Abstract
Background
Patients with psychosis often develop comorbid addiction, with a lifetime prevalence of ca. 50%. Dual diagnoses are considered hard to treat. Long-term integrated treatment programs might improve such patients’ outcomes, at least to a moderate extent, but they have not yet been adequately studied or implemented in Germany to date.
Methods
100 dual diagnosis patients participated in a single-center, randomized, controlled trial under standard hospital treatment conditions. They were randomly allotted to two groups. Patients in the intervention group were admitted to a specialized open hospital ward, where they were given integrated treatment, including disorder-specific group therapy. Their treatment was continued with further disorder-specific group therapy in the outpatient setting. Patients in the control group were admitted to an open general psychiatric ward and received treatment as usual, but no disorder-specific treatment either during their hospitalization or in the subsequent outpatient phase. Follow-up examinations were performed three, six, and twelve months after inclusion. The primary outcome was defined as the changes in substance use and abstinence motivation. The secondary outcome consisted of the patients’ satisfaction with treatment and with life in general, retention rate, psychopathology, rehospitalizations, and global level of functioning.
Results
The patients in the intervention group developed higher abstinence motivation than those in the control group (p = 0.009) and transiently reduced their substance use to a greater extent (p = 0.039 at three months). They were also more satisfied with their treatment (group effect: p = 0.011). Their global level of functioning and their retention rate were also higher, but these differences did not reach statistical significance.
Conclusion
Low-threshold, motivational, integrated treatment programs with psycho-educative and behavioral therapeutic elements may be helpful in the treatment of dual diagnosis patients and should be more extensively implemented as part of standard hospital treatment. Larger-scale, methodologically more complex studies will be needed to identify subgroups of patients that respond to such treatments in different ways.
Patients with schizophrenic psychosis and a comorbid addiction disorder account for about 50% (lifetime prevalence) and 25–30% (6-month prevalence) of all patients with psychosis (1– 3). These cases are referred to as dual diagnosis patients. Often, young men of a low educational status are affected and the disease course tends to be unfavorable with a poor prognosis (2– 5): dual diagnosis patients experience psychotic relapses and emergency admissions more frequently, and their disorder is more prone to becoming chronic. Such patients often display aggressive behaviors directed towards themselves and others, and they have poorer sociorehabilitative results in the long term than other patients with psychosis (3, 5). Primarily because of their relatively poor compliance, dual diagnosis patients are regarded as difficult to treat. They often encounter therapeutic nihilism from health professionals (6).
On the basis of model projects and controlled studies, integrated therapeutic programs have been favored since the 1990s, in which the treatment is administered in an integrative setting and by a team that has experience and competence in treating both disorders (2, 3, 7– 9). Measures that have been described as successful in the long term are motivational, low-threshold programs that were conceived for a longer period and include psychoeducational, behavioral therapeutic, and occasionally family therapy elements. Although the 2006 S3 guideline for schizophrenia recommended implementing these programs in standard care (10), progress since then has been slow.
Recent randomized studies and meta-analyses have taken a more critical view of the effectiveness of integrated treatment programs: success seems low and only partial, so that the cost–benefit ratio is regarded with some skepticism (11– 15). In spite of indications of the clinical relevance of even small effects that may be stronger in subgroups, no indicators of the preconditions for the observed therapeutic responses have been identified thus far. Accordingly, the 2011 guideline from the UK National Institute for Health and Care Excellence (NICE, National Institute for Health and Clinical Excellence at the time) recommends offering therapeutic measures for both disorders but does not recommend any particular program and neither does it demand an integrated approach (16).
Most randomized studies are from the US or European countries, whose healthcare systems differ from Germany’s. From Germany, only one randomized study has been reported that evaluated a brief inpatient motivational intervention (MI) and showed that, subsequent to inpatient treatment, the MI group used the outpatient therapeutic services to a greater degree than controls. No benefits were observed regarding substance use (17). The present study evaluated a long-term, trans-sector, integrated treatment program for dual diagnosis patients with a follow-up period of 12 months under standard treatment conditions.
Methods
Study design and setting
This randomized controlled study was conducted from 1 January 2011 to 30 June 2013 at a single center in a large psychiatric hospital in a large German city, the LVR-Klinik Köln.
Sample, recruitment
We included in the study adult patients with schizophrenia, schizophreniform disorders, or schizoaffective disorders (DSM-IV codes 295.xx) who were able to give consent and were voluntarily admitted to inpatient treatment, who had also been diagnosed with comorbid substance misuse or dependence (DSM-IV codes: 303.90, 305.00, 304.xx, 305.xx) (e1). We excluded patients who had substantially reduced their substance use to less than once a month—for the main substance—within the three months preceding admission. We also excluded patients with additional neuropsychiatric disorders that would have interfered with the therapeutic measures. These included organic mental disorders, manifest brain injury as a result of substance misuse, or lowered intelligence.
For the power calculation we used the only existing randomized study from Germany that included dual diagnosis patients (17). We calculated a required sample size of n = 122 in a scenario of an assumed effect size of f=0.15, power of 0.95, and an expected dropout rate of 20%; f is the statistical measure that expresses the practical relevance of statistically significant results. Although the recruitment period was extended from 12 months planned originally to 18 months, we did not reach the intended sample size and were restricted to n = 100.
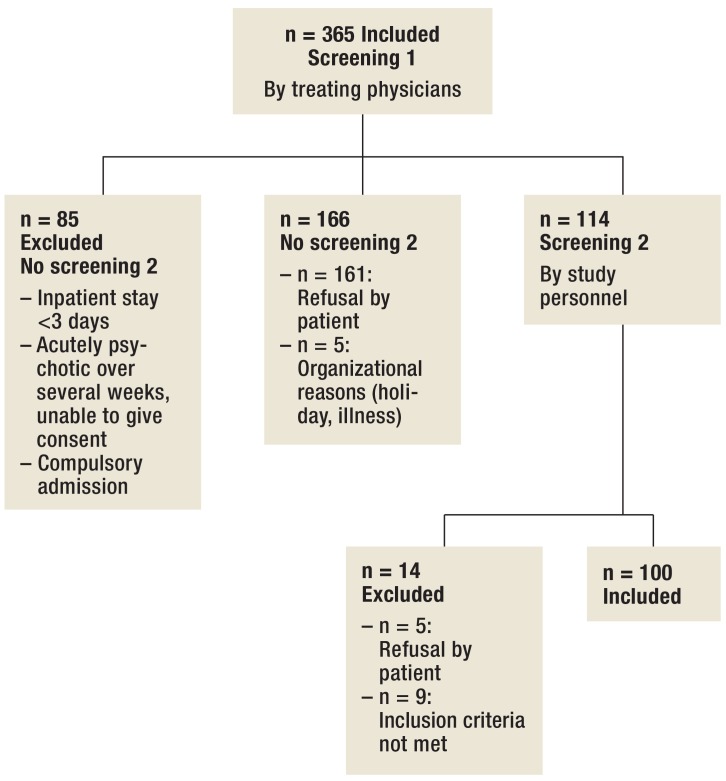
In the open wards, the treating physicians conducted the first screening step where possible within the first three days and, at the latest, within the first seven days of subjects’ inpatient stay. In the closed wards, patients were screened after they had clinically improved and before they were transferred to an open ward. With the patients’ consent, a member of the study group (S König) was notified, who checked the inclusion and exclusion criteria within three days where possible, and within seven days, at the latest. She also provided written and oral information and conducted the inclusion examinations for the second screening step ( Figure 1).
Figure 1.
Recruitment
Study protocol, study instruments, outcome
After the inclusion screening (baseline, t0), patients were randomly allocated to an open specialist ward with implemented integrated treatment (IntT) or to a different open general psychiatric ward, where they received treatment as usual (TAU). The eBox shows details on the randomization of the study participants to both therapeutic arms. At the end of their inpatient treatment, both the IntT patients and the TAU patients were offered continued treatment at the hospital’s own outpatient institute. Additionally, the IntT group was offered participation in disorder-specific outpatient therapy groups. Follow-up examinations were performed at three, six, and 12 months (t1–t3) after t0. The primary outcome was defined as changes in substance use and motivation for abstinence between t0 and t1; secondary outcome was defined as changes between t0 and t2 and between t0 and t3, respectively, and in all other variables considered in this study (Table 1).
eBox. Randomization method and results regarding secondary outcome.
-
Method:
Randomization
A colleague who was not involved in the screening examinations and assessments compiled the randomization list. Sheets of paper were marked with written numbers of 1 to 122 and inserted into envelopes. These were placed in a box, which was shaken to mix the envelopes. Subsequently, envelopes were pulled out alternately for the IntT and TAU groups. The group allocation was noted on the sheet and the number was written on the envelope, which was then sealed. The envelopes were kept safe in a locker, to which the colleague involved in screenings and assessments had no access (S König). Each patient received their identification number at the point of inclusion in the study. The group allocation was noted in the envelope with the subject’s ID number, so that patients were allocated by the same colleague who had compiled the randomization list opening the envelope and reading out the respective group allocation.
-
Results:
Secondary outcome
Readmission to hospital: The rate of readmissions to hospital in the follow-up period from t0 to t1 was 0.27 ± 0.55; from t1 to t2, 1.03 ± 4.36; and from t2 to t3, 0.71 ± 1.42. No between-group differences were observed (time effect: p = 0.123; group effect: p = 0.443; interaction: p = 0.909). The duration of inpatient or part-inpatient treatment from t0 to t1 was 32.4 days ± 25.1 days; from t1 to t2, 40.4 days ± 41.1 days; and from t2 to t3, 52.3 days ± 54.6 days. For this variable, no between-group difference was seen either (time effect: p = 0.000; group effect: p = 0.760; interaction: p = 0.298).
Satisfaction with life: The questionnaire for satisfaction with life (FLZ) showed a slight upward trend in the follow-up period for the entire sample (time effect: p = 0.015), but no difference between the groups (group effect: p = 0.261; interaction: p = 0.987).
Table 1. Study levels of analysis and instruments.
| Level | Instrument | t0 | t1 | t2 | t3 |
|---|---|---|---|---|---|
| Confirmation of diagnosis | Structured clinical interviews for DSM-IV: SKID-I, SKID-II (section on borderline personality disorder and antisocial personality disorder) (e2, e3) | x | / | / | / |
| Clinical data | Evaluation of patient file and semi-standardized clinical interview following the manual specifically developed for this study (time since first diagnosis, number of episodes, inpatient stays, medication) | x | / | / | / |
| Demographic data | Semi-standardized interview following the study manual (age, sex, school education and professional training, current employment, living circumstances, marital status, convictions) | x | x*1 | x*1 | x*1 |
| Primary outcome | |||||
| Consumption parameter*2 | 1. Standardized interview developed on the basis of the Addiction Severity Index (ASI) (e4), established in earlier studies of the working group (e5– e7) (frequency, average and maximum single and daily doses in the month preceding indpatient admission, time since most recent substance use relating to all substances consumed regularly > once a month) | x | x | x | x |
| 2. Interview by treating physician | x | x | x | x | |
| 3. Evaluation of patient file | x | x | x | x | |
| 4. Drug screening (urine sample) | x | x | x | x | |
| Motivation for abstinence | Self assessment: Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES), 19-item version 8A with 3 subscales: “recognition“ (awareness of problem), “ambivalence“ (ambivalence, increasing motivation in the narrower sense) and “taking steps“ (concrete efforts to remain abstinent) (e8) | x | x | x | x |
| Secondary outcome | |||||
| Adherence to therapy | Retention rate | / | x | x | x |
| Satisfaction with therapy | Self assessment: questionnaire on patient satisfaction (ZUF-8) (e9) | / | x | x | x |
| Readmissions to hospital*3 | Interview and evaluation of patient file | / | x | x | x |
| Global level of functioning | External assessment: Global Assessment of Functioning Scale (GAF) according to DSM-IV (e10) | x | x | x | x |
| Psychopathology (external assessment) | 1. Positive and Negative Syndrome Scale for Schizophrenia (PANSS) with 3 subscales: positive, negative, and general psychopathology scale (e11) | x | x | x | x |
| 2. Montgomery Asberg Depression Rating Scale (MADRS) (e12) | x | x | x | x | |
| Satisfaction with life | Self assessment: questionnaire on satisfaction with life (FLZ) (e13) | x | x | x | x |
DSM, Diagnostic and Statistical Manual of Mental Disorders
*1Change since previous examination
*2Not included if individual information sources were inconsistent (t 0) or if patients dropped out of the study (t 1 –t 3)
*3Readmission to hospital was defined as an inpatient stay of at least 36 hours or a part-inpatient stay of at least 5 days (17, e14).
t 0 , baseline; t 1, + 3 months; t 2, + 6 months; t 3, + 12 months
Intervention
The IntT group was offered two disorder-specific group therapies on the specialist ward, which took place once weekly over 60 minutes each. A motivational group, which was modified according to the MI principles (2, 17), as well as a manual-based psychoeducational group, in which the associations between the use of different substances and psychosis were addressed according to the psychoeducational training program for patients with a dual diagnosis of psychosis and dependence (KomPAkt, Komorbidität Psychose und Abhängigkeit psychoedukatives Training) for dual diagnosis patients (18). Both groups were provided with all the additional multimodal treatment elements that would be offered as standard treatment:
Individual therapeutic sessions
Psychoeducation about symptoms
Psychosis treatment, including pharmacotherapy
Occupational therapy
Exercise therapy
Relaxation training
Cognitive training.
In the outpatient treatment phase, subjects in the IntT group were offered to continue participation in the KomPAkt training program, in addition to the usual individual therapeutic sessions, and were able to participate once a week for 90 minutes in manual-based, disorder-specific cognitive behavioral therapy (KomPASs, Komorbidität Psychose und Abhängigkeit Skills [psychoeducational training for patients with a dual diagnosis of psychosis and dependence]) (19). The training tackles topics of relevance for dealing with both disorders:
How to identify potential risk situations and dysfunctional cognitions
Cognitive restructuring and resource activation
Anti-craving skills
Anti-stress skills
Training in general social competencies and special competencies to resist the temptation to consume
Dealing with crises
Health-promoting lifestyle.
All study patients received guideline-conform psychiatric treatment according to their treating doctors’ clinical discretion (10, e15).
Neither the duration of inpatient treatment nor the type and timespan of pharmacotherapy were set.
Statistical analysis
The t test or the χ 2 test were used to compare both groups of patients with regard to demographic and clinical characteristics at t0.
In order to analyze the outcome “substance use,” we selected an established approach from the literature (12). Accordingly, the amount that a patient consumed at t0 was defined as 100%: days of substance use per month × mean daily dose = estimated cumulative dose in the month immediately preceding inpatient admission. The extent of substance use as reported by the patients at follow-up was calculated as a percentage change on t0. The results were classified into five categories:
Abstinent/near abstinent (-100% to -80%)
Large decrease (-79% to -40%)
Small decrease (-39% to 0%)
Small increase (1% to 20%)
Large increase (>20%) (12).
We used the χ 2 test to analyze changes in the use of the main substance and in retention rates between treatment groups. To this end, the p-value of Pearson’s r×c contingency tables is calculated based on the exact distribution of the test statistic (module extract tests SPSS 22). The other outcome variables were studied by using analyses of variance with repeated measurements. All evaluations were done on an intention to treat (ITT) basis, using the last observation carried forward (LOCF) method. Wherever possible, effect sizes were calculated for the primary outcome.
We set the significance level at p ≤ 0.05. We used the statistical software package IBM SPSS Statistics (20/22) for Windows for the presented analyses.
Results
Sample
The sample consisted mainly of single unemployed men with a low educational status. Almost half of the patients had previous convictions. The IntT group had a poorer educational status and the TAU group a higher mean score as regards general psychopathology. Between-group differences did not reach significance in any other aspects (Table 2).
Table 2. Description of sample at t0: comparisons of groups by using statistical parameters.
| Characteristic | IntT (n = 50) | TAU (n = 50) | p |
|---|---|---|---|
| Sociodemographic | |||
| Sex (distribution): m/f | 43/7 | 41/9 | 0.39 3*1 |
| Age (mean ± SD) | 31.14 ± 8.90 | 30.8 ± 6.95 | 0.83 2*2 |
| Marital status (distribution): Single/married/partnered/divorced/other | 40/1/5/3/1 | 41/2/4/2/1 | 0.95 7*1 |
| Housing situation (distribution): No fixed abode/alone/with partner/with family/ other | 5/29/2/8/6 | 6/50/4/22/13 | 0.54 4*1 |
| School education (distribution): No final qualification/general secondary school (Hauptschule)/intermediate secondary school (Realschule)/university or technical college preparatory high school | 8/24/11/7 | 6/23/13/8 | 0.91 0*1 |
| Professional qualification (distribution): None/apprenticeship/degree/other | 31/14/0/5 | 26/23/1/0 | 0.0 35*1 |
| Current employment situation (distribution): Unemployed/apprenticeship/full-time/part-time/pension/ rehabilitation/other | 34/4/4/4/1/5/1 | 39/0/2/3/3/3 | 0.33 6*1 |
| Number of convictions (mean ± SD; range in parentheses) | 1.10 ± 1.63(0–7) | 0.84 ± 1.35(0–5) | 0.38 7*2 |
| Clinical | |||
| Diagnosis of psychosis (DSM-IV) (distribution): 295.30/295.60/295.40/295.70 | 42/1/4/3 | 42/0/4/4 | 0.76 7*1 |
| Diagnosis of substance-related disorder Main substance (distribution): Alcohol/amphetamines/cannabis/cocaine or crack cocaine/opiates/multiple | 6/5/36/1/1/1 | 9/3/31/2/4/1 | 0.60 7*1 |
| Main substance (distribution):Misuse/dependency | 20/30 | 29/21 | 0.10 9*1 |
| Number of diagnoses of substance-related disorder (mean ± SD; range in parentheses) | 1.5 ± 0.647(1–3) | 1.5 ± 0.735(1–4) | 1.0 0*2 |
| Intensity of consumption where the main substance is cannabis (IntT: n = 36; TAU: n = 31) | |||
| Estimated amount in the month preceding inpatient admission (grams) (mean ± SD) | 17.32 ± 23.16 | 21.80 ± 26.64 | 0.46 6*2 |
| Abstinence period (days between most recent consumption and inpatient admission) (mean ± SD) | 7.39 ± 9.28 | 5.68 ± 9.65 | 0.46 3*2 |
| Further axis 1 disorders (each mention equals one patient; DSM-IV codes in parentheses) | Major depression (296.23)Disturbed impulse control (312.30) Attention deficit/hyperactivity disorder (314.0) | Panic disorder or agoraphobia (300.01)Compulsive disorder (300.3) Pathological gambling (312.31) Attention deficit/hyperactivity disorder (314.0) | |
| Axis 2 disorders (each mention equals one patient) | Borderline (301.83) Antisocial personality disorder (301.7) | Borderline personality disorder (301.83) | |
| Time since the initial diagnosis of psychosis (months)(mean ± SD) | 65.73 ± 76.32 | 60.45 ± 59.78 | 0.70 1*2 |
| Number of psychotic episodes (mean ± SD) | 8.64 ± 10.76 | 5.6 ± 6.86 | 0.0 95*2 |
| Number of inpatient stays (mean ± SD) | 7.06 ± 8.78 | 4.88 ± 6.62 | 0.16 4*2 |
| Psychometric scores | |||
| PANSS positive scale (mean ± SD) | 18.54 ± 9.06 | 20.28 ± 9.30 | 0.34 5*2 |
| PANSS negative scale (mean ± SD) | 21.04 ± 8.97 | 23.66 ± 8.67 | 0.14 1*2 |
| PANSS general psychopathology scale (MW ± SD) | 38.88 ± 15.05 | 46.00 ± 18.63 | 0.0 38*2 |
| PANSS total scale (mean ± SD) | 78.46 ± 30.40 | 89.94 ± 34.53 | 0.0 81*2 |
| MADRS (mean ± SD) | 11.24 ± 6.40 | 12.52 ± 9.78 | 0.44 1*2 |
| GAF (mean ± SD) | 35.88 ± 5.82 | 35.26 ± 7.61 | 0.64 8*2 |
| SOCRATES “recognition“ (mean ± SD) | 27.40 ± 22.11 | 23.40 ± 19.55 | 0.34 0*2 |
| SOCRATES “ambivalence“ (mean ± SD) | 37.20 ± 26.35 | 36.40 ± 22.20 | 0.87 0*2 |
| SOCRATES “taking steps“ (mean ± SD) | 49.20 ± 30.50 | 52.40 ± 28.97 | 0.59 2*2 |
| FLZ (mean ± SD) | 2.78 ± 1.06 | 2.60 ± 1.01 | 0.38 6*2 |
IntT, integrated treatment program; TAU, treatment as usual; m, male; f, female; SD, standard deviation;
PANSS, Positive and Negative Syndrome Scale for Schizophrenia; MADRS, Montgomery Asberg Depression Rating Scale; GAF, Global Assessment of Functioning Scale; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale; FLZ. questionnaire on satisfaction with life
*1χ 2 test
*2t-test for non-dependent samples
Primary outcome
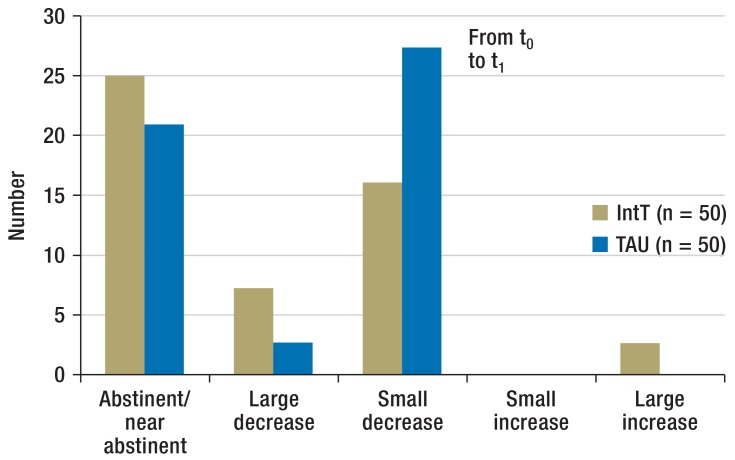
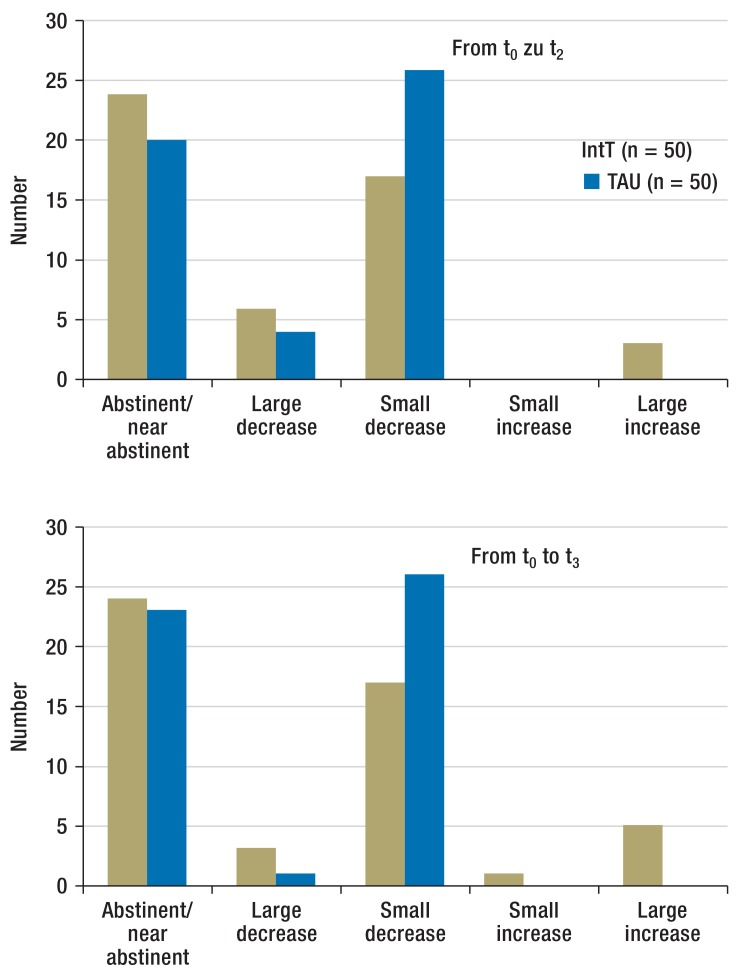
Substance use (Figure 2a): Both groups of patients reduced their levels of substance use during the course of the study. With regard to the main substance, the IntT group achieved in more cases abstinence, near-abstinence, or a large decrease in substance use at t1, whereas in the TAU group, a small decrease in substance use was more commonly observed. The difference between the IntT and TAU groups for t1 reached significance (exact significance: p = 0.039). The eTable shows the intensity of consumption of all main substances and time points/follow-ups.
Figure 2a.
Changes to the intensity of consumption of the main substance in the follow-up period from t0 to t1 Number of patients by class of change in consumption from baseline
IntT, integrated treatment program; TAU, treatment as usual
eTable. Substance use (main substances) in the control and intervention groups in the month preceding t0, t1, t2, and t3, with standard deviations and 95% confidence intervals.
| Cannabis (treatment per protocol) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 36 | 17.32 | 23.16 | 9.49 | 25.16 |
| t0 | TAU | 31 | 21.80 | 26.84 | 11.96 | 31.65 |
| t1 | IntT | 25 | 3.16 | 8.41 | –0.31 | 6.63 |
| t1 | TAU | 21 | 1.40 | 4.98 | –0.87 | 3.67 |
| t2 | IntT | 23 | 6.53 | 14.96 | 0.06 | 13.00 |
| t2 | TAU | 16 | 2.86 | 8.20 | –1.51 | 7.22 |
| t3 | IntT | 18 | 3.76 | 7.51 | 0.02 | 7.50 |
| t3 | TAU | 16 | 1.65 | 3.96 | –0.46 | 3.76 |
| Cannabis (intention to treat—last observation carried forward) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 36 | 17.32 | 23.16 | 9.49 | 25.16 |
| t0 | TAU | 31 | 21.80 | 26.84 | 11.96 | 31.65 |
| t1 | IntT | 36 | 8.85 | 19.04 | 2.41 | 15.29 |
| t1 | TAU | 31 | 7.96 | 18.36 | 1.22 | 14.70 |
| t2 | IntT | 36 | 10.85 | 20.86 | 3.79 | 17.91 |
| t2 | TAU | 31 | 9.33 | 18.72 | 2.47 | 16.20 |
| t3 | IntT | 36 | 9.40 | 18.91 | 3.00 | 15.80 |
| t3 | TAU | 31 | 8.71 | 18.26 | 2.02 | 15.41 |
| Alcohol (treatment per protocol) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 6 | 3635.17 | 2400.37 | 1116.13 | 6154.20 |
| t0 | TAU | 9 | 23328.67 | 59884.50 | –22702.63 | 69359.97 |
| t1 | IntT | 4 | 322.00 | 613.72 | –654.56 | 1298.56 |
| t1 | TAU | 4 | 188.72 | 161.21 | –67.81 | 445.24 |
| t2 | IntT | 3 | 498.33 | 644.96 | –1103.83 | 2100.50 |
| t2 | TAU | 4 | 276.00 | 167.97 | 8.73 | 543.28 |
| t3 | IntT | 2 | 5548.75 | 7033.95 | –57648.74 | 68746.24 |
| t3 | TAU | 2 | 368.00 | 520.43 | –4307.88 | 5043.88 |
| Alcohol (intention to treat—last observation carried forward) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 6 | 3635.17 | 2400.37 | 1116.13 | 6154.20 |
| t0 | TAU | 9 | 23328.67 | 59884.50 | –22702.63 | 69359.97 |
| t1 | IntT | 6 | 1714.67 | 2281.41 | –679.53 | 4108.86 |
| t1 | TAU | 9 | 22464.26 | 60223.08 | –23827.30 | 68755.82 |
| t2 | IntT | 6 | 1749.17 | 2251.20 | –613.32 | 4111.65 |
| t2 | TAU | 9 | 22503.06 | 60206.96 | –23776.11 | 68782.22 |
| t3 | IntT | 6 | 3376.42 | 4118.21 | –945.38 | 7698.22 |
| t3 | TAU | 9 | 22513.28 | 60202.97 | –23762.82 | 68789.38 |
| Amphetamines (treatment per protocol) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 5 | 25.41 | 21.87 | –1.74 | 52.57 |
| t0 | TAU | 3 | 2.87 | 2.90 | –4.33 | 10.07 |
| t1 | IntT | 4 | 9.88 | 13.97 | –12.35 | 32.10 |
| t1 | TAU | 1 | 0.00 | – | * | * |
| t2 | IntT | 3 | 11.17 | 16.81 | –30.59 | 52.93 |
| t2 | TAU | 1 | 0.00 | – | * | * |
| t3 | IntT | 3 | 15.33 | 12.66 | –16.12 | 46.79 |
| t3 | TAU | 0 | – | – | * | * |
| Amphetamines (intention to treat—last observation carried forward) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 5 | 25.41 | 21.87 | –1.74 | 52.57 |
| t0 | TAU | 3 | 2.87 | 2.90 | –4.33 | 10.07 |
| t1 | IntT | 5 | 10.95 | 12.33 | –4.36 | 26.26 |
| t1 | TAU | 3 | 2.70 | 3.11 | –5.02 | 10.42 |
| t2 | IntT | 5 | 9.75 | 13.20 | –6.63 | 26.13 |
| t2 | TAU | 3 | 2.70 | 3.11 | –5.02 | 10.42 |
| t3 | IntT | 5 | 12.25 | 11.27 | –1.75 | 26.25 |
| t3 | TAU | 3 | 2.70 | 3.11 | –5.02 | 10.42 |
| Opiates (treatment per protocol) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 1 | 0.40 | – | * | * |
| t0 | TAU | 4 | 0.94 | 0.57 | 0.02 | 1.85 |
| t1 | IntT | 1 | 0.20 | – | * | * |
| t1 | TAU | 1 | 0.75 | – | * | * |
| t2 | IntT | 0 | – | – | * | * |
| t2 | TAU | 1 | 0.75 | – | * | * |
| t3 | IntT | 0 | – | – | * | * |
| t3 | TAU | 1 | 0.75 | – | * | * |
| Opiates (intention to treat—last observation carried forward) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 1 | 0.40 | – | * | * |
| t0 | TAU | 4 | 0.94 | 0.57 | 0.02 | 1.85 |
| t1 | IntT | 1 | 0.20 | – | * | * |
| t1 | TAU | 4 | 0.69 | 0.19 | 0.38 | 0.99 |
| t2 | IntT | 1 | 0.20 | – | * | * |
| t2 | TAU | 4 | 0.69 | 0.19 | 0.38 | 0.99 |
| t3 | IntT | 1 | 0.20 | – | * | * |
| t3 | TAU | 4 | 0.69 | 0.19 | 0.38 | 0.99 |
| Cocaine/crack cocaine (treatment per protocol) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 1 | 137.25 | – | * | * |
| t0 | TAU | 2 | 76.00 | 62.22 | –483.07 | 635.07 |
| t1 | IntT | 0 | – | – | * | * |
| t1 | TAU | 1 | 4.00 | – | * | * |
| t2 | IntT | 0 | – | – | * | * |
| t2 | TAU | 1 | 4.00 | – | * | * |
| t3 | IntT | 0 | – | – | * | * |
| t3 | TAU | 0 | – | – | * | * |
| Cocaine/crack cocaine (intention to treat—last observation carried forward) in grams | ||||||
| Time point/follow-up | Group | n | Mean | SD | Lower limit | Upper limit |
| t0 | IntT | 1 | 137.25 | – | * | * |
| t0 | TAU | 2 | 76.00 | 62.22 | –483.07 | 635.07 |
| t1 | IntT | 1 | 137.25 | – | * | * |
| t1 | TAU | 2 | 18.00 | 19.80 | –159.89 | 195.89 |
| t2 | IntT | 1 | 137.25 | – | * | * |
| t2 | TAU | 2 | 17.5 | 20.5 | –166.74 | 201.74 |
| t3 | IntT | 1 | 137.25 | – | * | * |
| t3 | TAU | 2 | 17.5 | 20.5 | –166.74 | 201.74 |
IntT, integrated treatment program; TAU. treatment as usual; SD, standard deviation; n, number of patients; t0. baseline. t1. +3 months; t2. +6 months; t3. +12 months* Confidence interval cannot be calculated because cell frequency is too low
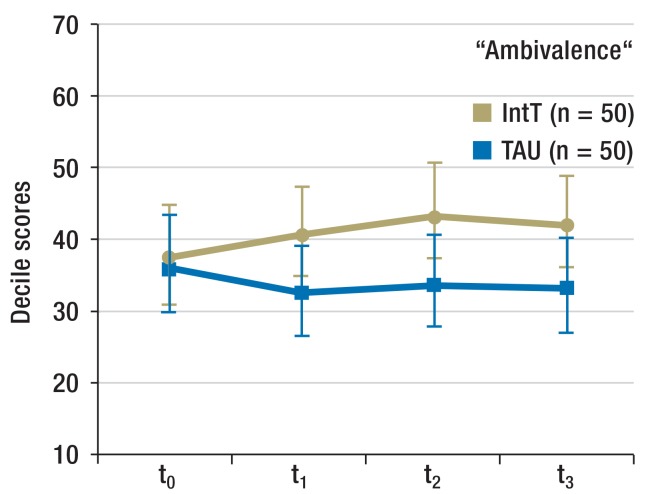
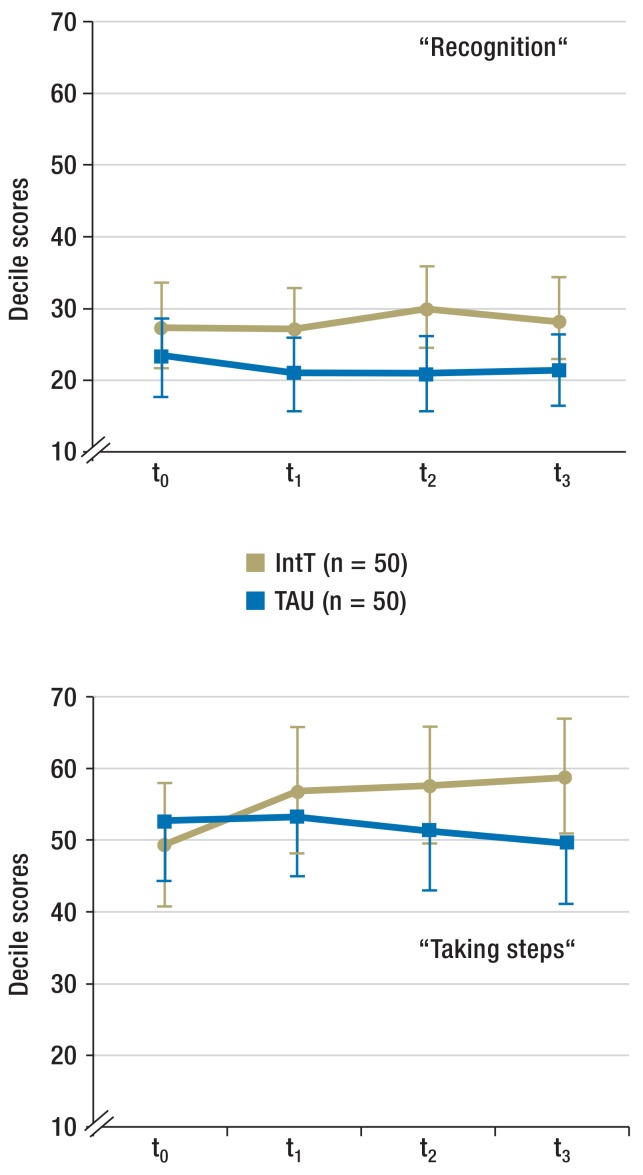
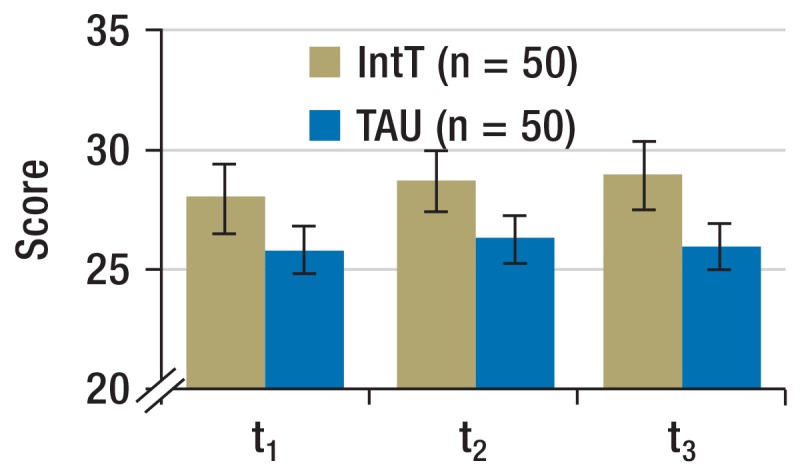
Motivation for abstinence (Figure 2b and eFigure 1): The motivation for abstinence was determined by using the SOCRATES (Stages of Change Readiness and Treatment Eagerness Scale) questionnaire consisting of three subscales that each capture different aspects of patients’ motivation. These are “recognition” (indicator for awareness of problem), “ambivalence” (indicator for the process of balancing two options—namely, to remain or become abstinent versus continued substance use), and “taking steps” (indicator for the concrete efforts to remain abstinent).
Figure 2b.
Motivation for abstinence
Scale readings from SOCRATES questionnaire (means, 95% confidence intervals)
IntT, integrated treatment program; TAU, treatment as usual; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale;
t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
eFigure 2.
Motivation for abstinence
Scale values of the SOCRATES questionnaire (means, 95% confidence intervals)
IntT, integrated treatment program; TAU, treatment as usual; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale; t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
For the period t0 to t1, the SOCRATES questionnaire did not reflect any change over time nor any difference between the groups for “recognition” (time effect p = 0.191; group effect: p = 0.208; interaction time × group: p = 0.276). “Ambivalence” increased in the IntT group (time effect: p = 0.768; group effect: p = 0.324; interaction: p = 0.009; effect size f = 0.129). For “taking steps” a tendency to increase over time was seen, but no between-group difference (time effect: p = 0.064; group effects: p = 0.986; interaction: p = 0.135).
Secondary outcome
Substance use: as regards consumption of the main substance, no differences between groups were seen for t2 and t3 (p = 0.158 and p = 0.345) (eFigure 2).
The change in cannabis consumption in the follow-up period from t0 to t1 was studied in an exploratory way. At t0, cannabis was defined as the main substance in 67 patients. The analysis of variance showed a reduction in substance use in both groups but no difference between IntT and TAU patients (time effect: p = 0.000; group effect: p = 0.818; interaction: p = 0.392). Because of small n-numbers, no further analyses for other substances were conducted.
Motivation for abstinence (Figure 2b and eFigure 1): When considering all follow-up points, “recognition” did not change over time nor between groups (time effect: p = 0.766; group effect: p = 0.082; interaction time × group: p = 0.322). “Ambivalence” increased in the IntT group (time effect: p = 0.524; group effect: p = 0.112; interaction: p = 0.007). Analysis of the within-subject contrasts showed that the interaction effect originated in the contrary development of both groups between t0 and t1 (p = 0.009). Scores for “Taking steps” increased more pronouncedly in the IntT group (group effect: p = 0.444; time effect: p = 0.229; interaction: p = 0.023). In the post-hoc comparison, however, no further differentiation was possible.
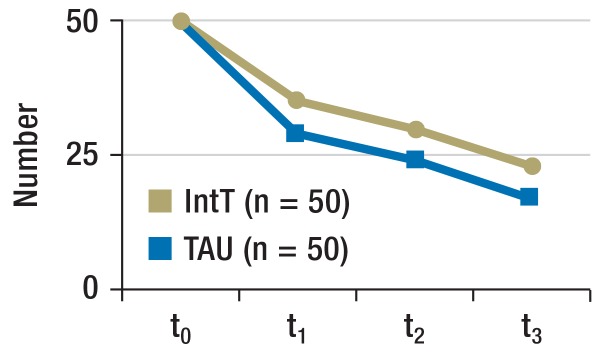
Retention rate (Figure 3a): The TAU group had a slightly poorer retention rate at t1. When all time points were considered, no difference was seen (p = 0.272). If only t1 was considered, the difference did not reach significance (p = 0.107).
Figure 3a.
Retention rate
Numbers of patients who remained in the program over the course of the study.
IntT, integrated treatment program; TAU, treatment as usual; t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
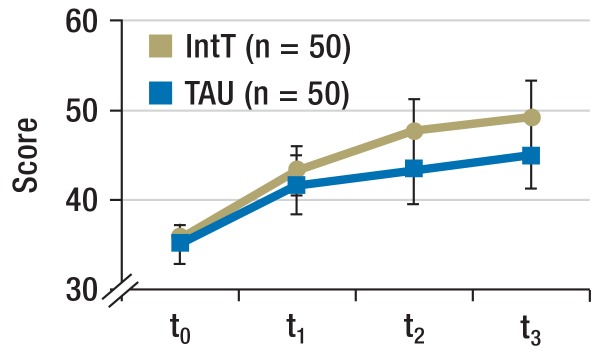
Satisfaction with treatment (Figure 3b): In the IntT group, patient satisfaction (ZUF-8) scores at all three follow-up points was higher (time effect: p = 0.009; group effect: p = 0.011; interaction: p = 0.189).
Figure 3b.
Satisfaction with treatment
Scale reading from the ZUF-8 questionnaire on patient satisfaction (mean, 95% confidence interval)
IntT, integrated treatment program; TAU, treatment as usual; t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
Global level of functioning (Figure 3c): During the follow-up period, the global assessment of functioning (GAF) score improved in the entire sample (time effect: p = 0.000). In the IntT group, the score increased more substantially after t2. The difference did not reach significance, however (group effect: p = 0.205; interaction: p = 0.094).
Figure 3c.
Global level of functioning
Scale readings from Global Assessment of Functioning (GAF) Scale (mean, 95% confidence interval)
IntT, integrated treatment program; TAU, treatment as usual; t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
Psychopathology: Psychotic symptoms (measured using the positive and negative syndrome scale for schizophrenia [PANNS]) improved in the total sample during the follow-up period (main effect of time in all subscales: p = 0.000). For negative symptoms and general psychopathology, the main effects were observed for the “group” factor (p = 0.027 and p = 0.016), but the interactions time × group did not reach significance (p = 0.406 and p = 0.807). Since the IntT group had lower scores for general psychopathology and tendentially also for negative symptoms at t0 (Table 2), an analysis of covariance was calculated, using the t0 value as the covariate. This analysis did not show up any main effects for the “group” factor in the follow-up period (negative symptoms: p = 0.074; general psychopathology: p = 0.219).
Depressive symptoms (measured using the Montgomery Asberg depression rating scale [MADRS]) showed a tendency towards improvement in the follow-up period for the total sample (time effect: p = 0.0031). However, no difference was seen between the groups (group effect: p = 0.163; interaction: p = 0.861).
The number of re-admissions to hospital and general satisfaction with life did not differ between groups. The eBox shows the results.
Discussion
Main results
This study investigated the treatment of a therapeutically challenging group of patients, who had psychosis as well as a substance use disorder. The study was conducted under conditions of standard care in a large, non-university affiliated, psychiatric hospital. The results imply that a long-term, motivational, integrated, trans-sector treatment program with cognitive behavioral elements offers at least small advantages compared with treatment as usual.
The patients in the integrated treatment program were more satisfied with their treatment, developed a greater motivation to remain abstinent, and controlled their consumption behavior more strictly, at least in the short term. Positive trends were seen in the global level of functioning and the retention rate. In the initial three months after inclusion in the treatment program, their consumption dropped more conspicuously and the retention rate tended to be better. The patients in the disorder-specific therapeutic program were on the whole more satisfied with their treatment. Their abstinence motivation seemed to increase over the entire follow-up period, and the relative benefit in terms of their global level of functioning became apparent only after six months. The latter results may indicate that patients in the integrated treatment program benefited from the longer-term treatment and therapeutic relationship on offer, although they continued to consume substances.
Comparison with other studies
The results are consistent with the available literature, which overall shows small and partial successes for motivational, integrated treatment programs for dual diagnosis patients (11– 14). Some of the results may indicate that at least certain positive effects of the integrated, disorder-specific treatment build up only over a longer time period. For this reason, a longer therapeutic and follow-up period will be required to stabilize a patient’s condition notably. This interpretation is consistent with follow-up studies and meta-analyses that described greater successes for treatment programs of more than one year’s duration compared with shorter treatment periods, as dual diagnosis patients stabilize and recover only gradually, over a period of several years (9, 20).
Strengths and limitations
The treatment program under evaluation used without exception standard hospital resources. The study is therefore transferable to standard healthcare services in Germany. It is of adequate methodological quality, with an adequate control group and randomization as well as conservative statistical analyses (ITT/LOCF). The same colleague (S König) conducted all inclusion screenings and assessments.
This being said, methodological limitations associated with limited research funds need to be borne in mind: we did not reach the sample size of n = 122 that was originally projected in our power calculation. The lower number of patients included may have contributed to the fact that some between-group differences did not reach significance. It was not possible to realize the original plan of blinding S König. Patients’ participation in the therapeutic measures was not documented systematically and adherence of the treatment to the manual was not controlled. For the primary outcome “substance use,” several information sources were consulted, including drug screening, but toxicological hair analyses were not conducted.
Assessment and outlook
The study results allow the conclusion that the widespread therapeutic nihilism regarding dual diagnosis patients is not justified. Long-term, motivational, psychosocial treatment programs have the potential to affect outcomes positively. However, the study also shows that it is not great therapeutic expectations that should be pursued but, rather, moderate aims in the sense of harm reduction approaches. The study results are so encouraging that further larger and methodologically more complex studies of the subject seem justified. In addition to confirming our results, such studies might aim to identify indicators for therapeutic successes and for subgroups that respond better to treatment. In parallel, integrated programs should be more widely implemented as part of standard care, and these should be developed further. Setting up special self-help groups among dual diagnosis patients (“double trouble groups”) (3, 21) would be highly desirable, as would the inclusion of promising family therapy elements (12, 22– 23).
Key Messages.
A long-term, trans-sector, motivational, disorder-specific treatment program with psychoeducational and behavioral therapeutic elements had slight positive effects relating to certain follow-up aspects in dual diagnosis patients.
The group that was put through a disorder-specific therapeutic program developed a higher motivation for abstinence during the course of the study and reduced consumption of substances to a greater degree, at least temporarily.
Patients who had received disorder-specific treatment were consistently more satisfied with the therapy and showed a positive trend in terms of their global level of functioning and the retention rate.
Participation in a disorder-specific treatment program did not affect readmission to hospital, psychopathology, and general satisfaction with life.
The implementation, evaluation, and optimization of motivational, integrated treatment programs for dual diagnosis patients should be progressed. However, expectations in terms of what successes the therapies can deliver should be realistic and moderate.
eFigure 2.
Changes in the intensity of consumption of the main substance in the follow-up periods t0 to t2 and t0 to t3
Number of patients by class of change in consumption, from baseline;
IntT, integrated treatment program; TAU, treatment as usual
t0, baseline; t1, + 3 months; t2, + 6 months; t3, + 12 months
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
This original article constitutes the early publication of parts of Susanne König’s doctoral dissertation at the medical faculty at the University of Cologne (Dr. rer. medic.)
Funding, ethics approval
The study was realized in cooperation with the psychiatric University Clinic, Cologne, and funded by a doctoral scholarship of the university clinic’s “Köln-Fortune” program. The doctoral candidate (S König) conducted the screening and baseline examinations and assessments. Apart from that, the treatment was continued under conditions of standard care.
The study was granted ethical approval by the ethics committee of the medical faculty at the University Clinic Cologne (application no: 10–216).
Study registration
The study was registered with ClinicalTrials.gov (U1111–1119–5851) and the German Clinical Trials Register (Deutsches Register Klinischer Studien; DRKS-ID: DRKS00000671).
Footnotes
Conflict of interest statement
Professor Gouzoulis-Mayfrank has received author fees for book publications on the subject of comorbidity, psychosis, and addiction from publishing companies Springer, Kohlhammer, and Steinkopff. She has received conference delegate fees and reimbursement of travel expenses as well as lecture fees from pharmaceutical companies Bristol-Myers Squibb, Servier, Otsuka, Janssen Cilag, Astra Zeneca, and Pfizer.
Ms König has received a doctoral scholarship from Köln-Fortune, the research funding program of the medical faculty at the University of Cologne.
Professor Schnell has received author fees from a book publication on the subject of comorbidity, psychosis, and addiction from publishing companies Springer, Kohlhammer, and Steinkopff. The Medical School Hamburg has covered conference delegate fees and travel expenses for him. He has received a lecture honorarium from the regional association for Westphalia–Lippe (LWL, Landschaftsverband Westfalen-Lippe).
Professor Daumann has received study support (third-party funding: doctoral scholarship from Köln-Fortune, the research funding program of the medical faculty at the University of Cologne.
Mr Koebke and Mr Schmitz-Buhl declare that no conflict of interest exists.
References
- 1.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 2.Drake RE, Mueser KT. Psychosocial approaches to dual diagnosis. Schizophr Bull. 2000;261:05–18. doi: 10.1093/oxfordjournals.schbul.a033429. [DOI] [PubMed] [Google Scholar]
- 3.Gouzoulis-Mayfrank E. Komorbidität Psychose und Sucht - Grundlagen und Praxis. Mit Manualen für die Psychoedukation und Verhaltenstherapie. 2. erweiterte Auflage unter Mitarbeit von Schnell T. Darmstadt. Steinkopff. 2007 [Google Scholar]
- 4.Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;(35 Suppl):93–100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 5.Mueser KT, Yarnold PR, Rosenberg SD, Swett C, Jr, Miles KM, Hill D. Substance use disorder in hospitalized severely mentally ill psychiatric patients: prevalence, correlates, and subgroups. Schizophr Bull. 2000;26:179–192. doi: 10.1093/oxfordjournals.schbul.a033438. [DOI] [PubMed] [Google Scholar]
- 6.Schnell T. Klinische Prognose schizophrener Patienten mit Cannabisabhängigkeit. Nervenarzt. 2014;85:1084–1092. doi: 10.1007/s00115-013-3926-1. [DOI] [PubMed] [Google Scholar]
- 7.Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24:589–608. doi: 10.1093/oxfordjournals.schbul.a033351. [DOI] [PubMed] [Google Scholar]
- 8.Drake RE, Mueser KT, Brunette MF, McHugo GJ. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360–374. doi: 10.2975/27.2004.360.374. [DOI] [PubMed] [Google Scholar]
- 9.Drake RE, O’Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J Subst Abuse Treatment. 2008;34:123–138. doi: 10.1016/j.jsat.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 10.DGPPN (Hrsg.) Band 1 - Behandlungsleitlinie Schizophrenie. Darmstadt: Steinkopff-Verlag; 2006. S3-Praxisleitlinien in Psychiatrie und Psychotherapie; pp. 225–227. [Google Scholar]
- 11.Cleary M, Hunt GE, Matheson SL, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev. 2008;23 doi: 10.1002/14651858.CD001088.pub2. CD001088. [DOI] [PubMed] [Google Scholar]
- 12.Barrowclough C, Haddock G, Wykes T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ. 2010;341 doi: 10.1136/bmj.c6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol Med. 2013;43:1499–1510. doi: 10.1017/S0033291712002255. [DOI] [PubMed] [Google Scholar]
- 14.Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD001088.pub3. CD001088. [DOI] [PubMed] [Google Scholar]
- 15.McLoughlin BC, Pushpa-Rajah JA, Gillies D, et al. Cannabis and schizophrenia. Cochrane Database Syst Rev. 2014;10 doi: 10.1002/14651858.CD004837.pub3. CD004837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE) Clinical Guideline 120. London: NICE; 2011. Psychosis with coexisting substance misuse: assessment and management in adults and young people. [PubMed] [Google Scholar]
- 17.Bechdolf A, Pohlmann B, Güttgemanns J, et al. Stadienabhängige Motivationsbehandlung bei Patienten mit der Doppeldiagnose Psychose und Sucht: Ergebnisse einer randomisierten Studie. Nervenarzt. 2012;83:888–896. doi: 10.1007/s00115-011-3331-6. [DOI] [PubMed] [Google Scholar]
- 18.Gouzoulis-Mayfrank E. KompAkt: Komorbidität Psychose und Abhängigkeit - Psychoedukatives Training. Komorbidität Psychose und Sucht - Grundlagen und Praxis. Mit Manualen für die Psychoedukation und Verhaltenstherapie. In: Gouzoulis-Mayfrank E, editor. 2. erweiterte Auflage unter Mitarbeit von Schnell T. Darmstadt: Steinkopff; 2007. pp. 75–103. [Google Scholar]
- 19.Schnell T. KomPASs: Komorbidität Psychose und Abhängigkeit - Skills Training. Komorbidität Psychose und Sucht - Grundlagen und Praxis. Mit Manualen für die Psychoedukation und Verhaltenstherapie. In: Gouzoulis-Mayfrank E, Gouzoulis-Mayfrank E, editors. 2. erweiterte Auflage unter Mitarbeit von Schnell T. Darmstadt: Steinkopff; 2007. pp. 105–172. [Google Scholar]
- 20.Baker AL, Thornton LK, Hides L, Dunlop A. Treatment of cannabis use among people with psychotic disorders: a critical review of randomised controlled trials. Curr Pharm Des. 2012;18:4923–4937. doi: 10.2174/138161212802884834. [DOI] [PubMed] [Google Scholar]
- 21.Magura S, Laudet AB, Mahmood D, Rosenblum A, Knight E. Adherence to medication regimens and participation in dual-focus self-help groups. Psychiatr Serv. 2002;53:310–316. doi: 10.1176/appi.ps.53.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeerdijk M, Keet R, Dekker N, et al. Motivational interviewing and interaction skills training for parents to change cannabis use in young adults with recent-onset schizophrenia: a randomized controlled trial. Psychol Med. 2012;42:1627–1636. doi: 10.1017/S0033291711002832. [DOI] [PubMed] [Google Scholar]
- 23.Mueser KT, Glynn SM, Cather C, et al. A randomized controlled trial of family intervention for co-occurring substance use and severe psychiatric disorders. Schizophr Bull. 2013;39:658–672. doi: 10.1093/schbul/sbr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.American Psychiatric Association (APA) DSM IV-TR. 4th ed. Washington DC: American Psychiatric Press; 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- e2.Wittchen HU, Zaudig M, Fydrich T. Achse I: Psychische Störungen/Achse II: Persönlichkeitsstörungen. Göttingen: Hogrefe; 1997. SKID-I und SKID-II Strukturiertes Klinisches Interview für DSM-IV. [Google Scholar]
- e3.Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV (Achse I und II) Göttingen: Hogrefe; 1997. Handanweisung zu SKID-I und-II. [Google Scholar]
- e4.McLellan AT, Luborsky L, Woody GE, et al. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- e5.Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug-free recreational ecstasy (MDMA) users. J Neurol Neurosurg Psychiatry. 2000;68:719–725. doi: 10.1136/jnnp.68.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Gouzoulis-Mayfrank E, Thimm B, Rezk M, Hensen G, Daumann J. Memory impairment suggests hippocampal dysfunction in abstinent ecstasy (MDMA) users. Progress Neuro-Psychopharmacol Biol Psychiatry. 2003;27:819–827. doi: 10.1016/S0278-5846(03)00114-3. [DOI] [PubMed] [Google Scholar]
- e7.Gouzoulis-Mayfrank E, Fischermann T, Rezk M, Thimm B, Hensen G, Daumann J. Memory performance in polyvalent MDMA (ecstasy) users who continue or discontinue MDMA use. Drug Alcohol Depen. 2005;78:317–323. doi: 10.1016/j.drugalcdep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- e8.Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The stages of change readiness and treatment eagerness scale (SOCRATES) Psychol Addict Behav. 1996;10:81–89. [Google Scholar]
- e9.Schmidt J, Lamprecht F, Wittmann WW. Zufriedenheit mit der stationären Versorgung. Entwicklung eines Fragebogens und erste Validitätsuntersuchungen. Psychother Psychosom Med Psychol. 1989;39:248–255. [PubMed] [Google Scholar]
- e10.Endicott J, Spitzer RL, Fliess JL, et al. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiat. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- e11.Kay SR, Fiszbein A, Lindenmayer JP, et al. Positive and negative syndromes in schizophrenia as a function of chronicity. Acta Psychiat Scand. 1986;74:507–518. doi: 10.1111/j.1600-0447.1986.tb06276.x. [DOI] [PubMed] [Google Scholar]
- e12.Schmidtke A, Fleckenstein P, Moises W, Beckmann H. Untersuchungen zur Reliabilität und Validität einer deutschen Version der Montgomery-Asberg Depression Rating Scale (MADRS) Schweiz Arch Neurol Psychiatr. 1988;139:51–65. [PubMed] [Google Scholar]
- e13.Fahrenberg J, Myrtek M, Schumacher J, Brähler E. Handanweisung. Göttingen: Hogrefe; 2000. Fragebogen zur Lebenszufriedenheit (FLZ) [Google Scholar]
- e14.Buchkremer G, Klingberg S, Holle R, et al. Psychoeducational psychotherapy for schizophrenic patients and their key relatives or care-givers: results of a 2-year follow-up. Acta Psychiat Scand. 1997;96:483–491. doi: 10.1111/j.1600-0447.1997.tb09951.x. [DOI] [PubMed] [Google Scholar]
- e15.Schmidt LG, Gastpar M, Falkai P, Gaebel W, editors. Köln: Deutscher Ärzte-Verlag; 2006. Evidenzbasierte Suchtmedizin - Behandlungsleitlinie Substanzbezogene Störungen. [Google Scholar]