Abstract
Purpose
Inflammation-based prognostic scores including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) are associated with oncologic outcomes in diverse malignancies. We evaluated the predictive value of pretreatment prognostic scores in differentiating nonmuscle invasive (NMIBC) and muscle invasive bladder cancer (MIBC).
Materials and Methods
Consecutive transurethral resection of bladder tumour (TURBT) cases from January 2011 to December 2013 were analysed retrospectively. Patient demographics, tumour characteristics and prognostic scores results were recorded. Receiver operating characteristics curves were used to determine prognostic score cutoffs. Univariate and multivariate binomial logistic regression analysis was performed to evaluate the association between variables and MIBC.
Results
A total of 226 patients were included, with 175 and 51 having NMIBC (stages Ta and T1) and MIBC (stage T2+) groups, respectively. Median age was 75 years and 174 patients were male. The NLR cutoff was 3.89 and had the greatest area under the curve (AUC) of 0.710, followed by LMR (cutoff<1.7; AUC, 0.650) and PLR (cutoff>218; AUC, 0.642). Full blood count samples were taken a median of 12 days prior to TURBT surgery. Multivariate logistic regression analysis identified tumour grade G3 (odds ration [OR], 32.848; 95% confidence interval [CI], 9.818-109.902; p=0.000), tumour size≥3 cm (OR, 3.353; 95% CI, 1.347-8.345; p=0.009) and NLR≥3.89 (OR, 8.244; 95% CI, 2.488-27.316; p=0.001) as independent predictors of MIBC.
Conclusions
NLR may provide a simple, cost-effective and easily measured marker for MIBC. It can be performed at the time of diagnostic flexible cystoscopy, thereby assisting in the planning of further treatment.
Keywords: Blood platelets, Lymphocytes, Neutrophils, Urinary bladder neoplasms
INTRODUCTION
Bladder cancer represents the ninth most common cancer worldwide and the most common malignancy of the urinary tract [1]. Approximately 75%-85% of patients present with nonmuscle invasive bladder cancer (NMIBC), for which transurethral resection of bladder tumour (TURBT) remains the standard first-line treatment [2]. Management drastically changes in muscle-invasive bladder cancer (MIBC) and may include radical cystectomy, radiotherapy and chemotherapy. At present, bladder cancer staging is most accurately performed with TURBT pathology specimens; however, errors in the staging process are common, with many patients upstaged at time of radical cystectomy [3].
Current theories suggest that cancer triggers a systemic inflammatory response, leading to changes in circulating inflammatory cells. The main changes include a neutrophilia with relative lymphocytopenia or thrombocytosis. These cells, along with the cytokines and chemokines they produce, play a role in the growth, maturation and differentiation of cells within the tumour microenvironment [4]. A number of inflammation-based prognostic scores that measure these changes, including preoperative neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), have been found to be associated with the oncologic outcomes in a range of diverse malignancies, including, but not limited to renal, colorectal, hepatic, breast and lung [5,6,7,8].
While previous studies establishing a relationship between elevated NLR and invasive bladder cancer have been published [9,10,11], these have neglected other, alternate inflammation-based scores including PLR and LMR. The aim of the current study was to evaluate the predictive value of pretreatment inflammation-based prognostic scores for differentiating muscle-invasive and non-muscle-invasive disease in patients undergoing TURBT surgery for primary bladder cancer.
MATERIALS AND METHODS
1. Patients
Consecutive patients with primary transitional cell bladder cancer who underwent TURBT between January 2011 and December 2013 at a large district general hospital were retrospectively reviewed. Patients with recurrent bladder tumours, nontransitional cell bladder carcinoma, metastatic disease, alternative cancer/haematological disorder diagnosis, evidence of active infection (including urinary tract infection) or lacking preoperative blood tests were excluded from the study. At time of surgery, all patients had undergone flexible cystoscopic evaluation and had negative urinalyses. Diagnosis of bladder cancer was confirmed by histology, and samples were categorised as NMIBC (stage pTa or T1) or MIBC (stage T2+). All specimens were confirmed to contain detrusor muscle for quality assurance; patients with specimens lacking detrusor muscle had a repeat TURBT at 6 weeks. Clinicopathologic variables recorded included age, sex, preoperative full blood count, tumour size, tumour grade, and multiplicity.
2. Blood analysis
Routine full blood counts were routinely collected as part of a preoperative protocol. Samples were collected in ethylenediaminetetraacetic acid anticoagulated tubes and analysed using Sysmex XE-2100 and XE-5000 Haematology Analysers (Sysmex UK, Milton Keynes, UK). Patients attending preoperative assessment clinic had concurrent urine dipstick and blood tests. Positive urine dipstick tests were sent for midstream urine microbiology and culture and antibiotics were prescribed. In these patients, repeat urine dipstick and blood tests were performed prior to the operation. Preoperative full blood counts within 60 days of TURBT were used for analysis. When multiple values existed for a patient, the sample values closest to the date or resection were analysed.
3. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. PLR was calculated as the absolute platelet count divided by the absolute lymphocyte count. LMR was defined as the absolute lymphocyte count divided by the absolute monocyte count. Receiver operating characteristics (ROC) curves were generated to determine cutoff points for each prognostic score. Patients were stratified into groups based upon the cutoff/threshold points, and characteristics compared using a chi-square test. Multivariate analysis was performed for the variables identified as statistically significant in univariate analysis, using logistic regression.
RESULTS
A total of 226 patients were included in the study, 175 and 51 with NMIBC and MIBC, respectively. The majority of the patients were male (174 of 226, 77.0%), with a median age of 75 years (interquartile range [IQR], 65-81 years). Of the NMIBC patients, 129 had Ta disease and 46 had T1 disease. Grade G1 of G2 disease was found in 128 of 175 (73.1%) NMIBC patients and 2 of 51 (3.9%) MIBC patients. Full blood count samples were taken a median of 12 days (IQR, 6-21 days) prior to TURBT surgery.
Using MIBC (stage T2 or greater disease) as a classification variable, ROC curves for the four inflammation-based prognostic scores, NLR, PLR, and LMR, are displayed in Fig. 1. Of the prognostic scores, NLR (threshold, >3.89) had the greatest area under the ROC curve (AUC) of 0.710 (sensitivity, 52.9%; specificity, 84.0%; p<0.0001), followed by LMR (threshold, <1.8; AUC, 0.650; sensitivity, 37.3%; specificity, 88.0%; p=0.0015). The threshold value for PLR was >218 with AUC of 0.642 (sensitivity. 37.3%; specificity. 87.4%; p=0.0022).
Fig. 1. Receiver operating characteristics curves for inflammation-based prognostic scores. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-tolymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
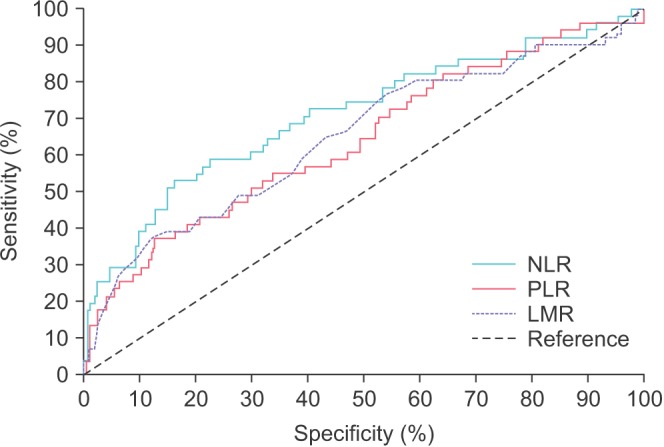
Patients were stratified into groups based upon the ROC threshold values. The patients' baseline characteristics and comparisons of the patients' clinicopathological characteristics stratified by NLR, PLR, and LMR are described in Table 1. Groups were compared using chi-square analysis, with p-values <0.05 considered significant.
Table 1. Baseline characteristics of patients, stratified by NLR, PLR, and LMR.
| Characteristic | Total | NLR | PLR | LMR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <3.89 | ≥3.89 | p-value | <218 | ≥218 | p-value | >1.8 | ≤1.8 | p-value | ||
| Age (y) | 0.019* | 0.164 | 0.008* | |||||||
| <70 | 82 (36.3) | 69 (40.6) | 13 (23.2) | 71 (38.4) | 11 (26.8) | 76 (40.0) | 6 (16.7) | |||
| ≥70 | 144 (63.7) | 101 (59.4) | 43 (76.8) | 114 (61.6) | 30 (73.2) | 114 (60.0) | 30 (83.3) | |||
| Sex | 0.490 | 0.521 | 0.903 | |||||||
| Male | 174 (77.0) | 129 (75.9) | 45 (80.4) | 144 (77.8) | 30 (73.2) | 146 (76.8) | 28 (77.8) | |||
| Female | 52 (23.0) | 41 (24.1) | 11 (19.6) | 41 (22.2) | 11 (26.8) | 44 (23.2) | 8 (22.2) | |||
| Tumour grade | 0.036* | 0.015* | 0.003* | |||||||
| G1/G2 | 132 (58.4) | 106 (62.4) | 26 (46.4) | 115 (62.2) | 17 (41.5) | 119 (62.6) | 13 (36.1) | |||
| G3 | 94 (41.6) | 64 (37.6) | 30 (53.6) | 70 (37.8) | 24 (58.5) | 71 (37.4) | 23 (63.9) | |||
| Tumour stage | 0.000* | 0.000* | 0.000* | |||||||
| Ta/T1 | 175 (77.4) | 146 (85.9) | 29 (51.8) | 153 (82.7) | 22 (53.7) | 156 (82.1) | 19 (52.8) | |||
| T2+ | 51 (22.6) | 24 (14.1) | 27 (48.8) | 32 (17.3) | 19 (46.3) | 34 (17.9) | 17 (47.2) | |||
| Tumour size (cm) | 0.029* | 0.000* | 0.002* | |||||||
| <3 (small) | 133 (58.8) | 107 (62.9) | 26 (46.4) | 119 (64.3) | 14 (34.1) | 120 (63.2) | 13 (36.1) | |||
| ≥3 (large) | 93 (41.9) | 63 (37.1) | 30 (53.6) | 66 (35.7) | 27 (65.9) | 70 (36.8) | 23 (63.9) | |||
| Multiplicity | 0.048* | 0.011* | 0.627 | |||||||
| Solitary | 149 (65.9) | 106 (62.4) | 43 (76.8) | 115 (62.2) | 34 (82.9) | 124 (65.3) | 25 (69.4) | |||
| Multiple | 77 (34.1) | 64 (37.6) | 13 (23.2) | 70 (37.8) | 7 (17.1) | 66 (34.7) | 11 (30.6) | |||
Values are presented as number (%).
NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
*p<0.05, statistically significant difference.
To identify factors associated with MIBC, binomial logistic regression analysis was performed, including prognostic scores, patient characteristics and tumour characteristics (Table 2). Univariate analysis identified age, tumour grade, tumour size, NLR, PLR, and LMR as significant predictors of muscle-invasive disease. The multivariate logistic regression model identified tumour grade (OR, 32.848; 95% CI, 9.818.109.902; p=0.000), tumour size (OR, 3.353; 95% CI, 1.347.8.345; p=0.009) and NLR (OR, 8.244; 95% CI, 2.488.27.316; p=0.001) as independent predictors of MIBC.
Table 2. Univariate and multivariate logistic regression analysis.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (>70 y) | 2.167 | 1.061-4.426 | 0.034* | 1.939 | 0.748-5.025 | 0.173 |
| Male sex | 0.643 | 0.319-1.300 | 0.219 | - | - | - |
| Tumour grade (G3) | 32.000 | 10.931-93.682 | 0.000* | 32.848 | 9.818-109.902 | 0.000* |
| Tumour size (≥3 cm, large) | 6.378 | 3.148-12.920 | 0.000* | 3.353 | 1.347-8.345 | 0.009* |
| Multiplicity (multiple) | 0.521 | 0.254-1.065 | 0.074 | - | - | - |
| NLR (≥3.89) | 5.664 | 2.872-11.169 | 0.000* | 8.244 | 2.488-27.316 | 0.001* |
| PLR (≥218) | 4.129 | 2.005-8.504 | 0.000* | 1.116 | 0.314-3.964 | 0.865 |
| LMR (≤1.8) | 4.105 | 1.935-8.710 | 0.000* | 0.695 | 0.188-2.568 | 0.586 |
OR, odds ratio; CI, confidence interval.
*p<0.05, statistically significant difference.
DISCUSSION
Bladder cancer is a heterogeneous disease and optimum management of bladder cancer is guided by accurate staging, for which pathological analysis is the gold standard. Staging error is extremely common, with reports demonstrate upstaging in up to 50% of patients at time of radical cystectomy [12,13]. Recently, researchers have looked to improve the staging process by combining histology, imaging modalities such as computed tomography or magnetic resonance imaging and molecular markers into predictive nomograms. Karakiewicz et al. [14] and Margel et al. [15] both presented nomograms to predict organ-confined disease precystectomy, the latter utilising molecular markers. However, novel variables, including laboratory analyses, are needed to improve the accuracy of these models [16].
There is increasing evidence that host inflammatory responses play a critical role in carcinogenesis, with inflammatory cells and innate immune system signalling molecules being involved in tumour progression [4]. This systemic inflammatory response leads to changes in relative levels of circulating leukocytes, providing a means to measure this response, in addition to circulating acute-phase proteins, e.g., C-reactive protein, fibrinogen, ferritin, albumin, etc. Thus, NLR, PLR, and LMR, indices that represent the systemic inflammatory response, have proven useful as potential prognostic factors in cancer [5,7].
In this present study, we examined the predictive value of four prognostic scores for invasive bladder cancer. Our results confirmed that NLR, is an independent predictor of MIBC. As expected, tumour grade and tumour size were also found to be independent predictors as both are well documented prognostic indicators in bladder cancer [17]. While PLR and LMR were not identified as independent predictors of MIBC, our study found an association between these factors and invasive disease on univariate analysis. To our knowledge, this is the first study to examine the relationship of LMR and invasive bladder cancer. Kaynar et al. [11] undertook limited analysis of PLR, and found no significant difference between mean PLR in NMIBC and MIBC (Mann-Whitney U test, p=0.810).
NLR was first proposed as a simple index to assess the systemic inflammatory response in critically ill patients [18]. It has the advantage over other markers of inflammation on the basis of low cost and ease of access, given that it comprises components of the routine full blood count assay, and can easily be performed prior to flexible cystoscopy or TURBT surgery. The association between NLR and invasive disease is complex and remains to be elucidated. A high NLR is likely to reflect an increased neutrophil-dependent inflammatory reaction and decreased lymphocyte-mediated antitumour immune response [19].
Clinically, patients must continue to have formal pathological diagnosis of MIBC through TURBT. However, one pitfall of TURBT is its inability to identify all cases of MIBC. In studies analysing patients undergoing radical cystectomy for clinical T1 disease after TURBT, as many as 48% of patients were to harbour muscle-invasive disease [13]. Good quality tumour resections are required for accurate pathological staging, and variables such as the presence of detrusor muscle in the tumour specimen can provide a proxy indicator for resection quality [20]. NLR provides a potential marker that may be used to identify patients at high risk for muscle-invasive disease. For example, a high NLR may guide the clinician towards planning further therapy; in these high risk patients, this could include early follow-up flexible cystoscopy or repeat TURBT to identify any residual muscle-invasive disease, missed during the first surgery. However, the use of NLR in a clinical setting does require further evaluation.
Our results agree with previous studies have examined the relationship between NLR and bladder cancer staging, summarised in Table 3. The consensus finding in these studies was elevated NLR in MIBC as compared to NMIBC; however, two research groups only performed univariate analysis [9,10,11]. In the papers examining patients postradical cystectomy, elevated NLR was associated with upstaging or extra-vesical disease [21,22,23]. It is important to note the wide range of ROC curve cutoff values for NLR in these publications. This variation may be explained by the nature of NLR as a nonspecific marker that may rise secondary to a number of insults, such as infection. A consensus NLR cutoff value remains to be determined.
Table 3. Previous studies comparing NLR and bladder cancer staging.
| Source | Patient | NLR | Analysis |
|---|---|---|---|
| Can et al. (2012) [9] | 182 Patients: NMIBC (n=80), MIBC (n=102) | ROC cutoff: 2.57 | NLR>2.57 was independent predictor of MIBC (OR, 2.78; 95% CI, 1.383-5.588; p=0.004*) |
| Ceylan et al. (2014) [10] | 198 Patients: NMIBC (n=162), MIBC (n=36) | Mean NLR: MIBC, 4.14±2.76; NMIBC, 3.36±2.88 | Mean NLR differed significantly between MIBC and NMIBC on Mann-Whitney U test (p=0.03*) |
| ROC cutoff: 3.96 | |||
| Kaynar et al. (2014) [11] | 291 Patients: NMIBC (n=192), MIBC (n=99) | Mean NLR: MIBC, 2.9±0.2; NMIBC, 2.4±0.1 | Mean NLR differed significantly between MIBC and NMIBC on Mann-Whitney U test (p=0.028*) |
| Significant correlation between NLR and MIBC on univariate analysis (r=0.138, p=0.031*) | |||
| Krane et al. (2013) [21] | 68 Patients with recurrent T1 disease or MIBC undergoing radical cystectomy | Mean NLR: 4.0±2.8 (overall) | NLR>2.5 was independent predictor of extravesical disease (RR, 3.18; 95% CI, 1.09-9.79; no p-value given) |
| Cutoff: 2.5 (as per previous publications) | |||
| Potretzke et al. (2014) [22] | 102 Patients undergoing radical cystectomy: NMIBC (n=25), MIBC (n=77) | Mean NLR: 4.33±0.87 (upstaged to ≥pT3) and 2.66±0.29 (≤pT2) | NLR (continuous variable) was independent predictor of upstaging (OR, 1.36; 95% CI, 1.01-1.84; p=0.04*) and extravesical dis- ease (OR, 1.5; 95% CI, 1.07-2.1; p=0.02*) at radical cystectomy |
| Viers et al. (2014) [23] | 899 Patients undergoing radical cystectomy: NMIBC (n=363), MIBC (n=524) | Cutoff: 2.7 (obtained visually) | NLR (continuous variable) was independent predictor of extravesical disease (OR, 1.07; 95% CI, 1.01-1.15; p=0.03*) and lymph node involvement (OR, 1.09; 95% CI, 1.02-1.16; p=0.02*) |
NLR, neutrophil-to-lymphocyte ratio; NMIBC, nonmuscle invasive bladder cancer; MIBC, muscle invasive bladder cancer; ROC, receiver operating characteristics; OR, odds ratio; CI, confidence interval; RR, relative risk.
NLR has been shown to be inversely associated with disease recurrence and progression in bladder cancer, first in a mixed cohort of patients undergoing radical cystectomy for MIBC and recurrent NMIBC. A number of authors have found patients with elevated NLR to an independent predictor recurrence-free, disease-specific and overall survival [21,23]. More recently, Mano et al. [24] examined a group of NMIBC patients and found an elevated NLR to be significantly associated with disease recurrence and progression. Ozyalvacli et al. [25] further limited their patient cohort to those with stage pT1 bladder tumours, and confirmed the association of NLR with disease recurrence and progression. This association of elevated NLR with invasive disease, disease recurrence and survival could signify a marker for a subset of patients with high risk, aggressive tumour biology.
We recognise the limitations in the retrospective nature of our study. While patients with concurrent inflammatory conditions (e.g., infection, haematological disorder) were omitted, the confounding ef fect of these cannot be completely excluded. In addition, preoperative blood tests were taken a median of 12 days prior to the TURBT surgery. While we excluded patients with blood tests more than 60 days before the date of surgery, it is possible that the full blood count changed during this time. However, the NLR study in patients undergoing radical cystectomy by Viers et al. [23] determined that NLR remains stable over a period as long as 90 days.
Furthermore, the main aim of our study was to differentiate muscle-invasive from superficial disease, as an aid to clinicians. The groups were separated into NMIBC (Ta/T1) and MIBC (T2+) as these differentiated patients who would be appropriately managed with TURBT as compared to radical surgery, radiotherapy or chemotherapy, as per current European Association of Urology guidelines [26]. It is noted that muscle-invasive, or T2+ disease does include a wide range of disease. This can include cancer that has invaded the muscularis propria (T2), up to adjacent structures (T4), such as the prostate, vagina or pelvic wall, and it is likely that the systemic inflammatory response increases with tumour stage. While the use of inflammation-based prognostic scoring to differentiate each stage of MIBC was beyond the scope of our study, it merits further study to identify patients most appropriate for radical surgery.
Despite these limitations, NLR appears to be a promising marker for invasive bladder cancer and may be a useful variable in future predictive nomograms. Larger, prospective studies are required to fully define the utility of NLR within a clinical setting.
CONCLUSIONS
Accurate pathological staging in bladder cancer is vital as it guides early management. Our comparison of pretreatment inflammation-based prognostic scores indicates that NLR is an independent predictor of muscle-invasive disease and may provide a simple, cost-effective and easily measured marker for MIBC. It can be performed at the time of diagnostic flexible cystoscopy, thereby assisting in the planning of further treatment and follow-up, including tumour resection and provision of intravesical therapy.
ACKNOWLEDGMENTS
Many thanks to Kirsty Moon for her assistance with the Northwick Park bladder cancer database.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dutta SC, Smith JA, Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001;166:490–493. [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Geuna E, Michalarea V, Guardascione M, Naumann U, Lorente D, et al. The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br J Cancer. 2015;112:1157–1165. doi: 10.1038/bjc.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Can C, Baseskioglu B, Yılmaz M, Colak E, Ozen A, Yenilmez A. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. 2012;89:468–472. doi: 10.1159/000343278. [DOI] [PubMed] [Google Scholar]
- 10.Ceylan C, Doluoglu OG, Keles I, Gazel E, Temucin T, Odabas O, et al. Importance of the neutrophil-to-lymphocyte ratio in muscle-invasive and non-muscle invasive bladder tumors. Urologia. 2014;81:120–124. doi: 10.5301/uro.5000031. [DOI] [PubMed] [Google Scholar]
- 11.Kaynar M, Yıldırım ME, Badem H, Cavis M, Tekinarslan E, Istanbulluoglu MO, et al. Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumour Biol. 2014;35:6601–6605. doi: 10.1007/s13277-014-1889-x. [DOI] [PubMed] [Google Scholar]
- 12.Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–149. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Ark JT, Keegan KA, Barocas DA, Morgan TM, Resnick MJ, You C, et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 2014;113:894–899. doi: 10.1111/bju.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karakiewicz PI, Shariat SF, Palapattu GS, Gilad AE, Lotan Y, Rogers CG, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 2006;176(4 Pt 1):1354–1361. doi: 10.1016/j.juro.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Margel D, Harel A, Yossepowitch O, Baniel J. A novel algorithm to improve pathologic stage prediction of clinically organ-confined muscle-invasive bladder cancer. Cancer. 2009;115:1459–1464. doi: 10.1002/cncr.24138. [DOI] [PubMed] [Google Scholar]
- 16.Bostrom PJ, Van Rhijn BW, Fleshner N, Finelli A, Jewett M, Thoms J, et al. Staging and staging errors in bladder cancer. Eur Urol Suppl. 2010;9:2–9. [Google Scholar]
- 17.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 19.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31–39. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Mariappan P, Finney SM, Head E, Somani BK, Zachou A, Smith G, et al. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int. 2012;109:1666–1673. doi: 10.1111/j.1464-410X.2011.10571.x. [DOI] [PubMed] [Google Scholar]
- 21.Krane LS, Richards KA, Kader AK, Davis R, Balaji KC, Hemal AK. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27:1046–1050. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 22.Potretzke A, Hillman L, Wong K, Shi F, Brower R, Mai S, et al. NLR is predictive of upstaging at the time of radical cystectomy for patients with urothelial carcinoma of the bladder. Urol Oncol. 2014;32:631–636. doi: 10.1016/j.urolonc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;33:67.e1–67.e7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Ozyalvacli ME, Ozyalvacli G, Kocaaslan R, Cecen K, Uyeturk U, Kemahlı E, et al. Neutrophil-lymphocyte ratio as a predictor of recurrence and progression in patients with high-grade pT1 bladder cancer. Can Urol Assoc J. 2015;9:E126–E131. doi: 10.5489/cuaj.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]


