A clinical cohort of 28 children with asymmetrical CP who received multiple pCIMT treatments gained and maintained new functional skills, supporting the conclusion that multiple pCIMT treatments can produce clinically important gains.
MeSH TERMS: cerebral palsy; combined modality therapy; paresis; restraint, physical; treatment outcome
Abstract
Pediatric constraint-induced movement therapy (pCIMT) is one of the most efficacious treatments for children with cerebral palsy (CP). Distinctive components of pCIMT include constraint of the less impaired upper extremity (UE), high-intensity therapy for the more impaired UE (≥3 hr/day, many days per week, for multiple weeks), use of shaping techniques combined with repetitive task practice, and bimanual transfer. A critical issue is whether multiple treatments of pCIMT produce additional benefit. In a clinical cohort (mean age = 31 mo) of 28 children with asymmetrical CP whose parents sought multiple pCIMT treatments, the children gained a mean of 13.2 (standard deviation [SD] = 4.2) new functional skills after Treatment 1; Treatment 2 produced a mean of 7.3 (SD = 4.7) new skills; and Treatment 3, 6.5 (SD = 4.2). These findings support the conclusion that multiple pCIMT treatments can produce clinically important functional gains for children with hemiparetic CP.
In 2004, Taub, Ramey, DeLuca, and Echols published the results of the first randomized controlled trial (RCT) testing the efficacy of pediatric constraint-induced movement therapy (pCIMT) for young children (ages 7 mo to 8 yr) with hemiparetic cerebral palsy (CP). The pCIMT protocol consisted of constructing a lightweight bivalve cast for the child’s nonhemiparetic upper extremity (UE) for continuous wear for 3 wk (with weekly removal to monitor skin integrity and UE mobility) and providing 6 hr of a systematic therapy protocol per day for 21 consecutive days. Pediatric therapists were trained to implement this version of pCIMT, which is now formally manualized and known as ACQUIREc (Case-Smith, DeLuca, Stevenson, & Ramey, 2012; DeLuca, Echols, & Ramey, 2007; DeLuca, Ramey, Trucks, Lutenbacher, & Wallace, 2013).
Taub et al.’s (2004) study found that children showed large-magnitude gains in the frequency and quality of their use of the hemiparetic arm and hand, acquired a mean of nine new motor skills, and demonstrated markedly higher rates of performance with the affected UE. Six months later, the children maintained—and some even increased—these gains. Later, a crossover phase offered all control participants the identical pCIMT intervention, and results replicated the original findings (DeLuca, Echols, Law, & Ramey, 2006). Since then, pCIMT has been the subject of at least 22 RCTs (e.g., Eliasson et al., 2014; Ramey, Coker-Bolt, & DeLuca, 2013; Ramey, DeLuca, Reidy, Wallace, & Trucks, 2013), including a multisite RCT using ACQUIREc therapy delivered in 3-hr and 6-hr daily dosages, both of which produced significant gains at 1 and 6 mo posttreatment (Case-Smith et al., 2012; DeLuca, Case-Smith, Stevenson, & Ramey, 2012).
In conventional therapy (non-CIMT) models, children with CP usually receive 1–2 hr of treatment once or twice a week, starting shortly after diagnosis (often in infancy) and continuing throughout childhood. These models continue to be used despite the fact that they have repeatedly been proven ineffective (Novak et al., 2013) and despite widespread recognition that the functional demands on children with CP change during development and their skill acquisition typically lags behind that of age-matched peers (Beckung, Carlsson, Carlsdotter, & Uvebrant, 2007; Himmelmann, Beckung, Hagberg, & Uvebrant, 2006; Novak et al., 2013; Rosenbaum et al., 2002; Sakzewski, Ziviani, & Boyd, 2009).
In contrast to conventional models of long-term, low-dosage therapy, pCIMT provides an intensive burst of several weeks or more of systematic treatment. This intensive treatment combines formal shaping techniques that use immediate, informative, and timely reinforcement for successively higher levels of performance with opportunity for repetitive practice (Bijou & Baer, 1961; Catania, 2007; DeLuca et al., 2007; Iversen & Lattal, 1991; Skinner, 1968). The constraint of the less impaired UE serves two purposes: (1) to promote shifting of the child’s attention and use to the more impaired UE and (2) to reduce competing motor and sensory input from the more functional UE. Like most pediatric therapy models, pCIMT is individualized with goals and activities to match the child’s current skill levels and developmental needs (Case-Smith, 2013; Case-Smith & O’Brien, 2010). The protocol used in this study matches the “signature form” of pCIMT (DeLuca et al., 2013; Ramey, DeLuca, & Coker-Bolt, 2013; Reiss, Wolf, Hammel, McLeod, & Williams, 2012).
What was unknown about pCIMT is whether more than one treatment of such a high-intensity therapy would produce additional benefits (Eliasson et al., 2014) and, if so, of what magnitude and for whom. Two published studies supported the value of at least a second treatment of pCIMT. The first was a case study of a 15-mo-old girl treated with pCIMT 6 hr/day for 21 consecutive days and then treated again 6 mo later at age 21 mo (DeLuca, Echols, Ramey, & Taub, 2003). After the first treatment, the child showed dramatic gains in new motor skills (e.g., reaching, general grasping, waving, independent sitting); after the second treatment, she displayed many new abilities in specific reach and hand manipulation activities and engaged in new self-help and play skills. pCIMT in the second treatment focused on shaping fine motor skills for activities of daily living, including holding a cup, self-feeding, and ball play. In the second study, Charles and Gordon (2007) reported findings from a clinical case series of eight children ages 8–11 yr who received a second course of pCIMT involving 6 hr of therapy for 10 days with the child’s nonhemiparetic UE constrained with a sling. Children benefited significantly after both treatments on the Jebsen–Taylor Hand Function Test and in speed and dexterity on the Bruininks–Oseretsky Test of Motor Proficiency.
The current study included a larger, more heterogeneous, and considerably younger clinical sample. Additionally, first-ever findings about the effects of a third pCIMT treatment are included for a subset of children. This study directly addresses one of the most important research questions identified by an international group in 2014 about the topic of pCIMT (Eliasson et al., 2014).
Method
Study Design and Enrollment
The design was a clinical series of 28 children whose families sought multiple treatments of pCIMT at a neuromotor research clinic that provided ACQUIREc therapy. The research clinic had institutional review board approval to collect pre- and posttreatment assessment data, daily clinical notes, and video documentation on all participants. Parents voluntarily granted written permission for their children to participate in this study to document the progression and effects of pCIMT. The clinic did not specifically solicit or recommend additional pCIMT treatments after the first treatment. Rather, when parents inquired about additional treatments, staff shared clinical experiences and peer-reviewed research findings known at the time. This study presents findings based on an entire cohort—that is, all children whose parents sought repeated pCIMT treatments over an 8-yr period of the clinic’s operations.
Children were screened individually for suitability (e.g., stable health, asymmetry between functional abilities of the two sides of the body, no frequent uncontrolled seizures) for pCIMT. The clinic did not exclude children with a diagnosis of quadriplegia or with comorbid conditions such as intellectual disabilities, autism spectrum disorder, challenging behaviors, or seizure disorders, but children with fragile health conditions (e.g., tube fed or dependent on respiratory assistance) were excluded.
pCIMT Intervention Protocol
The clinic administered ACQUIREc, the only manual-based version of pCIMT (DeLuca et al., 2007). ACQUIREc involves construction of a full arm-to-fingers lightweight cast worn continuously during the first 18 treatment days of a 20- or 21-day treatment over 4 wk. After cast removal, the last few days of therapy focus on bimanual therapy activities to promote integration of improved and new skills obtained earlier in treatment. All therapists in the clinic were formally trained in ACQUIREc, received active supervision from senior therapists, and maintained systematic daily progress notes.
Key components of pCIMT involve the use of a constraint, in this case a cast, shaping and repetitive task practice during treatment activities for many hours a day, many days a week, and for multiple weeks. Shaping and repetitive practice with reinforcement are grounded in learning theory, have a long history of efficacy, and were included in the development of CIMT for adults and children (Ramey & DeLuca, 2013; Woodbury, Fritz, Blanton, & Wolf, 2013). The amount of shaping versus massed or repetitive practice to include in pCIMT is often debated; for ACQUIREc, the primary emphasis is on shaping UE use and skills to be functional in the child’s overall repertoire, with repetitive practice used to increase automaticity and ease of performing new skills and to encourage their use in a variety of situations (i.e., to promote generalization and maintenance), and in this study involved 6 hr of daily therapy for 5 days/wk for 4 wk.
In ACQUIREc, therapists frequently and naturally transition between shaping and repetitive practice. What identifies shaping is the use of immediate and specifically informative feedback to the child through a process known as successive approximations—that is, the therapist helps the child advance to higher levels of performance in a given movement or activity (e.g., executing with greater consistency, accuracy, speed, strength, coordination, or complexity). Accordingly, ACQUIREc therapists are trained to approach all therapy and play activities as a means to accomplish shaping, which is success oriented. As needed, the therapist can use techniques such as direct facilitation, demonstration, and body stabilization for early task success.
After the child has shown progress in shaping, repetitive practice is important, and the therapist continues to use varied rewards (e.g., verbal praise, smiles, tangible tokens, offer of a favorite future activity) until the child reaches about 70%–80% competency at the current level. Once that competency level is reached, the therapist alters the task demands by withdrawing a small amount of support (e.g., when facilitation or verbal guidance has been used), changing the task to make it more challenging, or both. Reinforcement drops out for accomplishments at the obtained competence level and then occurs only when the child makes new advances under the new requirements. The therapist shifts from shaping and repetitive practice focused on one set of skills to other sets (on the basis of multiple treatment goals) to maintain the child’s high level of engagement and enjoyment throughout the day, relying on a play-based format and self-help skills (e.g., eating, drinking, dressing, cleaning up) as these naturally occur, but with a primary focus on using shaping techniques as much as possible.
The other necessary components of pCIMT, as recently defined (Ramey et al., 2013), include treatment within the child’s natural environment and a transition package for after-treatment generalization. All are key elements within the manualized ACQUIREc therapy model. In addition, the last 3 days of treatment include the removal of the constraint to incorporate bimanual arm and hand use.
Functional Assessments
In this article, we present data from two primary functional outcome measures: the Emerging Behaviors Scale (EBS; DeLuca et al., 2007; Taub et al., 2004) and the Pediatric Motor Activity Log (PMAL; DeLuca et al., 2007; Taub et al., 2004). These tools were used in the first RCT of pCIMT (DeLuca et al., 2006; Taub et al., 2004) and can be used with a wide range of ages and ability levels. The EBS is used to document and count the number of skills a child displays from a set of 31 discrete UE activities, including movement patterns and functional abilities (e.g., wrist extension, finger isolation, finger feeding, pushing buttons). To qualify as a skill, the movement or behavior must be documented through at least two independent sources. For example, the behavior could be documented on a video recording and then independently documented as an item passed on a standardized assessment tool. (Many of the children received additional standardized assessments, but they varied as a function of the child’s age and baseline functional level.) As another example, a new skill could be recorded in the therapist’s daily log, documented during a standardized assessment, and reported in writing by the parent.
The PMAL is a standardized tool in which parents rate their children’s abilities using a 6-point Likert scale on the subscales Quality of Movement (0 = no use, 5 = age-typical or normal use) and Amount of Use (0 = never, 5 = very frequently) for each of 22 arm and hand activities (e.g., holding a cup or bottle, waving good-bye, pushing a button to activate a toy, throwing a ball, putting an arm through a sleeve of clothing). Wallen, Bundy, Pont, and Ziviani (2009) reported PMAL psychometrics, and a translated version of the scale has been developed (Uswatte et al., 2012). In this study we used the version of the PMAL developed by the original authors of the tool because of its widespread use in other studies (DeLuca et al., 2006, 2007; Ramey, Coker-Bolt, & DeLuca, 2013; Taub et al., 2004).
Data Analysis
Data entry and analysis occurred in multiple stages. Clinical records were reviewed to identify any children who did not complete the entire course of pCIMT or who showed possible negative treatment effects, to complete post hoc Manual Ability Classification System (MACS) level ratings for use of arms and hands during typical performance handling objects (Eliasson et al., 2006), and to extract demographic information. Next, scores on all outcome measures were analyzed using an omnibus multivariate analysis of variance (MANOVA) to determine significant effects of multiple treatments. Univariate analyses of variance (ANOVAs) were then completed to compare changes in EBS and PMAL scores. To determine whether significant changes occurred across treatments, repeated-measures ANOVAs were conducted on outcome scores separately for the 20 children who received two pCIMT treatments and the 8 children who received three pCIMT treatments, recognizing that differences might exist between these groups. Finally, each child’s scores on the EBS were examined individually across treatment sessions to estimate the effects of each treatment on the child’s overall repertoire (i.e., gain in skills).
Results
Clinical Sample Characteristics
Table 1 presents demographic characteristics for the 28 children who received at least two treatments of pCIMT. The mean (M) interval between the first and second treatments was 13.2 mo (standard deviation [SD] = 6.7) for all 28 children and between the second and third treatments was 17.9 mo (SD = 9.8) for the 8 children who had three treatments. The majority (71%) had a primary diagnosis of CP; other diagnoses included stroke, acquired brain injury, and hemispherectomy. Most children functioned at MACS Level 3; levels range from 1 to 5 (higher numbers indicate greater impairment). Only 1 child had a MACS rating of 5, indicating that the child did not handle objects. This child was low functioning in multiple domains and had quadriplegia.
Table 1.
Participant Characteristics (N = 28)
| Characteristic | Received Two Treatments of pCIMT (n = 20) | Received Three Treatments of pCIMT (n = 8) |
| Mean age, mo (SD) | ||
| Treatment 1 | 30.1 (20.9) | 42.9 (49.6) |
| Treatment 2 | 44.6 (24.9) | 54.3 (49.1) |
| Treatment 3 | — | 72.1 (55.8) |
| Age range | ||
| Treatment 1 | 11 mo–8 yr, 3 mo | 13 mo–10 yr, 10 mo |
| Treatment 2 | 1 yr, 8 mo–10 yr, 2 mo | 24 mo–11 yr, 9 mo |
| Treatment 3 | — | 3 yr–15 yr, 1 mo |
| Gender, n (%) | ||
| Boys | 12 (60) | 2 (25) |
| Girls | 8 (40) | 6 (75) |
| Side of hemiparesis, n (%) | ||
| Right | 13 (65) | 4 (50) |
| Left | 7 (35) | 4 (50) |
| Diagnosis, n (%) | ||
| Cerebral palsy | 14 (70) | 6 (75) |
| Stroke | 4 (20) | 1 (13) |
| Acquired brain injury | 2 (10) | 0 |
| Hemispherectomy | 0 | 1 (13) |
| MACS level, n (%) | ||
| I (least impaired) | 0 | 0 |
| II | 6 (30) | 1 (13) |
| III | 10 (50) | 5 (63) |
| IV | 3 (15) | 2 (25) |
| V (most impaired) | 1 (5) | 0 |
| Mean interval between treatments (SD), mo | ||
| Between Treatments 1 and 2 | 14.8 (6.2) | 11.6 (4.9) |
| Between Treatments 2 and 3 | — | 17.9 (9.8) |
Note. Percentages may total more than 100% because of rounding. — = not applicable; MACS = Manual Ability Classification System; pCIMT = pediatric constraint-induced movement therapy; SD = standard deviation.
Outcomes
All 28 children completed the fully intended dosage level (6 hr/day of ACQUIREc therapy, 5 days/wk, for 4 consecutive weeks [20 or 21 days]). No child showed any negative effects of casting or high-intensity treatment. MANOVAs comparing change scores by treatment session showed statistical significance, F (6, 28) = 3.58, p = .003, indicating a differential response to the treatment sessions on at least one outcome measure. ANOVAs on EBS scores indicated a significant effect in favor of pCIMT Treatment 1, F (2, 28) = 11.67, p < .0001, with large gains in new behaviors after Treatment 1 (M = 13.2, SD = 4.2) and significant gains after Treatment 2 (M = 7.3, SD = 4.6) and Treatment 3 (M = 6.5, SD = 4.2). Analyses of PMAL subscale scores showed comparability of improvement across all three treatments, Quality of Movement, F (2, 28) = 0.8, p = .40, and Amount of Use, F (2, 28) = 2.8, p = .07, with significant benefits for all treatments.
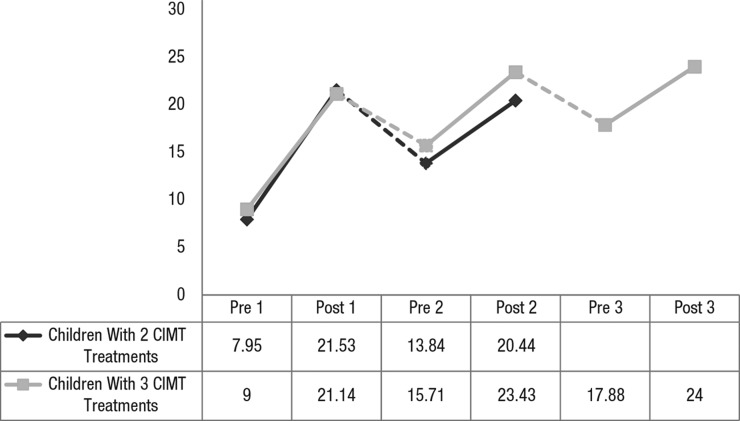
All repeated-measures ANOVAs reached statistical significance, indicating the benefit of treatment across time. Figure 1 shows that the patterns of gains in EBS scores are virtually identical across the first two treatments for children in both groups. Notably, between subsequent treatments, children did decline somewhat, but their performance did not drop to their initial baseline scores (M decline = 7.04 skills).
Figure 1.
Mean EBS scores across pCIMT treatments.
Note. Possible scores range from 0 to 31. Dotted lines indicate an intertreatment interval. CIMT = constraint-induced movement therapy; EBS = Emerging Behaviors Scale; pCIMT = pediatric constraint-induced movement therapy; pre 1 = pre–Treatment 1; post 1 = post–Treatment 1; pre 2 = pre–Treatment 2; post 2 = post–Treatment 2; pre 3 = pre–Treatment 3; post 3 = post–Treatment 3.
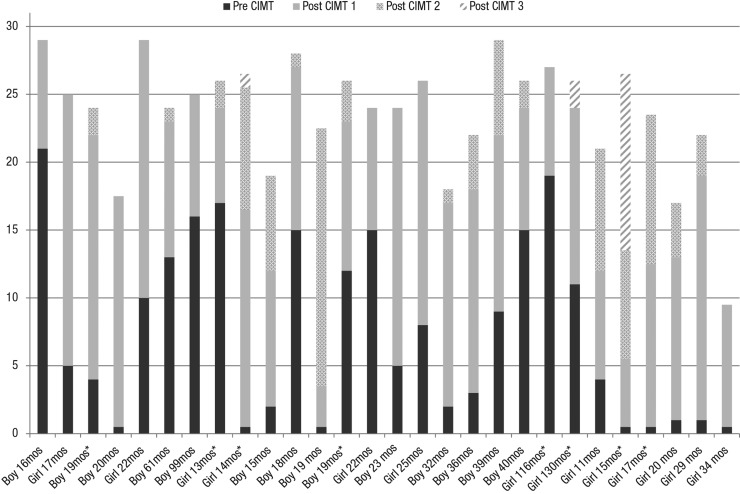
A clinically important concern is whether some children benefit more while other children benefit less or not at all from pCIMT. Figure 2 shows each child’s EBS scores pre– and post–Treatment 1 and final EBS scores after Treatment 2 for all 28 children and after Treatment 3 for 8 children. All 28 children showed significant gains in total EBS scores after Treatment 1, ranging from a low of 3 new skills to a high of 19. Twenty children (71%) developed at least 15 new skills after Treatment 1; only 2 children (7%) displayed gains of only 5 or fewer new skills. The majority of children (82%) demonstrated absolute gains on EBS from pretreatment to post–Treatment 2, and 63% demonstrated gains from pretreatment to post–Treatment 3, although the child’s final EBS score may not have exceeded that earned on a previous assessment occasion. Seventy-five percent of children showed some declines on EBS scores during the intertreatment interval, subsequent pCIMT treatments produced either partial recovery of these previously demonstrated skills or recovery plus acquisition of new skills to reach an even higher total score. See Figure 1 for further elaboration.
Figure 2.
Individual cumulative total EBS scores: two pCIMT treatments (n = 20) and three pCIMT treatments (n = 8).
The cumulative total EBS score indicates the highest number of emerging behaviors documented for each child and comprises each child’s pre-pCIMT Treatment 1 EBS score plus any additional items passed after subsequent treatments. The cumulative scores do not reflect the child’s absolute score changes from pretreatment to post–Treatments 2 or 3.
Note. An asterisk indicates that the child participated in three pCIMT treatments. EBS = Emerging Behaviors Scale; pCIMT = pediatric constraint-induced movement therapy.
Among the 10 children who did not show higher final EBS scores after Treatment 2, 8 had high post–Treatment 1 EBS scores—at least 24 out of a maximum 31. One child with a post–Treatment 1 score of 25 later had an identical score at pre– and post–Treatment 2. The other 7 children each showed some decline during the intertreatment interval and then showed significant reacquisition of skills after Treatment 2. Interestingly, these 8 children were among the 10 highest achievers in the clinical cohort.
We analyzed effects of gender, baseline EBS scores, MACS level, and age at which the child first received pCIMT on treatment responsiveness. None of these variables significantly predicted gains in skills. We note, however, that 24 children (86%) began treatment before age 40 mo, and only 3 were older than age 6 yr, perhaps limiting our power to detect significant age effects.
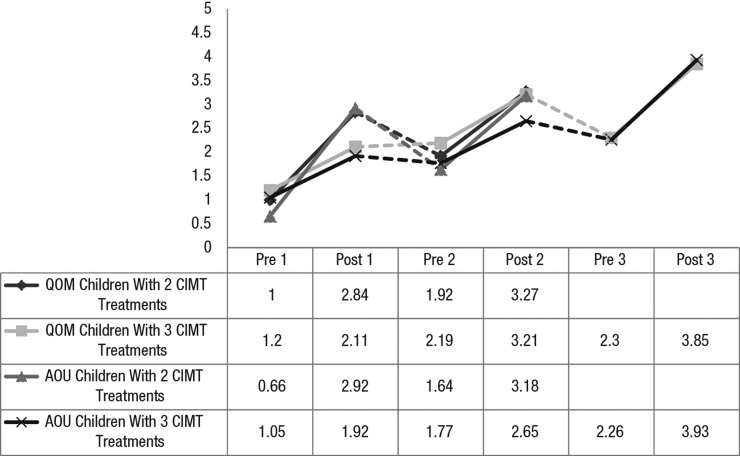
Figure 3 shows the PMAL subscale scores for Quality of Movement and Amount of Use. The children improved significantly and at comparable levels after each treatment. Some declines during the intertreatment intervals occurred, although the children maintained some of their benefits between treatments. Children who received three treatments showed higher PMAL scores at the end of Treatment 3 than those who received two treatments showed at the end of Treatment 2.
Figure 3.
Pediatric Motor Activity Log mean ratings across pCIMT treatments.
Note. Dotted lines indicate an intertreatment interval. AOU = Amount of Use subscale score; CIMT = constraint-induced movement therapy; pCIMT = pediatric constraint-induced movement therapy; pre 1 = pre–Treatment 1; post 1 = post–Treatment 1; pre 2 = pre–Treatment 2; post 2 = post–Treatment 2; pre 3 = pre–Treatment 3; post 3 = post–Treatment 3; QOM = Quality of Movement subscale score.
Discussion
All children in this clinical cohort of mostly young children benefited significantly from their first pCIMT treatment, and subsequent treatments produced significant gains for many children on both the EBS and PMAL. Children’s initial gains on the EBS were significantly greater after Treatment 1 compared with later treatments, although most children continued to gain clinically important new skills with each treatment. In addition, all treatments produced significant and comparable gains on the PMAL subscales Quality of Movement and Amount of Use. These gains cannot be attributed solely to the children’s increasing age or general development processes, as indicated by performance declines during the intertreatment intervals. Additionally, almost all children continued to receive conventional (lower dosage) therapy during the intertreatment period in clinic settings or early intervention or school-based programs. The fact that many of these children lost some skills and declined in their everyday use of the affected UE between treatments indicates that children may benefit from an improved posttreatment plan and additional environmental supports after receiving pCIMT to promote use of their newly acquired skills in everyday functional activities.
Among the approximately one-third of children whose final overall EBS scores did not improve with multiple treatments, the vast majority were among the highest scoring children in this sample. One possible reason for this finding is that these children were approaching ceiling levels on this particular tool; that is, the EBS may be insufficiently sensitive to the types of changes occurring in children with higher abilities (e.g., consistency, speed, strength, accuracy, coordination, smoothness).
Limitations
This study has several limitations. The sample was from a single clinic that developed the signature form of pCIMT, ACQUIREc (DeLuca et al., 2013; Ramey, DeLuca, & Coker-Bolt, 2013). Because their parents sought this treatment, these children may not be adequately representative of all children with asymmetrical CP. The parents had requested subsequent treatments on the basis of the perceived value of pCIMT, and the time between pCIMT sessions was a function of each family’s choice and schedule. Accordingly, these parents appeared highly invested in their child’s neuromotor progress, which may have contributed, in part, to the treatment benefits observed. Accordingly, no conclusions can be drawn about the most appropriate intervals between pCIMT sessions. In fact, the large range of such intervals (4–40 mo) may have been a factor that limited the benefits of subsequent pCIMT for some children. This factor warrants future research on repeated courses of pCIMT and other intensive therapies (e.g., bimanual).
Longitudinal research will facilitate a better understanding of the natural variation in development of UE skills in children with unilateral or asymmetrical CP. Such information would allow future researchers to interpret the magnitude and types of gains for children at different ages, thus providing an adjusted and more accurate assessment of the effects of repeated high-intensity treatments such as pCIMT. Finally, this sample was young, mostly in the toddler to preschool age range. Despite these limitations, this study is the first relatively large-scale clinical cohort sample with prospective assessment data on the effects of repeated pCIMT treatments.
Implications for Occupational Therapy Practice and Research
The results of this study have the following implications for occupational therapy practice and research:
Repeated pCIMT treatments for young children with asymmetrical CP can produce large and significant benefits; the largest gains may occur after the first treatment.
pCIMT can be clinically implemented and produce large benefits on repeated occasions for a wide range of children (age and ability levels).
Children and families can repeat the full course of high-intensity treatment with no negative effects.
Subsequent pCIMT treatments produce gains over and above those of the first pCIMT treatment for most children.
Future research needs to include more sensitive outcome measures for children who function at higher levels after Treatment 1 and to consider the optimal length of intertreatment intervals (perhaps varying as a function of the child’s age, ability level, and treatment goals).
Acknowledgments
This project was supported in part by the Virginia Tech Carilion Research Institute Neuromotor Research Clinic and the Eunice Shriver Kennedy National Institute of Child Health and Human Development, National Institutes of Health (1RO1HD074574 and 1RO1HD068345). The study is based on data collected at the former Pediatric Neuromotor Research Clinic at the University of Alabama at Birmingham. We gratefully acknowledge the contributions of participating children and families to this study. We dedicate this article to the lifetime contributions and memory of our colleague, Jane Case-Smith. She was a fervent supporter of pCIMT research and a well-admired collaborator. She encouraged and supported this work and other efforts on pCIMT for many years, and her legacy inspires us all to continue to investigate effects of varied forms of pCIMT and to work creatively to discover translational clinical models for the use of pCIMT to realize maximum benefits for all eligible children and their families.
Contributor Information
Stephanie C. DeLuca, Stephanie C. DeLuca, PhD, is Director, Neuromotor Research Clinic, Virginia Tech Carilion Research Institute, and Assistant Professor, Department of Pediatrics, Virginia Tech Carilion School of Medicine, Roanoke; Assistant Professor, Department of Psychology, Virginia Tech, Blacksburg; and Assistant Professor, Department of Rehabilitation and Wellness, Jefferson College of Health Sciences, Roanoke, VA; stephdeluca@vt.edu
Sharon Landesman Ramey, Sharon Landesman Ramey, PhD, is Distinguished Scholar and Professor, Virginia Tech Carilion Research Institute, and Professor, Department of Pediatrics, Virginia Tech Carilion School of Medicine, Roanoke; Professor, Department of Psychology, Virginia Tech, Blacksburg; and Professor, Psychiatry and Behavioral Medicine, Virginia Tech Carilion School of Medicine, Roanoke.
Mary Rebekah Trucks, Mary Rebekah Trucks, OTR/L, is Research Faculty and Senior Occupational Therapist, Virginia Tech Carilion Research Institute, Roanoke.
Dorian Ainsworth Wallace, Dorian Ainsworth Wallace, OTR/L, is Research Faculty and Senior Occupational Therapist, Virginia Tech Carilion Research Institute, Roanoke.
References
- Beckung E., Carlsson G., Carlsdotter S., & Uvebrant P. (2007). The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Developmental Medicine and Child Neurology, 49, 751–756. http://dx.doi.org/10.1111/j.1469-8749.2007.00751.x [DOI] [PubMed] [Google Scholar]
- Bijou S. W., & Baer D. M. (1961). Child development: A systematic and empirical therapy (Vol. 1). New York: Appleton-Century Crofts; http://dx.doi.org/10.1037/11139-000 [Google Scholar]
- Case-Smith J. (2013). Applying occupation and motor learning principles in pediatric CIMT: Theoretical foundations and conceptual framework. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 129–147). Bethesda, MD: AOTA Press. [Google Scholar]
- Case-Smith J., DeLuca S. C., Stevenson R., & Ramey S. L. (2012). Multicenter randomized controlled trial of pediatric constraint-induced therapy in children with cerebral palsy: 6-month follow-up. American Journal of Occupational Therapy, 66, 15–23. http://dx.doi.org/10.5014/ajot.2012.002386 [DOI] [PubMed] [Google Scholar]
- Case-Smith J., & O’Brien J. (2010). Occupational therapy for children (6th ed.). Maryland Heights, MO: Mosby/Elsevier. [Google Scholar]
- Catania A. C. (2007). Learning (4th ed). Cornwall-on-Hudson, NY: Sloan. [Google Scholar]
- Charles J. R., & Gordon A. M. (2007). A repeated course of constraint-induced movement therapy results in further improvement. Developmental Medicine and Child Neurology, 49, 770–773. http://dx.doi.org/10.1111/j.1469-8749.2007.00770.x [DOI] [PubMed] [Google Scholar]
- DeLuca S. C., Case-Smith J., Stevenson R., & Ramey S. L. (2012). Constraint-induced movement therapy (CIMT) for young children with cerebral palsy: Effects of therapeutic dosage. Journal of Pediatric Rehabilitation Medicine, 5, 133–142. http://dx.doi.org/10.3233/PRM-2012-0206 [DOI] [PubMed] [Google Scholar]
- DeLuca S. C., Echols K., Law C. R., & Ramey S. L. (2006). Intensive pediatric constraint-induced therapy for children with cerebral palsy: Randomized, controlled, crossover trial. Journal of Child Neurology, 21, 931–938. http://dx.doi.org/10.1177/08830738060210110401 [DOI] [PubMed] [Google Scholar]
- DeLuca S. C., Echols K., & Ramey S. L. (2007). ACQUIREc therapy: A training manual for effective application of pediatric constraint-induced movement therapy. Hillsborough, NC: MindNurture. [Google Scholar]
- DeLuca S. C., Echols K., Ramey S. L., & Taub E. (2003). Pediatric constraint-induced movement therapy for a young child with cerebral palsy: Two episodes of care. Physical Therapy, 83, 1003–1013. [PubMed] [Google Scholar]
- DeLuca S. C., Ramey S. L., Trucks M. R., Lutenbacher R., & Wallace D. A. (2013). ACQUIREc protocol: What we have learned from a decade of delivering a signature form of pediatric CIMT. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 129–147). Bethesda, MD: AOTA Press. [Google Scholar]
- Eliasson A. C., Krumlinde-Sundholm L., Gordon A. M., Feys H., Klingels K., Aarts P. B. M., . . . Hoare B.; European Network for Health Technology Assessment. (2014). Guidelines for future research in constraint-induced movement therapy for children with unilateral cerebral palsy: An expert consensus. Developmental Medicine and Child Neurology, 56, 125–137. http://dx.doi.org/10.1111/dmcn.12273 [DOI] [PubMed] [Google Scholar]
- Eliasson A. C., Krumlinde-Sundholm L., Rösblad B., Beckung E., Arner M., Ohrvall A. M., & Rosenbaum P. (2006). The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Developmental Medicine and Child Neurology, 48, 549–554. http://dx.doi.org/10.1017/S0012162206001162 [DOI] [PubMed] [Google Scholar]
- Himmelmann K., Beckung E., Hagberg G., & Uvebrant P. (2006). Gross and fine motor function and accompanying impairments in cerebral palsy. Developmental Medicine and Child Neurology, 48, 417–423. http://dx.doi.org/10.1017/S0012162206000922 [DOI] [PubMed] [Google Scholar]
- Iversen I. H., & Lattal K. A. (1991). The experimental analysis of behavior, Part 1: Techniques in behavioral and neural sciences. Oxford, England: Elsevier Science & Technology. [Google Scholar]
- Novak I., McIntyre S., Morgan C., Campbell L., Dark L., Morton N., . . . Goldsmith S. (2013). A systematic review of interventions for children with cerebral palsy: State of the evidence. Developmental Medicine and Child Neurology, 55, 885–910. http://dx.doi.org/10.1111/dmcn.12246 [DOI] [PubMed] [Google Scholar]
- Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.). (2013). Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators. Bethesda, MD: AOTA Press. [Google Scholar]
- Ramey S. L., & DeLuca S. C. (2013). Pediatric constraint-induced movement therapy: History and definition. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 19–39). Bethesda, MD: AOTA Press. [Google Scholar]
- Ramey S. L., DeLuca S. C., & Coker-Bolt P. (2013). Operationalizing pediatric CIMT: Guidelines for transforming basic principles and scientific evidence into clinical practice for individual children. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 115–128). Bethesda, MD: AOTA Press. [Google Scholar]
- Ramey S. L., DeLuca S. C., Reidy T. G., Wallace D. A., & Trucks M. R. (2013). Appendix A: Key findings from original research articles with functional and occupational outcomes of pediatric CIMT and related componential interventions. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 283–293). Bethesda, MD: AOTA Press. [Google Scholar]
- Reiss A. P., Wolf S. L., Hammel E. A., McLeod E. L., & Williams E. A. (2012). Constraint-induced movement therapy (CIMT): Current perspectives and future directions. Stroke Research and Treatment, 2012, 159391 http://dx.doi.org/10.1155/2012/159391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P. L., Walter S. D., Hanna S. E., Palisano R. J., Russell D. J., Raina P., . . . Galuppi B. E. (2002). Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. JAMA, 288, 1357–1363. http://dx.doi.org/10.1001/jama.288.11.1357 [DOI] [PubMed] [Google Scholar]
- Sakzewski L., Ziviani J., & Boyd R. (2009). Systematic review and meta-analysis of therapeutic management of upper-limb dysfunction in children with congenital hemiplegia. Pediatrics, 123, e1111–e1122. http://dx.doi.org/10.1542/peds.2008-3335 [DOI] [PubMed] [Google Scholar]
- Skinner B. F. (1968). The technology of teaching. East Norwalk, CT: Appleton-Century-Crofts. [Google Scholar]
- Taub E., Ramey S. L., DeLuca S., & Echols K. (2004). Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics, 113, 305–312. http://dx.doi.org/10.1542/peds.113.2.305 [DOI] [PubMed] [Google Scholar]
- Uswatte G., Taub E., Griffin A., Vogtle L., Rowe J., & Barman J. (2012). The Pediatric Motor Activity Log-Revised: Assessing real-world arm use in children with cerebral palsy. Rehabilitation Psychology, 57, 149–158. http://dx.doi.org/10.1037/a0028516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen M., Bundy A., Pont K., & Ziviani J. (2009). Psychometric properties of the Pediatric Motor Activity Log used for children with cerebral palsy. Developmental Medicine and Child Neurology, 51, 200–208. http://dx.doi.org/10.1111/j.1469-8749.2008.03157.x [DOI] [PubMed] [Google Scholar]
- Woodbury M. L., Fritz S. L., Blanton S., & Wolf S. L. (2013). History and development of CIMT. In Ramey S. L., Coker-Bolt P., & DeLuca S. C. (Eds.), Handbook of pediatric constraint-induced movement therapy (CIMT): A guide for occupational therapy and health care clinicians, researchers, and educators (pp. 3–18). Bethesda, MD: AOTA Press. [Google Scholar]