Abstract
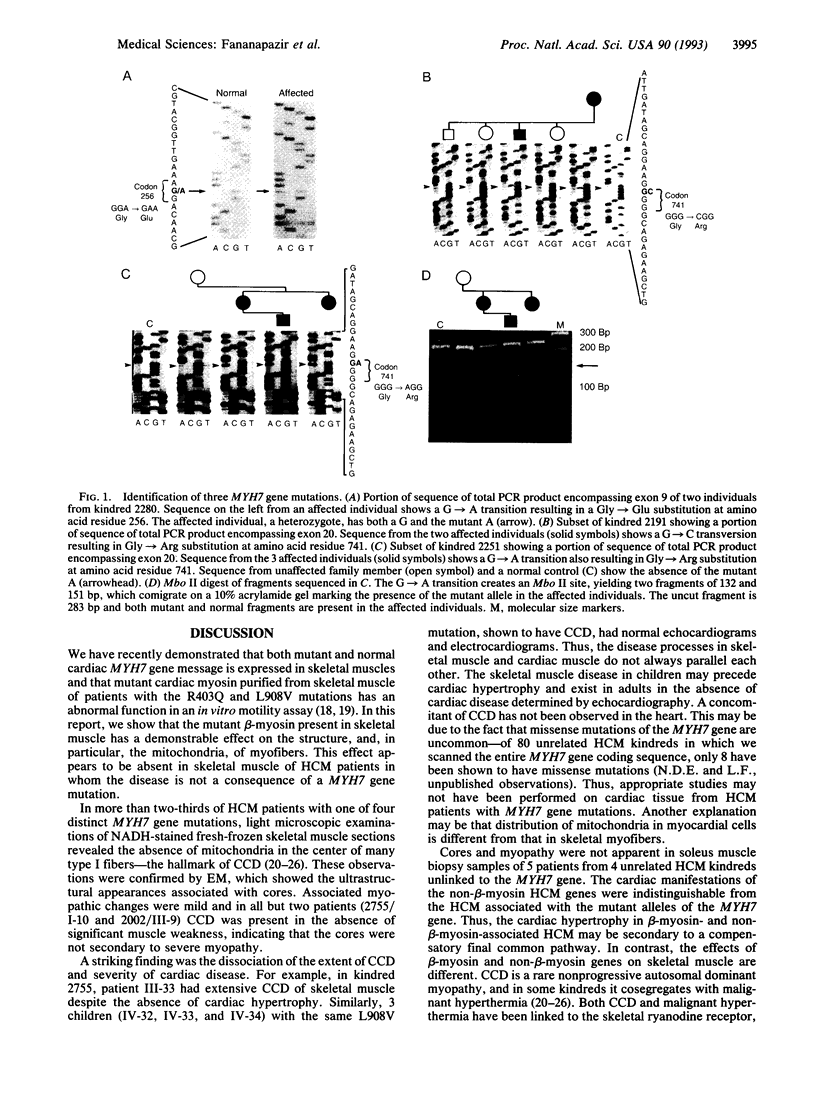
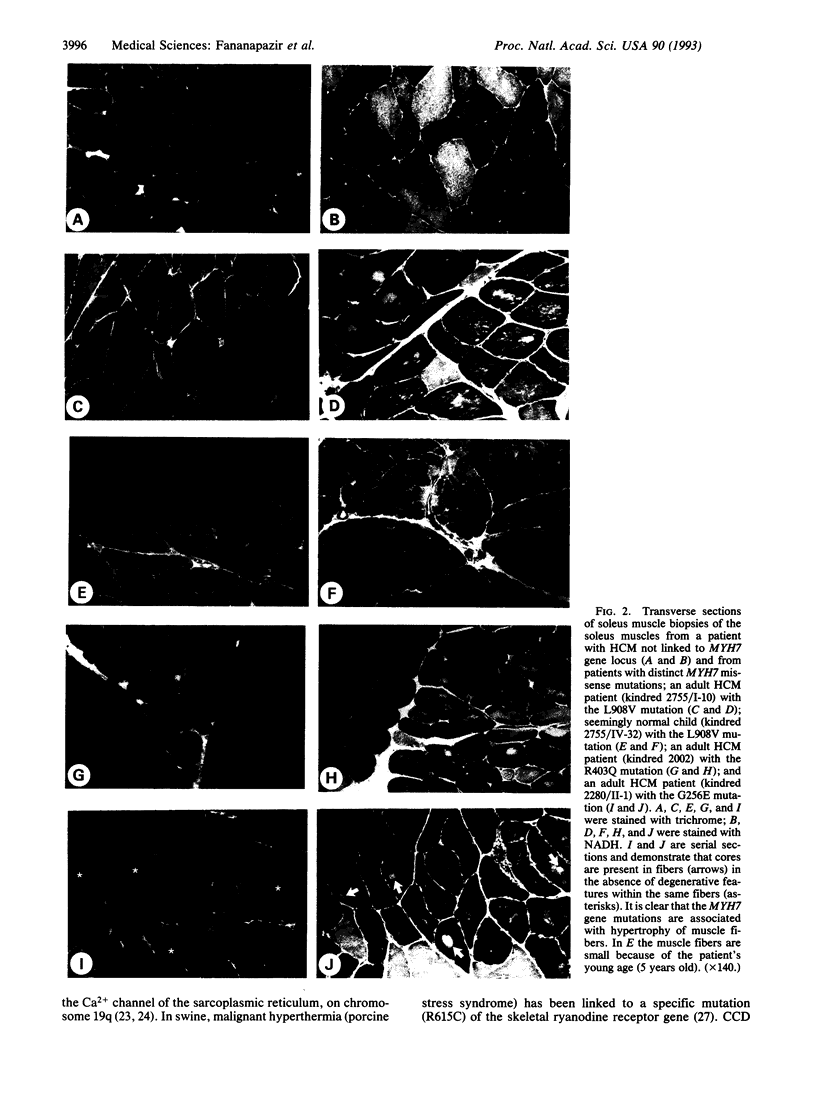
Hypertrophic cardiomyopathy (HCM) is an important cause of sudden death in apparently healthy young individuals. In less than half of kindreds with HCM, the disease is linked to the beta-myosin heavy-chain gene locus (MYH7). We have recently described two missense MYH7 gene mutations [Arg-403 to Gln (R403Q) and Leu-908 to Val (L908V)] and found that the mutant message is present in skeletal muscle soleus) and that the mutant beta-myosin obtained from soleus muscle has abnormal in vitro motility activity. Having identified a second kindred with the R403Q mutation, and 3 other kindreds with two additional mutations (G741R and G256E), we performed histochemical analysis of soleus muscle biopsies from 25 HCM patients with one of these four mutations. Light microscopic examination of the NADH-stained biopsies revealed the presence of central core disease (CCD) of skeletal muscle, a rare autosomal dominant nonprogressive myopathy characterized by a predominance of type I "slow" fibers and an absence of mitochondria in the center of many type I fibers. CCD was present in 10 of 13 patients with the L908V mutation, 5 of 8 patients with the R403Q mutation, 1 of 3 patients with the G741R mutation, and 1 patient with the G256E mutation. Mild-to-moderate myopathic changes with muscle fiber hypertrophy were present in 16 patients. Notably, CCD was present in 2 adults and 3 children with the L908V mutation who did not have cardiac hypertrophy. In contrast, soleus muscle samples from 5 patients from 4 kindreds in which HCM was not linked to the MYH7 locus showed no myopathy or CCD. Soleus muscle biopsies from 5 control subjects also showed normal histology. This work demonstrates that (i) MYH7-associated HCM is often a disease of striated muscle but with predominant cardiac involvement and (ii) a subset of HCM patients with MYH7 gene missense mutations have CCD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne E., Blumbergs P. C., Hallpike J. F. Central core disease. Study of a family with five affected generations. J Neurol Sci. 1982 Jan;53(1):77–83. doi: 10.1016/0022-510x(82)90081-8. [DOI] [PubMed] [Google Scholar]
- Caforio A. L., Rossi B., Risaliti R., Siciliano G., Marchetti A., Angelini C., Crea F., Mariani M., Muratorio A. Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: evidence of subclinical myogenic myopathy. J Am Coll Cardiol. 1989 Nov 15;14(6):1464–1473. doi: 10.1016/0735-1097(89)90383-5. [DOI] [PubMed] [Google Scholar]
- Epstein N. D., Cohn G. M., Cyran F., Fananapazir L. Differences in clinical expression of hypertrophic cardiomyopathy associated with two distinct mutations in the beta-myosin heavy chain gene. A 908Leu----Val mutation and a 403Arg----Gln mutation. Circulation. 1992 Aug;86(2):345–352. doi: 10.1161/01.cir.86.2.345. [DOI] [PubMed] [Google Scholar]
- Epstein N. D., Fananapazir L., Lin H. J., Mulvihill J., White R., Lalouel J. M., Lifton R. P., Nienhuis A. W., Leppert M. Evidence of genetic heterogeneity in five kindreds with familial hypertrophic cardiomyopathy. Circulation. 1992 Feb;85(2):635–647. doi: 10.1161/01.cir.85.2.635. [DOI] [PubMed] [Google Scholar]
- Epstein N. D., Lin H. J., Fananapazir L. Genetic evidence of dissociation (generational skips) of electrical from morphologic forms of hypertrophic cardiomyopathy. Am J Cardiol. 1990 Sep 1;66(5):627–631. doi: 10.1016/0002-9149(90)90492-j. [DOI] [PubMed] [Google Scholar]
- Fananapazir L., Chang A. C., Epstein S. E., McAreavey D. Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter, hemodynamic, and electrophysiological findings. Circulation. 1992 Sep;86(3):730–740. doi: 10.1161/01.cir.86.3.730. [DOI] [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V. K., Weiler J. E., O'Brien P. J., MacLennan D. H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991 Jul 26;253(5018):448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Haan E. A., Freemantle C. J., McCure J. A., Friend K. L., Mulley J. C. Assignment of the gene for central core disease to chromosome 19. Hum Genet. 1990 Dec;86(2):187–190. doi: 10.1007/BF00197703. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Miller R. G., Brownell A. K. Central core disease: ultrastructure of the sarcoplasmic reticulum and T-tubules. Muscle Nerve. 1989 Feb;12(2):95–102. doi: 10.1002/mus.880120203. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Homsher E., Wang F., Sellers J. R. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. Am J Physiol. 1992 Mar;262(3 Pt 1):C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Isaacs H., Heffron J. J., Badenhorst M. Central core disease. A correlated genetic, histochemical, ultramicroscopic, and biochemical study. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1177–1186. doi: 10.1136/jnnp.38.12.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J. A., McKenna W., Pare J. A., Solomon S. D., Holcombe R. F., Dickie S., Levi T., Donis-Keller H., Seidman J. G., Seidman C. E. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med. 1989 Nov 16;321(20):1372–1378. doi: 10.1056/NEJM198911163212005. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kausch K., Lehmann-Horn F., Janka M., Wieringa B., Grimm T., Müller C. R. Evidence for linkage of the central core disease locus to the proximal long arm of human chromosome 19. Genomics. 1991 Jul;10(3):765–769. doi: 10.1016/0888-7543(91)90461-m. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990 Feb 8;343(6258):559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Phillips M. S. Malignant hyperthermia. Science. 1992 May 8;256(5058):789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. G., Merril C. R. Affinity generation of single-stranded DNA for dideoxy sequencing following the polymerase chain reaction. Anal Biochem. 1989 May 1;178(2):239–242. doi: 10.1016/0003-2697(89)90631-3. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., 4th, Wallace D. C. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Sinha A. M., Friedman D. J., Nigro J. M., Jakovcic S., Rabinowitz M., Umeda P. K. Expression of rabbit ventricular alpha-myosin heavy chain messenger RNA sequences in atrial muscle. J Biol Chem. 1984 May 25;259(10):6674–6680. [PubMed] [Google Scholar]
- Smith E. R., Heffernan L. P., Sangalang V. E., Vaughan L. M., Flemington C. S. Voluntary muscle involvement in hypertrophic cardiomyopathy. A study of eleven patients. Ann Intern Med. 1976 Nov;85(5):566–572. doi: 10.7326/0003-4819-85-5-566. [DOI] [PubMed] [Google Scholar]
- Solomon S. D., Jarcho J. A., McKenna W., Geisterfer-Lowrance A., Germain R., Salerni R., Seidman J. G., Seidman C. E. Familial hypertrophic cardiomyopathy is a genetically heterogeneous disease. J Clin Invest. 1990 Sep;86(3):993–999. doi: 10.1172/JCI114802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Watkins H., Rosenzweig A., Hwang D. S., Levi T., McKenna W., Seidman C. E., Seidman J. G. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992 Apr 23;326(17):1108–1114. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Antozzi C., Rimoldi M., Morandi L., Villani F., Tiranti V., DiDonato S. Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 1991 Jul 20;338(8760):143–147. doi: 10.1016/0140-6736(91)90136-d. [DOI] [PubMed] [Google Scholar]






