Highlights
-
•
The thyroid gland is a rare site of clinically detectable tumor metastasis.
-
•
Preoperative distinction between primary and secondary thyroid tumors is difficult.
-
•
RCC disseminates in an unpredictable manner and can show late recurrences.
-
•
Metastasis should be considered if history for RCC and a thyroid nodule is present.
Keywords: Thyroid metastasis, Renal cell carcinoma
Abstract
Introduction
The thyroid gland is a rare site of clinically detectable tumor metastasis. As thyroid tumors are usually assumed to be primary in origin, its recognition as a secondary is difficult.
Presentation of case
We report a case of an 80-year old female who was referred to the Department of Surgery for a symptomatic thyroid nodule. Her medical history included a radical nephrectomy for renal cell carcinoma (RCC) nine years ago. During follow-up a pancreatic nodule was noted suggestive of a neuroendocrine tumor and the von Hippel-Lindau syndrome had to be ruled out. The fine-needle aspiration biopsy (FNAB) guided by ultrasound (US) of the thyroid nodule was inconclusive and a hemithyroidectomy and isthmectomy were performed. Histological examination revealed metastasis of a clear cell carcinoma.
Discussion
RCC disseminates in an unpredictable manner and can show late recurrences. Although secondary involvement of the thyroid gland by RCC is rare, it is still one of the more common neoplasms to metastasize to this site. There are no specific clinical features and few characteristic findings of metastatic thyroid carcinoma on imaging studies. FNAB is a useful procedure to diagnose metastatic thyroid cancer, but one should remain suspicious when the result for malignant cells is negative or indeterminate. After thyroidectomy the diagnosis of RCC is confirmed immunohistochemically. There is a clear survival benefit if a surgical approach to the thyroid metastasis is chosen.
Conclusion
Thyroid metastasis should be considered in patients with a thyroid nodule and positive history for RCC.
1. Introduction
The thyroid gland is a rare site of clinically detectable tumor metastasis [1], [2], [3], [4]. As thyroid tumors are usually assumed to be primary in origin, the distinction from a secondary is a clinical challenge [1], [5], [6], [7], [8].
The case report presented below was written according to the CARE guidelines [9].
2. Presentation of case
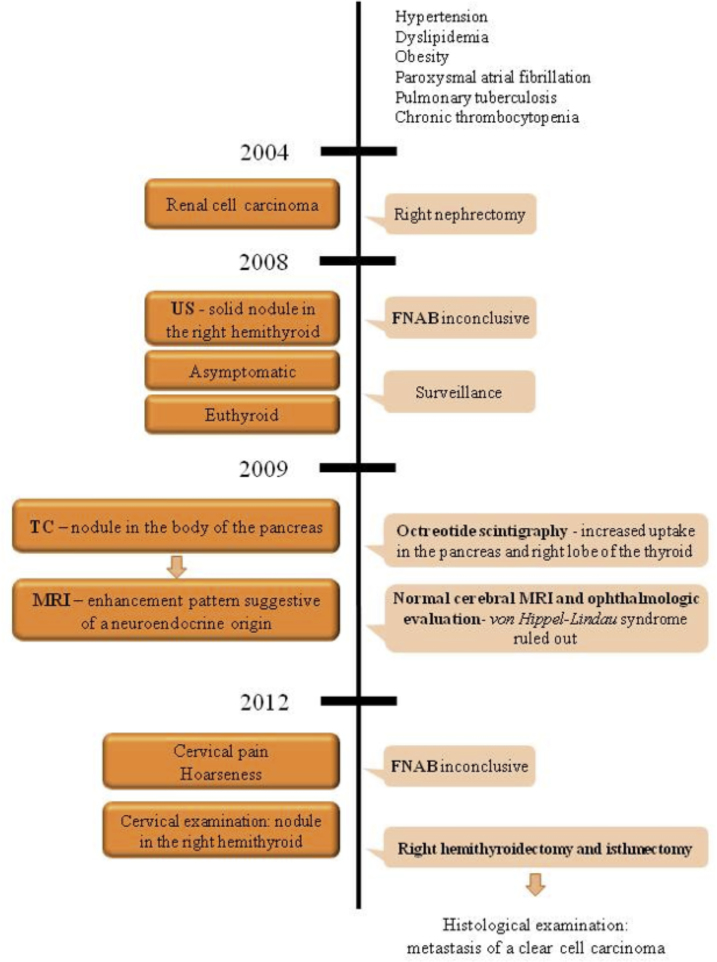
An 80-year-old white woman was referred to the Department of Surgery for a symptomatic thyroid nodule (Fig. 1). The nodule had been diagnosed four years earlier by US, which revealed a solid and well defined heterogeneous formation in the right hemithyroid, measuring 33 × 21 mm, containing an area of cystic degeneration. The patient was asymptomatic at that time, with a euthyroid status. FNAB guided by US of the thyroid nodule was performed and the cytological examination was inconclusive. Given the age of the patient, comorbidities and her asymptomatic status, surveillance was decided.
Fig. 1.
Patient medical history timeline and clinical case presentation.
Her medical history included hypertension, dyslipidemia, obesity, paroxysmal atrial fibrillation, pulmonary tuberculosis, chronic thrombocytopenia and RCC nine years ago, treated by a radical nephrectomy. The tumor removed was 5 cm in size; it was located in the upper pole of the right kidney and a clear cell carcinoma was reported, of Fuhrman nuclear grade 2. The patient’s postoperative course and subsequent recovery were uneventful. Neither postoperative chemotherapy nor interferon was proposed.
During follow-up, a computed tomography (CT) scan revealed a 20 mm vascularized nodule in the middle portion of the pancreas body (Fig. 2).
Fig. 2.
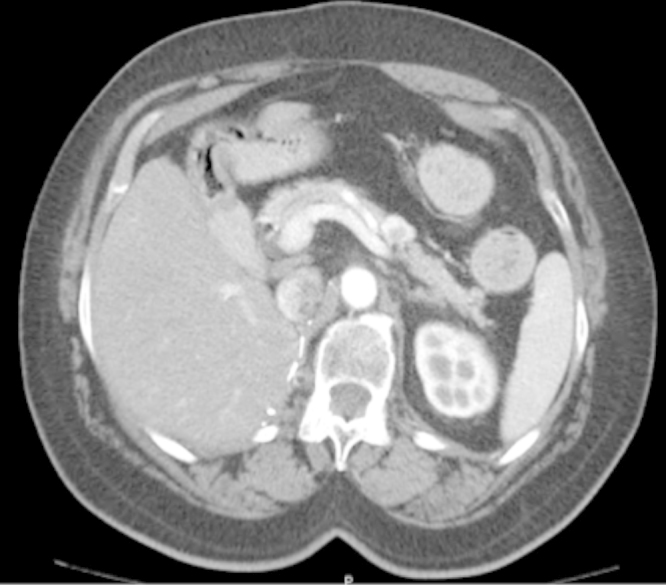
Computed tomography image of the nodule in the middle portion of the body of the pancreas.
A subsequent magnetic resonance imaging (MRI) identified a solid nodule in the transition between the pancreatic body and tail, measuring 20 × 13 mm, with an enhancement pattern suggestive of a neuroendocrine origin (Fig. 3). An octreotide scintigraphy was performed identifying a focus of anomalous hyper-expression of somatostatin receptors, with increased uptake in the pancreas and the right lobe of the thyroid. In order to rule out von Hippel-Lindau syndrome a cerebral MRI was done, as well as ophthalmologic evaluation, which were normal.
Fig. 3.
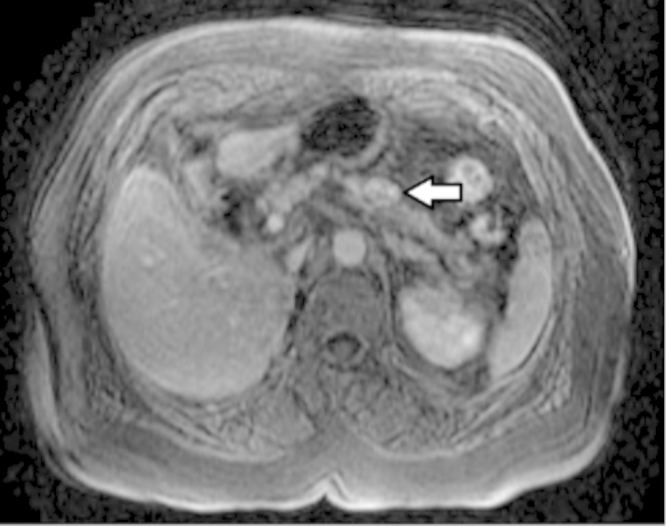
Magnetic resonance imaging showing a solid nodule in the transition between body and tail of the pancreas (arrow), with an enhancement pattern suggestive of a neuroendocrine origin.
In 2012 the patient remained euthyroid but the appearance of symptoms, such as cervical pain and hoarseness, led to a new FNAB guided by US that once again was inconclusive. Cervical examination revealed a nodule in the right lobe of the thyroid, measuring about 3 × 3 cm. Due to the symptoms, a hemithyroidectomy and isthmectomy was performed. The resection was carried out without difficulty after identifying superior and inferior right parathyroid glands and the right laryngeal recurrent nerve (Fig. 4). The postoperative course was uneventful. After 11 months, the patient was doing well and had not developed any additional metastasis. The follow-up CT-scan showed an increase in the size of the nodule in the body of the pancreas previously identified, and a new one in the head of the pancreas with similar characteristics. After the multidisciplinary team discussion no further investigation or treatment was proposed due to patient’s age and comorbidities.
Fig. 4.
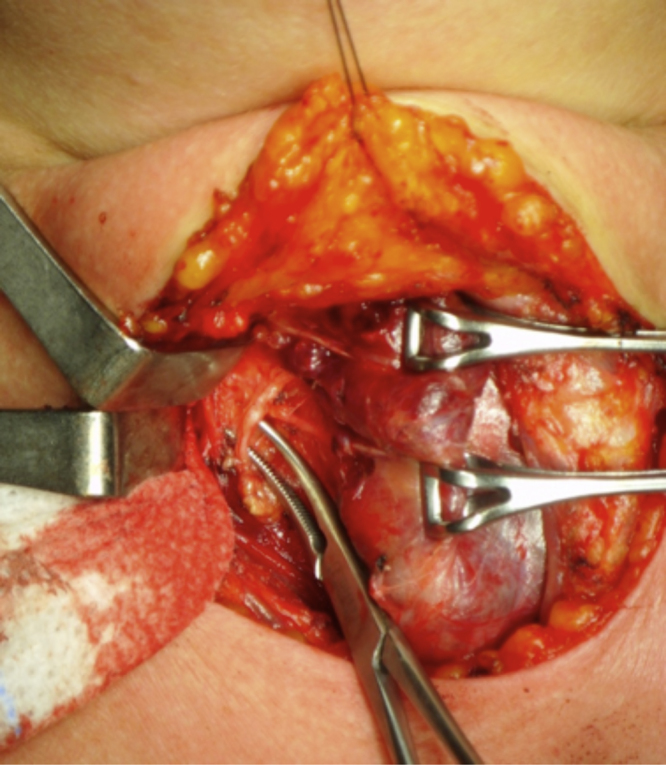
Dislocation of the right lobe of the thyroid with exposure of the right recurrent laryngeal nerve and the right inferior parathyroid.
2.1. Pathologic findings
The resected specimen weighed 26.7 g and revealed a well-defined yellowish nodule, with cystic areas, measuring 2.7 × 2.5 cm, was identified. Histological examination (Fig. 5) showed a clear cell carcinoma immunoreactive for vimentin and CD10, and negative for thyroglobulin, thyroid transcription factor-1 (TTF-1) and chromogranin, confirming the renal origin of the tumor.
Fig. 5.
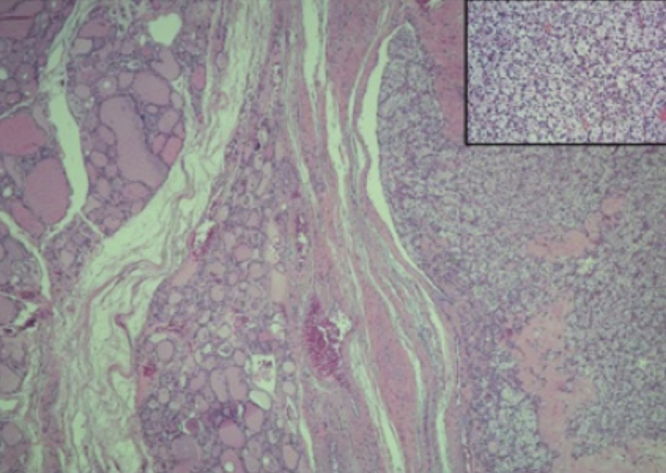
Well-demarcated nodule of metastatic RCC in the thyroid. Inset: tumor cells with clear cytoplasm and hypercromatic nuclei.
3. Discussion
RCC disseminates in an unpredictable manner and can show late recurrences [3], [5], [10], [11], [12], [13]. Although secondary involvement of the thyroid gland by RCC is rare, it is still one of the more common neoplasms to metastasize to this site. A thyroid mass can be the first clinical manifestation of RCC, even masquerading as a primary thyroid gland neoplasm [6], [11], [12], [14], [15], [16].
Despite the rich blood supply of the thyroid, it is a rare site of metastasis, which accounts for approximately 2% of thyroid malignancies [3], [16], [17]. Autopsy studies demonstrate a higher prevalence [2], [6], [18], with thyroid involvement in up to 24% of cases showing widespread malignant disease [2], [3], [4], [7], [11], [12], [13], [15], [16], [17], [19]. Most of the patients with metastatic thyroid tumor are asymptomatic at presentation [2], [17]. There are no specific clinical features and few characteristic findings of metastatic thyroid carcinoma on imaging studies such as US and CT [2], [3], [8], [13], [17]. The use of preoperative FNAB, with low morbidity and reasonable cost, has been emphasized as an effective and useful procedure for the diagnosis of metastatic thyroid cancer [2], [4], [17]. Contrary to the wide consensus that FNAB is an accurate diagnostic tool, Chung et al. reviewing the literature of metastasis reported a high false negative rate of 28.7% [15]. Thus, one should remain suspicious for metastatic disease to the thyroid gland when FNAB is negative or indeterminate for malignant cells [15].
After thyroidectomy, the diagnosis of RCC was confirmed by the positive immunohistochemical result for CD10 and vimentin [3], [14], [17] and negative for thyroglobulin, calcitonin and TTF-1 [3]. There is a clear survival benefit in surgical resection of the thyroid metastasis is chosen, with a mean 5-year survival rate of 30–60% [20]. However in general patients have a poor prognosis, since the presence of a thyroid metastasis is often the expression of widespread systemic disease [2], [4], [5], [14], [15], [17].
The possibility of von Hippel-Lindau disease (VHLD), an autosomal dominant syndrome characterized by the presence of multiple cysts and tumors was considered. The clinical presentation may be quite variable and the most common lesions include central nervous system or retinal hemangioblastoma, RCC, pheochromocytoma, pancreatic cysts or endocrine tumor, epididymal cystoadenoma or endolymphatic sac tumor [21]. In the present case, the diagnosis of pancreatic lesions suggesting a neuroendocrine nature in a patient with history of RCC motivated the investigation of other typical manifestations of VHLD. The diagnosis of VHLD in patients without family history of this syndrome, requires at least two hemangioblastomas of the central nervous system and/or the retina, or alternatively one hemangioblastoma of the central nervous system or of the retina associated with one visceral lesion [21]. Hemangioblastomas were not detected in this case. The age of presentation was not typical, nor were the clinical findings sufficient to warrant further investigation.
4. Conclusion
Preoperative distinction between primary and secondary thyroid tumors is difficult. Thyroid metastasis should be considered in patients with a thyroid nodule and positive history for RCC [6], [11], [12], [14], [15], [17]. Immunohistochemistry is essential for confirming the diagnosis [3], [14], [17]. The appearance of metastatic disease in the thyroid gland often indicates poor prognosis, because it indicates advanced disease. On the other hand, there are several reports suggesting that aggressive treatment for solitary thyroid metastasis may prolong survival [2], [4], [5], [14], [15], [17].
References
- 1.Dionigi G. Solitary intrathyroidal metastasis of renal clear cell carcinoma in a toxic substernal multinodular goiter. Thyroid Res. 2008;1(1):6. doi: 10.1186/1756-6614-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kihara M., Yokomise H., Yamauchi A. Metastasis of renal cell carcinoma to the thyroid gland 19 years after nephrectomy: a case report. Auris Nasus Larynx. 2004;31(1):95–100. doi: 10.1016/j.anl.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Medas F. Renal cell carcinoma metastasis to thyroid tumor: a case report and review of the literature. J. Med. Case Rep. 2013;7(1):265. doi: 10.1186/1752-1947-7-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakhjavani M.K. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79(3):574–578. doi: 10.1002/(sici)1097-0142(19970201)79:3<574::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.De Stefano R. Management of thyroid nodules as secondary involvement of renal cell carcinoma: case report and literature review. Anticancer Res. 2009;29(2):473–476. [PubMed] [Google Scholar]
- 6.Heffess C.S., Wenig B.M., Thompson L.D. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95(9):1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 7.Lehur P.A. Thyroid metastasis of clear-cell renal carcinoma. Can. Med. Assoc. J. 1983;128(2):154–156. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson L., Tervit G., Bloxham C. Metastatic renal cell carcinoma to the thyroid gland presenting 17 years after nephrectomy. Diagn. Histopathol. 2008;14(08):401–408. [Google Scholar]
- 9.Gagnier J., Kienle G., Altman D.G., Moher D., Sox H., Riley D.S. The CARE guidelines: consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2013;67(1):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Chin C.J. Metastasis from renal cell carcinoma to the thyroid 12 years after nephrectomy. Can. Med. Assoc. J. 2011;183(12):1398–1399. doi: 10.1503/cmaj.092152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.G. A case of metastatic renal cell carcinoma to thyroid gland. Chonnam Med. J. 2011;47(2):130–133. doi: 10.4068/cmj.2011.47.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sindoni A. Thyroid metastases from clear cell renal carcinoma 18 years after nephrectomy. Ann. Endocrinol. 2010;71(2):127–130. doi: 10.1016/j.ando.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Tunio M.A. Thyroid gland as an initial site of delayed metastasis from renal cell carcinoma: a case report. J. Solid Tumors. 2012;2:50–52. [Google Scholar]
- 14.Demir L. Metastases of renal cell carcinoma to the larynx and thyroid: two case reports on metastasis developing years after nephrectomy. Can. Urol. Assoc. J. 2012;6(5):E209–12. doi: 10.5489/cuaj.11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung A.Y. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22(3):258–268. doi: 10.1089/thy.2010.0154. [DOI] [PubMed] [Google Scholar]
- 16.Sindoni A.et al. Thyroid metastases from renal cell carcinoma: review of the literature. Sci. World J. 2010 doi: 10.1100/tsw.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo M. Thyroid metastasis of clear cell renal carcinoma: report of a case. Diagn. Cytopathol. 2009;37(10):759–762. doi: 10.1002/dc.21117. [DOI] [PubMed] [Google Scholar]
- 18.Madore P., Lan S. Solitary thyroid metastasis from clear-cell renal carcinoma. Can. Med. Assoc. J. 1975;112(6):719–721. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim T.Y. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin. Endocrinol. (Oxford) 2005;62(2):236–241. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 20.Machens A., Dralle H. Outcome after thyroid surgery for metastasis from renal cell cancer. Surgery. 2009;147(1):65–71. doi: 10.1016/j.surg.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Gouveia S. Doença de von Hippel-Lindau: da etiopatogenia ao tratamento. Rev. Port. Endocrinol., Diabetes Metab. 2012;7(02):28–35. [Google Scholar]