Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- ALD
alcoholic fatty liver disease
- LPS
lipopolysaccharide
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis.
The gut microbiota, a complex and diverse ecosystem, comprises at least 1013 to 1014 microbial cells. This microbiome represents genetic material more than 100 times the size of the human genome, and is called the “metagenome”. State‐of‐the‐art metagenomic technologies have revealed that the most abundant gut bacteria are members of the phyla Bacteroidetes and Firmicutes.1 Over the past decade, the intestinal microbiota have increasingly been recognized as a critical factor in the pathogenesis of both nonalcoholic and alcoholic fatty liver disease (NAFLD and ALD, respectively) in rodents and humans. This article provides a brief overview of the gut microbiota and corresponding fecal metabolomics in NAFLD and ALD.
The Gut Microbiota and NAFLD
Using germ‐free animal models, several research groups demonstrated that mice lacking gut microbiota are resistant to diet‐induced obesity, liver steatosis, and insulin resistance.2, 3 The data from animal studies support the concept that the contribution of the gut bacteria to NAFLD is multifactorial and may occur via regulation of energy homeostasis;4 modulation of choline5 and bile acid metabolism6; and/or the ability to generate bacteria‐derived toxins, eg, lipopolysaccharides (LPS)7 (Fig. 1). Small intestine bacterial overgrowth has also been linked to NASH pathogenesis.8 Elevated representation of Escherichia, alcohol‐producing bacteria, was observed in parallel with increased blood alcohol concentration in patients with NASH, suggesting a novel mechanism for the pathogenesis of NASH: gut microbiota enriched in alcohol‐producing bacteria (eg, E. coli) constantly produce more alcohol which, in turn, is known to play an important role in the disruption of intestinal tight junctions, hepatic oxidative stress, and liver inflammation.9 The gut bacteria may facilitate progression from simple steatosis to NASH. Dysbiosis (qualitative and quantitative alterations of gut microbiota) associated with the loss of NLRP3 and NLRP6 (NOD‐like receptor family, pyrin domain containing‐3 and −6) inflammasomes resulted in increased influx of LPS and bacterial DNA to the liver; these bacterial products stimulate TLR4 and TLR9 (Toll‐like receptor 4 and 9), respectively, leading to enhanced hepatic tumor necrosis factor‐α (TNF‐α) expression which drives NASH progression.10 The gut microbiota may also contribute to hepatic fibrosis via stimulation of TLR9‐dependent profibrotic pathways in hepatic Kupffer cells.11 An exciting advance in the field is the recent observation that gut microbiota transplantation from donor mice with NAFLD replicated the phenotype in wild‐type recipients, demonstrating that NAFLD is a potentially transmissible process.12
Figure 1.

The gut microbiota and NAFLD. The gut bacteria may contribute to NAFLD via multiple mechanisms and pathways: 1) production of bacteria‐derived toxins, eg, LPS, which may activate TLR‐4‐ and TLR‐9‐mediated proinflammatory cytokine production in the liver macrophages resulting in hepatocellular inflammation; 2) regulation of energy homeostasis involving increased fermentation of carbohydrates to SCFAs, selective suppression of Fiaf, a circulating lipoprotein lipase inhibitor, facilitating de novo hepatic lipogenesis and deposition of triglycerides in adipocytes and the liver; 3) modulation of choline metabolism (which is required for very low‐density lipoprotein synthesis and hepatic lipid export); 4) modulation of bile acid homeostasis; and/or 5) generation of endogenous EtOH, which is known to play an important role in the disruption of intestinal TJs, causing hepatic oxidative stress and inducing liver inflammation. Abbreviations: ChREBP, carbohydrate‐responsive element‐binding protein; Fiaf, fasting‐induced adipocyte factor; EtOH, ethanol; LPL, lipoprotein lipase; NAFLD, nonalcoholic fatty liver disease; SCFAs, short chain fatty acids; SREBP‐1c, sterol regulatory element‐binding transcription factor 1; TJs, tight junctions; TLR‐4, Toll‐like receptor‐4; VLDL, very low‐density lipoprotein.
Intestinal Dysbiosis and ALD
The importance of the gut‐liver axis and bacteria‐derived endotoxin, LPS, is well recognized in experimental and human ALD. Alcoholic endotoxemia is a multifactorial condition, with microbial dysbiosis and impaired intestinal integrity among the causal factors (Fig. 2). Several recent elegant studies have shown that alcohol consumption is associated with alterations in the gut microbiota community, metagenome, and metabolome (small‐molecule metabolites produced by microbiota), which may contribute to the abnormal gut‐liver axis, thereby exacerbating alcohol‐induced liver injury and inflammation. Our group was the first to demonstrate in a clinical study that chronic alcohol consumption altered the gut microbiota composition, and probiotic treatment improved moderate steatohepatitis in humans with alcoholism.13 In mice, chronic ethanol feeding caused a decline in the abundance of both Bacteriodetes and Firmicutes phyla, with a proportional increase in the gram‐negative Proteobacteria and gram‐positive Actinobacteria phyla (Fig. 3); these events were associated with disruption of the intestinal barrier, endotoxemia, liver steatosis, inflammation, and injury.14
Figure 2.
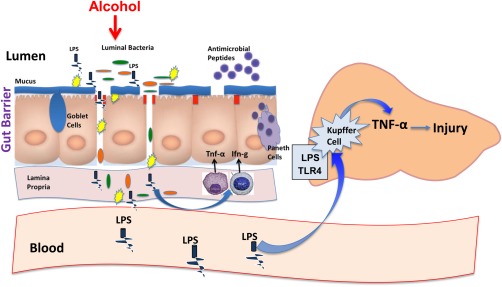
The gut‐liver axis and ALD. Alcohol consumption may cause dysbiosis and bacterial overgrowth. Intestinal bacteria alterations result in disruption of gut barrier integrity with the subsequent increase in gut permeability to bacteria‐derived pathogens, including LPS. In the liver, LPS causes hepatocellular inflammation by stimulating hepatic Kupffer cells to release proinflammatory cytokines via TLR‐4–mediated mechanisms leading to liver injury. Abbreviations: LPS, lipopolysaccharide; TLR‐4, Toll‐like receptor‐4; IFN‐γ, interferon gamma.
Figure 3.

The relative distribution of the bacterial phyla and genera in response to ethanol feeding and probiotic Lactobacillus GG (LGG) supplementation. The microbiome of the pair‐fed (PF), alcohol‐fed (AF) and alcohol+LGG fed (AF+LGG) mice are shown in the pie charts and color‐coordinated by genus and phylum. The different shades of color represent the different genera, and the common color spectrum (reds, purples, green and orange) represents the phyla. The outer ring around the pie charts also depicts the different phyla. The microbiomes of AF mice are characterized by greater abundance of Alcaligenes and Corynebacterium.
Metabolomics
There is great diversity in the metabolic actions and activity of different gut bacteria. Thus, a critical question related to liver disease is not only which bacteria are present, but what are the bacteria producing or utilizing that will impact the liver? This is not a new concept: gut‐derived toxins, such as ammonia and mercaptans, cause hepatic encephalopathy. Altering gut bacteria/bacterial metabolism with antibiotics, prebiotics (lactulose), and probiotics have all been used to treat hepatic encephalopathy. Our group and others have approached this issue using a mass spectrometry–based approach for metabolomics analysis. Metabolomics is the systematic analysis of unique chemical fingerprints from cellular processes represented by metabolite profiles that give an instantaneous snapshot of cellular physiology. Metabolomics can be performed on many types of samples ranging from feces to serum to liver tissue.15 Fecal metabolomic profiles have demonstrated certain consistent findings in liver disease, especially ALD. Abnormalities include a decrease in short‐chain fatty acids, decreased branched‐chain amino acids, altered bile acid profile, an increase in certain volatile organic compounds, and a decrease in certain long‐chain saturated fatty acids. Changes in other fecal metabolites can translate into important clinical effects. For example, the decreased short‐chain fatty acids can have an impact at multiple levels: they act as an energy source for the intestine; and butyrate (major short‐chain fatty acid) has epigenetic effects because it functions as a histone deacetylase (HDAC) inhibitor. Major decreases in amino acids are observed among the branched‐chain amino acids, and this is often reflected in a decrease in serum branched‐chain amino acids. This can have effects on muscle metabolism and on brain metabolism/function, to name only a few.
Modulation of the Gut Microbiota: New Therapeutic Strategies in the Management of NAFLD and ALD
The treatment of both NAFLD and ALD is challenging. Accumulating data suggest beneficial effects of prebiotics (nondigestible food substances that can promote growth of beneficial bacteria) and probiotics (live microorganisms that are favorable to the host) in preclinical animal and clinical human NAFLD and ALD. However, it is naive to think that one prebiotic or probiotic will treat all forms of fatty liver disease. There are many differences in the mechanisms leading to fatty liver caused by alcohol, fat, or fructose, and the microbiota changes may also be different. Further, there may be important host responses on the microbiota. Thus, ultimately there may be very individualized “microbiome therapy” based on fecal metagenome/metabolome profile, host influences, and other factors.
Potential conflict of interest: Nothing to report.
References
- 1. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022‐1023. [DOI] [PubMed] [Google Scholar]
- 2. Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet‐induced obesity in germ‐free mice. Proc Natl Acad Sci U S A 2007;104:979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabot S, Membrez M, Bruneau A, Gérard P Harach T, Moser M, et al. Germ‐free C57BL/6J mice are resistant to high‐fat‐diet‐induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010;24:4948‐4959. [DOI] [PubMed] [Google Scholar]
- 4. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101:15718‐15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A 2011;108(suppl 1):4523‐4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high‐fat‐diet‐induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007;50:2374‐2383. [DOI] [PubMed] [Google Scholar]
- 8. Ferolla SM, Armiliato, Couto CA, Ferrari TC. The role of intestinal bacteria overgrowth in obesity‐related nonalcoholic fatty liver disease. Nutrients 2014;6:5583‐5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601‐609. [DOI] [PubMed] [Google Scholar]
- 10. Henao‐Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome‐mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll‐like receptor 9 promotes steatohepatitis by induction of interleukin‐1beta in mice. Gastroenterology 2010;139:323‐34.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non‐alcoholic fatty liver disease in mice. Gut 2013;62:1787‐1794. [DOI] [PubMed] [Google Scholar]
- 13. Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol‐induced liver injury: a pilot study. Alcohol 2008;42:675‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bull‐Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi X, Wei X, Yin X, Wang Y, Zhang M, Zhao C, et al. Hepatic and fecal metabolomic analysis of the effects of Lactobacillus rhamnosus GG on alcoholic fatty liver disease in mice. J Proteome Res 2015;14:1174‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


