Abstract
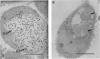
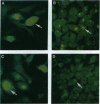
An in vitro cell culture system was used to study the effect of interferon gamma (IFN-gamma) on Chlamydia trachomatis growth and differentiation. The effect of IFN-gamma on chlamydiae was dose-dependent. IFN-gamma at 2 ng/ml completely inhibited chlamydial growth and differentiation; however, persistent infection was established when chlamydiae were cultured with IFN-gamma at 0.2 ng/ml. Persistent infection was characterized by the development of noninfectious atypical chlamydial forms from which infectious progeny could be recovered only when IFN-gamma was removed from the culture system. Analysis of persistently infected cells by immunofluorescent microscopy and immunoblotting with specific antibodies revealed that the atypical chlamydial forms had near-normal levels of the 60-kDa heat shock protein, an immunopathologic antigen, and a paucity of the major outer membrane protein, a protective antigen. Furthermore, steady-state levels of other outer membrane constituents, such as the 60-kDa cysteine-rich outer membrane protein and lipopolysaccharide, were greatly reduced. If IFN-gamma causes similar events to occur in vivo, then persistently infected cells could augment the pathogenesis of the chronic inflammatory sequelae that follow chlamydial infection by serving as depots of antigen capable of stimulating a sustained inflammatory response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Pearce J. H. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J Gen Microbiol. 1983 Jul;129(7):2001–2007. doi: 10.1099/00221287-129-7-2001. [DOI] [PubMed] [Google Scholar]
- Arno J. N., Ricker V. A., Batteiger B. E., Katz B. P., Caine V. A., Jones R. B. Interferon-gamma in endocervical secretions of women infected with Chlamydia trachomatis. J Infect Dis. 1990 Dec;162(6):1385–1389. doi: 10.1093/infdis/162.6.1385. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema M. A., Schumacher H. R., Hudson A. P. RNA-directed molecular hybridization screening: evidence for inapparent chlamydial infection. Am J Med Sci. 1991 Nov;302(5):261–268. doi: 10.1097/00000441-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Jeremias J., Ledger W. J., Witkin S. S. Interferon-gamma in the diagnosis and pathogenesis of pelvic inflammatory disease. Am J Obstet Gynecol. 1989 Jan;160(1):26–31. doi: 10.1016/0002-9378(89)90080-x. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Holland S. M., Hudson A. P., Bobo L., Whittum-Hudson J. A., Viscidi R. P., Quinn T. C., Taylor H. R. Demonstration of chlamydial RNA and DNA during a culture-negative state. Infect Immun. 1992 May;60(5):2040–2047. doi: 10.1128/iai.60.5.2040-2047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. P., McEntee C. M., Reacher M., Whittum-Hudson J. A., Taylor H. R. Inapparent ocular infection by Chlamydia trachomatis in experimental and human trachoma. Curr Eye Res. 1992 Mar;11(3):279–283. doi: 10.3109/02713689209001780. [DOI] [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. R., Weström L., Ahrons S., Ripa K. T., Svensson L., von Mecklenburg C., Henrikson H., Mårdh P. A. Chlamydia trachomatis infection of the Fallopian tubes. Histological findings in two patients. Br J Vener Dis. 1979 Dec;55(6):422–428. doi: 10.1136/sti.55.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D. L. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985 Nov-Dec;7(6):746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- Patton D. L., Kuo C. C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989 Mar;85(2):647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Moncada J., Dawson C. R., Sheppard J., Courtright P., Said M. E., Zaki S., Hafez S. F., Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988 Dec;158(6):1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M., Bailey R., Lesley A., Kajbaf M., Robertson J., Mabey D. Persisting inapparent chlamydial infection in a trachoma endemic community in The Gambia. Scand J Infect Dis Suppl. 1990;69:137–148. [PubMed] [Google Scholar]
- Whittum-Hudson J. A., Taylor H. R., Farazdaghi M., Prendergast R. A. Immunohistochemical study of the local inflammatory response to chlamydial ocular infection. Invest Ophthalmol Vis Sci. 1986 Jan;27(1):64–69. [PubMed] [Google Scholar]
- Yuan Y., Lyng K., Zhang Y. X., Rockey D. D., Morrison R. P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992 Jun;60(6):2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- de la Maza L. M., Plunkett M. J., Carlson E. J., Peterson E. M., Czarniecki C. W. Ultrastructural analysis of the anti-chlamydial activity of recombinant murine interferon-gamma. Exp Mol Pathol. 1987 Aug;47(1):13–25. doi: 10.1016/0014-4800(87)90003-7. [DOI] [PubMed] [Google Scholar]
- el-Asrar A. M., Van den Oord J. J., Geboes K., Missotten L., Emarah M. H., Desmet V. Immunopathology of trachomatous conjunctivitis. Br J Ophthalmol. 1989 Apr;73(4):276–282. doi: 10.1136/bjo.73.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]