Abstract
Objectives
Explore the association of self-rated health (SRH) with host-resistance to illness following exposure to a common cold virus and identify mechanisms linking SRH to future health status.
Methods
We analyzed archival data from 360 healthy adults (mean age = 33.07, SD = 10.69; 45.6% women). Each completed validated questionnaires assessing SRH (excellent, very good, good, fair, poor), socio-emotional factors and health practices; was subsequently exposed to a virus that causes the common cold; and monitored for 5 days for clinical illness (infection + objective signs of illness).
Results
Poorer SRH was associated in a graded fashion with greater susceptibility to developing clinical illness (good/fair vs. excellent: odds ratio [OR]=3.21, 95% confidence interval [CI]=1.47-6.99]; very good vs. excellent: OR=2.60, 95% CI=1.27-5.32), independent of age, sex, race, pre-challenge immunity (specific antibody), body mass, season, education, and income. Greater illness risk was not attributable to infection, but to increased likelihood of developing objective signs of illness once infected. Poorer SRH also correlated with poorer health practices, increased stress, lower positive emotions, and other socio-emotional factors. However, none of these (alone or together) accounted for the association between SRH and host-resistance. Additional data (separate study) indicated that history of having colds was unrelated to susceptibility and hence also did not account for the SRH link with immunocompetence.
Conclusions
Poorer SRH is associated with poorer immunocompetence, possibly reflecting sensitivity to sensations associated with premorbid immune dysfunction. In turn poorer immune function may be a major contributing mechanism linking SRH with future health.
Keywords: common cold, host-resistance, self-rated health, upper respiratory infection, viral-challenge
INTRODUCTION
Poor global self-ratings of health have been found to predict poor health trajectories in older adults, including increased risk of mortality (1,2). Strikingly, these relations withstand adjustments for baseline health status indicated by physical exams, medical records, hospitalizations, and self-reported functional disability and chronic conditions (3). Moreover, these associations are robust despite the most frequently used self-rated health (SRH) measure simply asking respondents to rate their health as falling into one of four or five categories, e.g., excellent, very good, good, fair, or poor.
Despite convincing evidence that a simple SRH assessment predicts poorer health in older adults, less clear is why SRH predicts declines in health above and beyond more objective measures of health status (4,5). Proposed mechanisms include individuals who report better health having healthier lifestyles (3) or differing in social and emotional factors known to promote health (6). These pathways, however, do not appear to account for the SRH-mortality link (6-8). Other explanations have focused on participants having access to bodily sensations not available to outside observers. For example, participants may be experiencing symptoms of illness that are not adequately assessed at baseline—a serious problem when studying the elderly who may suffer multiple infirmities. Alternatively, SRH may be sensitive to sensations associated with premorbid dysfunction of physiological systems that contribute to long-term health trajectories (5,9).
A physiological system that figures importantly into overall health is the immune system. The immune system is instrumental in responding to infectious pathogens (10), controlling the growth and metastasis of tumor cells (11-13) and producing and regulating inflammatory responses that influence risk for and progression of numerous diseases (14,15). SRH as a marker, or predictor of immunocompetence, would provide a possible pathway explaining its link with health trajectories. Indeed, poorer SRH has been found to be associated with mortality caused by infectious disease (7); with higher white blood cell counts—a marker of infection (16); and higher levels of circulating interleukin-6—a pro-inflammatory cytokine (17).
The present study uses data from 360 healthy young and middle-aged adults (median age = 30 years) to examine the association of SRH with functioning of the immune system in the face of a viral-challenge. Specific objectives are to: 1) evaluate SRH as an indirect marker of immunocompetence, which would suggest a potential pathway linking SRH to morbidity and mortality; 2) determine whether SRH predicts disease susceptibility in relatively young participants selected for good health (i.e., unlikely to have undetected symptoms of disease) at baseline; and 3) test the role of socio-emotional, and health lifestyle measures as explanations for a link between SRH and immunocompetence.
METHODS
Participants
The present analyses combine archival data from two viral-challenge studies conducted from 2000 to 2004 (Pittsburgh Mind-Body Center Study [PMBC]) and from 2007 to 2011 (Pittsburgh Cold Study 3 [PCS3]), respectively. The studies followed a common set of procedures which included a physical exam; blood and urine screenings; questionnaire assessments of SRH, demographics, and social and emotional factors; 14 consecutive days of evening telephone interviews; and subsequent participation in a viral-challenge trial. The total sample included 368 healthy adults, aged 18 to 55 years who were recruited from the Pittsburgh, PA metropolitan area via newspaper advertisements and community postings. Eight participants were excluded from the present analyses due to missing data on relevant covariates. Characteristics of the remaining 360 participants are reported in Table 1. All participants provided informed consent and received financial compensation for study participation. Study procedures were approved by the institutional review boards of the University of Pittsburgh and Carnegie Mellon University.
Table 1.
Sample Characteristics by Category of Self-Rated Health
| Self-Rated Health |
|||||||
|---|---|---|---|---|---|---|---|
| Sample (n = 360) |
Excellent (n=72) |
Very Good (n=191) |
Good Fair (n=97) |
X 2 | F (2, 357) | p | |
| Age (mean, SD) | 33.07 (10.69) | 33.36 (11.09) | 32.81 (10.90) | 33.03 (10.15) | 0.07 | .932 | |
| Female sex (%, n) | 45.6 (164) | 40.3 (29) | 47.1 (90) | 46.4 (45) | 1.03 | .599 | |
| White race (%, n) a | 61.1 (220) | 68.1 (49) | 58.6 (112) | 60.8 (59) | 1.96 | .376 | |
| PMBC (%, n) | 41.4 (149) | 44.4 (32) | 39.3 (75) | 43.3 (42) | 0.78 | .678 | |
| Pre-challenge Ab ≥4 (%, n) | 26.4 (95) | 23.6 (17) | 29.3 (56) | 22.7 (22) | 1.82 | .403 | |
| Body mass index (mean, SD) | 26.47 (6.49) | 25.82 (4.92) | 27.81 (6.32) | 30.00 (7.26) | 8.81 | .001 | |
| Season (%, n) | 8.98 | .175 | |||||
| Winter | 15.8 (57) | 11.1 (8) | 14.7 (28) | 21.6 (21) | |||
| Spring | 39.7 (143) | 41.7 (30) | 44.0 (84) | 29.9 (29) | |||
| Summer | 27.2 (98) | 26.4 (19) | 24.6 (47) | 33.0 (32) | |||
| Fall | 17.2 (62) | 20.8 (15) | 16.8 (32) | 15.5 (15) | |||
| Education (%, n) | 4.19 | .651 | |||||
| High school or less | 26.1 (94) | 34.7 (25) | 22.5 (43) | 26.8 (26) | |||
| Some college (no degree) | 27.8 (100) | 23.6 (17) | 29.3 (56) | 27.8 (27) | |||
| ≥2 yrs college + degree | 19.4 (70) | 16.7 (12) | 20.4 (39) | 19.6 (19) | |||
| Bachelor’s or greater | 26.7 (96) | 25.0 (18) | 27.7 (53) | 25.8 (25) | |||
| Household income, $1000 US (median, range) |
12.5 (2.5-162.5) | 7.5 (2.5-162.5) | 17.5 (2.5-112.5) | 17.5 (2.5- 137.5) | 1.33 | .263 | |
| Regular/heavy smoking (%, n) | 31.4 (113) | 25.0 (18) | 28.8 (55) | 41.2% (40) | 6.33 | .042 | |
| Heavy drinking (%, n) | 35.8 (129) | 33.3 (24) | 31.9 (61) | 45.4% (44) | 5.29 | .071 | |
| Low physical activity (%, n) | 58.9 (212) | 47.2 (34) | 57.6 (110) | 70.1% (68) | 9.22 | .010 | |
| Poor sleep (%, n) | 30.8 (111) | 20.8 (15) | 30.9 (59) | 38.1% (37) | 5.81 | .055 | |
| Parent status (%, n) | 33.3 (120) | 36.1 (26) | 33.0 (63) | 32.0% (31) | 0.34 | .842 | |
| Perceived stress (mean, SD) | 13.11 (6.18) | 11.72 (5.60) | 12.79 (5.90) | 14.77 (6.80) | 5.74 | .004 | |
| Sociability (mean, SD) | −0.02 (0.78) | −0.01 (0.81) | 0.06 (0.76) | −0.17 (0.79) | 2.67 | .071 | |
| Social integration (mean, SD) | 5.25 (1.90) | 5.22 (1.90) | 5.42 (1.89) | 4.94 (1.91) | 2.13 | .121 | |
| Positive emotional style (mean, SD) | 14.61 (4.21) | 16.12 (3.87) | 14.90 (4.17) | 12.95 (4.03) | 13.46 | .001 | |
| Emotional stability (mean, SD) | −0.01 (1.01) | 0.04 (1.07) | 0.03 (1.02) | −0.14 (0.96) | 1.10 | .335 | |
| Subjective social standing (mean, SD) | 4.46 (1.95) | 5.04 (2.12) | 4.36 (1.95) | 4.21 (1.75) | 4.31 | .014 | |
Non-white category included Black/African-American (31.7%; n = 114), Asian or Pacific Islander (1.7%; n = 6), Hispanic or Latino (1.1%; n = 4), Native American, Eskimo, or Aleut (0.6%; n = 2), and Other Race/Ethnicity (3.9%; n = 14).
Procedures
Screening
Volunteers were included for participation if they were in “good general health”, as determined by medical history and physical examination. They were excluded from study eligibility if they had diabetes, hepatitis, cardiovascular disease, chronic sinusitis, chronic bronchitis, asthma or any other chronic illness; abnormal clinical profiles discovered via urinalysis, complete blood count, or analysis of blood chemistry; previous hospitalization for flu-like illness; used steroids or immunosuppressants within the last 3 months; major nasal or otologic surgery; psychiatric disorder treated in the last year or psychiatric hospitalization in the last 5 years; were pregnant or currently lactating; seropositive for human immunodeficiency virus (HIV); on regular medication (except birth control); had a cold- or flu-like illness within 3 months of viral-challenge; reported any current acute illness; or were living with someone with a compromised immune system or chronic obstructive pulmonary disease. Baseline immunity to the challenge virus (viral specific antibody titers), demographics, and anthropometrics were also assessed at screening. To maximize the rate of infection, only volunteers with low levels of immunity to the virus (viral-specific antibody titers ≤4 [18-22]) at the medical screening were eligible for participation.
Baseline assessments
During the 8-12 week baseline period between screening and viral-challenge, volunteers meeting inclusion criteria completed questionnaire assessments of SRH and several socio-emotional variables found to predict colds in earlier studies: social integration (18), sociability (19), positive emotional style (20), neuroticism (20), subjective social status (21), psychological stress (23), and parent status (22). Three to five weeks pre-challenge, participants were interviewed by telephone on 14 consecutive evenings. Each interview assessed participants’ health behaviors (see below) and experiences of positive emotions during the last 24 hours, with resulting data being averaged (or summed) across the 14 days. Blood was drawn for assessment of baseline antibody levels to the challenge virus within 5 days of viral-exposure.
Quarantine
Participants were subsequently quarantined in separate rooms for 6 days (baseline [Day 0] and 5 post-challenge days). On Day 0 and prior to viral exposure, participants received an ear, nose, and throat examination, and provided a nasal wash specimen that was cultured for existing viral infection. Baseline objective measures of congestion (nasal mucociliary clearance time) and nasal mucus production were assessed. Five volunteers (not included in the N of 360) were excluded from participation at this point for reporting having a cold or cold-like symptoms, or retrospectively if a viral pathogen was later isolated from the baseline nasal wash.
After collection of Day 0 baseline data, participants were given nasal drops containing 100-300 50% tissue culture infective dose (TCID50) of rhinovirus (RV) 39, a virus that causes a common cold-like illness. Nasal clearance function and mucus production were again assessed on each of the 5 post-challenge days, and daily nasal wash samples were collected for virus culture. Approximately 28 days post-challenge, blood was collected to assay for antibody to RV39. On-site investigators were blinded to all interview, questionnaire and biological measures.
Measures
Self-rated health
SRH was measured using a single item from the Short Form Health Survey (24): In general, would you say your health is…, with response options of excellent, very good, good, fair, and poor.
Standard control variables
Six of the nine covariates were collected at screening: age (continuous), sex (female/male [reference]), race (dichotomized as white/other [reference]; see Table 1), education (represented as 3 dummy-coded variables [bachelor’s degree or higher as reference]: high school or less, some college, and ≥2 years of college with degree), household income (continuous), and body mass index (BMI; continuous weight [kg]/height [m]2). The remaining three control variables were season (represented as 3-dummy coded variables [fall as reference]: winter, spring, and summer) at viral-challenge, Study (PMBC or PCS3 [reference]), and viral-specific immunity (pre-exposure specific antibody to RV39 ≥4 or <4). Although having antibody titers ≤4 at screening was a criterion for inclusion, some participants (26.4%) evidenced titers >4 when pre-exposure levels were re-assessed (0-5 days pre-challenge). The apparent elevation in antibody could be due to assay error or natural exposure to the virus in the interim.
Health practices
Daily health practices were assessed during the 14 consecutive evening telephone interviews. Each evening, participants were asked whether in the past 24 hours they had smoked any tobacco products, consumed alcohol, and exercised long enough to work up a sweat. Positive endorsements were probed for quantities of tobacco products smoked, alcohol consumed, and minutes spent exercising, respectively. Sleep was assessed by asking participants what time they got into bed to sleep last night and what time they woke this morning, how much sleep they lost (minutes) because of difficulty falling asleep or waking up and not being able to return to sleep, and how much time (minutes) they spent intentionally awake.
To obtain maximal measurement reliability, each health practice variable was based on behavior over the 14-days. For analysis, behaviors were dichotomized based on whether participants met the following risk criteria: regular smoking (≥1 cigarette/day or if less than daily, ≥¾ pack on ≥50% of days); heavy drinking (≥15 drinks/week for men and ≥8 drinks/week for women) (25); poor sleep (average duration <7 hours or average sleep efficiency [percent time in bed spent sleeping] <90% (26); and low physical activity (<150 minutes/week) (27). For each variable, participants were given a score of 1 if they met the relevant criterion or 0 if they did not meet the criterion.
Socio-emotional variables
Three dimensions of personality were assessed: sociability (combination of extraversion, agreeableness, and positive relationship style) (19), emotional stability (inverse of neuroticism or negative emotional style), and positive emotional style. In the PMBC study, two of the components of sociability—extraversion and agreeableness—as well as emotional stability were assessed using items derived from Goldberg's Adjective Scale (18,29). Each personality dimension was represented by the 5 highest-loading items for the relevant factor, and internal reliabilities were α = .74 for extraversion and agreeableness, and α = .80 for emotional stability. In PCS3, extraversion, agreeableness, and emotional stability were measured using the relevant 10-item Big-Five subscales of the International Personality Item Pool (IPIP) (30), with internal reliabilities of α = .88 for extraversion and emotional stability, and α = .85 for agreeableness. Correlations between the adjective subscales and the IPIP have been reported as being between .72 and .84 for extraversion and emotional stability, and between .54 and .66 for agreeableness (31). To establish inter-study equivalency, standardized scores (z-scores) were computed for each personality subscale prior to inclusion in analysis. In both studies, the nine-item Positive Relationship with Others Scale (32) was used to assess the third component of sociability, positive relationship style (α = .75). To create a single, continuous sociability score, we summed the z-score transformations of the extraversion, agreeableness, and positive relationship style scales.
Positive emotional style (PES) was assessed in both studies during the daily interviews (see above). At the end of each interview, participants were asked how accurately (0=not at all accurate to 4=extremely accurate) each of 6 positive adjectives described how they felt during the past 24 hours. Adjectives represented three subcategories of positive emotion: vigor (lively, full-of-pep), well-being (happy, cheerful) and calm (at ease, calm). For each of the 14 days, daily positive mood scores were calculated by summing the ratings of the 6 adjectives. Internal-reliabilities for the 14 assessments ranged between 0.83 and 0.89. The summary measure of PES was created by averaging daily positive mood scores across the 14 days (20).
Additionally, two social factors were assessed that had been associated with host resistance in earlier studies, social integration (18) and subjective socioeconomic status (21). Social integration—the diversity of social roles in which one participates, was measured using the Social Network Index (SNI) (18) which assesses participation in 12 types of social roles: spouse/partner, child, son- or daughter-in-law, parent, other close family member, friend, neighbor, workmate, schoolmate, volunteer (e.g., charity or community work), religious group member, and member in groups without religious affiliations (e.g., social, recreational, professional). Participants were determined to hold a given social role if they interacted (in person or by telephone) with someone in the relevant relationship at least once every 2 weeks. Social integration was assessed by assigning one point for each endorsed social role and then summing the total number of roles (possible range, 0 to 12).
Subjective socioeconomic status was assessed using the MacArthur Scale of Subjective Social Status (21,33). Participants were presented with an illustration of a 9-rung ladder and instructed to interpret it as representing where people stand in the United States in terms of income, education, and occupational standing. They were then asked to rank their standing relative to other persons in the United States by placing an “X” on the appropriate rung of the ladder (possible range, 1 to 9)
Finally, psychological stress was assessed using the 10-item Perceived Stress Scale (PSS-10) (28), which assesses how unpredictable, uncontrollable, and overloaded respondents’ lives have been in the last month. Internal reliability for the PSS was α = .85.
Clinical colds
Volunteers were considered to have developed a clinical cold if they were both infected with the challenge virus and met illness criteria (18).
Infection was defined as recovery of the challenge virus in nasal lavage samples on any of the 5 post-challenge days (cultured using standard procedures) (34) or a 4-fold or greater rise in virus-specific serum neutralizing antibody titer from pre-exposure to 28-days post-exposure (using microtiter neutralizing assay) (35).
We assessed two objective markers of illness: increased nasal mucus production (weight) and decreased nasal mucociliary clearance function. Mucus production was assessed during each day in quarantine by collecting used tissues in sealed plastic bags, weighing the bags, and then subtracting the pre-use weights of the tissues and bags from the measured total (36). Mucociliary clearance function was assessed as the time required for a solution administered into the anterior nose to reach the nasopharynx (36). Baseline-adjusted daily scores for each measure were calculated by subtracting the appropriate baseline score from each of the 5 post-exposure daily scores, with negative values being re-scored to 0. The criterion for signs of illness required a total adjusted mucus weight (summed across the 5 post-challenge days) of ≥ 10 grams or an average adjusted post-challenge nasal clearance time of ≥ 7 minutes (18).
Data Analyses
The data were analyzed using IBM SPSS Statistics software, version 21, and figures were generated using Microsoft Excel 2010. Descriptive statistics are presented as means and standard deviations (SD) or as percentages. We compared continuous sample characteristics across the SRH groups by analysis-of-variance and frequencies of dichotomous variables by X2. For the main analyses, we used binary multiple logistic regression, fitting both unadjusted and adjusted (for nine standard control variables) models. We report odds ratios (OR), 95% confidence intervals (CI), and p values. Potential roles of health practices and socio-emotional variables are assessed by adding them as covariates to the adjusted model. All tests were two-tailed, with the criterion for statistical significance being set at p < .05.
Post-Hoc Testing of the Effect of History of Colds
A remaining issue in interpreting of our findings was whether it is possible that participants’ SRH was determined to some extent by their histories of colds and/or their perceptions of susceptibility to the common cold, and in turn that these factors predict susceptibility in the viral-challenge trial. Cold histories were not collected in PMBC or PCS3. Thus, the issue was addressed by analyzing archived data collected in Pittsburgh Cold Study 1 (PCS1), a similar viral-challenge trial. Although SRH was not assessed in PCS1, data collected on participants’ illness histories and perceptions of cold susceptibility permitted examination of whether these variables predicted cold risk.
Participants
The sample included 276 healthy adults, aged 18 to 55 years who were recruited from the Pittsburgh, PA metropolitan area via newspaper advertisements and community postings. One participant was excluded from the present analyses due to missing pre-challenge mucus weight data. The remaining 275 participants were 54.9% female, 81.5% white, and had an average age of 29.08 (SD = 9.06) years. All participants provided informed consent and received financial compensation for study participation. Study procedures were approved by the appropriate institutional review boards.
Procedures
The viral challenge protocol was identical to that described above for PMBC and PCS3, save for participants being experimentally challenged with either of two strains of rhinovirus: RV39 (n = 147) or Hanks (n = 128).
Measures
Clinical colds were defined as described earlier. PCS1 also included 8 of the 9 standard control variables that were assessed using the same methods as described above. The study (PMBC vs. PCS3) indicator variable was omitted since it was no longer relevant, and replaced by a virus indicator variable representing whether participants had been infected with RV39 or Hanks.
Recent cold history was assessed at the screening physical exam (6 weeks pre-quarantine) with the following open-ended items: “How many colds did you have in the past year?” and “What was the duration (days) of your last cold?” Participants also were asked to estimate how many colds they have in the average year. Perceived likelihood of developing a cold was assessed using the following item, administered on Quarantine Day 0, immediately following viral challenge: “On average, about one-third of the people in our studies develop colds. How likely do you think it is that you will develop a cold?” Response options were presented on a 5-point scale ranging from “not at all likely” to “extremely likely”. Data analyses were conducted using logistic regression as described earlier.
RESULTS
Self-Rated Health and Colds (PMBC & PCS3)
Twenty percent of participants (n = 72) rated their health as being excellent; 53% (n = 191) very good; 25% (n = 89) good; 2% (n = 8) fair; and 0% poor. Because few participants endorsed fair health and none endorsed poor health, SRH was recoded into a three-level categorical variable for analysis (excellent [reference], very good, and good or fair).
Table 1 presents characteristics of the sample (standard controls, health practices, socio-emotional variables) by the three categories of SRH. Groups did not differ on any of the standard control variables save for BMI, which increased in a graded fashion with decreasingly favorable SRH. The prevalences of all health behaviors (regular smoking, heavy drinking, poor sleep, and not meeting the recommended 150 minutes/week of physical exercise) except for heavy drinking increased in a graded fashion with less favorable SRH; a relative excess in heavy drinking appeared only for the good/fair group. Finally, in the case of socio-emotional factors, there were increases in perceived stress and decreases in PES and subjective socioeconomic status with less favorable SRH, as well as trends for decreasing sociability and social integration with decreasing SRH. These associations are all consistent with known relations of these variables with self-reported symptoms and health (20). By contrast, SRH groups did not differ on emotional stability or parent status.
Eighty percent of participants (n = 289) became infected with the challenge virus and 33% (n = 117) met criteria for a clinical cold. Figure 1 displays adjusted rates of colds by level of SRH, and Table 2 presents results of unadjusted and adjusted logistic regression analyses. Inspection of the raw odds ratios (Table 2, Model 1) reveals a graded association of SRH with cold risk: relative to participants who reported excellent health, those reporting good/fair health had almost 3 times the odds of meeting criteria for clinical illness and those reporting very good health, more than double the odds. Addition of the standard covariates did not appreciably influence the association (Table 2, Model 2).
Figure 1.
Cold rates by category of self-rated health (SRH). Percentages are adjusted for age, sex, race, pre-challenge antibody level, season, body mass index, educational attainment, and household income.
Table 2.
Multivariable Logistic Regression Analyses of Self-Rated Health with Odds of Developing a Cold Following Experimental Exposure to Rhinovirus (RV) 39
| Excellent (n = 72)a |
Very Good (n = 191) |
Good or Fair (n = 97) |
|||
|---|---|---|---|---|---|
| OR a (95% CI) | P Value | OR (95% CI) | P Value | ||
| Model 1 | Reference | 2.04 (1.06-3.93) | 0.034 | 2.91 (1.43-5.91) | 0.003 |
| Model 2 | Reference | 2.60 (1.27-5.32) | 0.008 | 3.21 (1.47-6.99) | 0.003 |
| Model 3 | Reference | 2.44 (1.18-5.05) | 0.016 | 2.85 (1.27-6.37) | 0.011 |
| Model 4b | Reference | 2.21 (1.06-4.64) | 0.035 | 2.85 (1.25-6.50) | 0.013 |
| Model 5b | Reference | 2.18 (1.03-4.60) | 0.042 | 2.65 (1.14-6.19) | 0.024 |
Model 1: self-rated health only
Model 2: adjusts for age, sex, race, pre-challenge Ab titer ≥4, study, season, body mass index, educational attainment, and household income.
Model 3: Model 1 + regular/heavy smoking, heavy drinking, low physical activity, and poor sleep.
Model 4: Model 1 + parent status, perceived stress, emotional stability, positive emotional style, sociability, social integration, and subjective social standing.
Model 5: Model 3 + Model 4.
Odds ratios (ORs) represent the incremental risk of developing a cold associated with reporting very good SRH and good or fair SRH, respectively, relative to the reference category (i.e., excellent SRH).
Sample was reduced to 359 (Excellent = 72; Very good = 191; Good or fair = 96) due to missing data.
We fit a set of additional models (one for each covariate) adding the interaction of SRH with the covariate to Model 2. The association of SRH with colds was consistent across levels of all the covariates (ps for all interactions >.09). Importantly, the association between SRH and colds was equivalent across studies (p >.66).
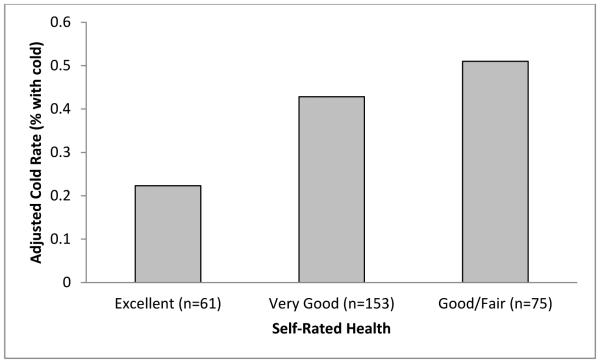
The increased risk of developing a cold associated with less favorable SRH may be due to increased susceptibility to infection and/or increased illness expression among the infected. Results of adjusted analyses showed no relation of SRH to infection (good/fair: OR = 0.60, 95% CI = 0.25-1.47, p = .265; very good: OR = 0.84, 95% CI = 0.38-1.86, p = .674). However, adjusted analyses limited to the 289 infected participants revealed graded associations of SRH with the odds of meeting criteria for both mucus production and nasal clearance time (see Figure 2 and Table 3).
Figure 2.
Cold rates among infected participants (n = 289) by category of self-rated health (SRH). Percentages are adjusted for age, sex, race, pre-challenge antibody level, season, body mass index, educational attainment, and household income.
Table 3.
Results of Logistic Regression Analyses Examining Associations of Self-Rated Health with the Odds of Meeting Objective Illness Criteria Among Infected Subjects (n = 289)
| Nasal Mucus Production Criterion a |
Nasal Mucociliary Clearance Criterion b |
Combined Cold Criteria c |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||||
| Self-Rated Health |
ORd
(95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P |
| Excellent (n=61) |
Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Very good (n=153) |
2.63 (1.23-5.59) |
.012 | 3.12 (1.37-7.13) |
.011 | 2.92 (0.97-8.75) |
.056 | 3.59 (1.12-11.47) |
.031 | 2.35 (1.19-4.63) |
.014 | 2.99 (1.40-6.36) |
.005 |
| Good or fair (n=75) |
3.22 (1.41-7.31) |
.005 | 3.25 (1.32-8.04) |
.007 | 7.13 (2.32-21.88) |
.008 | 7.98 (2.39-26.66) |
.001 | 3.84 (1.81-8.12) |
.001 | 4.19 (1.81-9.71) |
.001 |
Adjusted models control for age, sex, race, pre-challenge Ab titer ≥4, study, season, body mass index, educational attainment, and household income. For all analyses,
Total baseline-adjusted post-challenge mucus weight ≥10 grams.
Average baseline-adjusted post-challenge nasal clearance time ≥7 minutes.
Meets either of the above criteria.
Odds ratios (ORs) represent the incremental risk of meeting the specified illness criterion associated with reporting very good SRH and good or fair SRH, respectively, relative to the reference category (i.e., excellent SRH).
To determine whether the 4 health-related behaviors accounted for the association between SRH and cold risk, we added them to the logistic regression model including SRH and the standard covariates. Inclusion of these variables resulted in a 6.2% reduction in the OR associated with reporting very good SRH and an 11.2% reduction in the OR associated with good or fair SRH (Table 2, Model 3). Inclusion of the socio-emotional variables into the adjusted model with SRH resulted in a 15.3% reduction in the effect of reporting very good relative to excellent SRH, and a 13.1% reduction in the effect of reporting good or fair SRH relative to excellent (Table 2, Model 4).
In the final model, we added the four health-related behaviors and seven socio-emotional factors to the adjusted model with SRH. The increased odds of developing a cold with less favorable SRH remained robust, albeit somewhat reduced in size (very good vs. excellent, ΔOR = 16.5%; good or fair vs. excellent, ΔOR = 19.2%; Table 2, Model 5).
History of colds, self-rated health and host resistance (PCS1)
Forty percent (n = 111) of the participants met criteria for a cold. Table 4 displays descriptive data for the four cold history items and participant perceived likelihood of developing a cold, as well as the associations of these variables with cold risk. As apparent from the table, only the duration of participants’ most recent cold predicted disease susceptibility.
Table 4.
Results of Logistic Regression Analyses Examining Associations of Self-Reported History of Colds and Expectations of Illness with Risk of Developing a Cold in Pittsburgh Cold Study 1 Analysis.
| Descriptive Data |
Association with Colds |
|||||
|---|---|---|---|---|---|---|
| SRH Variable | n | Median | Range | ORa | 95% CI | p |
| Average number of colds per year | 270 | 2.0 | 0 to 10 | 1.13 | 0.91, 1.40 | .273 |
| Number of colds in past year | 271 | 1.0 | 0 to 10 | 1.04 | 0.83, 1.31 | .739 |
| Duration of most recent cold (days) | 242 | 4.0 | 0 to 42 | 1.14 | 1.02, 1.28 | .025 |
| Likelihood of getting a cold b | 275 | 1.0 | 0 to 4 | 1.21 | 0.91, 1.61 | .187 |
Analyses adjust for age, sex, race, virus, pre-challenge Ab, season, and body mass index
Response scale: 0 = not at all likely, 1 = a little likely, 2 = moderately likely, 3 = quite likely, 4 = extremely likely
DISCUSSION
We found that poorer SRH was associated with greater susceptibility to developing a common cold in healthy adults experimentally exposed to a cold virus. The association was graded, with risk increasing as SRH decreased, and was independent of sociodemographics, pre-challenge immunity (specific antibody titer) to the virus, BMI, season, and whether enrolled in the PMBC study or PCS3. The greater risk for illness was not attributable to the probability of infection, but to the likelihood of developing objective signs of illness once infected. Illness expression in colds is generally attributed to blunted down-regulation of local inflammatory response (37,38).
Poorer SRH also was associated with regular smoking, heavy drinking, less exercise, and poorer sleep, as well as with a greater BMI, a health marker possibly influenced by both poorer diet and less physical activity. Further, SRH correlated with a range of socio-emotional factors that have been found to predict colds in earlier studies, including less stress (23), higher levels of positive emotional style (20), and lower subjective socioeconomic status (21). However, none of these health-related behaviors or socio-emotional factors (alone or together) could account for more than 20% of the association of SRH with colds. Nonetheless, these variables may play a role in tying SRH to risk for diseases other that the common cold.
Unique to this study was the outcome--an assessment of immunocompetence in response to viral challenge. That we found SRH to predict immunocompetence suggests a possible pathway explaining associations of SRH with longevity. It also establishes this simple categorical measure of health as a risk factor for acute upper respiratory viral illnesses in younger adults, in addition to its known importance as a predictor of future health status in older persons.
Because we tested our hypotheses in a “healthy” younger adult sample, we were able to demonstrate that even after removing popular explanations for effects of SRH (baseline health, socio-emotional variables and health practices), it was still strongly associated with immunocompetence, a process that is central to health and accounts for a wide range of morbidities and mortality. These data are consistent with prospective epidemiologic findings from older adult samples which similarly fail to show that socioemotional variables or health practices are fundamental to explaining the association between SRH, disease and mortality (6-8).
The study is not without weaknesses. Although participants were chosen for good health, allergic status was not included as a criterion for exclusion. Allergic status could provide a basis for both SRH and host resistance. Findings from an earlier viral-challenge study, though, indicated no association between allergies and susceptibility to common cold viruses including RV (23). Moreover, in the current study, being bothered by allergies during the daily interviews was unrelated to cold risk (p=.79; data not reported). The skewed distribution of SRH in our study may limit the generalization of our results since older adult samples include a large percentage of participants reporting fair or poor health. This limited variation in SRH may also have resulted in our underestimating the real effect size. Finally, while we controlled for the most obvious socio-emotional explanations, there are other possibilities we have not covered, for example family history of illness or childhood illness.
A remaining issue critical to the interpretation of our findings is whether participants’ SRH was determined to some extent by their histories of colds and/or their perceptions of susceptibility to the common cold, and in turn that these factors predict susceptibility in the viral-challenge trial. We addressed this issue by analyzing archived data collected in PCS1, a similar viral-challenge trial, where we queried participants at baseline about their cold history. Although we did not obtain SRH assessments in PCS1, we were able to assess whether participants’ illness histories and perceptions of cold susceptibility predicted whether they developed a cold. Because neither the number of previous colds nor the perceived likelihood of getting a cold was associated with susceptibility to the viral-challenge, neither variable is likely to explain the association between SRH and cold susceptibility we found in the analyses of the PMBC/PCS3 data. The results do, however, leave the possibility that the duration of participants’ last cold is responsible for the association. This explanation is speculative because SRH was not measured in PCS1. Thus, we do not know whether SRH is associated with cold duration, let alone whether duration operates as either a mediator or confounder. Moreover, the possibility that the association of SRH and cold susceptibility is attributable to duration of the last cold seems unlikely given that the participants (by screening) could not have had a cold or flu-like illness within a minimum of 3 months of viral-challenge, and we assume most hadn’t had one within 6 months to 2 years. Hence to play a significant role in their assessment of SRH, the participants would need to be basing their current SRH on a relatively distant event.
Why, then, does SRH predict immunocompetence? It is possible that people may be indirectly sensitive to their general ability to resist disease. This explanation does not assume direct knowledge of whether the immune or other systems are operating at optimum efficiency, but rather that it may be possible to sense symptoms of their failure; for example, being aware of visceral sensations symptomatic of low grade infections or other premorbid disease processes.
In sum, SRH in relatively young healthy adults is associated with susceptibility to a viral-induced upper respiratory illness. This association is not attributable to demographics, prior immunity to the virus, health practices, or socio-emotional factors known to predict disease susceptibility and/or to be associated with SRH. The increasing evidence that SRH is a sensitive marker of disease risk, even in healthy young and middle-aged adults (39) suggests that physicians might integrate its use with more traditional indicators of disease risk. This may constitute an inexpensive, noninvasive method of screening for possible underlying disease susceptibility, especially related to immunocompetence.
Acknowledgements
The authors gratefully thank Dr. Ronald Turner, James Seroky, Juliane Banks, Ellen Conser and Chloe Detrick for their contributions to this research.
Sources of Funding
The study was funded by grants from the National Center for Complementary and Integrative Health (AT006694), the National Institute of Allergy and Infectious Diseases (AI066367) and National Heart, Lung, and Blood Institute (HL65111, HL65112); supplementary support was provided by the National Institutes of Health to the University of Pittsburgh Clinical and Translational Science Institute (UL1 RR024153, UL1 TR0005).
Abbreviations
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- HIV
human immunodeficiency virus
- IPIP
International Personality Item Pool
- PCS3
Pittsburgh Cold Study 3
- PMBC
Pittsburgh Mind-Body Center
- RV
rhinovirus
- SNI
Social Network Index
- SRH
self-rated health
- TCID50
50% tissue culture infective dose
Footnotes
Conflicts of Interest
The authors declare that they have no competing interests relevant to this article.
References
- 1.Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344–S353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- 2.Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality: additional studies, 1995 to 1998. Res Aging. 1999;21:392–401. [Google Scholar]
- 3.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 4.Idler EL, Hudson SV, Leventhal H. The meanings of self-ratings of health: A qualitative and quantitative approach. Res Aging. 1999;21:458–476. [Google Scholar]
- 5.Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan GA, Camacho T. Perceived health and mortality: a nine-year follow-up of the human population laboratory cohort. Am J Epidemiol. 1983;117(3):292–304. doi: 10.1093/oxfordjournals.aje.a113541. [DOI] [PubMed] [Google Scholar]
- 7.Benjamins MR, Hummer RA, Eberstein IW, Nam CB. Self-reported health and adult mortality risk: an analysis of cause-specific mortality. Soc Sci Med. 2004;59:1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Stenholm S, Pentti J, Kawachi I, Westerlund H, Kivimäki M, Vahtera J. Self-Rated Health in the Last 12 Years of Life Compared to Matched Surviving Controls: The Health and Retirement Study. PLoS ONE. 2014;9(9):e107879. doi: 10.1371/journal.pone.0107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossey JM, Shapiro MA. Self-rated health: A predictor of mortality among the elderly. AJPH. 1982;72:800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas AK, Lichtman AH, Pober JS. Cell Mol Immunol. W.B. Saunder Company; Philidelphia: 1991. [Google Scholar]
- 11.Burnet FM. Immunological Surveillance in Neoplasia. Immunol Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 12.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA. Karnofsky Memorial Lecture: the immunotherapy and gene therapy of cancer. J Clin Oncol. 1992;10:180–99. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. U S A. 2003;100(15):9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jylha M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J Clin Epidemiol. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Cohen HJ, Pieper CF, Harris T, Murali K, Rao K, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52A:M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 277;1997:1940–1944. [PubMed] [Google Scholar]
- 19.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Sociability and susceptibility to the common cold. Psychol Sci. 2003;14:389–395. doi: 10.1111/1467-9280.01452. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosom Med. 2003;65:652–657. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Alper C, Adler N, Treano JJ, Turner RB. Objective and Subjective Socioeconomic Status and Susceptibility to the Common Cold. Health Psychol. 2008;27(2):268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 22.Sneed R, Cohen S, Turner RB, Doyle WJ. Parenthood and host resistance to the common cold. Psychosom Med. 2012;74:567–573. doi: 10.1097/PSY.0b013e31825941ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress in humans and susceptibility to the common cold. NEJM. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr., Snow KK, Kosinksi M, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide. 1993 [Google Scholar]
- 25.Fact Sheets-Alcohol use and Your Health Centers for Disease Control and Prevention Web Site. 2014 http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. Updated August 19, 2014. Accessed December 1, 2014.
- 26.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.How much physical activity do adults need? Centers for Disease Control and Prevention Web Site. 2011 http://www.cdc.gov/physicalactivity/everyone/guidelines/adults.html. Updated March 3, 2014. Accessed December 1, 2014.
- 28.Cohen S, Kamarck K, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 29.Goldberg LR. The development of markers for the Big-Five factor structure. Psychol Assess. 1992;4:26–42. [Google Scholar]
- 30.Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, Gough HG. The international personality item pool and the future of public-domain personality measures. J Res Pers. 2006;40(1):84–96. [Google Scholar]
- 31.Saucier G, Goldberg LR. In: Big Five Assessment. De Raad B, Perugini M, editors. Hogrefe & Huber; Boston, MA: 2002. pp. 29–58. Chapter 2, Assessing the Big Five: Applications of 10 psychometric criteria to the development of marker scales. [Google Scholar]
- 32.Ryff C. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J Pers Soc Psychol. 1989;57:1069–1081. [Google Scholar]
- 33.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physical health: Preliminary data in healthy white women. Health Psychol. 2000;19:585–591. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 34.Gwaltney JM, Jr., Colonno RJ, Hamparian VV, Turner RB. Schmidt NJ, Emmons RW, editors. Rhinovirus. Diagnostic procedures for viral, rickettsial and chlamydial infections. 1998;6:579–614. [Google Scholar]
- 35.Al Nakib W, Tyrrell DAJ. Lennett EH, Halnen P, Murphy FA, editors. Picornviridae: Rhinoviruses—common cold viruses. Laboratory Diagnosis of Infectious Diseases: Principles and Practice. 1988;2:723–742. [Google Scholar]
- 36.Doyle WJ, McBride TP, Swarts JD, Hayden FG, Gwaltney JM., Jr The response of the nasal airway, middle ear and Eustachian tube to provocative rhinovirus challenge. Am J Rhinol. 1988;2:149–154. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 37.Proud D, Gwaltney JM, Jr, Hendley JO, Dinarello CA, Gillis S, Schleimer RP. Increased levels of interleukin-1 are detected in nasal secretions of volunteers during experimental rhinovirus colds. J. Infect. Dis. 1994;169:1007–1013. doi: 10.1093/infdis/169.5.1007. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Z, Tang W, Ray A, Wu Y, Einarsson O, Landry ML, Gwaltney J, Jr, Elias JA. Rhinovirus stimulation of interleukin-6 in vivo and in vitro: evidence for NF-kB dependent transcriptional activation. J. Clin. Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenholm S, Pentti J, Kawachi I, Westerlund H, Kivimäki M, Vahtera J. Self-rated health in the last 12 years of life compared to matched surviving controls: the Health and Retirement Study. PloS one. 2014;9:e107879. doi: 10.1371/journal.pone.0107879. [DOI] [PMC free article] [PubMed] [Google Scholar]