Abstract
Purpose
To examine the clinical, demographic, and anthropometric patient characteristics of secondary pseudotumor cerebri syndrome in children and adolescents based on the recently revised diagnostic criteria.
Design
Retrospective observational case series.
Methods
Patients seen at a tertiary children's hospital for pseudotumor cerebri syndrome were classified as having either primary idiopathic (n = 59) or secondary pseudotumor cerebri syndrome (n = 16), as rigorously defined by recently revised diagnostic criteria. Outcomes included body mass index Z-scores (BMI-Z), height and weight Z-scores, demographics, and clinical features at presentation, such as headache, sixth nerve palsy, and cerebrospinal fluid (CSF) opening pressure.
Results
In this cohort, the associated conditions and exposures seen in definite secondary pseudotumor cerebri syndrome included tetracycline-class antibiotics (n = 11), chronic kidney disease (n = 3), withdrawal from chronic glucocorticoids (n = 1), and lithium (n = 1). Other associations observed in the possible secondary pseudotumor cerebri syndrome group included Down syndrome, vitamin A derivatives, and growth hormone. In comparison with primary pseudotumor cerebri syndrome, definite secondary pseudotumor cerebri syndrome patients were on average older (15.0 vs 11.6 years; P = .003, Mann-Whitney test). According to US Centers for Disease Control (CDC) classifications, 79% of children with secondary pseudotumor cerebri syndrome were either overweight or obese (36% overweight [n = 5] and 43% obese [n = 6]), as compared to 32% nationally.
Conclusions
Even when a potential inciting exposure is identified for pediatric pseudotumor cerebri syndrome, the possible contribution of overweight and obesity should be considered.
Pseudotumor Cerebri Syndrome is an Umbrella term for the constellation of symptoms caused by elevated intracranial pressure with normal cerebrospinal fluid constituents and brain parenchyma. In adults, primary pseudotumor cerebri syndrome, also known as idiopathic intracranial hypertension, tends to affect obese female individuals of childbearing age who present with headache and papilledema. Pediatric pseudotumor cerebri syndrome shares some, but not all, features of adult pseudotumor cerebri syndrome. Both female sex and obesity are more strongly associated with pseudotumor cerebri syndrome in older, but not younger, pediatric patients.1,2 The clinical presentation of pediatric pseudotumor cerebri syndrome may also vary with age, with a greater number of younger patients presenting without symptoms, as compared to adolescents.3,4
Although many cases of pseudotumor cerebri syndrome are idiopathic (ie, primary pseudotumor cerebri syndrome), a number of medications and medical conditions have been associated with the disease (ie, secondary pseudotumor cerebri syndrome).5 Disease resolution following removal of the presumed trigger has been demonstrated for commonly reported offenders including tetracycline-related antibiotics,6 withdrawal from chronic glucocorticoid therapy,7,8 growth hormone,9 and retinoids.10 Presumed causality was further supported in several of these studies, in which rechallenge exposures resulted in recurrence of pseudotumor cerebri syndrome (eg, growth hormone and tetracycline antibiotics). Comprehensive lists of secondary pseudotumor cerebri syndrome associations have been reviewed elsewhere.5,11–16
Many of the secondary associations in pediatric pseudotumor cerebri syndrome are unique to or more prevalent in childhood, such as growth hormone supplementation9 and acne therapy involving tetracycline or retinoid medications.10,17,18 Few studies have directly compared primary and secondary pediatric pseudotumor cerebri syndrome, which limits our current understanding of how these subgroups could differ with regard to clinical presentation, treatment response, and pathophysiology. It is thus unclear whether certain demographics might predispose a child to developing secondary pseudotumor cerebri syndrome or whether the risk factors of obesity and female sex are as important for secondary pseudotumor cerebri syndrome as in primary pseudotumor cerebri syndrome.
The purposes of this study were (1) to identify “true” cases of pediatric pseudotumor cerebri syndrome through rigorous application of the recently revised diagnostic criteria, (2) to examine the secondary pseudotumor cerebri syndrome associations seen in a pediatric group, and (3) to compare the initial presentation of primary and secondary pseudotumor cerebri syndrome in children. In particular, we hoped to characterize similarities or differences between the 2 subgroups of pseudotumor cerebri syndrome that might generate new insights into the disease pathogenesis.
Methods
Study Design and Definitions
This study was a retrospective chart review. The following protocol was approved by the Institutional Review Board at The Children's Hospital of Philadelphia (IRB# 13-010158), including waivers of consent and of Health Insurance Portability and Accountability Act (HIPAA) authorization. Criteria for inclusion in this study are outlined in Figure 1. Patient charts were identified via an electronic medical record search for ICD-9 code 348.2 and a pediatric neuro-ophthalmologist's database (G.T.L.) from a tertiarycare children's hospital (The Children's Hospital of Philadelphia, diagnoses from July 1, 1993 to April 16, 2013, initial n = 363). Only patients aged 2-18 years were included in the study, as we considered the pathophysiology of any diagnosis in infancy to be distinct. Cases of pseudotumor cerebri syndrome were categorized through manual chart review using recently revised diagnostic criteria for pseudotumor cerebri syndrome.5
Figure 1.
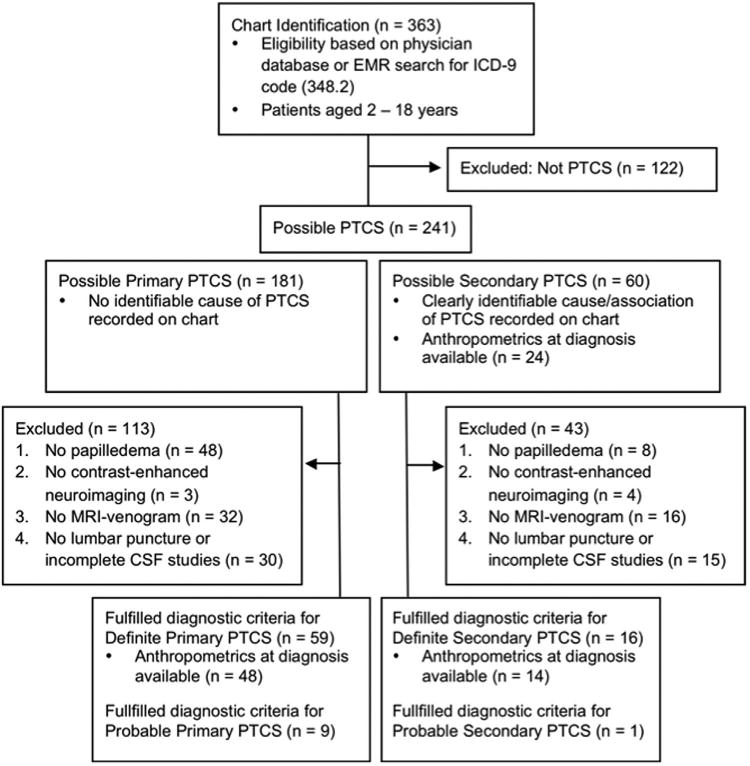
Criteria for patient inclusion in study using the recently revised diagnostic criteria for pseudotumor cerebri syndrome (PTCS). Cases that clearly did not meet the revised diagnostic criteria were considered as not PTCS and excluded from the study. The remaining cases were considered as possible PTCS, of which those cases meeting all revised diagnostic criteria were labeled as definite PTCS. Cases lacking an elevated opening pressure but otherwise meeting all criteria were labeled as probable PTCS.
Cases that clearly did not meet the revised diagnostic criteria or represented ICD-9 codes entered in error were considered as not pseudotumor cerebri syndrome; these cases were hence excluded from the study. Following their exclusion, the remaining pool of cases was defined as possiblepseudotumor cerebri syndrome. Next, the revised diagnostic criteria5 were strictly applied. Cases excluded were those that did not fulfill all of the definite pseudotumor cerebri syndrome criteria, typically because their medical records lacked necessary diagnostic studies (eg, lumbar punctures not completed, cerebrospinal fluid [CSF] results not recorded, or magnetic resonance venography [MRV] studies not done for atypical patients [ie, male patients or nonobese female patients]). Cases were excluded in the order of the criteria as numbered in Figure 1 (eg, a patient without neuroimaging was excluded before a patient lacking lumbar puncture results). Cases of “typical pseudotumor cerebri syndrome patients” (ie, obese and female) did not require an MRV study for inclusion in the definite pseudotumor cerebri syndrome pool.5 Patients fulfilling all diagnostic criteria except for the requirement of elevated opening pressure on lumbar puncture were identified as probable pseudotumor cerebri syndrome (n = 10).5 Thus, cases meeting all revised diagnostic criteria were considered to be definite pseudotumor cerebri syndrome (n = 75, of which 59 had primary pseudotumor cerebri syndrome and 16 had identifiable secondary associations).
For both possible and definite cases of pseudotumor cerebri syndrome, primary and secondary subgroups were defined. Primary pseudotumor cerebri syndrome (also known as idiopathic intracranial hypertension) was defined as cases of intracranial hypertension that had no reasonably identifiable cause (aside from obesity) and included young, thin children and patients of both sexes. Secondary pseudotumor cerebri syndrome was defined according to published lists of confirmed and suspected causes of pseudotumor cerebri syndrome or conditions associated with pseudotumor cerebri syndrome.11,13,15,16 If an obese adolescent female had been exposed to tetracycline antibiotics within 3 months preceding the onset of pseudotumor cerebri syndrome symptoms, we defined this as secondary pseudotumor cerebri syndrome attributable to tetracycline. We chose this 3-month exposure window because the average duration of antibiotic treatment prior to pseudotumor cerebri syndrome diagnosis was approximately 3 months in previous studies,6,19 and to strengthen the temporal likelihood of exposure-disease association. If the patient had a chronic medical condition (eg, chronic kidney disease or Down syndrome) with published associations to pseudotumor cerebri syndrome, and that condition was present at the time of pseudotumor cerebri syndrome diagnosis, then we defined that case as secondary pseudotumor cerebri syndrome associated with that condition.20,21 The various conditions associated with secondary pseudotumor cerebri syndrome were identified for the group with definite pseudotumor cerebri syndrome (n = 16), as well as for a larger cohort of possible secondary pseudotumor cerebri syndrome (n = 60) that included the definite pseudotumor cerebri syndrome cases as well as cases that did not have complete chart evidence to fulfill the revised diagnostic criteria. For patients with 2 or more potential secondary causes of or associations with pseudotumor cerebri syndrome, manual chart review and reassessment by 1 of the authors (G.T.L.) were employed to determine the most likely primary cause or association based on proximity of exposure and magnitude of the expected effect. For example, if a patient had a remote history of retinoid use but developed pseudotumor cerebri syndrome less than 3 months after starting tetracycline, the secondary pseudotumor cerebri syndrome was attributed to the antibiotic.
Patient Characteristics of Interest, Data Management, and Data Analysis
For the definite cases of pseudotumor cerebri syndrome, we noted age, height, and weight measurements from the electronic medical record at the time of pseudotumor cerebri syndrome diagnosis (defined as within 3 months of the date of initial diagnosis), when these data were available. Anthropometric Z-scores for body mass index (BMI-Z) were calculated from these data according to the national pediatric dataset published by the US Centers for Disease Control (CDC). Overweight and obese were defined according to the CDC classifications of overweight (BMI-Z ≥1.04 and <1.64) and obese (BMI-Z ≥1.64) in children. BMI-Z scores were used to quantify degree of obesity since, as a continuous measure, these values provide more information than prior categorical designations of normal weight, overweight, and obese. Other assessment parameters collected from the time of initial presentation included sex, population ancestry, CSF opening pressure, Snellen visual acuity, and the presence of various clinical features (headache, visual symptoms, sixth cranial nerve palsy). Study data were collected and managed securely using HIPAA-compliant REDCap (Research Electronic Data Capture) tools23 hosted at The Children's Hospital of Philadelphia.
Statistical comparisons between the primary and secondary pseudotumor cerebri syndrome groups used either Mann-Whitney tests or Fisher exact tests, as indicated in the legends in Tables 1 and 2. Statistical analysis of the secondary pseudotumor cerebri syndrome group used the 1-sample Wilcoxon signed rank test with the theoretical median Z-score equaling zero (ie, in comparison with a theoretical average Z-score of zero) or with the theoretical median Z-score equaling published national averages as referenced in the text. Additionally, a 1-proportion test was performed comparing the frequency of overweight and/or obese children in this study to the null value of the reported national frequency in all children, as referenced in the text. Standard deviations (SD) and interquartile intervals (IQI) were calculated, as appropriate.
Table 1. Patient Characteristics of Definite Secondary Pseudotumor Cerebri Syndrome in Children at Presentation.
| Presumed Secondary Cause of PTCS (n) | Mean Age (y) ± SD | Sex (% Female) | Median BMI-Z [IQI] (n) | Clinical Features | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sixth Nerve Palsy | Headache (n) | Blurry Vision, Double Vision, or Visual Obscurations (n) | Median CSF Opening Pressure, mm CSF [IQI] (n) | ||||
| Antibiotics (11) | 15.5 ± 2.0 | 82% | 1.58 [1.29-1.87] (9) | 36% | 82% (9) | 82% (9) | 430 [345-550] (11) |
| Chronic kidney disease (3) | 12.8 ± 5.2 | 67% | 1.42 [1.10-1.46] (3) | 0% | 100% (3) | 33% (1) | 360 [343-425] (3) |
| Withdrawal from chronic glucocorticoids (1) | 17.5 | 100% | 1.73 | No | Yes | No | 294 |
| Lithium (1) | 14.0 | 100% | 2.46 | No | No | Yes | 280 |
BMI-Z = body mass index Z-score; CSF = cerebrospinal fluid; IQI = interquartile interval; PTCS = pseudotumor cerebri syndrome.
Table 2. Patient Characteristics of Definite Secondary Pseudotumor Cerebri Syndrome vs Primary Pseudotumor Cerebri Syndrome in Children at Presentation.
| Characteristic at Time of Diagnosis of PTCS | Secondary PTCS (All Causes Considered Together) | Primary PTCS | P Valuea |
|---|---|---|---|
| Mean age (y) ± SD (n) | 15.0 ± 2.8 (16) | 11.6 ± 4.3 (59) | .003 |
| Sex, % female (n) | 81% (13) | 66% (39) | .36 |
| Population ancestry, self-reported (% for each group) | 31% African-American, 69% white | 22% African-American, 71% white, 7% unknown or not reported | - |
| Median BMI-Z [IQI] | 1.54 [1.18-2.02] | 1.58 [0.76-2.09] | .79 |
| Median height-Z [IQI] | -0.38 [−1.03 to 1.13] | 0.63 [−0.12 to 1.31] | .06 |
| Median weight-Z [IQI] | 1.40 [1.06-2.02] | 1.68 [0.91-2.19] | .65 |
| Sixth nerve palsy (n) | 31% (5) | 34% (20) | 1.00 |
| Median CSF opening pressure, mmCSF[IQI] | 365 [306-535] | 390 [355-475] | .70 |
| Presence of headache (n) | 81% (13) | 75% (44) | .44 |
| Presence of visual symptoms (n) | 69% (11) | 54% (32) | 1.00 |
| Visual acuity: % patients with 20/20 or better in both eyesb (n) | 50% (8) | 34% (19) | .24 |
| Visual acuity: % patients with 20/50 or worse in either eyeb (n) | 19% (3) | 9% (5) | .36 |
| Asymptomatic (incidental papilledema found on ophthalmologic examination) (n) | 0% | 12% (7) | .33 |
BMI-Z = body mass index Z-score; CSF = cerebrospinal fluid; IQI = interquartile interval; PTCS = pseudotumor cerebri syndrome.
P values for age, BMI-Z, height-Z, weight-Z, and CSF opening pressure as calculated from Mann-Whitney test. P values for sex, sixth nerve palsy, headache, visual symptoms, and asymptomatic patients as calculated from Fisher exact test.
Best-corrected visual acuity when available, otherwise uncorrected visual acuity.
Results
Of 363 Patients seen for Presumed Pseudotumor Cerebri syndrome at our hospital, 60 patients had possible secondary pseudotumor cerebri syndrome. As shown in Figure 2 (Top), the secondary associations from most to least common were tetracycline-class antibiotics (52%, n=31), Down syndrome (12%, n = 7), retinoids and other vitamin A derivatives (10%, n = 6), growth hormone (8%, n = 5), chronic kidney disease (7%, n = 4), withdrawal from chronic glucocorticoids (5%, n = 3), lithium (3%, n = 2), cerebral venous sinus thrombosis (2%, n = 1), and anemia (2%, n = 1).
Figure 2.
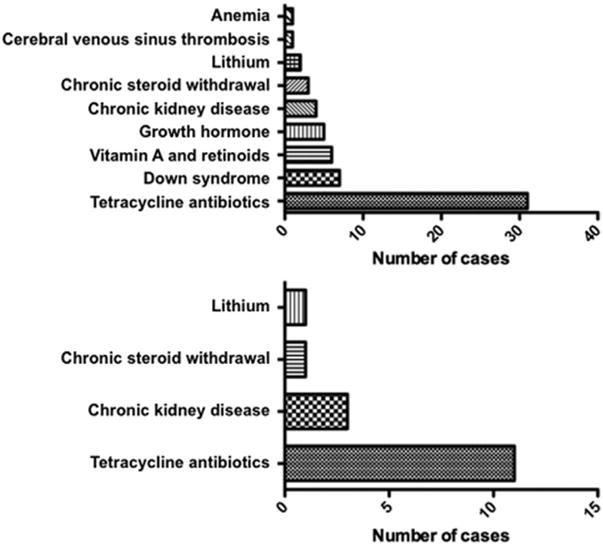
Associated conditions and causes of secondary pseudotumor cerebri syndrome (PTCS) in children. (Top) For all possible cases of secondary PTCS (including those that did not have complete chart evidence fulfilling the revised diagnostic criteria; n = 60). (Bottom) For cases of definite secondary PTCS (ie, fulfilling revised diagnostic criteria; n = 16).
A total of 75 patients had adequate chart documentation to establish a diagnosis of definite pseudotumor cerebri syndrome based on the revised diagnostic criteria.5 These patients ranged in age from 3 to 18 years (mean 12.3 years [SD = 4.2]) and were mostly female (69%, n = 52). Fifty-nine patients did not have an identifiable etiology for their disease and were defined as having primary pseudotumor cerebri syndrome (79% of definite pseudotumor cerebri syndrome cases), whereas 16 patients had secondary pseudotumor cerebri syndrome (21% of definite pseudotumor cerebri syndrome cases). In this select cohort of definite secondary pseudotumor cerebri syndrome, the most common attributable cause of pseudotumor cerebri syndrome was tetracycline-class antibiotics (69%, n = 11), which was similar to the larger group that did not have complete chart evidence to fulfill the revised diagnostic criteria (see above). The indication for antibiotics was most frequently for acne or skin-related infections (n = 5 for acne or skin-related conditions, 2 for Lyme, 4 for unknown indications). Minocycline was the most common antibiotic (n = 8 for minocycline, 2 for doxycycline, 1 for tetracycline). The average duration of antibiotic treatment before pseudotumor cerebri syndrome symptom onset was between 1 and 2 months. Other secondary associations seen in the group of definite secondary pseudotumor cerebri syndrome included chronic kidney disease (19%, n = 3), withdrawal from chronic glucocorticoids (6%, n = 1), and lithium (6%, n = 1) (Figure 2, Bottom). Patient characteristics of the children with definite secondary pseudotumor cerebri syndrome at initial presentation are presented in Table 1.
As outlined in Table 2, children with definite secondary pseudotumor cerebri syndrome, which was mostly attributable to tetracycline use, were typically older adolescents and female. The secondary pseudotumor cerebri syndrome group was significantly overweight with a median BMI-Z of 1.54 (definite secondary pseudotumor cerebri syndrome vs theoretical average BMI-Z score of zero, P = .0006; definite secondary pseudotumor cerebri syndrome vs 1 published estimate of the national average BMI-Z score for a similar age range24 of 0.44, P = .005) and mean BMI-Z of 1.53. According to the CDC classifications of overweight and obese in children, 79% of children with definite secondary pseudotumor cerebri syndrome were either overweight or obese (n = 11), including 43% who were obese (n = 6). These percentages differ significantly from a recent national survey,25 which reported 32% of all children aged 2-19 years as overweight or obese (P = .0002), including 17% as obese (P = .0094). The median height-Z of —0.38 for definite secondary pseudotumor cerebri syndrome was not statistically significantly different from the theoretical average or a reported national average of 0.2.24 Of note, the median BMI-Z did not change when the analysis was expanded from the definite secondary pseudotumor cerebri syndrome cases to all possible secondary pseudotumor cerebri syndrome cases (median BMI-Z [IQI]: definite secondary pseudotumor cerebri syndrome, 1.54 [1.18-2.02]; possible secondary pseudotumor cerebri syndrome, 1.54 [0.80-1.95]). The prevalence of overweight and obese children was also similar between these 2 groups (possible secondary pseudotumor cerebri syndrome: 71% overweight or obese [n = 17] including 46% obese [n = 11], vs definite secondary pseudotumor cerebri syndrome: numbers as above).
Secondary vs primary definite pseudotumor cerebri syndrome were compared with regard to patient demographics, anthropometrics, and clinical characteristics (Table 2 and Figure 3). As noted earlier, tetracycline use accounted for the majority of secondary cases. In this study population, children with definite secondary pseudotumor cerebri syndrome were on average older (15.0 vs 11.6 years; P = .003, Mann-Whitney test) and also tended to be female (81% vs 66%; P = 0.36, Fisher exact test) compared to children with definite primary pseudotumor cerebri syndrome. We found that median BMI-Z, weight-Z, and CSF opening pressures were similar between the 2 groups. Secondary pseudotumor cerebri syndrome children tended to be short for age while the primary pseudotumor cerebri syndrome children were normal to tall for age, but this difference did not reach statistical significance (median height-Z: —0.38 for secondary vs 0.63 for primary pseudotumor cerebri syndrome, P = .06). Clinical presentations were also similar. The ratios of patients with a sixth nerve palsy or symptomatic headache at presentation were similar between the primary and secondary pseudotumor cerebri syndrome groups. Half of the secondary pseudotumor cerebri syndrome patients and a third of the primary pseudotumor cerebri syndrome patients had 20/20 Snellen visual acuity at presentation. While a small fraction (12%) of the primary pseudotumor cerebri syndrome patients were asymptomatic at diagnosis, for whom papilledema was incidentally detected on routine ophthalmologic examination, none of the secondary pseudotumor cerebri syndrome patients were asymptomatic.
Figure 3.
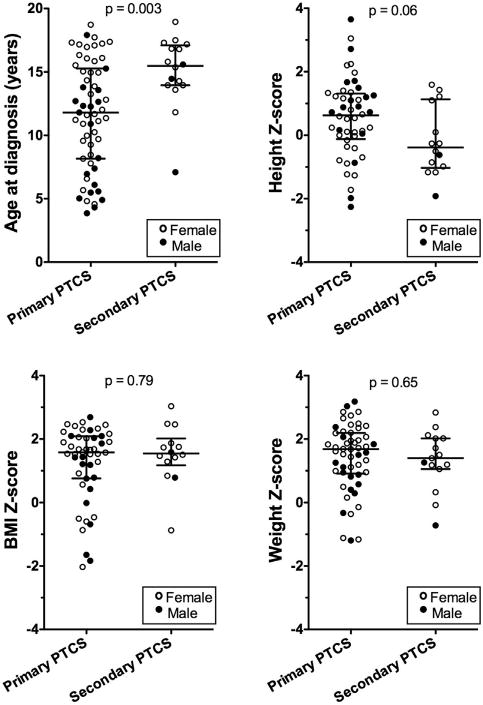
Age and anthropometrics in definite primary and secondary pseudotumor cerebri syndrome (PTCS) at initial presentation. Age (Top left) and Z-score data for height (Top right), weight (Bottom right), and body mass index (BMI) (Bottom left) are plotted with median and interquartile intervals. P values as calculated from Mann-Whitney test for definite primary PTCS vs definite secondary PTCS.
Discussion
Various Etiologies For Pseudotumor Cerebri syndrome have been proposed since the first recognition of the condition. Despite a number of reviews and studies that discuss secondary pseudotumor cerebri syndrome associations, to our knowledge none have systematically compared primary pseudotumor cerebri syndrome with secondary pseudotumor cerebri syndrome in children. The majority of studies on pediatric pseudotumor cerebri syndrome have been case reports or series comparing clinical data across different age groups, weight classes, or sets of symptoms. Furthermore, the diagnostic criteria for pseudotumor cerebri syndrome were recently revised5 with the goal of improving the accuracy of establishing a diagnosis. In this study, children with pseudotumor cerebri syndrome were classified as having primary pseudotumor cerebri syndrome or secondary pseudotumor cerebri syndrome and were then studied via comparative analysis. To our knowledge, this study began with the largest consideration of suspected cases of pediatric pseudotumor cerebri syndrome (n = 363).
After defining our groups of primary and secondary pediatric pseudotumor cerebri syndrome, we demonstrate that children with secondary pseudotumor cerebri syndrome, particularly in those cases attributable to tetracycline use, are typically overweight or obese at initial presentation. In contrast, prior studies have suggested that children of normal weight are more likely than overweight children to have secondary pseudotumor cerebri syndrome associations,26 and similarly only a minority of children with minocycline-induced pseudotumor cerebri syndrome were obese at diagnosis in another study.6 Our observation that children with secondary and primary pseudotumor cerebri syndrome are similarly overweight may perhaps reflect how antibiotics or other stimuli could serve as inciting factors for developing pseudotumor cerebri syndrome in a child already predisposed to the disease by being overweight. For example, 1 study in predominantly overweight or obese adults demonstrated that hyperandrogenism was associated with an earlier age of onset of pseudotumor cerebri syndrome.27 Another recent study suggested that adipose tissue–driven mineralocorticoid activity may increase CSF secretion,28 which could represent a common pathogenetic mechanism for a number of described secondary pseudotumor cerebri syndrome associations as well as for obesity. Although our post hoc analysis was not sufficiently powered to see whether the primary and secondary pseudotumor cerebri syndrome groups were statistically identical in terms of BMI-Z scores, to our knowledge there has not been another study collating the experience of secondary pseudotumor cerebri syndrome children as compared with primary pseudotumor cerebri syndrome cases.
Otherwise, the initial clinical presentation was remarkably similar between primary and secondary pseudotumor cerebri syndrome. Similarities included the incidence of 20/20 vision or sixth cranial nerve palsy at presentation, presence of headache, and CSF opening pressures. Although there were several asymptomatic cases of primary pseudotumor cerebri syndrome and no asymptomatic cases of secondary pseudotumor cerebri syndrome at presentation, this difference did not reach statistical significance. Sixth nerve palsy is an infrequent abnormality in adult pseudotumor cerebri syndrome29 but is seen in 8%-60% of pediatric pseudotumor cerebri syndrome cases.4,30 In summary, based on the characteristics studied here, secondary pseudotumor cerebri syndrome patients are generally not more symptomatic than primary pseudotumor cerebri syndrome patients at presentation.
Since the recently updated pseudotumor cerebri syndrome diagnostic criteria require an MRV for all patients except obese female patients in order to satisfy the diagnosis of definite pseudotumor cerebri syndrome, our definite pseudotumor cerebri syndrome groups might have been biased toward heavier patients. However, this notion is countered by the observation that both the median BMI-Z and the relative prevalence of overweight and obesity are similar between our larger group of possible secondary pseudotumor cerebri syndrome cases and the definite secondary pseudotumor cerebri syndrome group (ie, including those girls in whom obesity eliminated the need for MRV to satisfy “definite pseudotumor cerebri syndrome” diagnostic criteria). Thus our results suggest that a possible bias toward heavier female individuals in the definite pseudotumor cerebri syndrome criteria is not sufficient to explain the significant overweight of these children.
Tetracycline-class antibiotics represented the major category (69%) of definite secondary pseudotumor cerebri syndrome. When primary and secondary pseudotumor cerebri syndrome cases were combined, as in most previous studies, tetracycline antibiotics accounted for 15% of all definite pseudotumor cerebri syndrome cases. This latter figure is consistent with published estimates of 4%-43%.2,26,30–32 Interestingly, the upper estimate of tetracycline associations was noted in a study where the majority of cases were over age 11 years,26 whereas the lower end was observed in a study with an upper age limit of 13 years.31 This trend might reflect either a propensity for older, adolescent children to develop pseudotumor cerebri syndrome or/ and increased use of tetracyclines by this older group that is in part attributable to onset of pubertal acne. In fact, female sex and obesity may serve as risk factors that predispose children to developing pseudotumor cerebri syndrome when they are treated with tetracyclines.18 One might posit that overweight or obese adolescents with associated hyperandrogenemia experience more acne as a result and may thus be more likely to use antibiotic or retinoid medications to treat the acne—hence compounding their risk factors for developing pseudotumor cerebri syndrome. On a related note, many medications associated with pseudotumor cerebri syndrome also induce obesity, such as chronic steroid therapy, lithium, and oral contraceptives, among others. In these cases, it would not be feasible to distinguish whether the weight gain effect of these drugs or the drugs themselves was the major driver of the secondary pseudotumor cerebri syndrome. We had hoped to examine the effect of weight gain on developing secondary pseudotumor cerebri syndrome; unfortunately, our study group did not have a sufficient number of prior measurements to draw meaningful conclusions about weight gain prior to diagnosis. In future studies, change in BMI-Z score as seen over time in the electronic medical record may be a useful metric for investigating the relationship of weight change and incident pseudotumor cerebri syndrome.
Of note, no patients were rechallenged with tetracyclines or other presumed pseudotumor cerebri syndrome triggers after their disease resolution in order to verify causality, as was done elsewhere.11 Hence it is possible that we overdiagnosed secondary pseudotumor cerebri syndrome in patients who may have had primary pseudotumor cerebri syndrome and coincidental risk factors for secondary pseudotumor cerebri syndrome. Published reports suggest that 53%-78% of pediatric pseudotumor cerebri syndrome patients have underlying illnesses or exposures associated with the diagnosis, although it should be noted that the higher figure included obesity as an underlying condition.15,33 Here, we find that only 21% of children with definite pseudotumor cerebri syndrome have identifiable secondary causes or associations (excluding overweight and obesity), suggesting that we did not overestimate these secondary associations.
This study is limited in part by its retrospective design, incomplete medical record documentation leading to fewer patients meeting the revised diagnostic criteria, and similarly limited electronic documentation of height and weight, which may have biased our anthropometric measurements toward those patients receiving much or all of their medical care through our hospital network. It should also be noted that there were several cases of papilledema secondary to cerebral venous sinus thrombosis that were not coded as pseudotumor cerebri syndrome at our institution prior to the publication of the revised diagnostic criteria5; hence this secondary cause is likely more prevalent here than is reported by this study. Some of the common secondary associations seen in our larger, suspected pseudotumor cerebri syndrome cohort were not seen in the definite pseudotumor cerebri syndrome group, including vitamin A derivatives and growth hormone. These associations were not represented in our definite pseudotumor cerebri syndrome group because these patients had incomplete medical records or testing to confirm the diagnosis retrospectively. Nevertheless, they highlight important secondary associations particular to the pediatric population. Since the majority of our secondary pseudotumor cerebri cases were related to tetracycline use, it is not possible to generalize our conclusions to patients with secondary pseudotumor cerebri syndrome from these and other etiologies not observed in this study. Future prospective studies that clearly define secondary causes and associations may better capture the spectrum of secondary pseudotumor cerebri syndrome and refine the generalizability of our observations.
This study also used a single tertiary referral base for pediatric neuro-ophthalmology, which may be a source of referral bias or selection bias for patients with more severe disease and/or comorbidities. Since one purpose of this study was to employ a more restrictive definition of pseudotumor cerebri syndrome, we did not identify patients without papilledema who may in fact have had pseudotumor cerebri syndrome without papilledema. In addition, our secondary pseudotumor cerebri syndrome group may have missed children who did not undergo comprehensive medical evaluation at diagnosis to look for causes of secondary pseudotumor cerebri syndrome. The secondary pseudotumor cerebri syndrome group was older on average than the primary pseudotumor cerebri syndrome group, so it is possible that the slight differences in clinical profile and anthropometrics were influenced by the known age-related differences in clinical presentation of pseudotumor cerebri syndrome (ie, younger children with pseudotumor cerebri syndrome are more likely than older patients to present asymptomatically, be of normal weight, and be of male sex). In spite of these limitations, this report corroborates prior studies on pseudotumor cerebri syndrome on several topics, including the increased risk for pseudotumor cerebri syndrome conferred from obesity and female sex in older patients and the types of secondary pseudotumor cerebri syndrome associations seen in children. We also quantify for the first time the various similarities in demographics and clinical presentation between primary and secondary pediatric pseudotumor cerebri syndrome, including the importance of overweight and obesity in secondary pseudotumor cerebri syndrome related to tetracycline use.
A full history of possible exposures and related medical conditions should always be taken when pseudotumor cerebri syndrome is considered in the differential diagnosis in both thin and overweight children alike. A comprehensive, history- and examination-driven diagnostic evaluation is also advisable to identify any potentially treatable underlying conditions. However, even when a potential inciting exposure is identified, the possible contribution of overweight and obesity should be considered as well. Finally, the diagnosis of either primary or secondary pseudotumor cerebri syndrome in an overweight or obese child represents an opportunity to recommend intensive lifestyle modification; thus, efforts should be made to document weight status in all pediatric and adolescent patients with this complaint.
Grace L. Paley, MD, PhD, attended medical and graduate school at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, PA. She is currently completing a transitional internship at Mercy Hospital St. Louis and then will begin ophthalmology residency at Washington University in St. Louis, MO. Dr Paley is a founding member of the Pediatric Pseudotumor Cerebri Syndrome Collaborative Group, a multidisciplinary clinic and research group at The Children's Hospital of Philadelphia.
Acknowledgments
E.K.B. acknowledges grant support (5UL1TR000003-08) from the Clinical and Translational Science Award program at the National Institutes of Health (Bethesda, Maryland); G.T.L. acknowledges previous consultancy for Ipsen (Cambridge, Massachusetts), academic lecture fees (University of California, Los Angeles), and book royalties from Elsevier (London, UK) for co-authoring the textbook “Neuro-Ophthalmology: Diagnosis and Management”; S.E.M. acknowledges grant support (5-K12-DK-94723-2 [PI: Steven Willi]) from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, Maryland). Contributions of authors: design of study (G.L.P., C.A.S., S.E.M., G.T.L.); data collection and management (G.L.P., C.A.S., E.K.B., M.R.C.); data analysis (G.L.P., C.A.S., S.E.M.); data interpretation (G.L.P., C.A.S., S.E.M., G.T.L.); manuscript preparation (G.L.P.); manuscript review and approval (all authors); responsibility for the integrity of the study and manuscript (all authors).
Footnotes
All authors have completed and submitted the icmje form for disclosure of potential conflicts of interest.
References
- 1.Balcer LJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52(4):870–872. doi: 10.1212/wnl.52.4.870. [DOI] [PubMed] [Google Scholar]
- 2.Gordon K. Pediatric pseudotumor cerebri: descriptive epidemiology. Can J Neurol Sci. 1997;24(3):219–221. doi: 10.1017/s031716710002182x. [DOI] [PubMed] [Google Scholar]
- 3.Bassan H, Berkner L, Stolovitch C, Kesler A. Asymptomatic idiopathic intracranial hypertension in children. Acta Neurol Scand. 2008;118(4):251–255. doi: 10.1111/j.1600-0404.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 4.Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999;127(2):178–182. doi: 10.1016/s0002-9394(98)00386-9. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 6.Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126(1):116–121. doi: 10.1016/s0002-9394(98)00063-4. [DOI] [PubMed] [Google Scholar]
- 7.Levine A, Watemberg N, Hager H, et al. Benign intracranial hypertension associated with budesonide treatment in children with Crohn's disease. J Child Neurol. 2001;16(6):458–461. doi: 10.1177/088307380101600617. [DOI] [PubMed] [Google Scholar]
- 8.Liu GT, Kay MD, Bienfang DC, Schatz NJ. Pseudotumor cerebri associated with corticosteroid withdrawal in inflammatory bowel disease. Am J Ophthalmol. 1994;117(3):352–357. doi: 10.1016/s0002-9394(14)73145-9. [DOI] [PubMed] [Google Scholar]
- 9.Malozowski S, Tanner LA, Wysowski D, Fleming GA. Growth hormone, insulin-like growth factor I, and benign intracranial hypertension. N Engl J Med. 1993;329(9):665–666. doi: 10.1056/NEJM199308263290917. [DOI] [PubMed] [Google Scholar]
- 10.Fraunfelder FW, Fraunfelder FT, Corbett JJ. Isotretinoin-associated intracranial hypertension. Ophthalmology. 2004;111(6):1248–1250. doi: 10.1016/j.ophtha.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DI. Pseudotumor cerebri. Neurol Clin. 2004;22(1):99–131. doi: 10.1016/S0733-8619(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DI. The pseudotumor cerebri syndrome. Neurol Clin. 2014;32(2):363–396. doi: 10.1016/j.ncl.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Lessell S. Pediatric pseudotumor cerebri (idiopathic intracranial hypertension) Surv Ophthalmol. 1992;37(3):155–166. doi: 10.1016/0039-6257(92)90134-f. [DOI] [PubMed] [Google Scholar]
- 14.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52(6):597–617. doi: 10.1016/j.survophthal.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Scott IU, Siatkowski RM, Eneyni M, Brodsky MC, Lam BL. Idiopathic intracranial hypertension in children and adolescents. Am J Ophthalmol. 1997;124(2):253–255. doi: 10.1016/s0002-9394(14)70798-6. [DOI] [PubMed] [Google Scholar]
- 16.Wall M. Idiopathic intracranial hypertension. Semin Ophthalmol. 1995;10(3):251–259. doi: 10.3109/08820539509060979. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005;6(1):29–37. doi: 10.2165/00128071-200506010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Quinn AG, Singer SB, Buncic JR. Pediatric tetracycline-induced pseudotumor cerbri. J AAPOS. 1999;3(1):53–57. doi: 10.1016/s1091-8531(99)70095-9. [DOI] [PubMed] [Google Scholar]
- 19.Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand. 2004;110(6):408–411. doi: 10.1111/j.1600-0404.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 20.Esmaili N, Bradfield YS. Pseudotumor cerebri in children with Down syndrome. Ophthalmology. 2007;114(9):1773–1778. doi: 10.1016/j.ophtha.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Mourani CC, Mallat SG, Moukarzel MY, Akatcherian CY, Cochat P. Kidney transplantation after a severe form of pseudotumor cerebri. Pediatr Nephrol. 1998;12(9):709–711. doi: 10.1007/s004670050532. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber DR, Leonard MB, Shults J, Zemel BS. Acomparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2014;99(9):3208–3216. doi: 10.1210/jc.2014-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brara SM, Koebnick C, Porter AH, Langer-Gould A. Pediatric idiopathic intracranial hypertension and extreme childhood obesity. J Pediatr. 2012;161(4):602–607. doi: 10.1016/j.jpeds.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38(9):972–976. doi: 10.3109/02713683.2013.799214. [DOI] [PubMed] [Google Scholar]
- 28.Salpietro V, Mankad K, Kinali M, et al. Pediatric idiopathic intracranial hypertension and the underlying endocrine-metabolic dysfunction: a pilot study. J Pediatr Endocrinol Metab. 2014;27(1-2):107–115. doi: 10.1515/jpem-2013-0156. [DOI] [PubMed] [Google Scholar]
- 29.Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients Brain. 1991;114(Pt 1A):155–180. [PubMed] [Google Scholar]
- 30.Babikian P, Corbett J, Bell W. Idiopathic intracranial hypertension in children: the Iowa experience. J Child Neurol. 1994;9(2):144–149. doi: 10.1177/088307389400900208. [DOI] [PubMed] [Google Scholar]
- 31.Dhiravibulya K, Ouvrier R, Johnston I, Procopis P, Antony J. Benign intracranial hypertension in childhood: a review of 23 patients. J Paediatr Child Health. 1991;27(5):304–307. doi: 10.1111/j.1440-1754.1991.tb02544.x. [DOI] [PubMed] [Google Scholar]
- 32.Phillips PH, Repka MX, Lambert SR. Pseudotumor cerebri in children. J AAPOS. 1998;2(1):33–38. doi: 10.1016/s1091-8531(98)90107-0. [DOI] [PubMed] [Google Scholar]
- 33.Youroukos S, Psychou F, Fryssiras S, Paikos P, Nicolaidou P. Idiopathic intracranial hypertension in children. J Child Neurol. 2000;15(7):453–457. doi: 10.1177/088307380001500706. [DOI] [PubMed] [Google Scholar]


