could reflect different loci and/or mechanisms of gain control for the two responses.
Our study was not designed to study directly the relation between microsaccades and attention. It is likely that monkeys shift their attention to the color cue just after it appears, whether overtly in the central cue condition or covertly in the eccentric cue condition. If, indeed, attention is directed toward the color cue, then our interpretation that microsaccades are important for scanning of the fixation target is similar to the idea that the degree of overt attention to a visual stimulus near the fovea is the main driver of microsaccade rate. Later in the trial, it is possible that monkeys do not continuously attend the color cue as they do in other behavior tasks (Hafed and Clark 2002). Because we did not control attention, interpretation of our data in the attention framework is difficult.
Overall, our results support the notion that microsaccades and saccades play similar roles (Hafed and Krauzlis 2012; Otero-Millan et al. 2013). Both are directed toward targets of interest, but at different scales. The microsaccades probably allow scanning the fixation target to extract as many details as possible. From the perspective of the information in the external stimulus, microsaccades allow the brain to gather information about the visual stimuli that fall near the fovea (Ko et al. 2010; McCamy et al. 2014b). The effect of reward on microsaccade rate suggests a strategy of gathering more information about stimuli that have a greater perceived value.
Our results demonstrate the importance of monitoring eye movements during experiments that manipulate either the nature of a visual stimulus or the size of the reward (see also discussion in Hafed 2013). Microsaccades engage large parts of the visual-motor processing loop and either cause (Bair and O'Keefe 1998; Hafed et al. 2009; Martinez-Conde et al. 2000, 2002) or modulate (Chen et al. 2015) neural responses in visual and motor areas. Responses to microsaccades could be confused with visual, motor, or other responses. For example, activity in delay periods that is attributed to factors such as movement preparation, reward, or working memory might be related to microsaccades. The interactions we have found between the location of visual cues and the size of the reward suggest caution in the interpretation of experiments that do not measure microsaccades, especially because the motivational state of the animal is hard to deduce a priori in some experiments. It may be possible to rule out the confounding effects of microsaccades by verifying the consistency of the temporal response profiles across time, in the face of variable microsaccade timing. It would be still better to leverage the trial-by-trial randomness of the timing of microsaccades and correlate neural activity directly with the movements (Hafed et al. 2009; Martinez-Conde et al. 2000).
Conclusion.
The effects of reward and cue location on the rate of microsaccades solidify the link between microsaccades and foveal visual processing. The effect of reward expectation might be linked mechanistically to effects of overt attention. Our results point to similar roles in visual processing for microsaccades and saccades. Both facilitate detailed visual processing by changing the images that fall on the fovea, but they operate at different spatial scales.
GRANTS
This research was supported by the Howard Hughes Medical Institute, National Eye Institute Grant EY-03878, and the Human Frontiers Science Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J. and S.G.L. conception and design of research; M.J. and S.T. performed experiments; M.J. analyzed data; M.J. and S.G.L. interpreted results of experiments; M.J. and S.G.L. prepared figures; M.J. and S.G.L. drafted manuscript; M.J., S.T., and S.G.L. edited and revised manuscript; M.J., S.T., and S.G.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Ruffner and S. Happel for technical assistance.
REFERENCES
- Bair W, O'Keefe LP. The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis Neurosci 15: 779–786, 1998. [DOI] [PubMed] [Google Scholar]
- Chen CY, Hafed ZM. Postmicrosaccadic enhancement of slow eye movements. J Neurosci 33: 5375–5386, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ignashchenkova A, Thier P, Hafed ZM. Neuronal response gain enhancement prior to microsaccades. Curr Biol 25: 2065–2074, 2015. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res 43: 1035–1045, 2003. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966. [DOI] [PubMed] [Google Scholar]
- Gao X, Yan H, Sun HJ. Modulation of microsaccade rate by task difficulty revealed through between- and within-trial comparisons. J Vis 15: 3, 2015. [DOI] [PubMed] [Google Scholar]
- Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron 77: 775–786, 2013. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Res 42: 2533–2545, 2002. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323: 940–943, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Ignashchenkova A. On the dissociation between microsaccade rate and direction after peripheral cues: microsaccadic inhibition revisited. J Neurosci 33: 16220–16235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Microsaccadic suppression of visual bursts in the primate superior colliculus. J Neurosci 30: 9542–9547, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Similarity of superior colliculus involvement in microsaccade and saccade generation. J Neurophysiol 107: 1904–1916, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Lovejoy LP, Krauzlis RJ. Modulation of microsaccades in monkey during a covert visual attention task. J Neurosci 31: 15219–15230, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicheur H, Zozor S, Campagne A, Chauvin A. Microsaccades are modulated by both attentional demands of a visual discrimination task and background noise. J Vis 13: 18, 2013. [DOI] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. Reward action in the initiation of smooth pursuit eye movements. J Neurosci 32: 2856–2867, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I, Gur M, Snodderly DM. Saccades and drifts differentially modulate neuronal activity in V1: effects of retinal image motion, position, and extraretinal influences. J Vis 8: 19, 2008. [DOI] [PubMed] [Google Scholar]
- Kawano K, Miles FA. Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. J Neurophysiol 56: 1355–1380, 1986. [DOI] [PubMed] [Google Scholar]
- Ko HK, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat Neurosci 13: 1549–1553, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubrock J, Kliegl R, Rolfs M, Engbert R. When do microsaccades follow spatial attention? Atten Percept Psychophys 72: 683–694, 2010. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA 99: 13920–13925, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3: 251–258, 2000. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron 49: 297–305, 2006. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Otero-Millan J, Macknik SL. The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat Rev Neurosci 14: 83–96, 2013. [DOI] [PubMed] [Google Scholar]
- McCamy MB, Macknik SL, Martinez-Conde S. Different fixational eye movements mediate the prevention and the reversal of visual fading. J Physiol 592: 4381–4394, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCamy MB, Otero-Millan J, Di Stasi LL, Macknik SL, Martinez-Conde S. Highly informative natural scene regions increase microsaccade production during visual scanning. J Neurosci 34: 2956–2966, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Kobayashi Y. Fixational saccade-related activity of pedunculopontine tegmental nucleus neurons in behaving monkeys. Eur J Neurosci 40: 2641–2651, 2014. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Langston RE, Martinez-Conde S. An oculomotor continuum from exploration to fixation. Proc Natl Acad Sci USA 110: 6175–6180, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J Vis 8: 21, 2008. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Normal performance and expression of learning in the vestibulo-ocular reflex (VOR) at high frequencies. J Neurophysiol 93: 2028–2038, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M, Kliegl R, Engbert R. Toward a model of microsaccade generation: the case of microsaccadic inhibition. J Vis 8: 5, 2008. [DOI] [PubMed] [Google Scholar]
- Siegenthaler E, Costela FM, McCamy MB, Di Stasi LL, Otero-Millan J, Sonderegger A, Groner R, Macknik S, Martinez-Conde S. Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. Eur J Neurosci 39: 287–294, 2014. [DOI] [PubMed] [Google Scholar]
- Simons D, Lleras A, Martinez-Conde S, Slichter D, Caddigan E, Nevarez G. Induced visual fading of complex images. J Vis 6: 1093–1101, 2006. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res 142: 284–291, 2002. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Cook G. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 150: 1459–1460, 1965. [DOI] [PubMed] [Google Scholar]