Abstract
Fluorine NMR spectroscopy is a powerful tool for studying biomolecular structure, dynamics, and ligand binding, yet the origins of 19F chemical shifts are not well understood. Herein, we use electronic structure calculations to describe the changes in 19F chemical shifts of 2F- and 4F-histidine/(5-methyl)-imidazole upon acid titration. While the protonation of the 2F species results in a deshielded chemical shift, protonation of the 4F results in an opposite, shielded chemical shift. The deshielding of 2F-histidine/(5-methyl)-imidazole upon protonation can be rationalized by concomitant decreases in charge density on fluorine and a reduced dipole moment. These correlations do not hold for 4F-histidine/(5-methyl)-imidazole, however. Molecular orbital calculations reveal that for the 4F species, there are no lone pair electrons on the fluorine until protonation. Analysis of a series of 4F-imidazole analogues, all with delocalized fluorine electron density, indicates that the deshielding of 19F chemical shifts through substituent effects correlates with increased C-F bond polarity. In summary, the delocalization of fluorine electrons in the neutral 4F species, with gain of a lone pair upon protonation may help explain the difficulty in developing a predictive framework for fluorine chemical shifts. Ideas debated by chemists over 40 years ago, regarding fluorine's complex electronic effects, are shown to have relevance for understanding and predicting fluorine NMR spectra.
Introduction
While we have a robust framework for understanding and predicting NMR spectra for 1H and 13C nuclei, the origins of 19F chemical shifts are not as well understood. Fluorine nuclei are known to be sensitive probes of local environment,1-6 and biosynthetic incorporation of fluoro-labeled amino acids into proteins allows the use of 19F-NMR for studies of protein structure, dynamics, and ligand binding.7-23 Despite the sensitivity of 19F-NMR and the breadth of literature on proteins labelled with 19F-amino acids, there is still no unifying theoretical basis for predicting fluorine chemical shifts in proteins. When scientists wish to determine which fluorine chemical shift in a protein comes from a certain residue, it is necessary to make mutants at every fluorinated site, one-by-one eliminating fluorine NMR signals.16,17,23,24 Such work is extremely time-intensive. This report is a step toward developing a quantitative predictive framework5,25,26 for 19F chemical shifts, which will enable greater chemical insight. Such an advance would be a breakthrough, streamlining experiments and realizing the full potential of 19F NMR spectroscopy.
Experimental work over four decades ago used substituent effects to build an understanding of fluorine electronic structure through 19F NMR spectroscopy. Studies of the fluorine chemical shifts of substituted aromatic systems led scientists to postulate fluorine hyperconjugation,27 and donation of fluorine p electrons to the pi aromatic system (p-π donation).28 Later experiments extended to substituted aliphatic systems, investigating polar and resonance effects on aliphatic fluorine chemical shifts.4 This present work examines effects of fluorine electron delocalization on chemical shifts, through analysis of molecular orbitals calculated with DFT.
Previous theoretical efforts have also advanced our understanding of fluorine chemical shifts. This includes the assignment of fluorine signals from 5-fluorotryptophan residues in the solid-state NMR spectrum of the membrane-bound ion channel peptide gramicidin A by Sternberg et al.,29 using ab initio calculations and semi-empirical bond polarization parameters for chemical shift calculations. Coupling calculated 19F chemical shifts with Molecular Dynamics (MD) simulations, the authors were able to describe multiple conformations of tryptophan side chains in gramicidin A. Analysis by Lau and Gerigof the fluorine chemical shifts of dihydrofolate reductase (DHFR) labelled with 6-fluorotryptophan suggested that differences in fluorine chemical shifts are a sum of the following factors: hydrogen bonding, short-range interactions, electric fields and local magnetic anisotropies.3 Additionally, Dalvit and Vulpetti classified different fluorine-containing functional groups based on fluorine electron density and their interactions with proteins.30 Their experimental and theoretical results showed that the most-shielded fluorine atoms are most likely to form interactions with hydrogen bond donors of a protein. On the other hand, more deshielded fluorine atoms interact with hydrophobic side chains and carbonyl carbons. These efforts in the past decade are significant steps toward understanding 19F-NMR spectra. Still, a framework for the a priori assignment and prediction of fluorine chemical shifts remains to be developed.
Toward providing a better theoretical basis for predicting 19F-chemical shifts, we have sought to explain the physical origins for a long-standing mystery regarding the 19F chemical shifts of fluorohistidine isomers upon acid titration. Yeh et al. studied 19F and 1H NMR spectra for 2-fluoro- and 4-fluoro- histidine, -imidazole, and –(5-methyl)-imidazole in aqueous, basic, and acidic solutions.31 As expected, Yeh et al. found that the 1H chemical shifts for all fluoroisomersare deshielded upon protonation of the imidazole ring.31 Likewise, 2F-histidine and 2F-imidazoles exhibited a downfield (higher frequency) shift in the 19F signal upon acid titration. However, 4F-histidine and 4F-imidazoles exhibit an upfield (lower frequency) shift in the 19F signal at low pH.
We have identified electronic structure methods that can replicate the experimental 19F chemical shifts of 2- and 4-fluoro-histidine and –(5-methyl)-imidazole upon acid titration. Analysis provides a plausible explanation for the anomalous chemical shift changes observed. This system is a key place to start work on a rational framework for 19F chemical shifts. Since the NMR spectra of 2 & 4 fluorohistidines are nearly identical to their imidazole analogues, the discussion here focuses on 2- & 4- (5-methyl)-imidazole, given that they are small and easily amenable to computation. Understanding the physical origins of these specific chemical shift changes may provide clues that, in turn, will lead to a better understanding of 19F chemical shift differences in proteins.
2 Methods
All the input files were prepared in Gauss View 5,32 modifying the geometry provided in the 2F-histidine crystal structure33 for desired protonation states and C5 substitution. All the electronic calculations were performed using Gaussian 09 software.34 Optimized geometries, energies, 19F NMR shifts, electrostatic charge distributions, and Natural Chemical Shielding (NCS)35 calculations were performed with multiple methods to ensure general conclusions. Calculations were performed with the BHandHLYP and B3LYP density functionals and the MP2 method,36-40 using 6-311++G(3df,2p), aug-cc-pVTZ, and 6-31+G* basis sets and water solvation with SMD41 and CPCM42 solvent models. Negative frequencies were not observed for any molecule, indicating that the geometries are at energy minima. All reported values are from calculations using BHandHLYP40 hybrid density functional, 6-311++G(3df,2p) basis and SMD water solvation.41 Values from other methods are given in supplementary information, Table S1. Reported relative energies are free energies given from frequency calculations, setting the energy of the most stable tautomer to zero. NMR values are calculated with Gauge Independent Atomic Orbital (GIAO)43 method, and isotropic chemical shifts are reported. Molecular orbitals are visualized using Avogadro software.44 The standard reference for the 19F NMR values reported in Table 1 is C6F6, with shifts reported as σref- σcalc. The value of σref comes from an optimized geometry of hexafluorobenzene with BHandHLYP/6-311++G(3df,2p) method, and SMD model water solvation.
Table 1.
19F NMR shifts (experimental and calculated) and fluorine electrostatic potential (charge) for stable tautomers of 2-fluoro- and 4-fluoro-imidazole analogues.
| 2F isomers, τ-tautomers | 2F-His | 2F-MeIm | 2F-Im |
|---|---|---|---|
| ESP charge | -0.264 | -0.278 | -0.280 |
| 19F NMR shifts (calculated) | 54.96 | 53.62 | 52.50 |
| 19F NMR shifts (experimental) | 59.80 | 58.59 | 56.60 |
| 4F isomers, π-tautomers | 4F-His | 4F-MeIm | 4F-Im |
| ESP charge | -0.290 | -0.275 | -0.308 |
| 19F NMR shifts (calculated) | 16.54 | 13.83 | 21.97 |
| 19F NMR shifts (experimental) | 20.25 | 16.91 | 23.43 |
3 Results
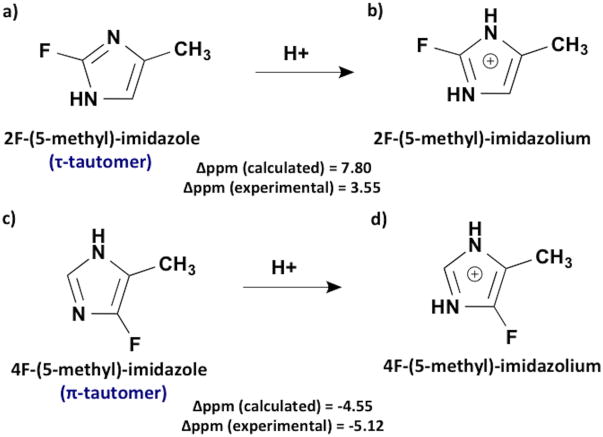
At neutral pH, the imidazole ring exists in two tautomeric forms: τ (protonation at N3) or π (protonation at N1) (Fig. 1), depending on which nitrogen is protonated.45 The tautomeric states of fluorohistidine/imidazoles have not been definitively determined by experiment, although the crystal structure suggests 2F-histidine is protonated at N3 (the τ-tautomer – Fig. 1a).33 Thus, we carried out calculations on both tautomers of each fluoroisomer. The π-tautomer of 4F-histidine/(5-methyl)-imidazole was found to be more stable than the τ-tautomer (by ∼15.0/25.8 kJ/mol using BHandHLPY/6-311++g(3df,2p) method). The two tautomers of 2F-(5-methyl)-imidazole were found to be nearly isoenergetic. However, we found that τ-tautomer of 2F-histidine is more stable than π-tautomer (by 5.3 kJ/mol), as it is in canonical histidine.45, 46 Our discussion from this point focuses on 2F- and 4F-(5-methyl)-imidazoles, since the simplified systems (relative to zwitterionic fluorohistidines) allow for more straightforward analysis, but shed light on experimental results for fluorohistidines.
Fig. 1.
Chemical structures and 19F chemical shifts (Δppm= δIm+ - δIm) for (a) neutral N3-H/τ-tautomer and (b) protonated 2F-(5-methyl)-imidazole; (c) neutral N1-H/π-tautomer and (d) protonated 4F-(5-methyl)-imidazole.
The results reported herein are for chemical shifts of the putatively more stable tautomers of each fluoro-isomer: τ-tautomer (N3-H) for 2F-(5-methyl)-imidazole (Fig. 1a) and π-tautomer (N1-H) for 4F-(5-methyl)-imidazole, shown in Fig. 1c. Reassuringly, we found that the direction of the 19F chemical shifts upon titration did not depend on the computational method used. The BHandHLYP/6-311++G(3df, 2p) calculations with SMD water solvation gave the best quantitative agreement with experimental Δppm values, where Δppm is the difference between the chemical shifts of the fluoroimidazolium and the fluoroimidazole (Δppm= δIm+ - δIm, given as Δ1 in Ref. 31).
Previous experimental work on the protonation of fluoropyridines, with results akin to 4F-(5-methyl)-imidazole, led researchers to postulate that anomalous values of Δppm arise from magnetic anisotropy.47 Although we cannot account for the contribution of magnetic anisotropy in the method used here, the isotropic values in the chemical shielding tensor are able to reproduce the magnitude and direction of the fluorine chemical shifts of fluoroimidazoles/histidines upon protonation.
For clues to the differences between 4F- vs 2F- 19F chemical shifts, we first looked at the charges on the fluorine atom to get a simple picture of the electron density available to shield the nucleus. In both the 2-fluoro and 4-fluoro cases, the charge on fluorine decreased upon protonation, as would be expected for systems gaining positive charge. For 2F-(5-methyl)-imidazole, the fluorine electrostatic charges calculated by the ChelpG procedure48 were -0.278 for neutral and -0.153 for protonated form. For 4F-(5-methyl)-imidazole, the charges on fluorine were -0.275 (neutral) and -0.164 (protonated). Using straightforward shielding arguments by electron density, the deshielded 19F chemical shifts for 2-fluorohistidine/imidazoles make sense, corresponding to reduced charge density. Meanwhile, the increase in shielding observed for 4F-imidazoliums is puzzling, given the decrease in charge density that would seem to indicate less shielding.
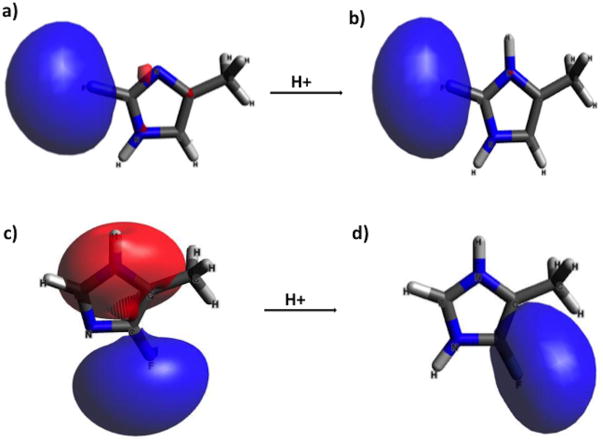
Next, we extended our analysis to Natural Chemical Shielding (NCS) analysis,35 which provides a breakdown of contributions from each molecular orbital to the overall isotropic and anisotropic chemical shift tensors. This was used to assess changes in shielding/deshielding from each molecular orbital from protonation of the fluoroimidazole rings. Out of 26 total molecular orbitals (MOs), 18 contribute to the 19F isotropic chemical shifts of 2F-(5-methyl)-imidazole and 4F-(5-methyl)-imidazole. In mapping the corresponding molecular orbitals of 2F-(5-methyl)-imidazole and 2F-(5-methyl)-imidazolium to determine NCS contributions, we found that the molecular orbitals of the neutral and protonated species were very similar. There are differences in contributions to the fluorine chemical shift from every MO, but many of them appear to cancel each other out. One would expect that electrons highly localized around fluorine would have the greatest contribution to shielding of the 19F nucleus. The calculated molecular orbital 2 (MO 2, the second-lowest in energy), visualized in Fig. 2a and 2b using Avogadro software, corresponds to a lone pair orbital on fluorine. Differences between the Natural Chemical Shielding of this MO in 2F-(5-methyl)-imidazole and 2F-(5-methyl)-imidazolium results in a deshielded shift (4.3 ppm downfield/higher frequency). The overall difference in chemical shift was calculated to be 7.8 ppm (experimentally31 it is 3.55 ppm), indicating that more than half of the change in the calculated chemical shift of 2F-(5-methyl)-imidazole upon acid titration can be attributed to changes in electron density in the fluorine lone pair molecular orbital. This corroborates with the calculated reduction in electrostatic charge on fluorine upon protonation, which leads to less shielding of the fluorine nucleus.
Fig. 2.
Lowest energy molecular orbitals containing fluorine electron density (MO 2) for a) 2F-(5-methyl)-imidazole b) 2F-(5-methyl)-imidazolium; c) lowest energy lone-pair like orbital (MO 5) for 4F-(5-methyl)-imidazole d) 4F-(5-methyl)-imidazolium (MO 2).
The shift in electron density away from the fluorine nucleus in 2F-(5-methyl)-imidazole can also be seen in changes in the dipole moment. The neutral species has a dipole moment of μ=5.2 Debye, whereas 2F-(5-methyl)-imidazolium shows a drastic change to 0.77 Debye, showing a reduction inpolarization toward the fluorine atom in the protonated species. However, the same pattern does not hold for 4F-(5-methyl)-imidazole. Neutral 4F-(5-methyl)-imidazole has a dipole moment of 7.1 Debye, and the dipole moment of 4F-(5-methyl)-imidazolium is only slightly reduced to 6.2 Debye. This may be seen as an indication that the electronic structure of the 4F-species is intrinsically different from the 2F-species. The authors of the pioneering experimental work on fluorinated imidazoles and histidines31 suggested that differences in pKa measured for 2- vs 4- fluoro-imidazole rings may be due to electronic structure differences that arise in 4-fluoro species, beyond σ-inductive effects.
In analyzing Natural Chemical Shielding (NCS) data for 4F-(5-methyl)-imidazolium, it was found that there are drastic changes in the molecular orbitals of 4F-(5-methyl)-imidazole compared to 4F-(5-methyl)-imidazolium. So much so, that comparison of NCS data for the neutral and protonated species of 4F-(5-methyl)-imidazole is difficult. The 2nd-lowest energy molecular orbital (MO 2) of 4F-(5-methyl)-imidazolium is a lone pair orbital (delocalized somewhat toward the methyl group, see Fig. 2d) that contributes to shielding of the 19F chemical shift. What is striking is that we observe no lone pair electrons on the fluorine for neutral 4F-(5-methyl)-imidazole. Fig. 2c shows the lowest energy molecular orbital (MO 5) that has any similarity to a lone pair orbital. As can be seen in the molecular orbital in Fig. 2c, while there is electron probability around the fluorine nucleus, the other lobe of the orbital encompasses the protonated nitrogen (N1) on the aromatic imidazole ring. The NCS data indicates that, relative to the shielding of MO 5 in the neutral species, MO 2 (lone pair) of 4F-(5-methyl)-imidazolium contributes more shielding (6.45 ppm) to the fluorine nucleus. Clearly, multiple orbitals contribute to chemical shielding, but these are the lowest-energy orbitals that have considerable electron density near the fluorine nucleus. The differences in chemical shielding provided by MO 2 (lone pair) of 4F-imidazolium and MO 5 (delocalized fluorine electrons) of neutral 4F-(5-methyl)-imidazole account for most of the change in chemical shift upon titration. Thus, protonation appears to trigger fluorine electron localization into a lone pair in 4F-imidazolium species, giving rise to higher shielding at low pH.
When fluorine electron density is delocalized, do fluorine chemical shifts correlate with charge?
To address the question of whether 4-fluoroimidazoles have “predictable” chemical shifts when they undergo minor perturbations in electron density, we evaluated and compared the calculated charges and chemical shifts for 4F-(5-methyl)-imidazole (4F-MeIm), 4F-histidine (4F-His), 4F-imidazole (4F-Im). For 4-fluoro-substituted species, electron density (charge) is lowest for 4F-MeIm (-0.275), slightly increases for 4F-His, and is highest for 4F-Im (-0.31). Table 1 summarizes the fluorine electrostatic potential (charge) alongside experimental and calculated fluorine chemical shifts. As can be seen, the 19F NMR shifts did not follow a predictable trend, with deshielded shifts corresponding to lower electron density. In fact, the chemical shifts are reversed: the most shielded 19F chemical shift, for 4F-MeIm, has lowest charge density, while the most deshielded, 4F-Im, has highest charge density. However, the fluorine chemical shifts (though not the charges) do fit chemical intuition. That is to say, that the most shielded fluorine chemical shift corresponds to the imidazole with the most electron donating substituent at C5. Note that the computationally-calculated NMR chemical shifts for the 4F-imidazole series match the experimental ordering of 19Fchemical shifts. Thus, without the calculated fluorine charge density, the experimental results don't “raise any eyebrows”.
The peculiar behaviour of 4F-imidazoles, in which chemical shift and electron density have reverse relationships relative to most NMR chemical shifts, is akin to that observed in aliphatic fluoride systems.4 Adcock and Abeywickrema performed in-depth studies of relationships between 19F substituent chemical shifts and fluorine electron density.4 They studied substituent effects on a fluorinated bicyclo-octane, and found that most aliphatic fluoride chemical shifts become deshielded with increasing electron density. This is in contrast to studies on phenyl fluorides, which showed through substituent effects that fluorine chemical shifts are deshielded as electron density decreases (“normal” behaviour). So, Adcock and Abeywickrema concluded that aliphatic fluorides may have “reverse” chemical shift effects, while aromatic fluorides behave normally. They postulated that the “reverse” behaviour of 19F chemical shifts reflects the polarization ofC-F σ bonds. To probe this in our data set, we looked at the charge separation between the carbon and fluorine electrostatic potential (ESP/charge). Indeed, the charge separation (polarity) of the C-F bond was greatest for the most deshielded shift, and least for the most shielded chemical shift. So, while charge of fluorine itself does not correlate with chemical shift for 4F-imidazole, which has all fluorine electron density delocalized, the extent of polarization does correlate in a reverse manner (polar bond = deshielding, less polar bond= shielding). This suggests that perhaps the reverse sigma effects seen for aliphatic systems also occur in aromatic systems in which there is full fluorine electron delocalization.
In contrast, the 2-fluoroimidazoles behaved normally, where more shielded 19F shifts can be correlated with increased electron density. The electron density on fluorine is observed to increase from 2F-His to 2F-MeIm to 2F-Im. In accordance with electron density, shielding of the 19F chemical shift is observed for the molecules in same order. However, there is no correlation between C-F charge separation and 19F chemical shift for the 2-fluoroimidazoles.
Discussion
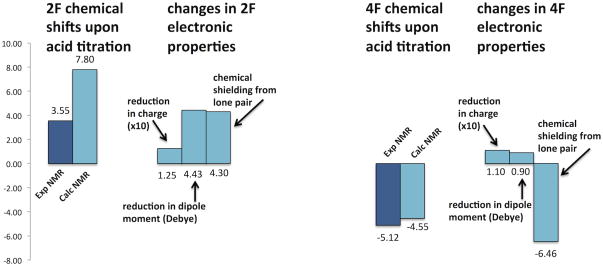
Considering only 2F-(5-methyl)-imidazole, the fluorine chemical shifts fit “chemical intuition”, with reduced fluorine electron density upon protonation leading to deshielding of the fluorine nucleus. Data from electronic structure calculations supports chemical intuition as well, with less negative fluorine electrostatic charges and reduced dipole moment coinciding with deshielding of the fluorine nucleus. In contrast, 4F-(5-methyl)-imidazole does not show the same correlations among indicators of electron density and fluorine chemical shift. Fig. 3 summarizes the correlations among properties calculated by DFT and the fluorine chemical shifts.
Fig. 3.
Summary of correlation between fluorine chemical shifts and electronic properties. Experimental and calculated 19F chemical shifts are given, alongside reduction in charge and dipole moment upon acid titration, and natural chemical shielding (NCS) contributions from the lone pairs of 2F-(5-methyl)-imidazolium& 2F-(5-methyl)-imidazole, and lone pair (MO 2) of 4F-(5-methyl)-imidazolium& MO 5 of 4F-(5-methyl)-imidazole.
The electronic structure of the π-tautomer (which is more stable) of 4-fluoroimidazole is unique compared to the other fluorinated imidazoles we considered. In all the molecular orbitals of 4F-(5-methyl)-imidazole that contain fluorine electron density, there is conjugation within the ring and/or overlap with the adjacent methyl group. See supplementary information (ESI) Fig. S1 for visualization of all molecular orbitals of 2F- and 4F-(5-methyl)-imidazole and –imidazolium. The electronic structure of 4F-(5-methyl)-imidazole appears to have effects along the lines of the fluorine p-π interaction proposed by Sheppard in 1965.28 Using this information, we can conclude that protonation of 4F-(5-methyl)-imidazole changes the electronic structure such that the lone pair electrons on fluorine are restored (localized), providing greater shielding to the fluorine nucleus.
We postulated the anomalous electronic structure of the 4F-imidazole moiety may arise from the electron-donating nature of the methyl (methylene) group adjacent to the fluorine in 4F-(5-methyl)-imidazole (4F-histidine), giving an effect akin to hyperconjugation. To test this, we performed calculations that replaced the methyl group with an electron-withdrawing trifluoromethyl group. Upon protonation, this species still exhibited higher shielding (by 5.09 ppm calculated by BHandHLYP/6-311++G(3d, 2p)), so we can conclude that the electronic structure is not dependent on the electron-donating or –with drawing character of the adjacent substituent on the imidazole ring.
The reverse behaviour of 19F NMR chemical shifts in substituted 4-fluoroimidazoles clearly relates these species with the behaviour of aliphatic fluorides. Studies of the 4F-His/Im/MeIM series also indicated an inverse relationship among electron density and shielding of chemical shifts. Calculated charges of fluorine and C4 support Adcock and Abeywickrema's hypothesis that “reverse” or abnormal fluorine chemical shift behaviour correlates with C-F bond polarity: deshielded shifts correspond to more polar C-F bonds (higher charge separation, provided in Table S2 of supplementary information).
It is interesting to note that the less stable τ-tautomer of 4F-(5-methyl)-imidazole has a lone pair orbital on fluorine and exhibits a deshielded chemical shift upon protonation (like 2F analoguesdo). Thus, for N1-protonated (π-tautomer), 4-fluoroimidazole moieties, the conjugated (delocalized) molecular orbitals of fluorine appear to be an inherent characteristic of the electronic structure. All of the systems analysed here support a hypothesis that when fluorines on an aromatic ring have no lone pair (i.e. completely delocalized electron density), the chemical shifts are abnormal or “reverse”. All aromatic fluorines with a lone pair, in the systems studied here, have 19F chemical shifts whose shielding/deshielding correlates with fluorine charge density. Further study with electronic structure methods is required to determine whether this is a general feature of 19F chemical shifts.
Conclusions
In summary, the electronic structure of 4F-(5-methyl)-imidazole (and histidine analogues) is unique, compared to the electronic structure of 2F-(5-methyl)-imidazole, 2F-(5-methyl)-imidazolium, and 4F-(5-methyl)-imidazolium analogues. Rather than a lone pair, all of the electrons and orbitals of fluorine in 4F-(5-methyl)-imidazole overlap with the aromatic ring and adjacent methyl group. Since shielding of the fluorine nucleus upon protonation was calculated and observed experimentally for 4F-(5-methyl)-imidazole, 4F-imidazole, and 4F-histidine, and calculated for 4F-(5-trifluoromethyl)-imidazole, it seems that the delocalized electronic structure has little dependence on substitution at C5. Instead, delocalized fluorine electron density appears to be an inherent characteristic of the π-tautomer (N1-H) of 4-fluoro-imidazoles. For 2F-(5-methyl)-imidazole species, predictions of deshielding from shifts of electron density away from the fluorine nucleus upon addition of positive charge are substantiated in the less-negative fluorine electrostatic potential (charge), significantly smaller dipole moment, and deshielding contributions from the fluorine lone pair of MO 2 (calculated by NCS analysis). Fig. 3 shows how the 19F-NMR chemical shifts of 2F-(5-methyl)-imidazole correlate with electronic structure properties, while only NCS data correlates well with the 19F-NMR chemical shifts of 4F-(5-methyl)-imidazole. Within a series of C5-substituted 4F-imidazoles, correlations were found between increased C-F bond polarity and deshielding of 19F chemical shifts. This abnormal or “reverse” relationship with bond polarity had been characterized previously only for aliphatic systems.4 Thus, when fluorine delocalization takes place in aromatic systems, fluorine chemical shift prediction may require more complex analysis, such as that provided by computational methods.
The concept of fluorine's complex electronic effects, such as hyperconjugation and lone pair back-donation, is not new,27 but the importance in terms of 19F-chemical shifts has been more difficult to nail down. The results here for 2F- and 4F-histidine/(5-methyl)-imidazole seem to suggest that when electron density is localized in a fluorine lone pair, changes in 19F NMR chemical shifts might be understood and predicted with the same chemical knowledge and intuition as 1H and 13C chemical shifts. However, when electronic effects lead to conjugation of all fluorine orbitals, a framework for understanding fluorine chemical shifts is not so straightforward. For understanding 19F chemical shifts in proteins, the next step will be to understand to what extent local environment induces fluorine lone pair localization/delocalization. With a database of proteins with known local environments and measured 19F chemical shifts, 49 and continued computational efforts, we may not be too far off from a unifying protocol for a priori prediction of 19F-NMR spectra in proteins.
Supplementary Material
Acknowledgments
We are grateful to Prof. Carl Frieden and Dr. Dana Schwartz for reading the manuscript and providing valuable suggestions. Financial support for the work comes from Wichita State University, Fairmount College of Liberal Arts and Sciences and K-INBRE startup funds under NIH National Institute of General Medical Sciences, P20 GM103418. Computing resources were funded by the National Science Foundation under Grant No. EIA-0216178 and Grant No. EPS-0236913, with matching support from the State of Kansas and the Wichita State University High Performance Computing Center.
Footnotes
Electronic supplymentry information (ESI) available: Calculated fluorine chemical shift with multiple method; molecular orbitals of fluoroimidazoles; input files for electronic structure calculation.
References
- 1.Zhao Y, Markopoulos G, Swager TM. J Am Chem Soc. 2014;136:10683–10690. doi: 10.1021/ja504110f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson EP, Eppler RK, Beaudoin JM, Dordick JS, Reimer JA, Clark DS. J Am Chem Soc. 2009;131:4294–4300. doi: 10.1021/ja806996q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau EY, Gerig JT. J Am Chem Soc. 2000;122:4408–4417. [Google Scholar]
- 4.Adcock W, Abeywickrema AN. J Org Chem. 1982;47:2945–2951. [Google Scholar]
- 5.Chen H, Viel S, Ziarelli F, Peng L. Chem Soc Rev. 2013;42:7971–7982. doi: 10.1039/c3cs60129c. [DOI] [PubMed] [Google Scholar]
- 6.Vulpetti A, Hommel U, Landrum G, Lewis R, Dalvit C. J Am Chem Soc. 2009;131:12949–12959. doi: 10.1021/ja905207t. [DOI] [PubMed] [Google Scholar]
- 7.Buer BC, Marsh ENG. Protein Sci. 2012;21:453–462. doi: 10.1002/pro.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh ENG, Suzuki Y. ACS Chem Biol. 2014;9:1242–1250. doi: 10.1021/cb500111u. [DOI] [PubMed] [Google Scholar]
- 9.Danielson MA, Falke JJ. Annu Rev Biophys Biomol Struct. 1996;25:163–195. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas CA, Talaty ER, Bann JG. Chem Commun. 2009;23:3366–3368. doi: 10.1039/b821952d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitevski-LeBlanc JL, Prosser RS. Prog Nucl Magn Reson Spectrosc. 2012;62:1–33. doi: 10.1016/j.pnmrs.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Yoder NC, Kumar K. Chem Soc Rev. 2002;31:335–341. doi: 10.1039/b201097f. [DOI] [PubMed] [Google Scholar]
- 13.Lau EY, Gerig JT. Biophys J. 1997;73:1579–1592. doi: 10.1016/S0006-3495(97)78190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renner C, Alefelder S, Bae JH, Budisa N, Huber R, Moroder L. Angew Chem Int Ed. 2001;40:923–925. [PubMed] [Google Scholar]
- 15.Merkel L, Budisa N. Org Biomol Chem. 2012;10:7241–7261. doi: 10.1039/c2ob06922a. [DOI] [PubMed] [Google Scholar]
- 16.Frieden C, Hoeltzli SD, Bann JG. Methods Enzymol. 2004;380:400–415. doi: 10.1016/S0076-6879(04)80018-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoeltzli SD, Frieden C. Biochemistry. 1994;33:5502–5509. doi: 10.1021/bi00184a019. [DOI] [PubMed] [Google Scholar]
- 18.Hoeltzli SD, Frieden C. Proc Natl Acad Sci USA. 1995;92:9318–9322. doi: 10.1073/pnas.92.20.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Frieden C. Proc Natl Acad Sci, USA. 2007;104:11993–11998. doi: 10.1073/pnas.0705253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong D, Lin CLS, d'Avignon DA, Lovey AJ, Rosenberger M, Li E. FEBS Lett. 1997;402:116–120. doi: 10.1016/s0014-5793(96)01509-8. [DOI] [PubMed] [Google Scholar]
- 21.Shu Q, Frieden C. J Mol Biol. 2005;345:599–610. doi: 10.1016/j.jmb.2004.10.057. [DOI] [PubMed] [Google Scholar]
- 22.Li E, Qiant SJ, Winter NS, d'Avignon A, Levin MS, Gordon JI. J Biol Chem. 1991;266:3622–3629. [PubMed] [Google Scholar]
- 23.Feeney J, McCormick JE, Bauer CJ, Birdsall B, Moody CM, Starkmann BA, Young DW, Francis P, Havlin RH, Arnold WD, Oldfield E. JAm Chem Soc. 1996;118:8700–8706. [Google Scholar]
- 24.Luck LA, Falke JJ. Biochemistry. 1991;30:4248–4256. doi: 10.1021/bi00231a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder FAA, Filatov M. Chem Soc Rev. 2010;39:578–590. doi: 10.1039/b811366c. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield E. Annu Rev Phys Chem. 2002;53:349–78. doi: 10.1146/annurev.physchem.53.082201.124235. [DOI] [PubMed] [Google Scholar]
- 27.Holtz D. Chem Rev. 1971;71:139–145. [Google Scholar]
- 28.Sheppard WA. J Am Chem Soc. 1965;87:2410–2420. [Google Scholar]
- 29.Sternberg U, Klipfel M, Grage SL, Witter R, Ulrich AS. Phys Chem Chem Phys. 2009;11:7048–7060. doi: 10.1039/b908236k. [DOI] [PubMed] [Google Scholar]
- 30.Dalvit C, Vulpetti A. Chem Med Chem. 2011;6:104–114. doi: 10.1002/cmdc.201000412. [DOI] [PubMed] [Google Scholar]
- 31.Yeh HJC, Kirk KL, Cohen LA. J Chem Soc Perkin Trans 2. 1975;2:928–934. [Google Scholar]
- 32.Dennington R, Keith T, Millam J. Semichem Inc Version 5.0. Shawnee Mission; KS: 2009. [Google Scholar]
- 33.Andra KK, Bullinger JC, Bann JG, Eichhorn DM. Acta Cryst. 2010;E66:o2713. doi: 10.1107/S1600536810038663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam J, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09 (Revision D.01) Gaussian, Inc.; Wallingford CT, USA: 2009. [Google Scholar]
- 35.Bohmann JA, Weinhold F, Farrar TC. J Chem Phys. 1997;107:1173–1184. [Google Scholar]
- 36.Head-Gordon M, Pople JA, Frisch MJ. Chem Phys Lett. 1988;153:503–506. [Google Scholar]
- 37.Hohenberg P, Kohn W. Phys Rev. 1964;136:B864–B871. [Google Scholar]
- 38.Kohn W, Sham LJ. Phys Rev. 1965;140:A1133–A1138. [Google Scholar]
- 39.Becke AD. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 40.Becke AD. J Chem Phys. 1993;98:1372–1377. [Google Scholar]
- 41.Marenich AV, Cramer CJ, Truhlar DG. J Phys Chem B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 42.Barone V, Cossi M. J Phys Chem A. 1998;102:1995–2001. [Google Scholar]
- 43.Wolinski K, Hinton JF, Pulay P. J Am Chem Soc. 1990;112:8251–8260. [Google Scholar]
- 44.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. J Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds WF, Peat IR, Freedman MH, Lyerla JR., Jr J Am Chem Soc. 1973;95:328–331. doi: 10.1021/ja00783a006. [DOI] [PubMed] [Google Scholar]
- 46.Bloomberg F, Maurer W, Rüterjans H. J Am Chem Soc. 1977;99:8149–8159. doi: 10.1021/ja00467a005. [DOI] [PubMed] [Google Scholar]
- 47.Giam CS, Lyle JL. J Am Chem Soc. 1973;95:3235–3239. [Google Scholar]
- 48.Breneman CM, Wiberg KB. J Comput Chem. 1990;11:361–373. [Google Scholar]
- 49.Fluorine chemical shift database, Laboratory of Dr. Carl Frieden, Washington University School of Medicine. http://biochem.wustl.edu/bmbnmr/Fluorine.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.