Abstract
Objectives
To engage a national advocacy group and local stakeholders for guidance in developing a bipolar disorder biobank through a web-based survey and a community advisory board.
Methods
The Depression and Bipolar Support Alliance and the Mayo Clinic Bipolar Biobank conducted a national web-based survey inquiring about interest in participating in a biobank (i.e., giving DNA and clinical information). A community advisory board was convened to guide establishment of the biobank and identify key deliverables from the research project and for the community.
Results
Among 385 survey respondents, funding source (87%), professional opinion (76%), mental health consumer opinion (79%), and return of research results (91%) were believed to be important for considering study participation. Significantly more patients were willing to participate in a biobank managed by a university or clinic (78.2%) than one managed by government (63.4%) or industry (58.2%; both p < 0.001). The nine-member community advisory board expressed interest in research to help predict the likelihood of bipolar disorder developing in a child of an affected parent and which medications to avoid. The advisory board endorsed the use of a comprehension questionnaire to evaluate participants' understanding of the study (e.g., longevity of DNA specimens, right to remove samples, accessing medical records) as a means to strengthen the informed-consent process.
Conclusions
These national survey and community advisory data support the merit of establishing a biobank to enable studies of disease risk, provided that health records and research results are adequately protected. The goals of earlier diagnosis and individualized treatment of bipolar disorder were endorsed.
Keywords: biobank, bipolar disorder, phenotype
Bipolar disorder is an impairing medical illness that is characterized by recurrent episodes of mania or hypomania and depression (1). Although bipolar disorder is highly heritable, with additive genetic effects contributing up to 85% of the variance in risk (2), the identity of all genes contributing to disease risk is not fully known, and diagnostic tests are not available. Both of these are important because onset of treatment for bipolar disorder is often delayed by more than a decade, frequently because of misdiagnosis (3, 4), and those with early onset generally have the longest delays to treatment, with overall poor outcomes (5, 6). In addition, pharmacogenomic predictors of treatment response (e.g., genetic variations influencing medication response phenotypes) could provide greater selectivity for treatment recommendations (7). Again, this is of great clinical value. Although multiple compounds from different classes (lithium, anticonvulsant mood-stabilizers, antipsychotics, and antidepressants) are available to treat bipolar disorder, no consensus exists for mood-stabilizer selection (e.g., sequence of treatment, use in combination), and there is no individualized risk assessment for adverse drug outcomes (e.g., rash, antidepressant-induced mania) (8). Biomarker-based treatment algorithms could enhance outcomes and mitigate ineffective or suboptimal treatment trials.
Mayo Clinic has established a bipolar disorder biobank using state-of-the-art research technology to enable clinical and biomarker studies of both disease risk and treatment response. The Bipolar Biobank was initiated in 2009, and key collaborators include the Lindner Center of HOPE/University of Cincinnati and the University of Minnesota. Administrative oversight has been provided by the co–principal investigators (MAF and JMB.) and their Executive Committee, the Mayo Clinic Biospecimen Trust Oversight Group, and the Center for Individualized Medicine (9). The design, infrastructure, aims and research uses of the biobank, along with demographics and clinical features of the first enrolled participants are described elsewhere (10)
Consultation or partnering with advocacy organizations can contribute to the development, operation, evaluation, and support of bipolar biobanks. Additionally, community support by key stakeholders—those who can benefit from or be affected by biobanking research (e.g., patients, parents, at-risk children, community mental health centers, hospital centers, public school systems)—can help guide the discussion on biobank development and provide the framework for and facilitate translation and dissemination of research back to the community. In this study, the Bipolar Biobank, during the first stages of its establishment, engaged with national and local communities to collect preference data to guide the development, operation, evaluation, and support of the biobank.
Methods
The study was approved by the Mayo Clinic Institutional Review Board.
National survey
The biobank partnered with the Depression and Bipolar Support Alliance (DBSA), a national grassroots organization created and led by people with depression or bipolar disorder. The DBSA provides information, empowerment, support, and inspiration for people with mood disorders. We created a web-based survey inquiring about interest in participating in a genomics biobank (i.e., donating DNA linked with phenotypic data). The survey was posted in the Consumer and Family Survey Center on the DBSA website (www.dbsalliance.org) during all of 2010 and 2011(during the initial phase of the biobank). Website visitors were asked questions related to their self-reported diagnosis, demographics, history of research participation, and factors that might influence participation in genomic research. Responses to various questions were recorded as yes/no, level of importance (very, somewhat, not very, not, unknown), level of agreement (strongly agree, agree, not sure, disagree, strongly disagree, unknown), or likelihood of participation (very, somewhat, not sure, unlikely, very unlikely, unknown) (Table 1). Data were summarized using frequency distributions. Chi-squared tests and McNemar tests for matched pairs were used for statistical analyses (SAS, version 9.3, Cary, NC, USA).
Table 1. Depression and Bipolar Support Alliance web-based survey questions.
| Questions | Answers |
|---|---|
| Familiarity or previous participation in clinical research | |
| Have you ever heard of clinical research? | Yes, no, not sure, unknown |
| Have you ever participated in a clinical research study, either for mental health or other health conditions? | |
| Have you ever been referred, or recommended, to participate in a clinical research study? | |
| Information that supports consideration for participation | |
| Who is paying for the study? | Very important, somewhat important, not very important, not important, unknown |
| What other professionals who are not conducting the study say about it? | |
| What other mental health consumers say about the study? | |
| Whether mental health consumers are part of the research team? | |
| Whether the study's results will be sent to you? | |
| If you participated in research that included your DNA, how important would it be to you to maintain control of your samples and personal information, including the right to withdraw from research? | |
| Personal reasons that may influence your participation | |
| I think it would be interesting? | Strongly agree, agree, not sure, disagree, strongly disagree, unknown |
| I would like to help others or myself in the long run? | |
| I am worried about confidentiality? | |
| I am worried that it might affect my insurance coverage? | |
| Information about the clinical research that may influence your participation | |
| If the clinical research was testing new medication(s) for your condition | Very likely to participate, somewhat likely to participate, not sure, unlikely to participate, very unlikely to participate, unknown |
| If the clinical research was testing a new talk therapy for your condition | |
| Required you to provide tissue or DNA sample for genetics research | |
| If there is financial compensation | |
| If you were asked, how willing would you be to contribute DNA (from a blood test or saliva sample) for research? | |
| How willing would you be to share your DNA and personal information with researchers working in the pharmaceutical industry? | |
| How willing would you be to share your DNA and personal information with DNA repositories stored at the National Institutes of Health (NIH) sponsored by the federal government? | |
| How willing would you be to share your DNA and personal information with researchers at a university or academic health center? | |
Development of community advisory board
A community advisory board based at Mayo Clinic in Rochester, Minnesota, was created during the initial establishment of the Biobank to review initial goals of the biobank and to advise investigators. The members were selected through direct invitations based on established community leadership position or acquaintance with members of the research team. We intended to have representation from community support groups (such as Depression/Bipolar Support Alliance, National Alliance for the Mentally Ill), patients with Bipolar disorder and different professionals in health care who interact with patients with bipolar disorder. The original nine-member community advisory board consisted of five men and four women, with an average age of 45.8 years. All members had personal (one member with established bipolar disorder diagnosis) or family experience with bipolar disorder. Occupations included attorney, clinical assistant, editor, engineer, physician assistant, registered nurse, sales consultant, and high school teacher.
The board met twice per year to evaluate milestones achieved [i.e., the sample size of the collection (first 100, first 500, first 1,000)] and problems encountered. The opportunity to build a bipolar disorder biobank resource was first introduced to the advisory board members, who were then asked their opinions about which important issues and outcomes the biobank should attempt to address. Actual biobank recruitment materials and consent documents were reviewed. The opinions of the community advisory board were recorded based on informal discussions lead by the research staff and principal investigator. A previously developed 10-item comprehension questionnaire to evaluate participants' understanding of the study was reviewed by the advisory board (Fig. 1). The comprehension questionnaire addressed the potential impact of the research (including no guarantee of symptom improvement), absence of placebo and study-specific treatment, longevity of DNA specimens, right to remove samples, accessing medical records, and clarification of family access to the genetic material in the event of a participant's death.
Fig. 1.
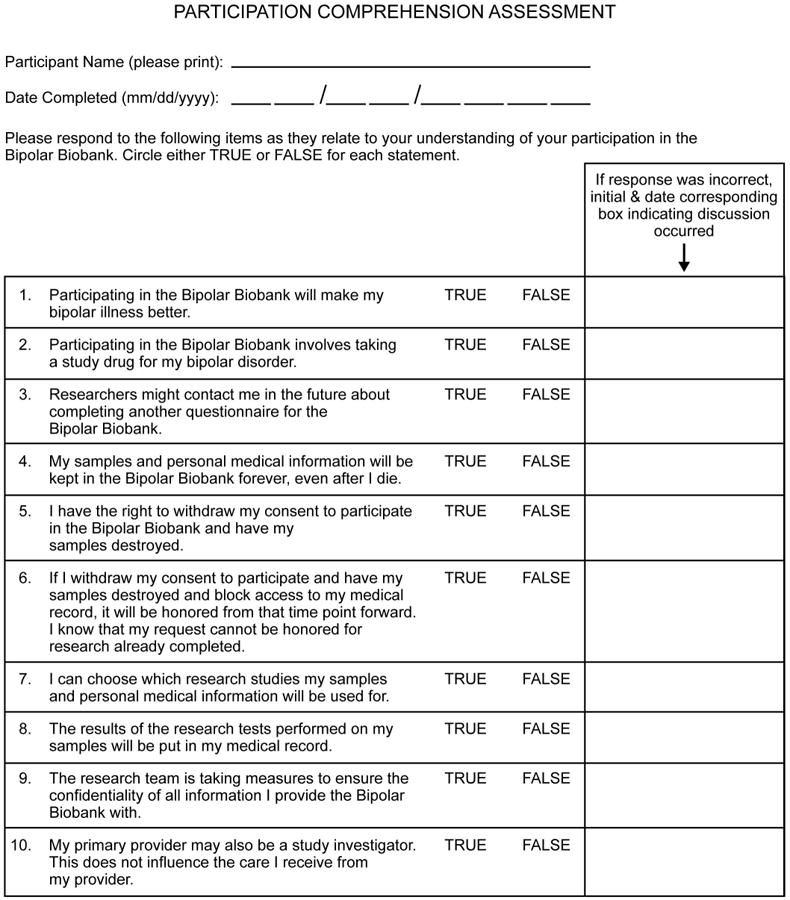
Participation comprehension assessment.
Results
National survey
A total of 385 persons completed the survey. The mean age of the respondents was 46.5 years (range: 14–72 years), 80% were female, and 90% were patients (10% were family or friend). Self-reported diagnoses included bipolar disorder (58%) and major depression (32%). Educational background included completion of high school (37%), college (36%), or advanced degree (22%), and unknown (5%). Of the respondents, 85% had heard of clinical research, 20% had participated in a clinical research study, either for mental health or other health conditions, and 19% had been referred or recommended to participate in a research study.
Features of biobank design that affected intent to participate in research included funding source, professional and consumer opinion, composition of the research team, and sharing the results of research. Specifically, factors that a majority of respondents believed to be important for their decision included: source of funding for the study (87%: very = 57%, somewhat = 30%); what professionals in the mental health field, but not involved in the study, thought about the merits of the study (76%: very = 31%, somewhat = 45%); what persons with mental illness thought about the merits of the study (79%: very = 36%, somewhat = 43%); whether mental health consumers are part of the research team (80%: very = 47%, somewhat = 33%); whether study results would be shared with them (91%: very = 62%, somewhat = 29%), and right to withdraw from the study (95%: very = 82%, somewhat = 13%).
Respondents rated several factors when considering research participation, including altruism, patient confidentiality, potential impact on insurance coverage, and type of study (e.g., pharmacologic, talk therapy, genetic). A majority of respondents agreed that their research participation would be based on whether they found the study to be interesting (84%: strongly agreed = 46%, agreed = 38%) and agreed to research participation if it would help others or themselves in the future (97%: strongly agreed = 76%, agreed = 21%). Much smaller percentages of participants worried about confidentiality in research participation (40%: strongly agreed = 17%, agreed = 23%) or worried that research participation might affect their insurance coverage (30%: strongly agreed = 14%, agreed = 16%).
Interest in research participation differed by the type of study. Likeliness to participate was lower for research investigating a new medication (62%: very = 28%, somewhat = 34%) than for a new talk therapy (87%: very = 68%, somewhat = 19%) or DNA sample for genetic research (70%: very = 36%, somewhat = 34%). Survey respondents (77%: very = 46%, somewhat = 31%) to participate in research that involved financial compensation. Specific to participating in research that involves genetics, the following statement was included in the survey: ‘To study mood disorders, DNA samples are needed from large numbers of people. This means that the researchers who are trying to collect samples might be asked to collaborate and share this information with other people or institutions who are conducting similar research studies.’ Given this, 86% of respondents were willing (very = 62%, somewhat = 24%) to participate in research that involved DNA from either a blood test or saliva sample. The percentage of patients willing to participate in a biobank managed by a university or clinic (78.2%) was significantly higher than that for a biobank managed by government (63.4%) or industry (58.2%; both p < 0.001) (Fig. 2).
Fig. 2.
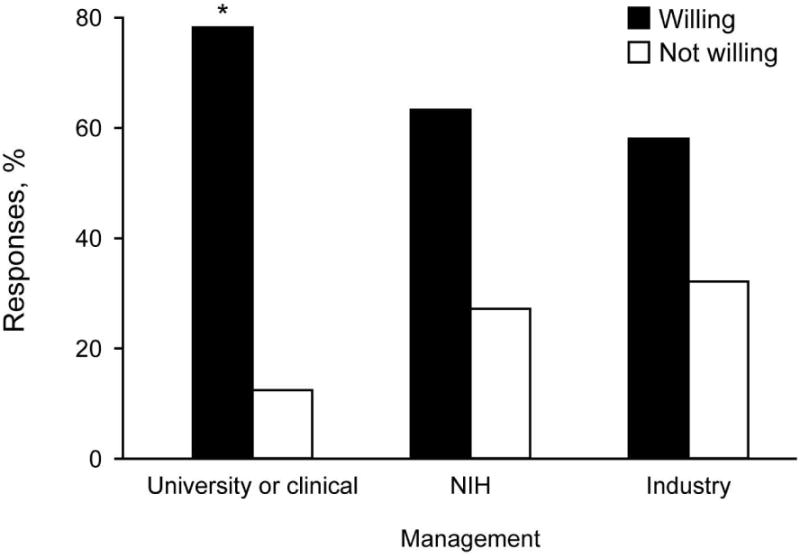
Willingness to contribute to a Bipolar Biobank by location or type of management (n = 385). For each category, 9.6% of respondents did not answer the question or their response is unknown. Respondents were significantly more willing to participate in a biobank managed by a university/clinic than by government [National Institutes of Health (NIH)] or industry (McNemar test for matched pairs, both p < 0.001). *p < 0.001 in a χ2 test of independence.
Development of community advisory board
With an understanding that science is dynamic and issues arise that can affect study recruitment and results, the advisory board expressed interest in research to help predict whether a child of a person with bipolar disorder was at risk for the condition and which medications certain patients should avoid. These comments aligned with our goal of studying risk to allow earlier diagnosis and individualized treatments. The advisory board believed the consent form was informative and that the 10-item comprehension questionnaire added a sense of protection and strengthened the informed consent process. After review of procedures, the advisory board believed that the current study participants' privacy, welfare, and health were adequately protected throughout. Recommendations were made to consider an electronic consent process and to develop an educational DVD about the biobank study, with the goal of engaging a younger audience and evaluating whether this mode of information delivery might be associated with greater understanding of informed consent.
Discussion
Results from our survey, facilitated by the largest national advocacy group for bipolar disorder, suggest that personal interest and altruism are major factors that drive potential participation in genomic research. Interest and altruism overcame concerns such as breach of confidentiality or potential effects on insurance coverage. Respondents were interested in biobank participation provided that both stakeholder groups—clinical researchers not associated with the actual study and patient stakeholders themselves—had endorsed the study. The differential interest in participating in research conducted by universities or clinics, compared with government or industry, may be related to a perceived conflict of interest or more local management or accessibility. Some similar studies show that patients have concerns about government or for-profit industry (11–13). The survey, however, did not allow for greater explanation as to choice of administrative oversight of the biobank.
To our knowledge, outside of Johns Hopkins and University of Michigan Prechter Bipolar Genetics Repository there are very few bipolar disease biobanks actively recruiting with simultaneous recruitment of healthy controls in the public domain (14, 15). The PGC (Psychiatric Genomics Consortium) is a large consortium of bipolar genomic data from multiple studies/sites and does not have a unified community advisory process (16). While there may be bipolar biospecimen repositories associated with clinical drug development and industry sponsored clinical trials in bipolar disorder, they have not focused on disease risk candidate genes or genome wide association studies. Given the sparsity of comparison bipolar biobank community advisory boards in the USA, we are unable to compare our results with other similar studies.
However, our results are comparable to national and international surveys about biobank participation. In a deliberative engagement model that relies on consulting the public through democratic deliberation, 78% of self-identified African Americans before, and 81% after, were very or somewhat interested in participating in a study that would collect DNA samples for future research (17). Overall willingness to participate in a biobank in a pan-European study was 46%; there was however great range of interest with highest and lowest level of willingness from Iceland (93%) and Turkey (24%) respectively (13). Further research is encouraged to clarify whether this variability in biobank participation is related to regional bias in the literature (most of the studies focus on north-western Europe), or concerns about security/privacy, or governance of biobanks. To assess the community support of Biobanks based on specific disease/research area, a community sample of 393 Michigan residents were engaged in a series of quantitative and qualitative surveys. The specific disease area to pursue and not pursue varied greatly; cancer research was the most supported research field (n = 266 pursue; n = 13 not pursue) and mental illness was the least supported research field (n = 1 pursue; n = 32 not pursue) (18). It is important to remember the composition of this random community sample with little interest to pursue mental illness biobanking versus our survey respondents who self-identified as having a mood disorder.
The community advisory board endorsed the biobank's goal of studying risk to allow earlier diagnoses and individualized treatment. Overall, the advisory board felt that the participants' privacy, welfare, and health were adequately protected. The comprehension assessment evaluating the adequacy of understanding, which was reviewed and validated by the community advisory board, has become part of the informed consent process for all research participants. The clinic-based community advisory board, taken from a small community of 100,000 people in a large referral medical practice, is unique in its composition. The community is highly educated, and most households are directly or indirectly linked to the health care industry. The advisory board engaged key members of the bipolar stakeholder community. We are aware of at least one patient with bipolar disorder who was a member of our advisory board. No active patients were involved in the development of the biobank. We anticipate having future community advisory board meetings, which will focus on review of our genomic research results and return of incidental research findings (19, 20).
Biobank-based research has the potential to transform the diagnosis and treatment of bipolar disorder. Earlier work from the DBSA encouraged increasing public health efforts to promote early diagnosis and treatment with adequate trials of mood-stabilizers for patients with frequent recurrences. This call to action was driven primarily by data suggesting that early manifestation of illness without accurate diagnosis is associated with great hardship for the individual and his/her family (3, 4). Early examples of genomic technology affecting psychiatric clinical practice, by prompting a US Food and Drug Administration drug safety communication revision, include the dosing guidelines for citalopram (cytochrome p450 2C19 poor metabolizer phenotype associated with QTc prolongation) (21) and preemptive genetic testing before carbamazepine use in patients of Chinese ancestry (HLA-B1502 association with Stevens-Johnson syndrome) (22, 23). Efforts are also under way to identify pharmacogenomic predictors of lithium response (24) and antidepressant-induced mania (25, 26).
The survey is limited by sample size, the survey not being pre-tested and lack of detailed demographics of survey respondents (both overall and compared with DBSA members who did not access the survey). While 80% of the survey participants were female, this is not uncommon health behavior knowing that females are more likely to seek health care for themselves and their household (27). However, through a nationally based consumer group—therefore, one of the strongest stakeholders in biobanking initiatives—this study provides a cross-sectional view of potential drivers of research participation. On the other hand, the methodology of selecting the advisory board members was not a random sampling method; instead, the members were selected through direct invitations.
Although the potential for biomarker-based personalized medicine is clear with regard to transforming clinical practice, several current barriers to full utilization must be addressed. Some barriers include the need for test results in real time to guide treatment selection at the point of care; development of an electronic medical record that can integrate large-scale genomic data with clinical decision support tools; and a wide array of financial, insurance, and ethical issues (28). Community involvement and discussion among people living with bipolar disorder will be critical for these advances in medicine.
Acknowledgments
We would like to thank Mayo Clinic Bipolar Biobank participants, as well as all the community advisory board members for their contribution to our research program.
Funding for the study was provided by the Marriott Foundation. The foundation had no further role in the study design, analysis or interpretation of the data, writing of the report, or decision to submit the paper for publication. Additional funding for this study included a National Human Genome Research Institute grant (P20HG007243, to BK).
MAF has received grant support from Assurex Health, Myriad, Pfizer, National Institute of Mental Health (R01 MH079261), National Institute of Alcohol Abuse and Alcoholism (P20AA017830), and the Mayo Foundation; has been a consultant to Janssen Global Services, LLC, Mitsubishi Tanabe Pharma Corporation, Myriad, Sunovion, and Teva Pharmaceuticals; has received CME/travel support/presentation from CME Outfitters Inc. and Sunovion. Mayo Clinic has a financial interest in AssureRx and the technology referenced in this publication/presentation. BK has received grant support from the National Institutes of Health (U01 HG004599, U01 HG006379, R01 DA014577, UL1 RR024150, and UL1 TR000135). SLM is a consultant to or member of the scientific advisory boards of Bracket, MedAvante, Naurex, Shire, and Sunovion; is a principal or co-investigator on studies sponsored by the Agency for Healthcare Research & Quality, AstraZeneca, Cephalon, Forest, Marriott Foundation, National Institute of Mental Health, Orexigen Therapeutics, Inc., Shire, and Takeda Pharmaceutical Company, Ltd.; is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent's assignee, University of Cincinnati, Cincinnati, OH, has received payments from Johnson & Johnson, which has exclusive rights under the patent.
Footnotes
Disclosures: AD, MN, LRS, JMB, and ASD have no disclosures or conflicts of interest to report.
References
- 1.Frye MA. Clinical practice. Bipolar disorder--a focus on depression. N Engl J Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- 2.Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41:33–40. doi: 10.1017/S003329171000084X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 4.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- 5.Leverich GS, Post RM, Keck PE, Jr, et al. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 6.Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71:864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 7.Severino G, Squassina A, Costa M, et al. Pharmacogenomics of bipolar disorder. Pharmacogenomics. 2013;14:655–674. doi: 10.2217/pgs.13.51. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 9.Olson JE, Ryu E, Johnson KJ, et al. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clin Proc. 2013;88:952–962. doi: 10.1016/j.mayocp.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElroy SL, Crow S, Biernacka JM, et al. Clinical phenotype of bipolar disorder with comorbid binge eating disorder. J Affect Disord. 2013;150:981–986. doi: 10.1016/j.jad.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL. Public opinion about the importance of privacy in biobank research. Am J Hum Genet. 2009;85:643–654. doi: 10.1016/j.ajhg.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollinger JM, Green RC, Kaufman D. Attitudes about regulation among direct-to-consumer genetic testing customers. Genet Test Mol Biomarkers. 2013;17:424–428. doi: 10.1089/gtmb.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaskell G, Gottweis H, Starkbaum J, et al. Publics and biobanks: Pan-European diversity and the challenge of responsible innovation. Eur J Hum Genet. 2013;21:14–20. doi: 10.1038/ejhg.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goes FS, Hamshere ML, Seifuddin F, et al. Genome-wide association of mood-incongruent psychotic bipolar disorder. Transl Psychiatry. 2012;2:e180. doi: 10.1038/tp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jen A, Saunders EF, Ornstein RM, Kamali M, McInnis MG. Impulsivity, anxiety, and alcohol misuse in bipolar disorder comorbid with eating disorders. Int J Bipolar Disord. 2013;1:13. doi: 10.1186/2194-7511-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemke AA, Halverson C, Ross LF. Biobank participation and returning research results: perspectives from a deliberative engagement in South Side Chicago. Am J Med Genet A. 2012;158A:1029–1037. doi: 10.1002/ajmg.a.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel DB, Platt T, Platt J, King SB, Kardia SL. Community perspectives on public health biobanking: an analysis of community meetings on the Michigan BioTrust for Health. J Community Genet. 2014;5:125–138. doi: 10.1007/s12687-013-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartzler A, McCarty CA, Rasmussen LV, et al. Stakeholder engagement: a key component of integrating genomic information into electronic health records. Genet Med. 2013;15:792–801. doi: 10.1038/gim.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig BA. Have we asked too much of consent? Hastings Cent Rep. 2014;44:33–34. doi: 10.1002/hast.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar Y, Kung S, Shinozaki G. CYP2C19 variation, not citalopram dose nor serum level, is associated with QTc prolongation. J Psychopharmacol. 2014;28:1143–1148. doi: 10.1177/0269881114543720. [DOI] [PubMed] [Google Scholar]
- 22.Khor AH, Lim KS, Tan CT, Wong SM, Ng CC. HLA-B*15:02 association with carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in an Indian population: a pooled-data analysis and meta-analysis. Epilepsia. 2014;55:e120–124. doi: 10.1111/epi.12802. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell PB, Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543–1546. doi: 10.2217/14622416.9.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchia M, Adli M, Akula N, et al. Assessment of Response to Lithium Maintenance Treatment in Bipolar Disorder: A Consortium on Lithium Genetics (ConLiGen) Report. PLoS One. 2013;8:e65636. doi: 10.1371/journal.pone.0065636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frye MA, McElroy SL, Prieto ML, et al. Clinical risk factors and serotonin transporter gene variants associated with antidepressant-induced mania. J Clin Psychiatry. 2015;76:174–180. doi: 10.4088/JCP.14m09127. [DOI] [PubMed] [Google Scholar]
- 26.Biernacka JM, McElroy SL, Crow S, et al. Pharmacogenomics of antidepressant induced mania: a review and meta-analysis of the serotonin transporter gene (5HTTLPR) association. J Affect Disord. 2012;136:e21–9. doi: 10.1016/j.jad.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–623. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 28.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


