Abstract
The purpose of this study was to create a prognostic model for distant metastasis in patients with locally advanced NPC who accept concurrent chemotherapy combined with intensity-modulated radiotherapy (CCRT) to identify high-risk patients who may benefit from neoadjuvant chemotherapy (NACT). A total of 881 patients with newly-diagnosed, non-disseminated, biopsy-proven locoregionally advanced NPC were retrospectively reviewed; 411 (46.7%) accepted CCRT and 470 (53.3%) accepted NACT followed by CCRT. Multivariate analysis demonstrated N2–3 disease, plasma Epstein–Barr virus (EBV) DNA > 4000 copies/mL, serum albumin ≤46 g/L and platelet count >300 k/cc were independent prognostic factors for distant metastasis in the CCRT group. Using these four factors, a prognostic model was developed, as follows: 1) low-risk group: 0–1 risk factors; and 2) high-risk group: 2–4 risk factors. In the high-risk group, patients who accepted NACT + CCRT had significantly higher distant metastasis-free survival and progression-free survival rates than the CCRT group (P = 0.001; P = 0.011). This simple prognostic model for distant metastasis in locoregionally advanced NPC may facilitate with the selection of high-risk patients who may benefit from NACT prior to CCRT.
Nasopharyngeal carcinoma (NPC) is endemic in Southeast Asia, North Africa, Alaska and the Mediterranean basin1. Due to its silent, deep-seated location and mild, non-specific symptoms, early detection is a challenge; 60–70% patients present with locally advanced NPC at diagnosis2. The standard therapy for non-disseminated NPC is radiotherapy; however, this strategy successfully controls disease in only 67%–77% of patients with advanced disease3. Intensity-modulated radiation therapy (IMRT) is now the primary radiotherapy modality in NPC as it provides better dose distribution and locoregional control4,5. Additionally, several clinical trials and meta-analysis demonstrated chemotherapy administered concurrently with radiotherapy (CCRT) is the most effective treatment and improves overall survival6,7,8,9,10. Nevertheless, over 20% of patients still experience distant metastasis after CCRT, necessitating exploration of other intensive treatment modalities for NPC11.
Addition of neoadjuvant chemotherapy (NACT) before CCRT may be a reasonable approach. Theoretically, NACT could reduce the tumor burden and kill occult micro-metastases, which may improve survival. A recent meta-analysis revealed NACT significantly reduced the risk of distant metastasis in NPC12. But published single arm or randomized phased II studies regarding the efficacy of NACT followed by CCRT in locally advanced NPC have provided conflicting results13,14,15. One possible reason for the lack of benefit is due to the inclusion criteria used in those studies, which was mainly based on patients’ clinical stages. However, the present NPC staging system is restricted in its diagnostic reach to the anatomical extent of the tumors, and may not accurately categorize patients at high risk of distant metastasis. As NACT may induce an unnecessary financial burden and delay CCRT, it is of utmost importance to identify high-risk patients who may obtain benefit from NACT before treatment.
Although the Tumor, Node, Metastasis (TNM) staging system is widely used to predict prognosis and guide therapy, accumulating data suggests circulating Epstein–Barr virus (EBV) DNA and several other serum markers as prognostic factors for distant metastasis in NPC16,17,18,19,20. Therefore, we retrospectively analyzed a large cohort of patients to evaluate the prognostic value of pretreatment clinical and laboratory factors and construct a prognostic score model to facilitate pretreatment decision-making regarding NACT in NPC.
Methods and Materials
Patients
We reviewed all cases of newly-diagnosed, biopsy-proven, non-metastatic NPC treated at Sun Yat-sen University Cancer Center using IMRT between October 2009 and February 2012. In all, 1811 cases were evaluated, of whom 1330 (73.4%) were diagnosed with stage III-IVb disease according to the 7th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) staging system21. Of these, 1044/1330 (78.5%) were treated with CCRT or NACT + CCRT; 163/1044 (15.6%) cases were subsequently eliminated due to incomplete laboratory data. Therefore, 881 patients were included in this analysis. The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, and was conducted in accordance with the Good Clinical Practice guideline. Informed consent was obtained from each patient for their consent to have their information used in research without affecting their treatment option or violating their privacy.
Two experienced radiologists separately evaluated all MRI scans to minimize heterogeneity, and two physicians specializing in head and neck cancer restaged all patients according to 7th edition of the UICC/AJCC. Any disagreements were resolved by consensus.
Laboratory measurements
Plasma EBV DNA, serum lactate dehydrogenase (LDH), serum alkaline phosphatase (ALP), serum albumin, leukocytes, hemoglobin and platelets were measured in all patients at the same time within 2 weeks before therapy. Blood counts were performed using a Sysmex XE-5000 automated hematology analyzer (Sysmex, Kobe, Japan). Serum LDH, ALP and albumin were measured using an automated immunoturbidmetric analyzer (7600-020; Hitachi High-Technologies, Tokyo, Japan). Patient plasma EBV DNA was measured by real-time quantitative PCR targeting the EBV BamH I-W region22,23.
Treatment
All patients were treated using IMRT with one fraction daily 5 days per week. Target volumes were delineated according to International Commission on Radiation Units and Measurements Reports 50 and 62. Clinical target volumes (CTV) were individually delineated based on the tumor invasion pattern. The prescribed radiation dose was: a total of 68–70 Gy in 30–33 fractions at 2.13–2.27 Gy/fraction to the planning target volume (PTV) of the gross tumor volume of the primary (GTV-P), 60–68 Gy to the nodal gross tumor volume PTV (GTV-N), 60 Gy to the PTV of CTV-1 (high-risk regions), and 54–56 Gy to the PTV of CTV-2 (low-risk regions and neck nodal regions). In total, 411/881 (46.7%) patients received CCRT, and 470 (53.3%) received NACT + CCRT. NACT was a platinum-based regimen of two or three drugs every 3 weeks for two or three cycles; 796/881 (90.4%) patients received a single-drug platinum-based CCRT regimen every 3 weeks for at least 2 cycles, or weekly for at least 3 cycles.
Follow-up
After treatment, patients were examined every 3 months during the first 2 years, and every 6 months thereafter for up to 5 years or until death. Median follow-up was 38.7 (range, 1.3–60.2) months. No patients were lost to follow-up. The following end points (time to first defining event) were assessed: distant metastasis-free survival (DMFS), progression-free survival (PFS), overall survival (OS) and local relapse-free survival (LRFS).
Statistical analysis
All calculations were performed using Statistical Package for the Social Sciences, version 20.0 (SPSS, Chicago, IL, USA). Grouping by EBV DNA, leukocyte count, platelet count, hemoglobin, LDH and ALP was performed using standard or published thresholds16,17,18,19,20. Serum albumin was analyzed as a binary variable using the median value of the CCRT group as a cut-off (≥median of high-serum albumin group and < median of low serum albumin group). The Chi-square test was used to analyze differences between the CCRT and NACT + CCRT groups. Two-tailed P-values < 0.05 were considered significant.
Step 1: Survival prediction. The CCRT group was used to determine the prognostic significance of pretreatment clinical and laboratory factors for distant metastasis in univariate (Kaplan–Meier method and log-rank test) and multivariable analysis (Cox proportional hazards model to test independent significance by backward elimination of insignificant explanatory variables).
Step 2: Model construction. A prognostic score model was created based on the independent prognostic factors identified in the CCRT group. The maximum score for each patient was equal to the total number of risk factors. The cut-off score to define the high-risk and low-risk groups for DMFS was identified using receiver-operating characteristic (ROC) curve analysis.
Step 3: Stratification survival analysis. The efficacy of NACT was assessed for each stratification of the entire cohort dichotomized by each individual prognostic factor and the prognostic score model.
Step 4: Multivariate survival analysis in the high-risk group. Multivariate analysis of the high-risk group was performed to confirm the benefit of NACT in addition to CCRT while controlling for host, tumor and laboratory parameters.
Results
Clinicopathological features and treatment outcomes
The clinicopathological characteristics of the 881 patients are presented in Table 1. Median age at diagnosis was 45 (range, 14–77) years; 98.9% of patients had type II or III disease, based on the World Health Organization (WHO) criteria.
Table 1. Characteristics of the 881 patients with NPC.
| Characteristic | CCRT | NACT + CCRT | P-value |
|---|---|---|---|
| No. of patients (%) | No. of patients (%) | ||
| Total | 411 | 470 | |
| Age, years | 0.083 | ||
| ≤50 | 278 (67.6) | 343 (73.0) | |
| >50 | 133 (32.4) | 127 (27.0) | |
| Sex | 0.699 | ||
| Male | 312 (75.9) | 362 (77.0) | |
| Female | 99 (24.1) | 108 (23.0) | |
| T classification | <0.001 | ||
| T1–3 | 338 (82.2) | 315 (67.0) | |
| T4 | 73 (17.8) | 155 (33.0) | |
| N classification | 0.002 | ||
| N0–1 | 296 (72.0) | 292 (62.1) | |
| N2–3 | 115 (28.0) | 178 (37.9) | |
| Clinical stage | <0.001 | ||
| III | 314 (76.4) | 252 (53.6) | |
| IV | 97 (23.6) | 218 (46.4) | |
| Serum EBV DNA, copies/mL | <0.001 | ||
| ≤4000 | 259 (63.0) | 186 (39.6) | |
| >4000 | 152 (37.0) | 284 (60.4) | |
| Leukocytes, k/cc | 0.015 | ||
| ≤10 | 384 (93.4) | 417 (88.7) | |
| >10 | 27 (6.6) | 53 (11.3) | |
| Hemoglobin, g/L | 0.513 | ||
| ≤120 | 26 (6.3) | 35 (7.4) | |
| >120 | 385 (93.7) | 435 (92.6) | |
| Platelets, k/cc | 0.012 | ||
| ≤300 | 361 (87.8) | 384 (81.7) | |
| >300 | 50 (12.2) | 86 (18.3) | |
| Serum albumin, g/L | 0.001 | ||
| ≤ 46 | 215 (52.3) | 300 (63.8) | |
| > 46 | 196 (47.7) | 170 (36.2) | |
| Serum alkaline phosphatase, U/L | 0.254 | ||
| ≤110 | 389 (94.6) | 436 (92.8) | |
| >110 | 22 (5.4) | 34 (7.2) | |
| Serum lactate dehydrogenase, U/L | 0.001 | ||
| ≤245 | 399 (97.1) | 431 (91.7) | |
| >245 | 12 (2.9) | 39 (8.3) | |
| Progression-free survival | 0.784 | ||
| Events | 76 (18.5) | 85 (18.1) | |
| Rate at 3 years, % | 82.0 | 82.3 | |
| Distant metastasis-free survival | 0.627 | ||
| Events | 49 (11.9) | 52 (11.1) | |
| Rate at 3 years, % | 88.0 | 89.2 | |
| Local relapse-free survival | 0.852 | ||
| Events | 31 (7.5) | 38 (8.1) | |
| Rate at 3 years, % | 93.1 | 91.9 | |
| Overall survival | 0.801 | ||
| Events | 32 (7.8) | 40 (8.5) | |
| Rate at 3 years, % | 93.9 | 92.4 |
Abbreviations: CCRT, concurrent chemoradiotherapy; NACT, neoadjuvant chemotherapy.
Patients with stage IV, T4 or N2–3 disease were more likely to receive NACT than patients with stage III (P < 0.001), T1–3 (P < 0.001) or N0–1 (P = 0.002) disease. High pretreatment plasma EBV DNA (> 4,000 copies/ml; P < 0.001), leukocyte count (P = 0.015), platelet count (P = 0.012) and serum LDH (P = 0.001), and low pretreatment albumin (P = 0.001) were significantly associated with NACT.
The biases towards selecting patients with bulky tumors or high EBV DNA for more aggressive treatment reflects the clinical decision-making preferences during the study period. Despite these variations, there was no significant difference in any end-point between treatment groups (Table 1).
Prognostic factors for metastasis in the CCRT group
Univariate analysis identified N2–3 disease (P = 0.003), plasma EBV DNA (P = 0.003), serum albumin (P = 0.009), leukocyte count (P = 0.012) and platelet count (P = 0.016) as significant prognostic factors for DMFS. Multivariate analysis confirmed all of these factors, except leukocyte count, were independent prognostic factors for DMFS in locally advanced NPC after CCRT (Table 2).
Table 2. Survival analysis of risk factors for distant metastasis in patients with NPC who accepted CCRT (n = 411).
| Factor | Distant metastasis-free survival rate at 3 years (%) | Univariate analysis | Multivariate analysis | P-value |
|---|---|---|---|---|
| P value | HR (95% CI) | |||
| Age, years | 0.398 | |||
| ≤50 | 89.1 | |||
| >50 | 85.5 | |||
| Sex | 0.185 | |||
| Male | 86.8 | |||
| Female | 91.7 | |||
| T classification | 0.068 | |||
| T1–3 | 89.4 | |||
| T4 | 81.2 | |||
| N classification | 0.003 | 0.005 | ||
| N0–1 | 90.5 | 1 | ||
| N2–3 | 81.6 | 2.246 (1.276–3.955) | ||
| Serum EBV DNA, copies/mL | 0.003 | 0.004 | ||
| ≤4000 | 91.7 | 1 | ||
| >4000 | 81.9 | 2.321 (1.317–4.089) | ||
| Leukocytes, k/cc | 0.012 | |||
| ≤10 | 89.0 | |||
| >10 | 73.4 | |||
| Hemoglobin, g/L | 0.978 | |||
| ≤120 | 88.5 | |||
| >120 | 87.9 | |||
| Platelets, k/cc | 0.016 | 0.007 | ||
| ≤300 | 89.5 | 1 | ||
| >300 | 76.2 | 2.531 (1.288–4.977) | ||
| Serum albumin, g/L | 0.009 | 0.008 | ||
| ≤46 | 84.2 | 2.290 (1.244–4.214) | ||
| >46 | 92.1 | 1 | ||
| Serum alkaline phosphatase, U/L | 0.681 | |||
| ≤110 | 88.2 | |||
| >110 | 85.0 | |||
| Serum lactate dehydrogenase, U/L | ||||
| ≤245 | 87.6 | 0.222 | ||
| >245 | 100 |
Abbreviations: CCRT, concurrent chemoradiotherapy; 95% CI, 95% confidence interval; HR, hazard ratio.
Prognostic score model for the CCRT group
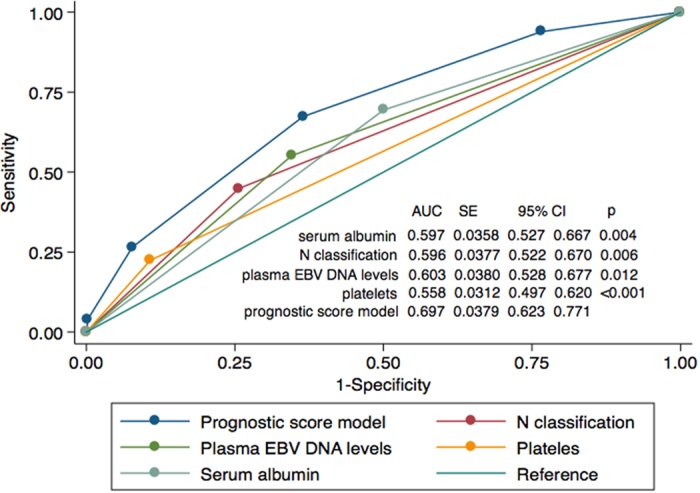
We constructed a prognostic score model for DMFS in patients with locally advanced NPC who accepted CCRT. Patients were sub-grouped by N classification, pretreatment plasma EBV DNA, serum albumin and platelet count. If a risk factor was present, a score of 1 was recorded (maximum score, 4). The ROC curves are shown in Fig. 1. The area under the curve (AUC) for the prognostic score model was 0.697; a score of 1.5 resulted in a sensitivity of 0.67 and specificity of 0.64 for DMFS.
Figure 1. Receiver-operating characteristic curves for distant metastasis in locally advanced NPC after concurrent chemotherapy (n = 411) based on the individual prognostic factors and prognostic score model.
P-values vs. prognostic score model.
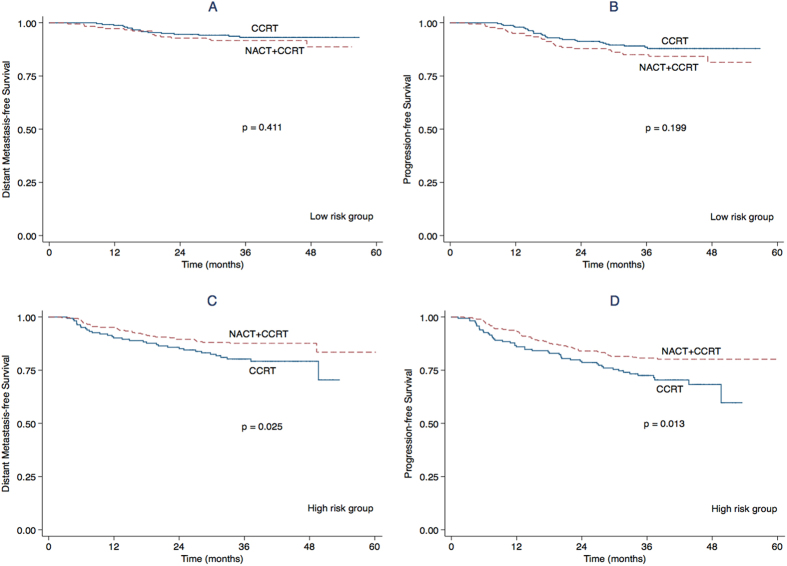
Thus, two risk stratification groups were obtained: 1) low-risk: total score 0–1 (246 patients); and 2) high-risk: total score 2–4 (165 patients). The 3-year DMFS rates for the low and high-risk groups were 93.1%, and 80.3% (P < 0.001) and the 3-year PFS rates were 88.5% and 72.5% (P < 0.001), respectively.
Stratification survival analysis
Based on the prognostic score model, we stratified all 881 patients with locally advanced NPC as low-risk and high-risk. High-risk patients who accepted NACT + CCRT had significantly better DMFS and PFS than high-risk patients who accepted CCRT (P = 0.025; P = 0.013; Fig. 2). However, NACT provided no additional survival advantage in the low-risk group or when patients were stratified by the individual prognostic factors (Table 3).
Figure 2. Kaplan-Meier distant metastasis-free survival curves and progression-free survival curves for patients with locally advanced NPC in the low-risk (A,B), and high-risk groups (C,D) stratified by the CCRT and NACT + CCRT.
Table 3. Stratification survival analysis of the CCRT group versus the NACT + CCRT group (n = 881).
| Subgroups | Distant metastasis-free survival |
Progression-free survival |
||
|---|---|---|---|---|
| Rate at 3 years (CCRT group vs. NACT + CCRT group) | P-value | Rate at 3 years (CCRT group vs. NACT + CCRT group) | P-value | |
| N classification | ||||
| N0–1 (n = 588) | 90.5 vs. 93.1 | 0.356 | 85.5 vs. 86.2 | 0.956 |
| N2–3 (n = 293) | 81.4 vs. 82.6 | 0.630 | 73.1 vs. 75.9 | 0.237 |
| Serum EBV DNA level, copies/mL | ||||
| ≤4000 (n = 445) | 91.7 vs. 94.5 | 0.281 | 85.8 vs. 87.0 | 0.802 |
| >4000 (n = 436) | 81.6 vs. 85.7 | 0.289 | 75.6 vs. 79.3 | 0.228 |
| Platelets, k/cc | ||||
| ≤300 (n = 745) | 89.5 vs. 90.2 | 0.814 | 83.1 vs. 82.8 | 0.979 |
| >300 (n = 136) | 76.2 vs. 84.7 | 0.264 | 73.9 vs. 80.1 | 0.363 |
| Serum albumin, g/L | ||||
| ≤46 (n = 366) | 84.2 vs. 89.4 | 0.070 | 77.3 vs. 82.1 | 0.158 |
| >46 (n = 515) | 92.1 vs. 88.8 | 0.197 | 87.2 vs. 82.8 | 0.322 |
| Prognostic score model | ||||
| Low risk (score 0–1, n = 427) | 93.1 vs. 91.6 | 0.411 | 88.5 vs. 85.0 | 0.199 |
| High risk (score 2–4, n = 454) | 80.3 vs. 87.6 | 0.025 | 72.5 vs. 80.7 | 0.013 |
Abbreviations: CCRT, concurrent chemoradiotherapy; NACT, neoadjuvant chemotherapy.
Multivariate analysis of the high-risk group
The covariates listed in Table 2 were included in multivariate analysis of the 454 patients in the high-risk group. Patients in the high-risk group who accepted NACT + CCRT had significantly higher DMFS and PFS (P = 0.001; P = 0.011) than high-risk patients who accepted CCRT alone (Table 4).
Table 4. Multivariate analysis of distant metastasis and tumor progression in high-risk patients with locally advanced NPC (n = 454).
| Variable | Distant metastasis-free survival |
Progression-free survival |
||
|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | |
| N classification (N2–3 vs. N0–1) | 0.001 | 2.490 (1.443–4.298) | 0.018 | 1.637 (1.087–2.465) |
| Chemotherapy (CCRT vs. NACT + CCRT) | 0.001 | 2.338 (1.408–3.881) | 0.011 | 1.657 (1.123–2.446) |
| Serum lactate dehydrogenase (>245 vs. ≤ 245 U/L) | 0.018 | 2.187 (1.141–4.191) | 0.077 | 1.702 (0.943–3.071) |
| Serum EBV DNA level (>4000 vs. ≤4000 copies/ml) | 0.009 | 2.376 (1.239–4.557) | ||
| Platelets (>300 vs. ≤ 300 k/cc) | 0.006 | 2.118 (1.245–3.604) | ||
| Serum albumin (≤46 vs. > 46 g/L) | 0.079 | 1.781 (0.935–3.392) | ||
Abbreviations: CCRT, concurrent chemoradiotherapy; NACT, neoadjuvant chemotherapy.
Discussion
Ongoing phase III trials (e.g. NCT01245959, NCT00828386, NCT01536223, NCT00201396) are examining the most effective NACT regimens to improve the relatively poor prognosis of locoregionally advanced NPC. To our knowledge, this is the first attempt to design a prognostic score model to select high-risk patients with locoregionally advanced NPC who may benefit from NACT before CCRT.
Some results of this study are consistent with previous findings and consensus. Bulky or extensive nodal disease were associated with a poorer DMFS after CCRT, indicating the need for more intensive combined primary treatments. However, in contrast to a previous study2, T classification was not prognostic for DMFS, which is reasonable given CCRT was used in this study. As concurrent chemotherapy with IMRT can achieve excellent locoregional control and OS in NPC4,5,6,7,8,9,10,11, the prognostic effect of T classification may have become less relevant; T classification is generally considered an indicator of local invasion.
High pretreatment plasma EBV DNA was validated as a prognostic factor for DMFS after CCRT in locoregionally advanced NPC. EBV and its gene products play a pathogenic role and have prognostic value in the non-keratinizing subtypes of NPC in patients from the endemic region. Numerous groups have confirmed circulating EBV DNA correlates with tumor stage, presence of residual disease or metastasis, and OS in NPC22,24,25,26. Additionally, plasma EBV DNA has prognostic value for poorer OS in stage III and IV NPC, indicating the potential of this biomarker to complement the TNM classification during treatment planning24.
Furthermore, a high pretreatment platelet count and lower serum albumin were associated with unfavorable DMFS after CCRT. These host-related factors reflect host-tumor interactions. Platelets are involved in hemostasis, angiogenesis, inflammation and wound healing, and may play a role in cancer biology by promoting primary tumor growth via facilitating angiogenesis and tumor invasion via platelet-derived microparticles or thrombin activity27. Platelets may also surround and protect circulating tumor cells from elimination by natural killer cells, and thus promote distant metastasis28. A high platelet count is an unfavorable prognostic factor in several solid tumors, including NPC20,27,28. Several studies have investigated anti-platelet therapy in cancer; nonsteroidal antiinflammatory drugs (NSAIDs) and selective inhibitors of arachidonic acid cyclooxygenase-2 (COXIBs) have been reported to effectively inhibit cancer initiation and progression29.
Serum albumin is regularly used as a biomarker of long-term nutritional status, and is also known to correlate with systemic inflammation, stabilize cell growth and DNA replication, buffer a variety of biochemical changes, and prevent development of sex hormone-induced cancers30. Associations have been reported between low serum albumin and increased disease severity, higher risk of disease progression and poorer OS in cancer30. Additionally, although most patients have normal serum albumin values at diagnosis, a lower pretreatment serum albumin to globulin level (<1.4) was associated with poorer OS in NPC31.
The major challenges in NPC are assessment of the risk of metastasis and development of preventive treatments. It has been shown inadequate to apply only the TNM staging system for treatment guidance, and use of biomarkers would probably enhance the power of clinical trials to obtain positive results. For example, the phase III trials (NCT00370890) is designed to evaluate the benefit of adjuvant chemotherapy in high-risk NPC patients, identified with detectable plasma EBV DNA six weeks after chemo-radiotherapy. However, up until now, no similar-designed study has been conducted regarding the use of NACT yet. Our prognostic score model combines several pretreatment clinical variables with the clinical implications of both tumor burden and host response. Using this model, patients could be separated into low-risk and high-risk groups with different survival outcomes and responses to NACT. Therefore, the prognostic model may complement the current clinical staging system and enable identification of patients who may benefit from more intensive therapy in addition to CCRT.
The major limitation of this study is its retrospective single-center design. However, we endeavored to control biases by striving to review all patients treated with IMRT during the study period. Nevertheless, a prospective study is necessary to validate the prognostic model.
In conclusion, the prognostic score model based on N classification, pretreatment plasma EBV DNA, platelet count and serum albumin provides a useful method of selecting patients with locoregionally advanced NPC who may benefit from more intensive treatment. Furthermore, addition of NACT to the standard CCRT regimen may provide most benefit in patients with two or more risk factors.
Additional Information
How to cite this article: Du, X.-J. et al. Neoadjuvant chemotherapy in locally advanced nasopharyngeal carcinoma: Defining high-risk patients who may benefit before concurrent chemotherapy combined with intensity-modulated radiotherapy. Sci. Rep. 5, 16664; doi: 10.1038/srep16664 (2015).
Acknowledgments
This work was supported by grants from Key Laboratory Construction Project of Guangzhou City of China (No.121800085), the Health & Medical Collaborative Innovation Project of Guangzhou City of China (201400000001), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10), the Science and Technology Project of Guangzhou City of China (No.14570006), and the Planned Science and Technology Project of Guangdong Province (No. 2013B020400004).
Footnotes
Author Contributions D.X.J. and T.L.L. conducted data analysis and drafted the manuscript. C.L., M.Y.P., G.R. and L.X. participated in data collection. S.Y., Z.M.S., K.T.B., S.J.Y. and L.A.H. participated in the design of the study and helped to perform the statistical analysis. M.J. conceived of the study, and participated in its design. All authors read and approved the final manuscript.
References
- Muir C., Waterhouse J. & Mack T. Cancer incidence in five continents. IARC Sci Publ. 5, 1–970 (1987). [PubMed] [Google Scholar]
- Chen L. et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol. 104(3), 331–7 (2012). [DOI] [PubMed] [Google Scholar]
- Yi J. L. et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten year experience of a single institution. Int J Radiat Oncol Biol Phys. 65, 161–68 (2006). [DOI] [PubMed] [Google Scholar]
- Lee N. et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 53, 12–22 (2002). [DOI] [PubMed] [Google Scholar]
- Kam M. K. et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 60, 1440–50 (2004). [DOI] [PubMed] [Google Scholar]
- Al-Sarraf M. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup Study 0099. J Clin Oncol. 16, 1310–1317 (1998). [DOI] [PubMed] [Google Scholar]
- Lin J. C. et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 21, 631–637 (2003). [DOI] [PubMed] [Google Scholar]
- Chan A. T. et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 20, 2038–2044 (2002). [DOI] [PubMed] [Google Scholar]
- Langendijk J. A., Leemans C. R., Buter J., Berkhof J. & Slotman B. J. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 22, 4604–4612 (2004). [DOI] [PubMed] [Google Scholar]
- Baujat B. et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 64, 47–56 (2006). [DOI] [PubMed] [Google Scholar]
- Wu F. et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 112(1), 106–11 (2014). [DOI] [PubMed] [Google Scholar]
- OuYang P. Y. et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 24(8), 2136–2146 (2013). [DOI] [PubMed] [Google Scholar]
- Hui E. P. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 27(2), 242–249 (2009). [DOI] [PubMed] [Google Scholar]
- Fountzilas G. et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 23(2), 427–435 (2012). [DOI] [PubMed] [Google Scholar]
- Huang P. Y. et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 48(10), 1038–1044 (2012). [DOI] [PubMed] [Google Scholar]
- Lin J. C. et al. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 350(24), 2461–2470 (2004). [DOI] [PubMed] [Google Scholar]
- Zhou G. Q. et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 82(3), e359–e365 (2012). [DOI] [PubMed] [Google Scholar]
- Li G. et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 31(4), 197–206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. et al. To build a prognostic score model containing indispensible tumour markers for metastatic nasopharyngeal carcinoma in an epidemic area. Eur J Cancer. 48(6), 882–8 (2012). [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. Y. & Xia Y. F. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol. 34(1), 39–45 (2013). [DOI] [PubMed] [Google Scholar]
- Edge S. B. & Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 17, 1471–1474 (2010). [DOI] [PubMed] [Google Scholar]
- Lo Y. M. et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 59(6), 1188–1191 (1999). [PubMed] [Google Scholar]
- Hou X. et al. Different clinical significance of pre- and post-treatment plasma Epstein-Barr virus DNA load in nasopharyngeal carcinoma treated with radiotherapy. Clin Oncol. 23(2), 128–33 (2001). [DOI] [PubMed] [Google Scholar]
- Leung S. F. et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 24(34), 5414–8 (2006). [DOI] [PubMed] [Google Scholar]
- Lin J. C. et al. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol. 19, 2607–2615 (2001). [DOI] [PubMed] [Google Scholar]
- Wang W. Y. et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer. 119(5), 963–70 (2013). [DOI] [PubMed] [Google Scholar]
- Nash G. F., Turner L. F., Scully M. F. & Kakkar A. K. Platelets and cancer. Lancet Oncol. 3(7), 425–30 (2002). [DOI] [PubMed] [Google Scholar]
- Jain S., Harris J. & Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 30(12), 2362–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter D. G. et al. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 33(1), 231–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. & Lis C. G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 9, 69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. J. et al. The Pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. Plos one. 9(4), e94437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]