Abstract
A practical protocol for the synthesis of N-arylated methyl 2-aminothiophene-3-carboxylate has been developed via Chan-Lam cross-coupling. The desired products were synthesized by cross-coupling of methyl 2-aminothiophene-3-carboxylate with both arylboronic acids and potassium aryltrifluoroborate salts in moderate to good yields. A broad range of functional groups was well tolerated.
Keywords: thiophene, cross-coupling, copper, amine, carboxylate, aryltrifluoroborate
Graphical Abstract

INTRODUCTION
Thiophene-containing compounds have attracted much attention because of their intrinsic electronic properties, which make them important in light-emitting diodes, field effect transistors, organic solar cells, and photovoltaic devices.1–5 Thiophene derivatives have also shown versatile pharmacological activities.6–14 In particular, substituted aminothiophenes, including 2-aminothiophene-3-carboxylate derivatives, appear to be of interest in medicinal chemistry and are represented in several classes of biologically active molecules.15–17
Traditionally, N-arylation of these systems was achieved through nucleophilic aromatic substitution of the amines with aryl halides, although activated substrates and strong conditions were necessary.18–20 Recently, the synthesis of biologically-active fused thiophenes employed a derivative of 2-aminothiophene 3-carboxylate in a low-yielding nucleophilic aromatic substitution.21 Additionally, a Buchwald-Hartwig approach to N-arylation has been reported to proceed with an ethyl 2-aminothiophene-3-carboxylate derivative.22 Although the use of commercially available aryl halides is advantageous, the need for expensive Pd catalyst systems, high reaction temperatures (100 °C), and inert conditions make an alternative approach to N-arylation of methyl 2-aminothiophene 3-carboxylate desirable.
Copper-mediated Chan-Lam coupling reactions23,24 provide an important entry to heteroatom arylation and heteroarylation. The development of these cross-coupling reactions has attracted much attention because the reactions can typically be conducted at room temperature in open air in the presence of stoichiometric copper salt. Because of their robust nature, the Chan-Lam coupling has been utilized in N-arylation using a variety of amines, amino acid esters, anilines, imidazoles and nitrogen heterocycles under these conditions.25 Herein, a Chan-Lam cross-coupling protocol of methyl 2-aminothiophene-3-carboxylate (1) is revealed, providing broad access to this important chemical architecture. The results constitute an effective method for this N-arylation using mild conditions, starting from both arylboronic acids and aryltrifluoroborates. The use of aryltrifluoroborates, although unprecedented in this context, is especially attractive as these reagents are known to be easy to handle, bench-stable solids with favorable physical and chemical properties.26–30
Results and discussion
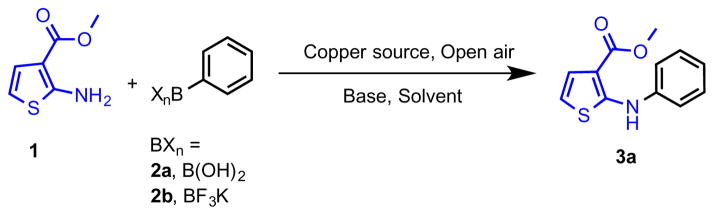
In initial screening, methyl 2-aminothiophene-3-carboxylate (1) was treated with phenylboronic acid (2a) or potassium phenyltrifluoroborate (2b) (Scheme 1) in the presence of various copper sources, bases, and solvents in an open flask.
Scheme 1.
Reaction of methyl 2-aminothiophene-3-carboxylate with phenylboronic acid and potassium phenyltrifluoroborate
Comprehensive results and all screenings employed are shown in Figures S1, S2, and S3 (Supporting Information). Various copper sources were screened, out of which Cu(OAc)2 provided product 3a with good conversion in the presence of Et3N as base in ClCH2CH2Cl at rt for 12 h. Unfortunately, attempts to drive these reactions to completion with longer reaction times (24 h) resulted in large quantities of diaryl substitution in preference to monoarylation. To minimize diaryl products, the reaction concentration was reduced to 0.03 M. Attempts to augment reactivity under either an oxygen or nitrogen atmosphere were made, however, open air was found to be most effective for obtaining good, reliable yields. With suitable conditions developed, various arylboronic acids were employed, and the scope of this coupling was evaluated (Table 1).
Table 1.
Substrate Scope of N-Arylation of Methyl 2-Aminothiophene-3-carboxylate with Arylboronic Acids
Reaction performed on 1.0 g (6.36 mmol).
The reaction proved to be relatively insensitive to the electronic nature of the boron substrates. Arylboronic acid with electron-donating substituents (e.g., 4-isopropyl-, 4-tert-butyl-, and 4-ethylbenzeneboronic acids) afforded N-arylated products 3b, 3c, and 3e, respectively, in modest to good yields. Substrates bearing electron-withdrawing moieties such as fluoro, cyano, formyl, and acetyl substituents also provided the desired products in good yields. In those instances where low yields were obtained (e.g., 3f), starting material was typically recovered. To ensure the scalability and efficiency of this coupling, a gram-scale reaction generating 3i was performed, and the desired product 3i was obtained in good yield (69%).
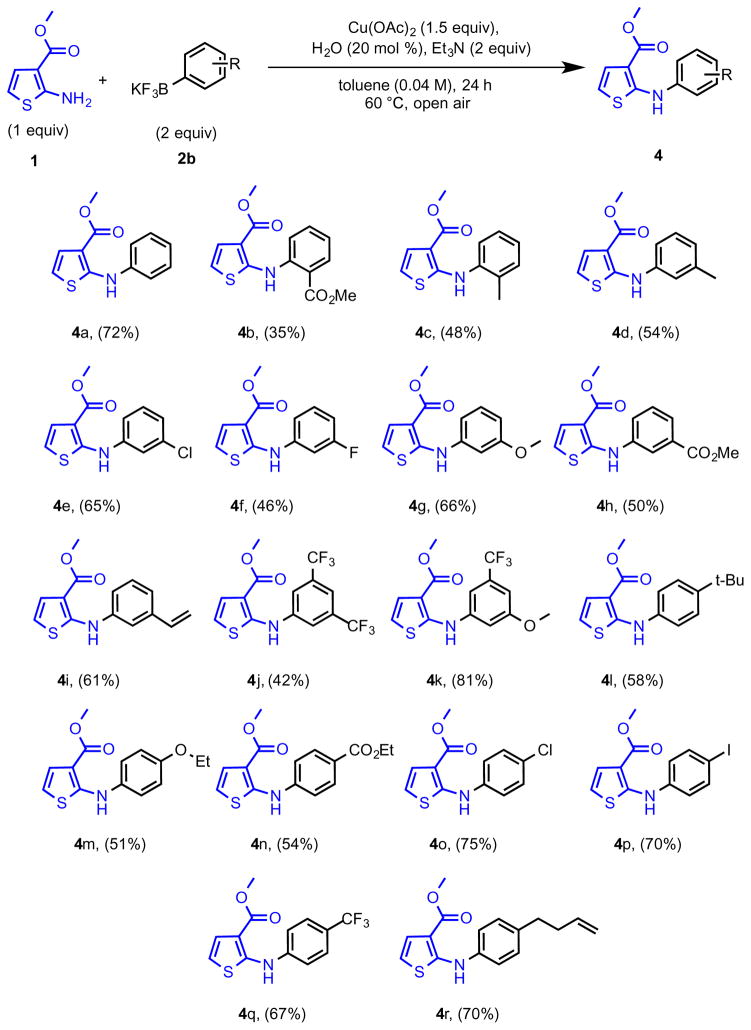
With the boronic acid scope demonstrated, the conditions developed for the coupling of 2-aminothiophene-3-carboxylate (1) were directly applied using potassium aryltrifluoroborates (2b). Unfortunately, these conditions resulted in only trace conversion. Fortunately, a slight modification of the reaction conditions identified a protocol for these substrates as well (Table S1: Supporting Information). Using the original copper source and base, switching the solvent to toluene with a few drops of water at 60 °C in open air afforded the desired product in good yield. The role of water in aryltrifluoroborate cross-couplings has been well documented.31–35 Thus, water is normally required to hydrolyze the trifluoroborates to the boronic acids, the former thus serving as a stable reservoir for the more reactive boronic acid analogues. In this manner, bench stable aryltrifluoroborates were successfully cross-coupled with 2-aminothiophene-3-carboxylate (1) (Table 2). For the substrates incorporating electron-donating groups [2-methyl (4c), 3-methyl (4d), 4-tert-butyl (4l), 4-ethoxy (4m)], modest yields were obtained under optimized conditions. The 3-carboxymethyl derivative (4h) provided a better yield as compared to the 2-carboxymethyl substrate (4b), perhaps owing to steric hindrance. The substrates containing 3-vinyl (4i) and 4-carboxyethyl (4n) groups also the afforded desired products in good yield. Other electron-withdrawing groups were also well tolerated under the reaction conditions. For example, 3-fluoro-(4f), 3,5-trifluoromethyl-(4j), 4-iodo-(4p), 4-trifluoromethyl-substituted aromatics (4q) were accessed in reasonable yields. 4-Trifluoroboratochlorobenzene provided 4o in higher yield than the 3-chloro-substituted substrate produced 4e. The 3-methoxy-5-trifluoromethyltrifluoroborate provided targeted product 4k in excellent yield. Unfortunately, the optimized reaction conditions did not extend well to heteroaryltrifluoroborates or boronic acids.
Table 2.
Substrate Scope of N-Arylation of Methyl 2-Aminothiophene-3-carboxylate with Aryltrifluoroborate
In conclusion, a practical approach to the synthesis of N-arylated aminothiophene carboxylate analogues has been developed involving mild conditions. A variety of functional groups were well tolerated using stable, commercially available aryltrifluoroborates as well as arylboronic acids and inexpensive copper acetate. Efforts toward screening the biological activities of these newly synthesized compounds are ongoing.
Supplementary Material
Acknowledgments
We are grateful to Higher Education Commission (HEC), Pakistan, for a scholarship (PIN No. 112-24510-2PS1-388) to Komal Rizwan, and the National Institute of General Medical Sciences (R01 GM-081376) for additional support. Frontier Scientific, Inc., provided the organoboron compounds used in this study. We thank Dr. Rakesh Kohli (University of Pennsylvania) for acquisition of HRMS spectra.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garnier F, Yassar A, Hajlaoui R, Horowitz G, Deloffre F, Servet B, Ries S, Alnot P. J Am Chem Soc. 1993;115:8716. [Google Scholar]
- 2.Garnier F, Hajlaoui R, Yassar A, Srivastava P. Science. 1994;265:1684. doi: 10.1126/science.265.5179.1684. [DOI] [PubMed] [Google Scholar]
- 3.Garnier F. Angew Chem Int Ed. 1989;101:529. [Google Scholar]
- 4.Dodabalapur A, Torsi L, Katz H. Science. 1995;268:270. doi: 10.1126/science.268.5208.270. [DOI] [PubMed] [Google Scholar]
- 5.Dodabalapur A, Katz HE, Torsi L. Adv Mater. 1996;8:853. [Google Scholar]
- 6.Chaudhary A, Jha K, Kumar S. J Adv Sci Res. 2012;3:3. [Google Scholar]
- 7.Mohan C, Bhargava G, Bedi PM. J Life Sci. 2009;1:97. [Google Scholar]
- 8.Sharma S, Athar F, Maurya MR, Azam A. Eur J Med Chem. 2005;40:1414. doi: 10.1016/j.ejmech.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJ, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ. J Med Chem. 2008;51:5522. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 10.Abdelhamid AO. J Heterocyclic Chem. 2009;46:680. [Google Scholar]
- 11.Laddha SS, Bhatnagar SP. Bioorg Med Chem. 2009;17:6796. doi: 10.1016/j.bmc.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Alagarsamy V, Raja Solomon V, Meenac R, Ramaseshu K, Thirumurugan K, Murugesan S. Med Chem. 2007;3:67. doi: 10.2174/157340607779317599. [DOI] [PubMed] [Google Scholar]
- 13.Wardakhan W, Abdel-Salam O, Elmegeed G. Acta Pharm. 2008;58:1. doi: 10.2478/v10007-007-0041-5. [DOI] [PubMed] [Google Scholar]
- 14.Connor DT, Sorenson RJ, Cetenko WA, Kerbleski JJ, Tinney FJ. J Med Chem. 1984;27:528. doi: 10.1021/jm00370a016. [DOI] [PubMed] [Google Scholar]
- 15.Gouda MA, Eldien HF, Girges MM, Berghot MA. Med Chem. 2013;3:228. [Google Scholar]
- 16.Castelli MP, Casu A, Casti P, Lobina C, Carai MA, Colombo G, Solinas M, Giunta D, Mugnaini C, Pasquini S, Tafi A, Brogi S, Gessa GL, Corelli F. J Pharmacol Exp Ther. 2012;340:529. doi: 10.1124/jpet.111.186460. [DOI] [PubMed] [Google Scholar]
- 17.Patch RJ, Baumann CA, Liu J, Gibbs AC, Ott H, Lattanze J, Player MR. Bioorg Med Chem Lett. 2006;16:3282. doi: 10.1016/j.bmcl.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Skerlj RT, Bastos CM, Booker ML, Kramer ML, Barker RH, Celatka CA, O’Shea TJ, Munoz B, Sidhu AB, Cortese JF, Wittlin S, Papastogiannidis P, Angulo-Barturen I, Jimenez-Diaz MB, Sybertz E. ACS Med Chem Lett. 2011;2:708. doi: 10.1021/ml200143c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornberger KR, Badiang JG, Salovich JM, Kuntz KW, Emmitte KA, Cheung M. Tetrahedron Lett. 2008;49:6348. [Google Scholar]
- 20.Gao M, Shi Z, Wang M, Zheng QH. Bioorg Med Chem Lett. 2013;23:1953. doi: 10.1016/j.bmcl.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Laing VE, Brookings DC, Carbery RJ, Simorte JG, Hutchings MC, Langham BJ, Lowe MA, Allen RA, Fetterman JR, Turner J, Meier C, Kennedy J, Merriman M. Bioorg Med Chem Lett. 2012;22:472. doi: 10.1016/j.bmcl.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 22.Yin J, Zhao MM, Huffman MA, McNamara JM. Org Lett. 2002;4:3481. doi: 10.1021/ol0265923. [DOI] [PubMed] [Google Scholar]
- 23.Lam PY, Clark CG, Saubern S, Adams J, Winters MP, Chan DM, Combs A. Tetrahedron Lett. 1998;39:2941. [Google Scholar]
- 24.Chan DMT, Monaco KL, Wang RP, Winters MP. Tetrahedron Lett. 1998;39:2933. [Google Scholar]
- 25.Qiao JX, Qiao JX, Lam PYS. Recent Advances in Chan-Lam Coupling Reaction: Copper-Promoted C-Heteroatom Bond Cross-Coupling Reactions with Boronic Acids and Derivatives. 2011:315. [Google Scholar]
- 26.Molander GA, Cavalcanti LN. J Org Chem. 2012;77:4402. doi: 10.1021/jo300551m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darses S, Genet JP. Chem Rev. 2008;108:288. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]
- 28.Doucet H. Eur J Org Chem. 2008;2008:2013. [Google Scholar]
- 29.Molander GA, Raushel J, Ellis NM. J Org Chem. 2010;75:4304. doi: 10.1021/jo1004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefani HA, Cella R, Vieira AS. Tetrahedron. 2007;63:3623. [Google Scholar]
- 31.Lennox AJ, Lloyd-Jones GC. J Am Chem Soc. 2012;134:7431. doi: 10.1021/ja300236k. [DOI] [PubMed] [Google Scholar]
- 32.Ting R, Harwig CW, Lo J, Li Y, Adam MJ, Ruth TJ, Perrin DM. J Org Chem. 2008;73:4662. doi: 10.1021/jo800681d. [DOI] [PubMed] [Google Scholar]
- 33.Molander GA, Ito T. Org lett. 2001;3:393. doi: 10.1021/ol006896u. [DOI] [PubMed] [Google Scholar]
- 34.Molander GA, Biolatto B. J Org Chem. 2003;68:4302. doi: 10.1021/jo0342368. [DOI] [PubMed] [Google Scholar]
- 35.Molander GA. J Org Chem. 2015;80:7837. doi: 10.1021/acs.joc.5b00981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.