Abstract
Background
Levodopa is the most effective therapy for Parkinson's disease (PD), but chronic treatment is associated with the development of potentially disabling motor complications. Experimental studies suggest that motor complications are due to non-physiologic, intermittent administration of the drug, and can be reduced with continuous delivery. Levodopa-carbidopa intestinal gel (LCIG) is a form of levodopa that can be delivered continuously through an intrajejunal percutaneous tube.
Methods
We performed a 12-week double-blind, double-dummy, double-titration, multi-center trial to evaluate the efficacy and safety of LCIG compared to optimized, oral, immediate-release levodopa-carbidopa (LC-IR) in advanced PD patients with motor complications. The primary endpoint was change from baseline to final visit in motor “Off” time. Motor “On” time without troublesome dyskinesia was the key secondary endpoint.
Findings
71 patients with advanced PD were randomized to receive continuous LCIG infusion plus placebo LC-IR capsules (n=37) or to receive LC-IR capsules plus continuous placebo LCIG infusion (n=34). Both groups were titrated to optimal effect. 93% of subjects (n=66) completed the trial. In comparison to LC-IR, LCIG significantly reduced “Off” time by a mean (±SE) of 1·91±0·57 hours (P=0·0015) and increased “On” time without troublesome dyskinesia by a mean of 1·86±0·65 hours (P=0·006). Adverse events were primarily related to the surgical procedure and the device, and while potentially serious, were not associated with residual deficit or mortality.
Interpretation
In comparison to standard oral LC-IR, LCIG significantly reduced “Off” time and increased “On” time without troublesome dyskinesia in patients with advanced PD. Adverse events were largely due to the procedure and the device. Benefits are of greater magnitude than have been obtained with medical therapies to date, and represent the first demonstration of the benefit of continuous levodopa delivery in a double-blind controlled study.
Keywords: Parkinson's disease, Levodopa/Carbidopa Intestinal Gel, Motor fluctuations
Introduction
Parkinson's disease (PD) is characterized by degeneration of dopamine neurons in the substantia nigra pars compacta (SNc) with resultant depletion of striatal dopamine leading to the core motor features of the disease. The mainstay of treatment is levodopa, the amino-acid precursor of dopamine. Virtually all PD patients have a beneficial response, and no present medical or surgical therapy has been shown in controlled trials to provide greater anti-parkinsonian benefit. However, chronic oral levodopa therapy is associated with the development of potentially disabling motor complications (motor fluctuations and dyskinesia) in the majority of patients.1 Motor fluctuations consist of an initial benefit after a dose of levodopa (“On” period) followed by a return of parkinsonian features (“Off” period) prior to the onset of benefit from the subsequent dose. Dyskinesias are levodopa-induced involuntary movements that typically occur during “On” periods. Higher doses of levodopa can reduce “Off” time but tend to increase dyskinesia, while a reduction in levodopa dose can reduce dyskinesia but tends to worsen “Off” time. In advanced PD patients, it can be difficult to find a dose of levodopa that satisfactorily controls “Off” time without inducing dyskinesia. Multiple classes of medication (dopamine agonists, COMT-inhibitors, MAO-B inhibitors) have been developed to try to reduce “Off” time, but they typically provide only modest benefit and are frequently complicated by worsening dyskinesia.2 Deep brain stimulation (DBS) is widely employed to improve both “Off” time and dyskinesia, but requires a neurosurgical intervention that is associated with potentially serious complications.3,4 The development of a levodopa formulation that provides benefits without inducing or worsening motor complications is a major unmet need in PD.
Clinical and laboratory evidence suggests that levodopa-induced motor complications are related to the non-physiologic restoration of brain dopamine with intermittent doses of standard oral levodopa.5 Striatal dopamine levels are normally maintained at a relatively constant level. This is not the case in PD, where in the absence of nigro-striatal terminals striatal dopamine levels are dependent on the peripheral availability of levodopa. Intermittent dosing with standard oral levodopa formulations provides fluctuating plasma levels due to erratic gastric emptying, variable jejunal absorption, and the short half-life of the drug (60-90 minutes).6,7 In the dopamine-depleted state, this variability in plasma levodopa concentration is translated into abnormal, fluctuating, striatal dopamine concentrations,8,9 which in turn are associated with non-physiologic intermittent or pulsatile stimulation of dopamine receptors. This results in gene and molecular changes in striatal neurons, neurophysiologic changes in the firing pattern of pallidal output neurons, and the development of motor complications.5 It has been hypothesized that continuous delivery of levodopa could restore brain dopamine in a more physiologic manner, and thereby avoid or reduce motor complications associated with traditional levodopa therapy.5,10 Indeed, continuous levodopa infusion has been reported to reduce both “Off” time and dyskinesia in open-label studies in patients with advanced PD.11–13 It has, however, proven difficult to develop oral or patch formulations that deliver levodopa in a continuous manner.
Levodopa-carbidopa intestinal gel (LCIG) (AbbVie Inc., North Chicago, IL) is a carboxymethylcellulose aqueous gel that can be delivered continuously to the proximal jejunum via a percutaneous gastrojejunostomy (PEG-J) tube connected to a portable infusion pump (CADD-Legacy® Smiths Medical, MN, USA). Pharmacokinetic studies show LCIG jejunal infusion provides relatively constant plasma levodopa levels with less variability than oral formulations,14,15 and open label studies report a marked reduction (improvement) in “Off” time without worsening of dyskinesias.16–19 Despite the lack of double blind trials, LCIG is approved for use in 43 countries. However, open label interventional studies in advanced PD patients have frequently not been confirmed in double blind trials.20 We present the results of the first prospective, double-blind, placebo-controlled study evaluating the safety and efficacy of continuous LCIG infusion in patients with advanced PD. This is also the first double-blind controlled trial testing the hypothesis that continuous delivery of levodopa can reduce “Off” time without worsening dyskinesia.
Methods
Study design
The study was a 12-week prospective, multi-center, placebo-controlled, parallel group, double-blind, double-dummy, double-titration study. Candidates were patients with advanced PD complicated by “Off” periods that could not be satisfactorily controlled with “optimized” medical therapy. “Optimized” was defined as an adequate trial in the judgment of the investigator of levodopa-carbidopa, a dopamine agonist, and at least one other class of anti-parkinsonian therapy (COMT inhibitor, MAO-B inhibitor). Following confirmation by an independent Enrolment Steering Committee that the subject was an appropriate candidate, patients signed an informed consent that was approved by the IRB at each participating site. Subjects were then hospitalized for jejunal placement of a PEG-J tube under local anesthesia using endoscopic and/or fluoroscopic guidance and randomly assigned to treatment with either a) over-encapsulated immediate release levodopa-carbidopa 25/100 (LC-IR) plus placebo LCIG gel infusion, or b) LCIG infusion plus over-encapsulated placebo LC-IR.
Subjects
Male and female patients of any race who were at least 30 years of age with a diagnosis of PD consistent with United Kingdom Brain Bank criteria were eligible to participate. Patients had to be receiving stable doses of levodopa for at least four weeks prior to enrollment, and to be experiencing recognizable “On” and “Off” periods with a minimum of three hours of “Off” time per day based on a home diary assessment21. Subjects receiving sustained release levodopa-carbidopa, Stalevo®, or other formulations of levodopa were permitted into the study but had to be converted to equivalent doses of LC-IR and to have been on stable doses for at least four weeks prior to entry. Concurrent anti-parkinsonian drugs (except apomorphine) were permitted if patients were on stable doses for four weeks prior to randomization, and the dose was not changed during the study. Exclusion criteria included atypical or secondary parkinsonism, previous neurosurgical treatment for PD, clinically significant medical, psychiatric or laboratory abnormalities in the judgment of the investigator, or any condition that might interfere with absorption, distribution, metabolism, or excretion of study drug or contradict placement of an intrajejunal PEG-J tube.
Randomization and Masking
Eligible subjects who signed an informed consent were randomized to treatment group in a 1:1 ratio according to a central, computer-generated, pre-determined, randomization code. Randomization was stratified by site, with a mixed block size of 2 or 4. An interactive voice response system (IVRS) supported by a contracted vendor generated the randomization schedule and assigned subjects to treatment groups. Subjects were enrolled by site investigaors. All participants and investigators were masked to group assignment. Those analyzing data were masked until after the database was locked. Simultaneous titration of both active and placebo therapy was performed for patients in both groups in order to maintain the integrity of the blind (see details below), but masking of subjects and investigators was not formally evaluated..
Dosing
Both LCIG and LC-IR were initially administered at the subject's total daily levodopa dose prior to randomization. LCIG was delivered as an aqueous intestinal gel (containing 20 mg/mL levodopa and 5 mg/mL carbidopa monohydrate solution) in 100 gram cassettes or matching placebo gel (sodium carboxymethylcellulose solution alone) administered as a morning bolus (5-10 ml) followed by continuous infusion at a constant rate for the remainder of each patient's waking day (approximately 16 hours). The infusion was stopped overnight. LC-IR capsules containing 25/100 mg of carbidopa/levodopa or matching placebo were initially administered in divided doses over the course of their waking day (approximately 16-hours) beginning at the same time as the infusion and at the same dose and frequency as at baseline. There was a four-week titration period, during which dosing for patients in either group could be adjusted once daily during the first two weeks (during the in-patient hospital stay) and weekly during weeks 3 and 4 (during scheduled outpatient visits). LCIG could be adjusted by changing the infusion rate in 100 mg daily increments; LC-IR could be adjusted by increasing one or more doses by 100 mg but the dosing frequency could not be changed. Changes in dose were made solely based on investigator judgment; subjects could not change the dose or titration rate on their own. Any change in the dosage of an active intervention in a given subject had to be matched by a corresponding change in the placebo treatment, so that both treatments (active and placebo) for each patient were adjusted at the same time. In this way the blind was maintained for patients in both groups. Dosage adjustment could be made for patients in both the LCIG or LC-IR treatment groups so that all patients were titrated to their optimal state. The titration period was followed by an eight-week maintenance period during which patients were maintained on stable doses of their assigned treatment. Open label LC-IR could be used as rescue therapy for persistent “Off” episodes for patients in either group.
Visits and Evaluations
Visits were performed at baseline, and weeks 1, 2, 3, 4, 6, 8, 10, and 12. For three consecutive days prior to baseline visit and each visit beginning at week 2, patients completed a 24 hour home-diary assessment of motor status at 30-minute intervals, recording if they were “Off”, “On” without dyskinesia, “On” with non-troublesome dyskinesia, or “On” with troublesome dyskinesia or asleep.21 Prior to entry into the study patients were trained in the use of the home diary, and had to have at least 75% concordance with investigator rating and at least 75% compliance in completion of home diary. Additional evaluations at each visit included vital signs, Unified Parkinson Disease Rating Scale (UPDRS; Part II in the “On” state, and Part III in the “On” state approximately 2-4 hours after an oral dose),22 Parkinson Disease Questionnaire (PDQ-39),23 EuroQual quality of life-5 Dimensions (EQ-5D),24 Zarit care-giver burden interview (ZBI),25 and investigator-rated clinical global impression (CGI-I). Safety assessments were performed at each visit. Plasma concentrations of levodopa were obtained in the first 20 subjects at weeks 4, and 12 at 12, 16, 17, and 18 hours post-initiation of intestinal gel and the next day prior to infusion and at 1, 1.33, 1.67, 2, 2.33, 2.67, 4, 4.33, 4.67, 8, 8.33 and 8.67 hours after start of infusion. For the remaining subjects, sampling was performed at week 6 prior to initiation of intestinal gel infusion and at 1, 2, 4, and 8 hours after start of infusion,
Outcome measures and Statistical analyses
The primary efficacy endpoint was the change between baseline and final visit (week 12) in the mean number of “Off” hours collected on the home diary during the three days prior to each visit, normalized to a 16 hour waking day. An important secondary outcome was change from baseline to final visit in “On” time without troublesome dyskinesia. Other secondary outcomes measures in hierarchical order of analysis included change from baseline in PDQ-39 summary index, CGI-I, UPDRS part II (Activities of Daily Living subscore), UPDRS part III (Motor subscore), ZBI score, and EQ-5D summary index.
The primary endpoint was analyzed using an analysis of covariance (ANCOVA) model including effects for treatment group and country, with baseline “Off” time, and average daily rescue levodopa dose as covariates. Missing data were imputed using the last observation carried forward. A mixed model repeated measures (MMRM) was performed as a sensitivity analysis which included baseline as a fixed-effect covariate; treatment, country, and time (scheduled assessment visits) as fixed-effect (categorical) factors, and interaction between time and treatment as well as between time and baseline. An unstructured matrix was used for the covariance of the within-subject repeated measures. Pre-specified hierarchical testing and a Gatekeeping procedure were used to maintain the family-wise error rate at 0·05. The hierarchical testing method uses a fixed sequence approach that allows testing of each of the null hypotheses at a significance level of 0.05 without adjustment, as long as the null hypotheses are hierarchically ordered and pre-defined.26 Claims of statistical significance stop as soon as the first null hypothesis in the testing sequence is not rejected (p-value > 0.05). Inter- and intra-subject coefficients of variation for levodopa plasma concentrations were estimated using a linear mixed-effects model. For safety data, the incidence of adverse events (AEs) and serious AEs (SAEs) were summarized. The Full Analysis data set, consisting of all randomized subjects with data for baseline and at least 1 post-baseline assessment was used for all efficacy analyses. The Safety dataset consisted of all randomized patients who underwent the PEG-J procedure.
Sample size was estimated based on previous open label trials and indicated that 31 subjects per group would provide 90% power to detect a difference between the LCIG and LC-IR groups of 2·5±2·85 hours in “Off” time with alpha=0·05 and a dropout rate of 5%. Two identical studies were originally planned and initiated. After discussion with regulatory authorities, the protocols and statistical analysis plan were amended to combine the studies while they were ongoing, prior to database lock and analysis of any data.
Role of Sponsor
The study was registered at ClinicalTrials.gov (NCT00357994 and NCT00660387). AbbVie Inc. funded the study and was responsible for data collection, monitoring, and statistical analysis. The authors were responsible for study design, interpretation of data, and writing the manuscript. Authors had full access to all data in the study. AbbVie participated in the study design, reviewed the manuscript and provided comments for author consideration, and approved the submission of the manuscript; however, the authors made the final decision on the content. The corresponding author had the final responsibility for the decision to submit for publication.
Results
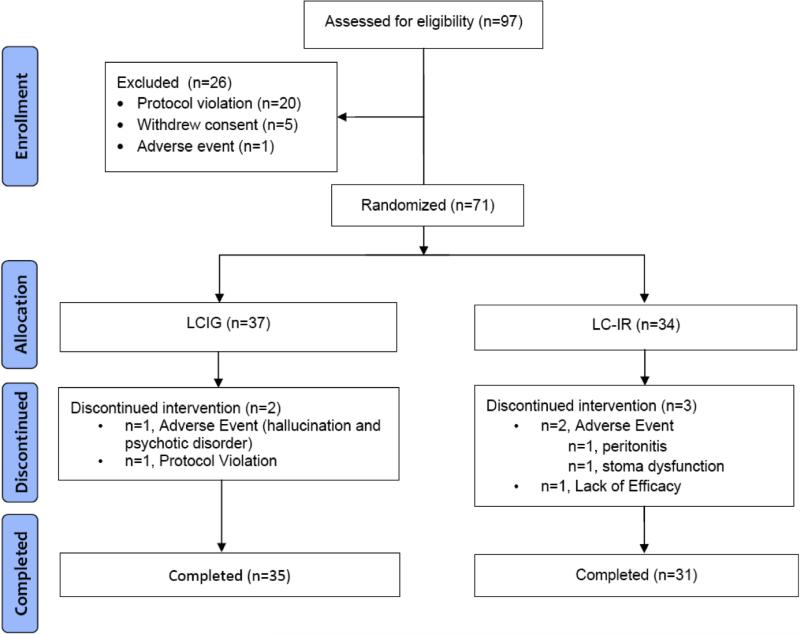
Twenty-six centers in the United States, New Zealand, and Germany participated in the study. Seventy-one patients met entry criteria, were approved by the enrollment steering committee, signed an IRB-approved informed consent, and were randomly assigned to a treatment group (LCIG=37, LC-IR=34). The mean number of patients per Center was 2.8, and 34 patients were enrolled in the 5 largest sites. A total of 66 patients (LCIG=35; LC-IR=31) completed the trial. A CONSORT diagram is provided in Figure 1. Baseline characteristics are summarized in Table 1; there were no significant differences between treatment groups. Titration to stable dose was achieved in a mean of 7 days for LCIG subjects and 8 days for LC-IR subjects; 90% were titrated to stable doses in ≤ 9 days.
Figure 1.
CONSORT Diagram
Table 1.
Baseline Characteristics
| Baseline Characteristic | LCIG (N=37) | LC-IR (N=34) |
|---|---|---|
| Mean age, years (SD) | 63·7 (9·5) | 65·1 (6·8) |
| Male, n (%) | 24 (64·9) | 22 (64·7) |
| White, n (%) | 35 (94·6) | 31 (91·2) |
| Mean duration of PD, years (SD) | 10·0 (4·6) | 11·8 (5·6) |
| Mean “Off” time, h/d (SD)a | 6·3 (1·7) | 7·0 (2·1) |
| Mean “On” time without dyskinesia, h/d (SD)a | 6·3 (2·7) | 5·6 (3·2) |
| Mean “On” time with non-troublesome dyskinesia, h/d (SD)a | 2·4 (1·8) | 2·2 (2·2) |
| Mean “On” time without troublesome dyskinesia, h/d (SD) | 8·7 (2·0) | 7 ·8 (2·5) |
| Mean “On” time with troublesome dyskinesia, h/d (SD)a | 1·0 (1·6) | 1·2 (1·7) |
| UPDRS, mean (SD)a | ||
| Part I | 1·8 (1·7) | 1·8 (1·8) |
| Part II | 11·6 (6·9) | 11·8 (7·0) |
| Part III | 18·1 (9·9) | 22·5 (11·7) |
| Total | 31·5 (15·6) | 35·8 (18·9) |
| PDQ-39a | 35·1 (18·0) | 38·6 (17·9) |
| Mean Mini-Mental State Exam (SD) | 28·7 (1·4) | 28·9 (1·4) |
| Mean daily levodopa dose, mg (SD) | 1005·4 (373·6) | 1123·5 (477·9) |
| Anti-Parkinsonian Medication Use, n (%) | ||
| Dopamine agonist | 22 (59.5) | 26 (76.5) |
| COMT inhibitor | 18 (48.6) | 15 (44.1) |
| MAOB inhibitor | 15 (40.5) | 6 (17.6) |
Full Analysis data set: N=36 for LCIG, N=33 for LC-IR; “On” time without troublesome dyskinesia = “On” time without dyskinesia + “On” time with non-troublesome dyskinesia.
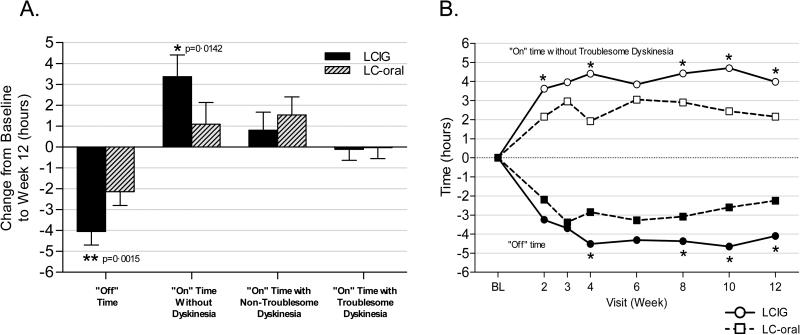
The efficacy analyses performed in hierarchical order demonstrated statistically significant results for “Off” time, “On” time without troublesome dyskinesia, PDQ-39 summary index, CGI-I score, and UPDRS Part II score. Efficacy results are summarized in Table 2. In comparison to LC-IR, LCIG treatment provided significantly greater reduction (improvement) in “Off” time between baseline and final visit, the primary endpoint (difference between groups was −1·91±0·57 hours; P=0·0015). LCIG treatment was also associated with significantly greater improvement than LC-IR in “On” time without troublesome dyskinesia, the important secondary endpoint (difference between groups 1·86±0·65 hours; P=0·0059), as well as in “On” time without any dyskinesia (difference between groups 2·28±0·90hrs; P=0·0142; Figure 2a). Results at each time point are provided in Figure 2b. We utilized a large number of sites to facilitate enrollment in this complex study, but there was no Center effect in the analysis. The results of the primary analysis were confirmed by the MMRM sensitivity analysis. The benefits of LCIG compared with standard LC-IR were reflected by significant improvement in the activities of daily living subscale of the UPDRS (Part II), and measures of quality of life (Table 2). No significant difference between treatment groups was detected for UPDRS Part III (motor subscale). Levodopa doses are shown in Table 2; the change from baseline levodopa dose and the amount of rescue levodopa employed were greater in the LC-IR group. Intra-subject variability in plasma levodopa concentration was less for LCIG-treated (21%) than LC-IR-treated (67%) subjects.
Table 2.
Summary of Efficacy Findings
| Assessment | LCIG N = 35 | LC-Oral N = 31 | Treatment Difference |
|---|---|---|---|
| Primary Efficacy Measure | |||
| “Off” time, hrs/day | |||
| Mean change from baseline (SE) | −4·04 (0·65) | −2·14 (0·66) | −1·91 (0·57)** |
| Important Secondary Efficacy Measure | |||
| “On” time without troublesome dyskinesia, hrs/day | |||
| Mean change from baseline (SE) | +4·11 (0·75) | +2·24 (0·76) | +1·86 (0·65)** |
| Other Endpoints | |||
| “On” time without dyskinesia, hrs/daya | |||
| Mean change from baseline (SE) | +3·37 (1·04) | +1·09 (1·05) | +2·28 (0·90)* |
| “On” time with non-troublesome dyskinesia, hrs/daya | +0·81 (0·86) | +1·54 (0·86) | −0·73 (0·74) |
| Mean change from baseline (SE) | |||
| “On” time with troublesome dyskinesia, hrs/daya | −0·11 (0·52) | −0·03 (0·52) | −0·08 (0·45) |
| Mean change from baseline (SE) | |||
| PDQ-39 Summary Index | |||
| Mean change from baseline (SE) | −10·9 (3·3) | −3·9 (3·2) | −7·0 (2·8)* |
| CGI-Ib | |||
| Mean score at final (SE) | 2·3 (0·4) | 3·0 (0·4) | −0·7 (0·3)* |
| UPDRS Part IIc | |||
| Mean change from baseline (SE) | −1·8 (1·3) | +1·3 (1·3) | −3·0 (1·1)** |
| UPDRS Part IIIc | |||
| Mean change from baseline (SE) | −1·5 (2·4) | −2·9 (2·4) | +1·4 (2·1) |
| EQ-5D | |||
| Mean change from baseline (SE) | +0·05 (0·04) | −0·02 (0·04) | +0·07 (0·04) |
| Zarit Burden Interview | |||
| Mean change from baseline (SE) | −2·8 (3·7) | +1·7 (3·3) | −4·5 (3·1) |
| Levodopa total daily dose | |||
| Mean change from baseline (SE) | +91·7 (96·6) | +249·7 (94·9) | −158·0 (83·3) |
| Levodopa rescue dose | |||
| Overall mean, mg (SD) | 139.8 (81./3) | 180.6 (156.2) |
“On” time without troublesome dyskinesia = “On” time without dyskinesia + “On” time with non-troublesome dyskinesia.
Measure not part of hierarchical analysis
CGI-I, 1= very much improved, 2=much improved, 3=minimally improved, 4=no change, 5=minimally worse, 6=much worse, 7=very much worse.
UPDRS was completed in the “On” state
P<0 05
P<001
+ = increase in score, − = reduction in score
Figure 2. Diary measures.
A. Home Diary Results: Change between baseline and Week 12 in the various PD motor states. B. Home Diary Results: PD motor states at each visit. For each variable, data shown are the average from the symptom diary for the 3 consecutive days prior to the clinic visit, normalized to a 16-hour waking day. “On” time without Troublesome Dyskinesia = “On” time without dyskinesia + “On” time with non-troublesome dyskinesia. N = 35 (LCIG), 31 (LC-IR). *P<0·05 between treatment groups.
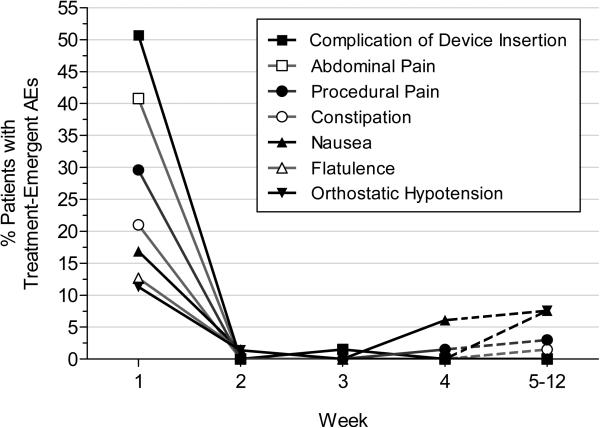
AEs were reported in 35 (94·6%) LCIG patients and 34 (100%) LC-IR patients; it should be noted that patients in both groups received PEG-J placement. SAEs occurred in 13.5% and 20.6% respectively (Table 3). Three AEs resulted in study termination; one LCIG-treated patient had psychosis, one LC-IR patient had peritonitis and pneumonia, and one LC-IR subject had a post procedural discharge. Most AEs were related to the surgical procedure or the device, were mild to moderate in severity, occurred almost exclusively within the first week, and resolved in all cases; there were no deaths (see details in Table 3 and in Figure 3). It should be noted, however, that 2/71 (2.8%) patients discontinued from the study due to complications of surgery and that 63 (88.7%) experienced device-related complications including tube dislocations 17 (23.9%), PEG-J insertion complications 15 (21.1%), stoma insertion complications 7/71, pump malfunctions, 6/71 (8.5%), and pneumoperitoneum 5/71 (7.0%). Symptoms consistent with the possibility of polyneuropathy were recorded in four patients (LCIG-1, LC-IR-3); no cases of Guillain-Barre syndrome were reported. There were no clinically significant laboratory abnormalities.
Table 3.
Summary of Adverse Events (AEs) and Device Complications N (%)
| Overall, N (%) | LCIG N = 37 | LC-Oral N = 34 | Total N = 71 |
|---|---|---|---|
| Any AE | 35 (94·6) | 34 (100·0) | 69 (97·2) |
| Serious AE* | 5 (13·5)a | 7 (20·6)b | 12 (16·9) |
| Abdominal pain | 19 (51·4) | 11 (32·4) | 30 (42·3) |
| Nausea | 11 (29·7) | 7 (20·6) | 18 (25·4) |
| Procedural Pain | 11 (29·7) | 12 (35·3) | 23 (32·4) |
| Constipation | 8 (21·6) | 7 (20·6) | 15 (21·1) |
| Incision Site Erythema | 7 (18·9) | 4 (11·8) | 11 (15·5) |
| Flatulence | 6 (16·2) | 4 (11·8) | 10 (14·1) |
| Dyskinesia | 5 (13·5) | 4 (11·8) | 9 (12·7) |
| Orthostatic Hypotension | 5 (13·5) | 8 (23·5) | 13 (18·3) |
| Depression | 4 (10·8) | 1 (2·9) | 5 (7·0) |
| Fall | 4 (10·8) | 4 (11·8) | 8 (11·3) |
| Insomnia | 4 (10·8) | 4 (11·8) | 8 (11·3) |
| Pneumoperitoneum | 4 (10·8) | 1 (2·9) | 5 (7·0) |
| Post-procedure Discharge | 4 (10·8) | 3 (8·8) | 7 (9·9) |
| Wound Infection | 4 (10·8) | 8 (23·5) | 12 (16·9) |
| Device complication | 34 (91·9) | 29 (85·3) | 63 (88·7) |
| Intestinal tube comp | 14 (37·8) | 12 (35·3) | 26 (36·6) |
| Leakage | 2 | 1 | 3 |
| Insertion complication | 3 | 1 | 4 |
| Dislocation | 8 | 9 | 17 |
| Occlusion | 5 | 4 | 9 |
| Unintentional removal | 0 | 1 | 1 |
| PEG-J comp | 11(29·7) | 12 (35·3) | 23 (32·4) |
| Breakage | 1 | 0 | 1 |
| Insertion complication | 8 | 7 | 15 |
| Dislocation | 2 | 3 | 5 |
| Occlusion | 0 | 1 | 1 |
| Connection issue | 1 | 3 | 4 |
| Unintentional removal | 0 | 1 | 1 |
| Pump comp | 5 (13·5) | 8 (23·5) | 13 (18·3) |
| Breakage | 1 | 0 | 1 |
| Malfunction | 3 | 3 | 6 |
| Occlusion | 1 | 2 | 3 |
| Stoma comp | 15 (40·5) | 15 (44·1) | 30 (42·3) |
| Leakage | 2 | 1 | 3 |
| Insertion complication | 2 | 5 | 7 |
| Dislocation | 0 | 1 | 1 |
| Connection issue | 0 | 1 | 1 |
SAEs included:
2 events of confusional state, and 1 event each of pneumoperitoneum, complication of device insertion, catheter site cellulitis, hypersomnia, delusions, hallucinations, mutism, and psychotic disorder
2 events of pheumonia, and 1 event each of neutropenia, abdominal pain, peritonitis, postprocedural complication, elevated body temperature, depressed level of consciousness, mental status change, psychosis, and orthostatic hypotension. More than 1 could be in the same individual.
Figure 3. Incidence of treatment-emergent adverse events (AEs) reported by >10% of patients during any time interval.
Week 5-12 time point summarizes AEs initiating over multiple weeks.
Discussion
We demonstrate in a prospective double-blind, double-dummy, double-titration study that, in comparison with intermittent doses of immediate release oral levodopa (LC-IR), continuous intrajejunal infusion of levodopa gel (LCIG) provides a significant reduction in “Off” time in patients with advanced PD. Importantly, this benefit of LCIG is also associated with a significant increase in “On” time without troublesome dyskinesia. “Off” time in LCIG-treated patients was reduced by 1·91 hours in comparison to standard oral levodopa, and by 4 hours in comparison to baseline. This magnitude of benefit is greater than has been achieved with medical therapies evaluated in double-blind studies in which there was no increase in troublesome dyskinesia,2 and is of similar magnitude to that reported with DBS in open label studies.3
Treatment was optimized for patients in both treatment groups. Thus, it is unlikely that the greater reduction in “Off” time seen in the LCIG group was due to disproportionate levodopa dosing in the LCIG group. Indeed, there was a greater increase from baseline in total daily levodopa dose in the LC-IR group, and there was no difference between the groups in UPDRS motor scores. A summary of the study rationale and results is provided in the panel on “Research in Context”.
Research in Context
Background
Chronic treatment with standard levodopa/carbidopa is associated with motor complications in the majority of patients with Parkinson's disease (PD). These can be a source of disability, and represent the major reason for surgical therapy in PD patients. Laboratory studies suggest that motor complications are related to fluctuating plasma levels of levodopa and might be avoided with continuous delivery of the drug5. However, it has proven difficult to accomplish this with long-acting oral or patch formulations. Levodopa-carbidopa intestinal gel (LCIG) is a novel formulation of levodopa that is administered by continuous intra-intestinal infusion (duodopa®) to provide relatively constant plasma levodopa levels.
Systematic Review
We performed a Pubmed search and an extensive literature review on August 15, 2013 under the search terms of “duodopa”, “levodopa carbidopa intestinal gel” “continuous levodopa infusion”, “continuous levodopa delivery” and “continuous dopamine stimulation” with no restriction on date or language. There were no double-blind, placebo-controlled parallel group trials assessing the safety and efficacy of LCIG or any other form of continuous levodopa delivery in patients with Parkinson's disease and motor complications.
Interpretation
We performed a 12-week double-blind, double-dummy, placebo-controlled, double-titration parallel group trial comparing continuous infusion of LCIG to optimized treatment with standard LC-IR. In comparison to optimized LC-IR, continuous intraintestinal LCIG infusion provided a significant reduction in “Off” time, significant increase in “On” time without troublesome dyskinesia, and significant improvement in measures of quality of life. Benefits were of a greater magnitude than have been achieved in placebo-controlled trials with available medical therapies for “the treatment of Off” time, and in a similar range as reported with Deep Brain Stimulation3. The study provides the first double-blind data evaluating the safety and efficacy of continuous levodopa delivery as a treatment strategy for Parkinson's disease. These results are consistent with the concept of continuous dopaminergic stimulation as a therapy for PD; future longer-term studies are required to test the potential for LCIG to reverse established dyskinesia.
Great efforts were employed to maintain the integrity of the blind. Patients were randomized, all investigators and subjects were blinded as to treatment group, titration was performed simultaneously for both active and placebo treatments such that any change in dose of one form of drug delivery had to be matched by a comparable change in the other during the titration period, no change in dosage was permitted during the maintenance phase, and the pump was locked so that the dose couldn't be modified by the patient. We did not perform formal evaluations to assess masking of subjects or investigators; there were no reports of unblinding during the study.
Evidence in dopamine-lesioned rodents and primates indicates that intermittent oral levodopa dosing induces molecular changes in striatal neurons, and physiologic changes in pallidal neurons that are associated with the development of motor complications.5 These can be avoided with more continuous or long-acting dopaminergic therapies. We believe that the significant reduction in “Off” time and significant increase in “On” time without worsening of troublesome dyskinesia observed in the LCIG group in patients with advanced PD was due to restoration of brain dopamine in a more physiologic manner than can currently be achieved with intermittent oral administration of the drug. The possibility that benefits were simply due to bypassing gastric emptying has been considered, but LCIG and LC-IR have comparable bioavailability in pharmacokinetic studies14, and we believe that the continuous levodopa delivery is a more reasonable explanation.
Continuous levodopa delivery has been reported to reduce dyskinesia as well as off time in open label studies.12 Indeed, LCIG subjects in the present study had a significant improvement in both “off” time and “On” time without dyskinesia (Figure 2). However, the present study was designed to assess the effect of LCIG on “Off” time. Accordingly, subjects were selected based on having > 3 hours “Off” time per day, and had very low baseline levels of dyskinesia. This precluded determining if LCIG also provides a benefit with respect to established dyskinesia. Further studies to assess the effect of LCIG on dyskinesia are required.
AEs were primarily related to the surgical procedure or the device and included pneumoperitoneum, peritonitis, pump malfunction, obstruction of catheter, tube displacement, and the need for additional procedures to repair or replace the catheter. These primarily occurred within the first two weeks and were not associated with residual deficit. Further, serious device-related AEs were fewer than have been reported in the literature27 which may reflect a benefit of increased experience with the procedure. Polyneuropathy and Guillain-Barre syndrome have been reported with LCIG infusion,28 but neuropathy has also been reported in association with oral levodopa,29 and a specific relationship to LCIG treatment has not been established. In the present study, Guillain-Barre syndrome was not encountered in any patient, and symptoms potentially related to neuropathy were only reported in one LCIG subject compared to three LC-IR subjects. An open-label, long-term safety study is currently underway.
LCIG represents a potentially important therapeutic advance in the management of PD patients with motor complications, and represents an alternative to DBS that avoids the need for a neurosurgical procedure, although LCIG does require an intervention that is associated with potentially serious complications. The study was 12 weeks in duration, and longer-term studies are required to better assess safety, to evaluate the effect of LCIG on dyskinesia, and to determine what level of expertise is required to manage patients who have this procedure. Similar reductions in “Off” time have been reported in open label studies with continuous subcutaneous delivery of apomorphine,30,31 but this procedure has not been evaluated in a double-blind trial and it is associated with troublesome skin nodules as well as the side effects of dopamine agonists. There are presently no trials directly comparing LCIG infusion with DBS and apomorphine infusion, and randomized studies are awaited.
There are several limitations to the study. Because of the complexity of the study we utilized a large number of sites which only had limited numbers of subjects, but statistical analyses showed no center effect. We did not conduct a formal evaluation of the blind, and as with all effective therapies there is the possibility that a beneficial response could cause unblinding however, there were no reports of unblinding and the study was designed so as to minimize this risk. The study was only 12 weeks in duration, which precludes an evaluation of the complications associated with LCIG infusion and the J-tube that might develop after this time period. The relatively short duration of the study and the patient population that was studied also prevent an evaluation of the potential of LCIG treatment to reduce established dyskinesia. Finally, it should be noted that the procedure can be associated with potentially serious adverse events and is a rather complex procedure that likely will need to be performed in specialty centers.
In summary, this study demonstrates that LCIG provides a therapeutic option for patients with advanced PD who suffer “Off” episodes that cannot be satisfactorily controlled with standard medical therapies. The present study also represents the first double-blind study to provide data consistent with the concept of continuous dopaminergic stimulation as a treatment for the motor complications of PD. Longer term studies to determine if continuous levodopa infusion reduces dyskinesia in addition to off time are required to prove this hypothesis. In the final analysis, the value of LCIG as a treatment for PD patients with motor complications will ultimately be determined by trials that provide a full assessment of its relative safety, efficacy, and cost in comparison to other available therapies such as DBS.
Supplementary Material
Acknowledgements
This study was funded by AbbVie Inc. We would like to thank Nathan Rustay of AbbVie who provided technical assistance in the preparation of this manuscript.
AJE is supported by the K23 Research Scholars mentored career development awards (NIMH, 1K23MH092735), has received grant support from CleveMed/Great Lakes Neurotechnologies, Davis Phinney Foundation, and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board for Solvay, Abbott (AbbVie), Chelsea Therapeutics, TEVA, Eli Lilly, Impax, and Solstice Neurosciences; and honoraria from Novartis, the American Academy of Neurology, and the Movement Disorders Society.
DGS is an investigator in studies funded by Abbott Laboratories, the American Parkinson Disease Association, the Michael J. Fox Foundation for Parkinson Research, the National Multiple Sclerosis Society, the RJG Foundation, and NIH grants 5F30NS065661, 1F31NS076017, 5K08NS060948, 5R01MH082304, 5K01NS069614, 1R01NS064934, 5P50 - NS037406, 1F31NS081963, 1K23NS080912, 1U18NS082132, 2U10NS044547 and 1R25NS079188; has a clinical practice and is compensated for these activities through the University of Alabama Health Services Foundation; has served as a consultant for or received honoraria from Teva Neurosciences, Serina Therapeutics, Lundbeck, Solvay, Abbott (AbbVie), the Michael J. Fox Foundation for Parkinson Research, Partners Healthcare, the University of Michigan, The University of Virginia, The University of Pittsburg, the Mayo Clinic, The Pennington Research Center, the University of Kansas, the University of Arizona, the National Institutes of Health, Balch and Bingham LLC, the American Academy of Neurology, North Shore Hospital, the Thomas Hartman Foundation, the Bachmann - Strauss Foundation, Nupathe Inc., Bradley Arrant Boult Cummings, and he has received royalties for publications from McGraw Hill, Inc.
HHF has received research support from Abbott (AbbVie), Acadia, Biotie Therapeutics, EMD-Serono, Huntington Study Group, Ipsen, Merz Pharmaceuticals, Michael J. Fox Foundation, Movement Disorders Society, National Parkinson Foundation, NIH/NINDS, Novartis, Parkinson Study Group, and Teva, but has no owner interest in any pharmaceutical company; and has received honoraria from Cleveland Clinic CME, Northwestern University CME, Ipsen, Merz Pharmaceuticals, and US World Meds.
AV is a paid consultant for CVS Caremark's Pharmacy & Therapeutics committee and Abbott (AbbVie), and has been a scientific advisory board member for Abbott (AbbVie).
AA has received honoraria for consulting services and symposia from Abbott (AbbVie), Boheringer Ingelheim, GSK, Lundbeck, UCB, Novartis and Merck Serono.
AAO, WZR, KC, and JB are current employees of AbbVie Inc. and own AbbVie stock and/or stock options.
YP is a current employee of Astellas Pharma, but was an employee of Abbott (now AbbVie) when these studies were completed and owns Abbott and AbbVie stock.
KLW and RL are current employees of Amgen, but were employees of Abbott (now AbbVie) when these studies were completed and own Abbott and AbbVie stock.
Funding: The study was funded by AbbVie Inc and registered at ClinicalTrials.gov (NCT00660387 and NCT0357994).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
CWO interpreted data, wrote the report, and approved the final draft. KK interpreted data, contributed to writing the report, and approved the final draft. PO collected and interpreted data, contributed to writing the report, and approved the final draft. AJE collected and interpreted data, contributed to writing the report, and approved the final draft. DGS collected and interpreted data, contributed to writing the report, and approved the final draft. HHF collected and interpreted data, contributed to writing the report, and approved the final draft. AV collected and interpreted data, contributed to writing the report, and approved the final draft. AAO contributed to study design, contributed to writing the report, analyzed and interpreted data, and approved the final draft. KLW contributed to writing the report, interpreted data, and approved the final draft. WZR contributed to study design, analyzed and interpreted data, contributed to writing the report, and approved the final draft. YP contributed to study design, analyzed and interpreted data, contributed to writing the report, and approved the final draft. KC contributed to study design, interpreted data, contributed to writing the report, and approved the final draft. JB contributed to study design, interpreted data, contributed to writing the report, and approved the final draft. RAL contributed to study design, interpreted data, contributed to writing the report, and approved the final draft. AA interpreted data, contributed to writing the report, and approved the final draft.
Conflicts of interest
CWO has received consultancy fees from Abbott (AbbVie), Novartis/Orion, Impax, Ceregene, and Teva/Lundbeck, AstraZeneca, Biotie/Synosia, CHDI, Civitas, LZ Therapeutics, Medivation, Neuroderm, Pharma2B, Phytopharm, Siena, Synagile, UCB, Otsuka, Upsher Smith, and US World Med, has stock in Clintrex, and is on scientific advisory board of the Michael J Fox Foundation. KK has been a consultant to the National Institutes of Health (NINDS), the US FDA, the USVA, Abbott (AbbVie), Acorda, Aptiv, AstraZeneca, Auspex, Biogen Idec, Biotie, Biovail, Boehringer Ingelheim, Ceregene, CHDI, Civitas, Clintrex, Cynapsus, Endo, EMD Merck Serono, Genzyme, Impax, Intec, Ipsen, Isis, Knopp, Lilly, Link Medicine, Lundbeck, LZ Therapeutics, Medivation, Merck, Merz, Neotope/Elan Pharmaceuticals, Novartis, Orion, Otsuka, Pharm2B, Phytopharm, Roche, Schering-Plough, Siena Biotech, Synosia, Solvay, Synagile, Sofinnova, Teva, UCB Pharma, Upsher-Smith, Vaccinex, Vectura, and Xenoport, has received grant/research support from Medivation, Michael J Fox Foundation, National Institutes of Health (NEI, NINDS, NIA, NICHD), Neurosearch, and Pfizer, and has acted as a legal consultant for Pfizer, Thompson Hine, and the Welding Rod Litigation Defendants.
PO has acted as a study investigator in AbbVie-sponsored studies, has received compensation from AbbVie, Boehringer-Ingelheim, Britannia, Cephalon, GSK, Ipsen, Lundbeck, Nordic Infucare, and UCB for serving as a consultant and/or lecturer, and has received honoraria from Movement Disorder Society.
References
- 1.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, Stern MB, Sethi K. Scientific and Clinical Basis for the treatment of PD – 2009. Neurology. 2009;72(21 Suppl 4):S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 3.The Deep Brain Stimulation for PD study group Deep brain stimulation of the subthalamic nucleus or globus pallidus pars interna in Parkinson's disease. New Eng J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 4.Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S2–41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Obeso JA, Stocchi F. Continuous Dopamine Receptor Stimulation in the Treatment of Parkinson's Disease: Scientific Rationale and Clinical Implications. Lancet Neurology. 2006;5:677–687. doi: 10.1016/S1474-4422(06)70521-X. [DOI] [PubMed] [Google Scholar]
- 6.Nutt JG, Woodward WR, Beckner RM, et al. Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetcs and pharmacodynamics of levodopa in parkinsonian patients. Neurology. 1994;44:913–919. doi: 10.1212/wnl.44.5.913. [DOI] [PubMed] [Google Scholar]
- 7.Hardoff R, Sula M, Tamir A, et al. Gastric emptying time and gastric motility in patients with Parkinson's disease. Mov Disord. 2001;16:1041–7. doi: 10.1002/mds.1203. [DOI] [PubMed] [Google Scholar]
- 8.Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72:1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- 9.de la Fuente-Fernandez R, Sossi V, Huang Z, et al. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain. 2004;127:2747–54. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- 10.Bibbiani F, Costantini LC, Patel R, Chase TN. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol. 2005;192:73–8. doi: 10.1016/j.expneurol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Kurlan R, Rubin AJ, Miller C, Rivera-Calimlim L, Clarke A, Shoulson I. Duodenal delivery of levodopa for on-off fluctuations in parkinsonism: preliminary observations. Ann Neurol. 1986;20:262–5. doi: 10.1002/ana.410200213. [DOI] [PubMed] [Google Scholar]
- 12.Sage JI, Trooskin S, Sonsalla PK, Heikkila R, Duvoisin RC. Long-term duodenal infusion of levodopa for motor fluctuations in parkinsonism. Ann Neurol. 1988;24:87–9. doi: 10.1002/ana.410240116. [DOI] [PubMed] [Google Scholar]
- 13.Stocchi F, Vacca L, Ruggieri S, Olanow CW. Infusion of levodopa methyl ester in patients with advanced PD: A clinical and pharmacokinetic study. Arch of Neurol. 2005;62:905–10. doi: 10.1001/archneur.62.6.905. [DOI] [PubMed] [Google Scholar]
- 14.Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol. 2003;26:156–63. doi: 10.1097/00002826-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Nyholm D, Odin P, Johansson A, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson's disease patients. AAPS J. 2013;15:316–23. doi: 10.1208/s12248-012-9439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson's disease--long-term experience. Acta Neurol Scand. 2001;104:343–8. doi: 10.1034/j.1600-0404.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- 17.Antonini A, Isaias IU, Canesi M, et al. Duodenal levodopa infusion for advanced Parkinson's disease: 12-month treatment outcome. Mov Disord. 2007;22:1145–9. doi: 10.1002/mds.21500. [DOI] [PubMed] [Google Scholar]
- 18.Eggert K, Schrader C, Hahn M, et al. Continuous jejunal levodopa infusion in patients with advanced Parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol. 2008;31:151–66. doi: 10.1097/wnf.0b013e31814b113e. [DOI] [PubMed] [Google Scholar]
- 19.Devos D, French DUODOPA Study Group Patient profile, indications, efficacy and safety of duodenal levodopa infusion in advanced Parkinson's disease. Mov Disord. 2009;24:993–1000. doi: 10.1002/mds.22450. [DOI] [PubMed] [Google Scholar]
- 20.Alterman RL, Tagliati M, Olanow CW. Open-label surgical trials for Parkinson's disease: Time for reconsideration? Ann Neurol. 2011;70:5–8. doi: 10.1002/ana.22453. [DOI] [PubMed] [Google Scholar]
- 21.Hauser RA, Friedlander J, Zesiewicz TQ. A Home Diary to Assess Functional Status in Patients with Parkinson's Disease with Motor Fluctuations and Dyskinesia Clinical. Neuropharmacology. 2000;23:75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Elton RL, Members of the UPDRS Development Committee . The Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Macmillan Healthcare Information; Florham Park: 1987. pp. 153–63. [Google Scholar]
- 23.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–8. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 24.Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ-5D--a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2000;69:67–73. doi: 10.1136/jnnp.69.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–7. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 26.Huque M, Alosh M. A flexible fixed-sequence testing method for hierarchically ordered correlated multiple endpoints in clinical trials. Journal of Statistical Planning and Inference. 2008;138:321–335. [Google Scholar]
- 27.Nyholm D. Duodopa® treatment for advanced Parkinson's disease: A review of efficacy and safety. Parkinsonism Relat Dis. 2012;18:916–29. doi: 10.1016/j.parkreldis.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Jugel C, Ehlen F, Taskin B, Marzinzik F, Müller T, Klostermann F. Neuropathy in Parkinson's disease patients with intestinal levodopa infusion versus oral drugs. Mov Disord. doi: 10.1371/journal.pone.0066639. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology. 2011;77:1947–50. doi: 10.1212/WNL.0b013e31823a0ee4. [DOI] [PubMed] [Google Scholar]
- 30.Hughes AJ, Bishop S, Kleedorfer B, et al. Subcutaneous apomorphine in Parkinson's disease: response to chronic administration for up to five years. Mov Disord. 1993;8:165–70. doi: 10.1002/mds.870080208. [DOI] [PubMed] [Google Scholar]
- 31.Antonini A, Isaias IU, Rofolfi G, et al. A 5-year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol. 2011;258(4):579–85. doi: 10.1007/s00415-010-5793-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.