SUMMARY
Tumorigenesis is associated with increased glucose consumption and lipogenesis, but how these pathways are interlinked is unclear. Here, we delineate a pathway in which EGFR signaling, by increasing glucose uptake, promotes N-glycosylation of sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP) and consequent activation of SREBP-1, an endoplasmic reticulum-bound transcription factor with central roles in lipid metabolism. Glycosylation stabilizes SCAP and reduces its association with Insig-1, allowing movement of SCAP/SREBP to the Golgi and consequent proteolytic activation of SREBP. Xenograft studies reveal that blocking SCAP N-glycosylation ameliorates EGFRvIII-driven glioblastoma growth. Thus, SCAP acts as key glucose-responsive protein linking oncogenic signaling and fuel availability to SREBP-dependent lipogenesis. Targeting SCAP N-glycosylation may provide a promising means of treating malignancies and metabolic diseases.
Keywords: SCAP, N-glycosylation, SREBP-1, EGFR signaling, glioblastoma, Insig-1
Graphical Abstract
INTRODUCTION
Elevated lipogenesis is a common patho-physiological characteristic of cancer and metabolic diseases (Guo et al., 2013; Menendez and Lupu, 2007; Moon et al., 2012; Ru et al., 2013; Tang et al., 2011). In these processes, a critical regulatory role is played by sterol regulatory element-binding proteins (SREBPs), a family of transcription factors that control the expression of genes important for the uptake and synthesis of cholesterol, fatty acids and phospholipids (Goldstein et al., 2006; Jeon and Osborne, 2012; Nohturfft and Zhang, 2009). There are two mammalian SREBP genes, SREBF1 and SREBF2. SREBP-1a and −1c, which are encoded by SREBF1 with different N-terminus (~20 amino acids) owing to their mRNAs being transcribed from different transcriptional start sites, mainly regulate the expression of genes required for fatty acid synthesis. SREBP-2 is encoded by SREBF2 and is responsible for the synthesis of cholesterol (Goldstein et al., 2006; Horton et al., 2002; Horton et al., 2003). Recent evidence shows that the nuclear form of SREBP-1 is highly upregulated in a variety of malignancies (Ettinger et al., 2004; Guo et al., 2009b; Li et al., 2014). Targeting SREBP-1 has become a promising therapeutic strategy to treat cancer and other metabolic syndromes (Griffiths et al., 2013; Guo et al., 2014; Kamisuki et al., 2009; Tang et al., 2011).
SREBP-1 and SREBP-2 are synthesized as inactive precursors bound to the membrane of the endoplasmic reticulum (ER) through two transmembrane domains (Goldstein et al., 2006). SREBP activation requires proteolytic release of an N-terminal fragment that constitutes a basic helix-loop-helix transcription factor (Goldstein et al., 2006; Wang et al., 1994). Currently, post-translational activation of SREBPs is best understood in the context of cellular cholesterol homeostasis (Radhakrishnan et al., 2008; Sun et al., 2007). When cholesterol level is high, it binds to SREBP-cleavage activating protein (SCAP), inducing a conformational change that promotes binding to ER-anchored insulin induced gene protein (Insig), thus preventing Golgi transport and activation of SREBPs (Adams et al., 2004; Sun et al., 2007; Yang et al., 2002). When the cholesterol concentration drops, the SCAP/SREBP complex dissociates from Insig, allowing vesicular transport to the Golgi where SREBPs are exposed to proteases that release the transcriptionally active N-terminal fragment (Goldstein et al., 2006; Nohturfft et al., 2000; Sun et al., 2007).
Glucose is a major resource for de novo lipid synthesis. In cancer cells, elevated glucose consumption is often accompanied by increased lipogenesis (Guo et al., 2013; Menendez and Lupu, 2007), and the link between glucose supply and SREBP-1 activation seems common in both physiological and patho-physiological conditions (Guillet-Deniau et al., 2004; Hasty et al., 2000; Horton et al., 1998; Kaplan et al., 2008). In a previous study we showed that epidermal growth factor receptor (EGFR) via PI3K/Akt signaling activated SREBP-1 in glioblastoma (GBM) cells (Guo et al., 2009b; Guo et al., 2011). While a number of studies have demonstrated that elevated EGFR signaling is coupled with enhanced glucose uptake and lipogenesis in tumorigenesis (Babic et al., 2013; Cloughesy et al., 2014; Guo et al., 2009a; Guo et al., 2009b), the molecular mechanisms that underlie the cross-talk between the altered glucose and lipid metabolism in tumorigensis remain largely unknown. In this study, we test the hypothesis that SCAP acts as a key glucose-responsive protein to integrate oncogenic signaling and fuel availability for modulation of SREBP-dependent lipogenesis.
RESULTS
Glucose activates SREBPs via upregulation of SCAP
To investigate how glucose and sterol signaling are integrated during SREBP activation, human GBM U87 cells that have been shown to contain a high SREBP-1 activity (Guo et al., 2009b; Guo et al., 2011), were grown in the absence or presence of glucose and sterols (cholesterol/25-hydroxycholesterol), and SREBP processing was analyzed by western blot and immunofluorescence microscopy. We found that in the absence of glucose, only the ER-bound SREBP-1 precursor could be clearly detected, even in cells that were deprived of sterols by removal of serum (Figure 1A). While glucose supplement modestly activated SREBP-1 in the presence of sterols (as assessed by the appearance of the N-terminal cleavage fragment of SREBP-1), maximal SREBP-1 and SREBP-2 cleavage required both glucose supplement and low sterols in the absence or presence of serum (Figures 1A and S1A). Moreover, glucose activated SREBP-1 and SREBP-2 in a dose- and time-dependent manner and increased the protein levels of fatty acid synthase (FASN) and low-density lipoprotein receptor (LDLR), the genes encoding both of which are downstream targets for SREBP-1 (Figures 1B, 1C, S1A and S1B) (Bennett et al., 1995; Yokoyama et al., 1993). Furthermore, real-time qPCR analysis showed that glucose stimulation enhanced both SREBP-1a and −1c expression and activated the expression of SREBP-1 or SREBP-2 regulated genes involved in lipid metabolism (Figures S1C). Using confocal fluorescence imaging, we found that stimulation of U87 cells with glucose promoted nuclear translocation of transgenic GFP-SREBP-1 (Figures 1D and S1D). Translocation of endogenous SREBP-1 into the nucleus in response to glucose stimulation was demonstrated by immunofluorescence (Figures 1E and S1E). In contrast, under glucose-deprived condition, even with removal of sterols, SREBP-1 was still retained in the ER membrane as shown by its co-staining with protein disulfide isomerase (PDI), an ER membrane protein (Figures 1E and S1E) (Uehara et al., 2006). These data suggest that glucose is required for SREBP activation under conditions of sterol deprivation.
Figure 1. Glucose activates SREBPs via upregulation of SCAP.
(A) Western blot analysis of U87 cells cultured in serum-free media with or without glucose (5 mM) in the presence or absence of sterols (10 µg/ml 25-hydroxycholesterol, and 10 µg/ml cholesterol) for 12 hr.
(B, C) Western blot analysis of U87 cells cultured in serum-free media with different dose of glucose for 12 hr (B) or stimulated with 5 mM glucose at indicated times (C).
(D, E) Confocal microscopy images show GFP-SREBP-1 (green), which was derived from a cDNA lacking the exon 1 of SREBP-1 (encodes the identical amino acid sequence between SREBP-1a and −1c) (D) or endogenous SREBP-1 (E, red) subcellular localization in U87 cells cultured in serum-free media with or without glucose (Gluc) for 12 hr. Nuclei were stained by DAPI (blue). Scale bars, 10 µm. The nucleus intensity of GFP-SREBP-1 (D) or endogenous SREBP-1 (red) (E) was quantified over 30 cells by using ImageJ. Red lines in the quantification graphs show mean ± SEM (n = 30). Significance was determined by an unpaired student t test.
(F) Western blot analysis of membrane and nuclear extracts from U87 cells cultured in serum-free media with or without glucose (5 mM) for 12 hr.
(G, H) Western blot analysis of membrane, nuclear extracts or total cell lysates from HEK293T cells co-transfected with GFP-SCAP or GFP vector and full length (FL) Flag-SREBP-1a, −1c or HA-SREBP-2 plasmids (G) or from U87 cells after knockdown of SCAP in comparison with shRNA control (shCtrl) in serum-free media with or without glucose (5 mM) for 12 hr (H). P, precursor of SREBPs; N, N-terminus of SREBP-1. C, C-terminus of SREBP-2.
See also Figure S1.
SREBP stability, transport to the Golgi and cleavage require formation of a complex between SREBP and SCAP (Nohturfft et al., 2000; Rawson et al., 1999; Sakai et al., 1998). We found that glucose-induced cleavages of SREBP-1 and SREBP-2 were accompanied by increase of SCAP protein levels in GBM cells (Figures 1F, S1A and S1B). Similar results were observed in various other cancer cell lines in which both SREBP-1 and −2 were activated (see Figures S1F). HEK293T cells expressing GFP-SCAP and full length Flag-SREBP-1a, −1c or HA-SREBP-2 displayed glucose-dependent upregulation of GFP-SCAP and activation of all three SREBP isoforms, which were detected by increased N-terminal fragment of epitope-tagged SREBP in the nuclear fraction under conditions of glucose supplement (Figures 1G and S1G). We found that knockdown of SCAP reduced glucose-mediated activation of SREBP-1 and SREBP-2 (Figure 1H).
Because liver X receptor (LXR), a member of the nuclear receptor family of transcription factors, promotes the expression of SREBP-1c upon activation (Repa et al., 2000), and glucose is able to activate LXR in hepatocytes (Mitro et al., 2007), we sought to examine whether LXR possibly involves in glucose-activated SCAP/SREBP signaling. As shown in Figures S1A–S1C, our data show that both the protein and mRNA levels of ATP-binding cassette activating proteins ABCA1 and ABCG1, which are major downstream targets of LXR (Zelcer and Tontonoz, 2006), were not significantly upregulated by glucose supplement in U87 cells within 12 hr, demonstrating that LXR was not strongly activated by glucose in GBM cells. In contrast, SCAP protein and SREBP-1 and SREBP-2 cleavage were enhanced by glucose stimulation (Figure S1A and S1B). Together, these data suggest that LXR was not involved in glucose-mediated SREBP signaling pathway.
Collectively, these data demonstrate that glucose availability constrains SREBP activity by controlling the levels of SCAP.
Glucose promotes SCAP N-glycosylation and enhances its stability
To explore the mechanisms of how glucose enhances SCAP protein levels and activates SREBP, the intermediate metabolite, pyruvate or lactate of the glycolysis pathway or N-acetylglucosamine (GlcNAc) of the hexosamine biosynthetic pathway (HBP) (Figure 2A), was added to GBM U87 cells in glucose- and sterol-free medium, respectively. The data showed that GlcNAc was as effective as glucose at enhancing SCAP protein levels and promoting SREBP-1 cleavage, while pyruvate and lactate had no effect (Figure 2B). Upon exposure of cells to an inhibitor of HBP (azaserine, AZA) or to an inhibitor of N-glycan synthesis (tunicamycin, Tuni), both SCAP protein levels and SREBP-1 cleavage were reduced, while an inhibitor of O-glycosylation (BADGP) had no effect (Figures 2C and S2A–S2D). Immunofluorescence imaging showed that glucose-induced Golgi and nuclear translocation of SREBP-1 were largely blocked by AZA and tunicamycin (Figures 2D and S2E). As expected, addition of GlcNAc restored AZA treatment-reduced SCAP protein levels and SREBP-1 and SREBP-2 cleavages in U87 cells (Figure S2F). These data suggest that the effects of glucose on SCAP and SREBP were likely mediated by an induction of protein N-glycosylation.
Figure 2. Glucose promotes SCAP N-glycosylation and enhances its stability.
(A) Schematic model shows the glucose metabolism divided into glycolysis, de novo lipid synthesis, oxidative pentose phosphate pathway (PPP) and hexosamine synthesis pathway (HBP) for glycosylation modification. The key enzymes controlling HBP and glycosylation modifications and their specific inhibitors are shown in the scheme. Pyr, pryruvate; Lac, lactate; TCA, tricarboxylic acid cycle; Fruc-6-P, fructose-6-phosphate; GlcNAc, N-acetylglucosamine; AZA, azaserine; Tuni, tunicamycin; GFAT, fructose-6-phosphate amidotransferase; DPAGT1, dolichyl-phosphate (UDP-GlcNAc) GlcNAc-1-P transferase; OGT, O-glycosylation transferase.
(B, C) Western blot analysis of U87 cell lysates which were derived from cells cultured in serum-free media with or without glucose (5 mM), pyruvate (10 mM), lactate (10 mM) or GlcNAc (20 mM) (B), or in combination with AZA (100 µM), tunicamycin (Tuni, 1 µg/ml) or BADGP (1 mM) for 12 hr (C).
(D) Confocal microscopy images of SREBP-1 sub-cellular localization in relation with the Golgi protein marker Giantin and nuclear DAPI staining in U87 cells with same treatment procedure as panel C. Scale bars, 10 µm.
(E) Western blot analysis of cell membrane fractions for protease-protected N-glycosylation fragment (a.a. 540–707) of SCAP (upper panel) or total GFP-SCAP protein levels (lower panel) from HEK293T cells transiently transfected with GFP-SCAP for 24 hr and then cultured in serum-free media with or without glucose (5 mM) or GlcNAc (20 mM) for another 12 hr. The numbers on the left side of blot indicate the number of N-glycosylation on SCAP protein (upper panel) (Nohturfft et al., 1998). For details, please see supplemental experiment procedures.
(F, G) Western blot analysis of SCAP N-glycosylation (upper panel) or GFP-SCAP protein levels (lower panel) from HEK293T cells transiently transfected with GFP-SCAP for 24 hr and then cultured in serum-free media with or without GlcNAc (20 mM) or glucose (5 mM) in the presence or absence of AZA (100 µM) (F) or with or without glucose in combination with tunicamycin (1 µg/ml) (G) for 12 hr.
(H) Western blot analysis of cell membrane fractions from U87 cells cultured in serum-free media with or without glucose (5 mM) in combination with tunicamycin (1 µg/ml) and protease inhibitor E64D (10 µg/ml)/Pepstatin A (10 µg/ml)/Leupeptin (10 µg/ml) or proteasome inhibitor MG132 (50 µM) for 12 hr.
(I) Western blot analysis of cell membrane fractions from U87 cells cultured in serum-free media with or without glucose (5 mM) in the presence or absence of MG132 (50 µM) for 6 hr.
(J, K) Western blot analysis of cell membrane fractions from HEK293T cells transfected with indicated amounts of plasmid expressing the wild-type GFP-SCAP (NNN) or mutant GFP-SCAP (QQQ) for 24 hr (J), then treated with or without MG132 (50 µM) for another 4 hr (K). The levels of GFP-SCAP-NNN or -QQQ were quantified by ImageJ and normalized with PDI. The results are shown as mean ± SD (n = 3). Significance was determined by an unpaired Student t test. * p < 0.01.
See also Figure S2.
Nohturfft et al. showed that SCAP protein carries three N-linked oligosaccharides at asparagine (N) positions N263, N590 and N641 and those mutations of one or two of these asparagines had no apparent effect on the function of SCAP. However, a version of SCAP with all three asparagines mutated to glutamine (NNN to QQQ) could not be detected, suggesting that N-glycosylation is important for the stability of SCAP (Nohturfft et al., 1998). Therefore, we asked whether glucose might control the levels of SCAP by affecting its glycosylation. Furthermore, the role for N-glycosylation in SCAP/SREBP function has not been extensively explored.
We adopted the approach of Nohturfft et al. and examined the effects of glucose on SCAP N-glycosylation in HEK293T cells and CHO cells (Nohturfft et al., 1998). SCAP contains a luminal region (a.a. 540–707) with two N-glycosylation sites that is protected from proteolysis when intact membranes are treated with trypsin (Nohturfft et al., 1998). This luminal fragment has a molecular weight of ~30 kDa that is small enough to allow the resolution of individual glycosylated variants of SCAP by SDS-PAGE (Figures 2E, upper panel and S2G). Glucose deprivation for 12 hr resulted in two weaker bands that correspond to SCAP fragments carrying one or two oligosaccharides in HEK293T cells expressing GFP-SCAP (Figure 2E, upper panel), indicating that the level of SCAP N-glycosylation was decreased under low glucose condition that was correlated with reduced total GFP-SCAP protein (Figures 2E, lower panel and S2H). Removal of the oligosaccharides by treatment with endoglycosidase (PNGase) reduced the apparent molecular weight to the un-glycosylated form of SCAP (Nohturfft et al., 1998). Notably, in the presence of glucose, high amount of fully glycosylated form of SCAP and total GFP-SCAP protein were detected (Figures 2E, S2G and S2H), which were associated with elevated SREBP activation (Figures 1F, 1G and S1G). Supplementing the culture medium with GlcNAc enhanced SCAP glycosylation and increased total GFP-SCAP protein to a level similar to that found upon glucose treatment (Figures 2E). Furthermore, AZA or tunicamycin treatment blocked glucose-promoted SCAP N-glycosylation and reduced GFP-SCAP levels in HEK293 cells and endogenous SCAP levels in CHO cells (Figures 2F, 2G, S2G and S2H), and addition of GlcNAc restored AZA treatment-mediated reduction of SCAP N-glycosylation and total GFP-SCAP protein to levels similar to cells exposed to glucose supplement alone (Figure 2F). In GBM cells treated with tunicamycin, the endogenous SCAP protein level was reduced (Figures 2H and S2C). Co-treatment with MG132, a proteasome inhibitor, but not protease inhibitors (combination of E64D, Pepstatin and Leupepsin), restored the SCAP protein to a level similar to that when cells exposed to glucose alone (Figures 2H and 2I). Taken together, these data demonstrate that glucose deprivation-led reduction of SCAP protein levels is caused by proteasome-dependent degradation.
To examine the effect of N-glycosylation on SCAP stability, we generated a mutant form of SCAP by replacing all three asparagines (N263, N590 and N641) with glutamines (NNN to QQQ). We added a GFP tag to the N-terminal end of SCAP and used a strong CMV promoter to drive its expression in HEK293T cells. This enabled us to produce measurable amount of GFP-SCAP-QQQ protein, overcoming the problem of Nohturfft et al. faced (Nohturfft et al., 1998). Compared with the wild-type protein (GFP-SCAP-NNN), lower levels of GFP-SCAP-QQQ were detected (Figure 2J), suggesting that the un-glycosylated SCAP proteins are less stable and presumably are more susceptible to proteasomal degradation. Indeed, treatment of cells with MG132 significantly restored GFP-SCAP-QQQ protein levels (Figure 2K).
Glucose-mediated glycosylation promotes SCAP trafficking to the Golgi leading to SREBP activation
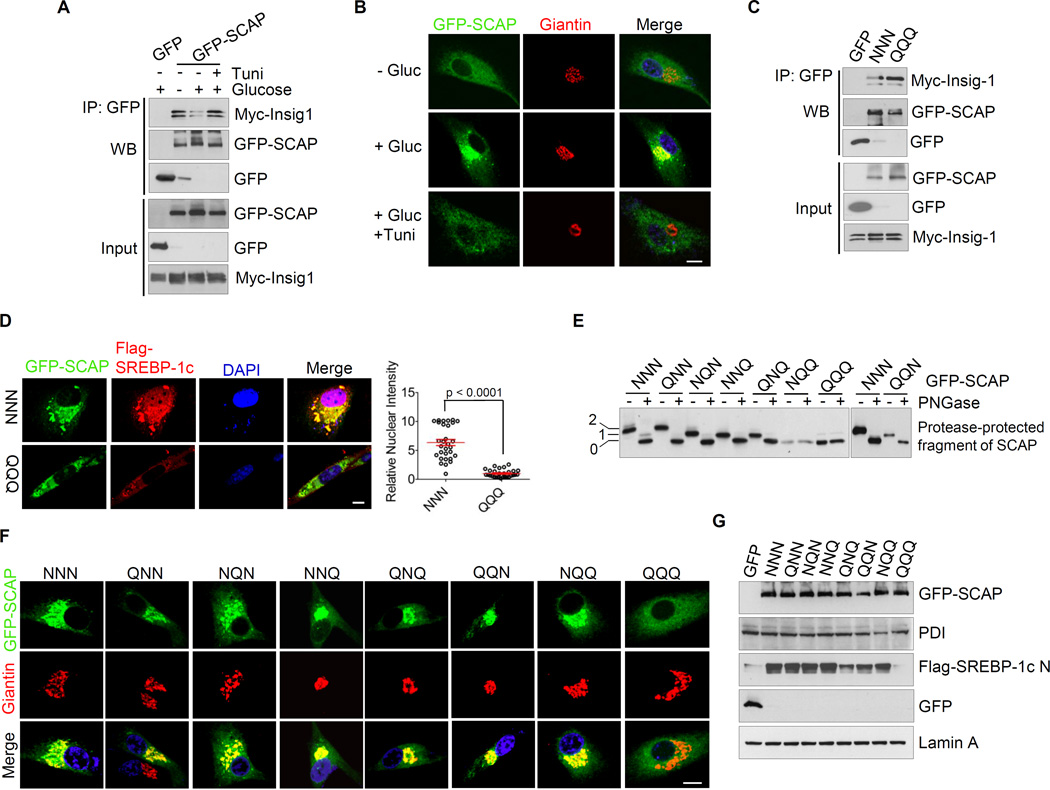
We wondered whether N-glycosylation altered the association of SCAP with Insig and thereby promoted SCAP/SREBP trafficking to the Golgi in the absence of sterols. To address this, HEK293T cells were co-transfected with Myc-Insig-1 and GFP-SCAP or GFP-vector to determine their interaction in response to glucose and tunicamycin treatment. We found that glucose treatment reduced the association of GFP-SCAP with Myc-Insig-1 compared with glucose-deprived condition (Figure 3A). Further, blocking N-glycosylation by tunicamycin restored the binding of GFP-SCAP to Myc-Insig-1 (Figure 3A). These data suggest that N-glycosylation modification by glucose reduced the binding of SCAP to Insig-1. To test if this affected the SCAP/SREBP trafficking to the Golgi, the subcellular distribution of GFP-SCAP was examined by confocal microscopy. The data show that glucose stimulation promoted the GFP-SCAP trafficking to the Golgi, which was blocked by inhibition of N-glycosylation with tunicamycin (Figures 3B and S3A).
Figure 3. N-glycosylation promotes SCAP trafficking to the Golgi leading to SREBP-1 activation.
(A) Total or immunoprecipitated as indicated of cell lysates from HEK293T cells transfected with GFP-SCAP or GFP vector together with Myc-Insig-1 without or with glucose (5 mM) stimulation in the presence or absence of tunicamycin (1 µg/ml) for 12 hr were immunoblotted using the indicated antibodies.
(B) Confocal microscopy images show GFP-SCAP trafficking related to the Golgi marker Giantin in response to glucose (5 mM) in the presence or absence of tunicamycin (1 µg/ml) in U87 cells for 12 hr. Scale bars, 10 µm.
(C) Immunoblot analysis of total or immunoprecipitated as indicated of cell lysates from HEK293T cells transfected with wild-type GFP-SCAP (NNN) or mutant GFP-SCAP (QQQ) together with Myc-Insig-1 in response to glucose (5 mM) for 12 hr using the indicated antibodies.
(D) Confocal microscopy images show SCAP and SREBP-1 trafficking in U87 cells transfected with wild-type GFP-SCAP (NNN) or mutant GFP-SCAP (QQQ) together with Flag-SREBP-1c (full length, FL) in response to glucose (5 mM) for 12 hr. Scale bars, 10 µm. The nucleus intensity of Flag-SREBP-1c was quantified over 30 cells by using ImageJ. The results are shown as mean ± SEM (n = 30). Significance was determined by an unpaired student t test.
(E) Western blot analysis of the N-glycosylation levels of SCAP mutant in HEK293T cells transfected with different mutant of GFP-SCAP in comparison with wild-type (NNN).
(F) Confocal microscopy images show the trafficking of different SCAP mutants in U87 cells transfected with wild-type (NNN) or different mutant GFP-SCAP under glucose (5 mM) treatment in serum-free media. Scale bars, 10 µm.
(G) Western blot analysis of membranes and nuclear extracts from HEK293T cells transfected with wild-type or different mutant GFP-SCAP together with Flag-SREBP-1c (full length) in response to glucose (5 mM) stimulation in serum-free media.
See also Figure S3.
Data with transient expression of GFP-SCAP-QQQ mutant and Myc-Insig-1 in HEK293T cells further supported the notion that N-glycosylation reduces SCAP binding to Insig-1 and subsequent SCAP/SREBP trafficking to the Golgi. We found that GFP-SCAP-QQQ displayed stronger association with Myc-Insig-1 compared with GFP-SCAP-NNN (Figure 3C). While GFP-SCAP-NNN promoted trafficking of Flag-SREBP-1c to the Golgi and subsequent translocation to the nucleus, the GFP-SCAP-QQQ mutant failed to do so (Figures 3D and S3B).
The contribution of individual N-glycosylation sites to SCAP function and trafficking was further investigated in HEK293T and U87 cells expressing the GFP-SCAP protein harboring single, double or triple mutations. Our data showed that none of the single or double mutations in SCAP impaired the trafficking and activation of Flag-SERBP-1c in response to glucose stimulation (Figures 3E–3G, S3C and S3D). These data were consistent with the early studies by the Brown and Goldstein laboratory which demonstrated that mutations of one or two N-glycosylation sites produced negligible effects on SREBP-2 activation (Nohturfft et al., 1998). Interestingly, we found that the triple mutant GFP-SCAP-QQQ was unable to traffic to the Golgi and failed to activate epitope-tagged SREBP-1a, −1c and SREBP-2 (Figures 3F, 3G, S3C and S3E–S3G). Thus, even though maintenance of a single glycosylation site on SCAP appeared to be sufficient for its biological function (Nohturfft et al., 1998), complete abolition of all glycosylation sites on SCAP disrupted the exit pathway for the SCAP/SREBP complex from ER to the Golgi.
Elegant studies by Brown and Goldstein laboratory established that sterols are critical factors in the regulation of SCAP trafficking and SREBP activation (Goldstein et al 2006). We conducted similar studies and found that incubation of cells with sterols could block glucose-mediated SCAP trafficking and SREBP-1 nuclear translocation (Figure S3H and S3I). Overexpressing Myc-Insig-1 or increasing sterol concentration reduced glucose-mediated SREBP-1 activation (Figure S3J and S3K). While these data are consistent with the early work of Brown and Goldstein laboratory, the present findings suggest that glucose is indispensable for SCAP/SREBP trafficking and function. Our data support that N-glycosylation relieves SCAP association with Insig, leading to SCAP/SREBP trafficking to Golgi and subsequent SREBP activation; and sterols could antagonize glucose function on SCAP trafficking and SREBP activation.
EGFR signaling activates SREBP-1 by enhancing the glucose uptake, SCAP protein levels and N-glycosylation
EGFR signaling has been shown to enhance glucose uptake and activate SREBP-1 (Babic et al., 2013; Guo et al., 2009a; Guo et al., 2009b). Based on our observation that SREBP activation in sterol-deprived condition was fully depended on glucose (Figure 1), we tested whether EGFR-mediated SREBP-1 activation also required glucose. As shown in Figure 4A, EGF stimulation was unable to promote the nuclear translocation of SREBP-1 in the absence of glucose, and addition of glucose could restore EGF-mediated SREBP-1 nuclear translocation. Western blotting showed that EGF stimulation had no effect on SREBP-1 cleavage in glucose-free media, even though EGFR-PI3K-Akt-mTOR signaling pathway was strongly activated as evident by the upregulation of p-EGFR, p-Akt and p-S6 (Figures 4B and S4A). In contrast, EGF stimulation in the presence of glucose promoted the cleavage of SREBP-1 (Figures 4B and S4A).
Figure 4. EGFR signaling via promoting glucose uptake upregulates SCAP and activates SREBP-1.
(A, B) Confocal microscopy images of SREBP-1 subcellular distribution (A), or western blot analysis of SREBP-1 cleavage (B) in U87/EGFR cells in response to EGF (50 ng/ml) in the presence or absence of glucose (5 mM) for 12 hr. Scale bars, 20µm. SREBP-1 nucleus intensity was quantified over 30 cells by using ImageJ. The results are shown as mean ± SEM (n = 30). Significance was determined by an unpaired student t test.
(C) Glucose uptake analysis of U87/EGFR cells in response to EGF (50 ng/ml) by using 14C-glucose isotope. The results are shown as mean ± SEM (n=3). Significance was determined by an unpaired student t test.
(D) Western blot analysis of cell membrane and nuclear extracts from U87/EGFR cells in response to EGF (50 ng/ml) stimulation in the presence or absence of glucose (5 mM) for 12 hr.
(E) Western blot analysis of U87/EGFR cells after knockdown of SCAP in comparison with shRNA control (shCtrl) in response to EGF (50 ng/ml) stimulation in the presence of glucose (5 mM) for 12 hr.
See also Figure S4.
Using 14C-glucose radioisotope measurements, we found that EGF stimulation led to significant increase of glucose uptake in U87/EGFR cells (Figure 4C). While EGF stimulation did not appear to affect the SCAP protein level in the absence of glucose, additive effect of glucose and EGF-dependent enhancement of SCAP expression was observed (Figures 4D and S4A). Moreover, the EGF-induced changes in SCAP expression was correlated with SREBP-1 activation, as reflected by the increased appearance of the SREBP-1 N terminal fragment in the nuclear fraction (Figures 4D and S4A). Moreover, increase in glucose uptake by insulin in HepG2 cells, also upregulated SCAP protein and activated SREBP-1 (Figures S4B and S4C), suggesting that the activation of glucose-SCAP-SREBP signaling pathway could be induced by various other growth factors. We then knocked down the expression of SCAP in U87/EGFR cells in order to examine whether the effect of EGF on SREBP-1 activation was mediated by the Glucose-SCAP axis. As shown in Figure 4E, knockdown of SCAP completely abolished EGF-mediated activation of SREBP-1.
Inhibition of the HBP pathway by AZA or suppression of N-glycosylation by tunicamycin blocked EGF-mediated nuclear translocation of SREBP-1, whereas the O-glycosylation inhibitor BADGP treatment failed to do so (Figure 5A). Western blotting showed that AZA or tunicamycin treatment blocked EGFR signaling-dependent increase in SREBP-1 cleavage, whereas no changes were observed by BADGP treatment (Figure 5B). Moreover, EGF-mediated upregulation of SCAP was reduced by AZA or tunicamycin treatment, but not by the BADGP treatment (Figure 5B). We incubated U87/EGFR cells with 14C-glucose and measured isotope labeled lipid products, and found that AZA or tunicamycin treatment significantly reduced EGFR signaling-dependent increase in de novo lipid synthesis (Figure 5C). These results demonstrate that N-glycosylation was a key mediator for EGFR signaling-mediated increase in SCAP level and subsequent activation of SREBP-1.
Figure 5. EGFR signaling activates SREBP-1 via upregulation of SCAP N-glycosylation.
(A, B) Confocal microscopy images of SREBP-1 translocation (A), or western blot analysis of SCAP levels and SREBP-1 cleavage (B) in U87/EGFR cells cultured in serum-free media in response to EGF (50 ng/ml) in the presence or absence of AZA (100 µM), tunicamycin (1 µg/ml) or BADGP (1 mM) with glucose (5 mM) for 12 hr. Scale bars, 20 µm. SREBP-1 nucleus intensity was quantified over 30 cells by using ImageJ. The results are shown as mean ± SEM (n = 30). Significance was determined by an unpaired student t test.
(C) De novo lipid synthesis analysis in U87/EGFR cells cultured in serum-free media treated with or without EGF (50 ng/ml) in combination with AZA (100 µM) or tunicamycin (1 µg/ml) for 12 hr, then adding 0.5 µCi 14C-labeled glucose for 2 hr followed by lipid extraction and analysis by scintillation counter. The results are presented as mean ± SEM (n=3). Significance was determined by an unpaired student t test. *p < 0.05 and **p < 0.01.
(D) Western blot analysis of total protein (upper panel) and N-glycosylation levels (lower panel) of GFP-SCAP and SREBP-1 nuclear form (upper panel) in LN229/EGFR cells transiently expressing GFP-SCAP after the stimulation of EGF (50 ng/ml) in the presence or absence of tunicamycin (1 µg/ml) for 12 hr.
(E) Western blot analysis of U87/EGFR cells transfected with GFP, wild-type GFP-SCAP (NNN) or triple mutant GFP-SCAP (QQQ) after the stimulation of EGF (50 ng/ml) in serum-free media for 12 hr.
We then assessed the effect of EGF stimulation on SCAP glycosylation. Stimulation of cells with EGF led to the increase of the total and the N-glycosylated GFP-SCAP protein when compared with control and both were blocked by tunicamycin (Figure 5D). In correlation, EGF stimulation also upregulated SREBP-1 activity, which was inhibited by tunicamycin (Figure 5D, upper panel). Similar to the studies in Figure 3G, we measured the effect of EGF on SREBP-1 function in U87/EGFR cells expressing GFP-SCAP-NNN or GFP-SCAP-QQQ. We found that cells expressing GFP-SCAP-NNN showed enhanced SREBP-1 activation in response to EGF stimulation, whereas cells expressing GFP-SCAP-QQQ showed reduced SREBP-1 activation even below the levels observed with cells expressing GFP alone (as control) (Figure 5E). Interestingly, biochemical studies showed that the other signaling components for EGFR, e.g. p-EGFR, p-AKT and p-S6, were not affected by overexpression of either GFP-SCAP-NNN or GFP-SCAP-QQQ in U87/EGFR cells (Figure 5E). Taken together, our data show that EGFR-dependent SREBP-1 activation is mediated by upregulation of SCAP and its N-glycosylation through enhancing glucose uptake.
Impairment of SCAP N-glycosylation suppresses GBM tumor growth
To determine the effect of EGFR on SCAP/SREBP-1 function in tumorigenesis, we employed a cell culture model with stable expression of EGFRvIII, a constitutively active form of EGFR with enhanced activation of PI3K-Akt signaling that leads to aggressive tumorigenesis (Guo et al., 2009b; Huang et al., 1997). Western blot revealed that U87/EGFRvIII cells displayed elevated SCAP protein levels and enhanced SREBP-1 activation associated with upregulated p-Akt and p-S6 levels (Figure 6A). Based on this observation, we asked whether EGFRvIII-mediated tumorigenesis is mediated by enhanced SCAP expression and its glycosylation.
Figure 6. Impairment of SCAP N-glycosylation suppresses tumor growth and significantly prolongs overall survival of GBM-bearing mice.
(A) Western blot analysis of SCAP levels and SREBP-1 cleavage in U87 vs. U87-EGFRvIII cells.
(B–D) The effects of shRNA-mediated knockdown of SCAP in U87/EGFRvIII cells analyzed by western blot (B), subcutaneous tumor growth (1×106 cells/mouse), which are shown as mean ± SEM (n=8) (C), or overall survival of intracranial tumor bearing-mouse (1×105 cells/mouse) assessed by Kaplan-Meier analysis (D).
(E–H) The effects of wild-type GFP-SCAP (NNN) or mutant GFP-SCAP (QQQ) compared with the control GFP expression in U87/EGFRvIII cells analyzed by western blot (E), subcutaneous tumor growth (1×106 cells/mouse), which are shown as mean ± SEM (n=5) (F), luminescence imaging of intracranial tumor (1×105 cells/mouse) at day 14 after implantation, which results are shown as mean ± SEM (n=7) (G) or GBM bearing-mouse overall survival assessed by Kaplan-Meier analysis (H). Significance was determined by an unpaired student t test. * p< 0.05; ** p < 0.01. Survival was analyzed by Log-rank test.
We established a stable U87/EGFRvIII cell line with knockdown of SCAP (U87/EGFRvIII-shSCAP). Western blotting showed that knockdown of SCAP reduced SREBP-1 activation (Figure 6B), and which significantly reduced tumor growth in mice flank compared with the shRNA control group (Figure 6C). To assess the potential of these modified GBM cells to form orthotopic tumors, shSCAP or shControl cells were implanted into mouse brain. A Kaplan-Meier plot showed that reducing SCAP level significantly prolonged the overall survival of GBM-bearing mice (Figure 6D).
We next examined whether impairment of SCAP N-glycosylation had any effect on GBM tumorigenesis. For this purpose, GFP-SCAP-NNN, GFP-SCAP-QQQ, or GFP (as control) was transiently expressed in U87/EGFRvIII cells. The data showed that the expression of GFP-SCAP-NNN enhanced whereas GFP-SCAP-QQQ reduced SREBP-1 activation, compared with cells transfected with GFP (Figure 6E), suggesting that GFP-SCAP-QQQ had a dominant negative effect on SREBP-1 activation. Next, these transfected cells were implanted into mouse flanks. As shown in Figure 6F, GFP-SCAP-NNN expression promoted GBM tumor growth compared with the GFP control; whereas GFP-SCAP-QQQ expression significantly reduced the GBM tumor growth.
Finally, to determine the effect of SCAP N-glycosylation on GBM intracranial tumor growth, we used a U87/EGFRvIII cell line with stable expression of luciferase, which allows the visualization of tumor in mouse brain using luminescence imaging (Wojton et al., 2013). The data showed that the expression of GFP-SCAP-NNN promoted tumor growth whereas the expression of GFP-SCAP-QQQ significantly reduced tumor growth (Figure 6G). Moreover, overexpression of GFP-SCAP-NNN reduced the overall survival of GBM-bearing mice (Figure 6H). Inversely, impairment of SCAP N-glycosylation significantly prolonged the overall survival of mice (Figure 6H). Collectively, these data demonstrate that N-glycosylation on SCAP is a critical factor for EGFRvIII-induced GBM tumorigenesis.
DISCUSSION
Malignant cells, in general, have high rates of de novo lipid synthesis (Currie et al., 2013; Guo et al., 2013; Menendez and Lupu, 2007). Since solid tumors often reside in an environment with fluctuant nutrient supply (Ackerman and Simon, 2014; Gullino et al., 1967; Hirayama et al., 2009), they must develop adaptive mechanisms to preserve energy for maintenance of tumor cell survival under conditions of low glucose supply. Proper gauging of glucose levels and lipid synthesis is not only critical for tumor growth and survival, but also for normal cell function under stress conditions. In this study, we demonstrate that SCAP acts as a key glucose-responsive protein to orchestrate fuel availability and sterol levels for control of SREBP function in lipid synthesis. We show that glucose controls N-glycosylation of SCAP, which is indispensable for SCAP/SREBP trafficking from ER to the Golgi and for the nuclear activation of SREBPs (Figure 7). This finding renovates our current understanding of the regulation of SREBP function in physiology and patho-physiology. Elegant studies by Brown and Goldstein demonstrated that sterols act as negative regulators for SCAP/SREBP trafficking (Goldstein et al., 2006), and ~5% decrease of cholesterol in ER membrane is sufficient to trigger the activation of SREBP (Radhakrishnan et al., 2008). Such a tight regulation mechanism necessitates the participation of additional cellular factors to coordinate with sterols for fine tune of SREBP function in normal and patho-physiological conditions. Our study unravels the important function of glucose in controlling lipid metabolism in tumorigenesis, linking EGFR signaling to glucose uptake and SCAP/SREBP activation (Figure 7).
Figure 7. Schematic diagram illustrating EGFR signaling via enhancing glucose uptake promotes SCAP N-glycosylation leading to SREBP-1 activation.
(A) Signaling cascade for EGFR stimulation and glucose uptake to SCAP N-glycosylation and SREBP-1 activation.
(B) Without glucose, unglycosylated SCAP binds to Insig, leading to retention of SREBP in the ER. Under this condition, changes in sterol level are insufficient to trigger exit of the SCAP/SREBP complex from the ER. In the presence of glucose, N-glycosylation of SCAP leads to increased stability of SCAP and its dissociation from Insig. Thus, the SCAP/SREBP complex can traffic to the Golgi, resulting in SREBP cleavage and its nuclear function. Sterols block glucose-mediated SCAP/SREBP trafficking and subsequent SREBP activation. Elevated EGFR signaling causes increased glucose uptake and SCAP/SREBP activation to promote tumor growth.
Activation of SREBP relies on SCAP-escorted trafficking from ER to the Golgi (Espenshade et al., 1999; Goldstein et al., 2006). Sterols serve as negative regulators enhancing SCAP binding to Insig to retain the SCAP/SREBP complex in ER membrane (Goldstein et al., 2006). In this study, we show that in glucose-deficient condition, removal of sterols was unable to relieve SCAP binding to Insig-1 and trigger exit of SCAP/SREBP from ER. Supplement of glucose promoted SCAP N-glycosylation and SCAP/SREBP trafficking to the Golgi and consequent SREBP activation. We show that the interaction between SCAP and Insig-1 is reduced when SCAP is glycosylated. Studies by Brown and Goldstein have shown that Insig is a sterol sensor that negatively regulates SCAP interaction with SREBP (Goldstein 2006). Our data are consistent with their idea, as we show that even in the presence of glucose, sterol still exerts its control of SCAP/SREBP trafficking and subsequent SREBP activation. Thus, glucose functions as a prerequisite activator for control of SCAP trafficking and SREBP activation. Since sterol can still block SCAP/SREBP signaling complex, future studies will be required to examine how glucose communicates with sterol in orchestrating the cross-talk between energy supply and lipid metabolism in physiology and cancer biology.
There are three N-glycosylation sites on SCAP, one located in loop 1 (N263) and two in loop 7 (N590 and N641), and both loops reside in the inner lumen of ER (Nohturfft et al., 1998). Interestingly, mutation in any one or two sites of N-glycosylation was unable to affect SCAP trafficking and SREBP function. This observation was consistent with previous report by Nohturfft et al., who demonstrated that as long as one N-glycosylation site is present on SCAP, its control of SREBP-2 is unperturbed (Nohturfft et al., 1998). Here we tested the hypothesis that the presence of any single glycosylation site on SCAP is sufficient for SCAP/SREBP trafficking and activation. We found that even though presence of a single glycosylation site on SCAP appeared to be sufficient for its biological function, complete abolition of all glycosylation sites on SCAP disrupted the exit pathway for the SCAP/SREBP complex from ER to the Golgi. This further supports the notion that specific recognition of the glycosylation motif may provide a mechanism for modulation of the Insig/SCAP/SREBP interaction. Clearly, identification of the binding motif for Insig or SCAP for N-linked oligosaccharide will require further studies.
Our previous studies revealed that SREBP-1 is activated by EGFR-PI3K-Akt signaling pathway, but mTORC1 seems not involved (Guo et al., 2009b; Guo et al., 2011). Studies by other investigators reported that PI3K-Akt-mTORC1 signaling regulates SREBP-1 activation (Du et al., 2006; Porstmann et al., 2005; Yecies et al., 2011). Therefore, the mechanisms that underlie the oncogeneic signaling to SREBP-1 function remain unclear. In the present study, we show that in the absence of glucose, EGF cannot activate SREBP-1, even though other downstream signaling components (e.g. p-Akt, p-S6) remain upregulated. This suggests that EGFR-mediated control of SREBP-1 is fully dependent on glucose. We also examined mTORC1 activity by checking its downstream effectors p-S6 and total S6 levels in the absence or presence of serum. The data show that the levels of p-S6 and total S6 were not significantly changed by glucose withdrawal within 12 hr time window. In contrast, the levels of SCAP and SREBP-1 and SREBP-2 activation were reduced. Moreover, in the absence of glucose, even growth factor (EGF or insulin) strongly activated mTORC1, but failed to activate SCAP/SREBP signaling. Take together, these data demonstrate that glucose, not mTORC1, plays a critical role in regulating SCAP/SREBP signaling.
Our study revealed that EGFR signaling via promoting glucose uptake enhances SCAP N-glycosylation and its protein levels to activate SREBP-1. This finding provides a plausible explanation for the conundrum faced by the tumor cells: Under rich nutrient environment, oncogenic signaling via hijacking glucose import-SCAP N-glycosylation-SREBP-1 activation metabolic pathway enhances lipogenesis and promotes rapid tumor growth. Once the glucose level drops, SCAP loses N-glycosylation and disconnects oncogenic signaling with SREBP-1, leading to reduced lipogenesis and thereby preserving limited energy sources for tumor cell survival. These data demonstrate the plasticity and survival capability of tumor cells in facing harsh nutrient microenvironment.
Complete understanding of the molecular mechanisms of SCAP/SREBP trafficking and its relationship to fuel supply and growth signaling may help design effective therapeutic strategies to target lipid metabolism in cancer and other metabolic syndromes.
EXPERIMENTAL PROCEDURES
More detailed procedures were described in the supplemental Experimental Procedures.
Plasmids
GFP-SCAP wild-type plasmid is a gift from Dr. Peter Espenshade. Original plasmids for SCAP mutants were provided by Drs. Nohturfft, Brown and Goldstein (Nohturfft et al., 1998). 2 × HA-SREBP-2 (full length) plasmid is a gift from Dr. John Shyy. Adenovirus containing GFP-SREBP-1 (full length) were produced and amplified as described (Dif et al., 2006).
Cell Culture and Transfection
U87, U87EGFR, U87EGFRvIII (Guo et al., 2009b), HEK293T, U87EGFRvIII-luc and other cancer cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro) supplemented with 5% HyClone fetal bovine serum (FBS; Thermo Scientific). All cell culture were supplemented with 1% penicillin/streptomycin/glutamine and cells incubated at 5% CO2 at 37°C. Transfection of plasmids was performed using X-tremeGENE HP DNA Transfection Reagent (Roche) following the manufacturer’s instructions.
Coimmunoprecipitation
Coimminoprecipitation assay was performed as described previously (Yang et al., 2002).
Preparation of Cell Membrane Fractions and Nuclear Extracts
Cell membrane and nuclear fractions were isolated as described previously (Nohturfft et al., 1998).
Detection of SCAP N-glycosylation
The detection of SCAP N-glycosylation was performed according to the method described previously (Nohturfft et al., 1998).
Xenograft Mouse Model and Mouse Survival
GBM cells (1×106 cells) were implanted into the flank of athymic nu/nu female mice (6–8 weeks old) subcutaneously. Mice were sacrificed by euthanasia when tumor size reached the limitation and tumors were isolated and weighed. For intracranial xenograft models, 1×105 GBM cells in 4 µl of PBS were stereotactically implanted into mouse brain. Mice were then observed until they became moribund, at which point they were sacrificed. All animal procedures were approved by the Subcommittee on Research Animal Care at Ohio State University Medical Center.
Mouse Luminescence Imaging
Mice implanted with GBM cells expressing luciferase were injected with Luciferin (Perkin Elmer) solution (15 mg/ml in PBS, dose of 150 mg/kg) by an intraperitoneal route for about 5–15 min. Mice were placed into a clear Plexiglas anesthesia box (2.0–3.0% isoflurane) that allowed unimpeded visual monitoring of the animals. Animals were then placed on non-fluorescent black paper on the imaging platform of an IVIS Lumina II to reduce background noise. The imaging chamber was continuously infused with 1–1.5% of isoflurane, and imaging platform was heated at 37°C to keep the mice warm. Animals were imaged 10 min after Luciferin injection to ensure consistent photon flux. The imaging experiments were conducted at OSU Small Animal Imaging Core.
Statistical Analysis
Statistical analyses were performed by Excel or GraphPad Prism5. All data are analyzed by 2-tailed t-test as well as by ANOVA as appropriate. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. Mike S. Brown and Joseph L. Goldstein for their careful reading of the manuscript and helpful comments. We thank Dr. Paul Mischel for GBM cell lines. We thank Dr. S. Jaharul Haque for helpful comments. This work was supported by NIH grants NS072838 and NS079701 to DG, NS064607, CA163205, CA150153 to BK, AG28614 to JM; American Cancer Society Research Scholar Grant RSG-14-228-01–CSM to DG. We also appreciate the support from the Ohio State Neuroscience Core and OSUCCC start-up funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
D.G. conceived the ideas. C.C., and D.G. designed the experiments. C.C., P.R., F.G., J.L., J.Y., X.W., X.C., V.E., P.H., J.Y.G., E.L., and D.G. performed the experiments. C.C., B.K., A.C., and D.G. analyzed the data. C.C., A.N., J.M., and D.G. wrote the manuscript, and all authors reviewed and approved the manuscript for publication.
REFERENCES
- Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends in cell biology. 2014;24:472–478. doi: 10.1016/j.tcb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. The Journal of biological chemistry. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- Babic I, Anderson ES, Tanaka K, Guo D, Masui K, Li B, Zhu S, Gu Y, Villa GR, Akhavan D, et al. EGFR Mutation-Induced Alternative Splicing of Max Contributes to Growth of Glycolytic Tumors in Brain Cancer. Cell metabolism. 2013;17:1000–1008. doi: 10.1016/j.cmet.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Lopez JM, Sanchez HB, Osborne TF. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. The Journal of biological chemistry. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular Fatty Acid Metabolism and Cancer. Cell metabolism. 2013 doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem J. 2006;400:179–188. doi: 10.1042/BJ20060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Kristiana I, Wong J, Brown AJ. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Molecular biology of the cell. 2006;17:2735–2745. doi: 10.1091/mbc.E05-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, Nelson CC. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer research. 2004;64:2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC, Konisti S, Peck B, Miess H, East P, et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013;1:3. doi: 10.1186/2049-3002-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet-Deniau I, Pichard AL, Kone A, Esnous C, Nieruchalski M, Girard J, Prip-Buus C. Glucose induces de novo lipogenesis in rat muscle satellite cells through a sterol-regulatory-element-binding-protein-1c-dependent pathway. Journal of cell science. 2004;117:1937–1944. doi: 10.1242/jcs.01069. [DOI] [PubMed] [Google Scholar]
- Gullino PM, Grantham FH, Courtney AH. Glucose consumption by transplanted tumors in vivo. Cancer research. 1967;27:1031–1040. [PubMed] [Google Scholar]
- Guo D, Bell EH, Chakravarti A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncology. 2013;2:289–299. doi: 10.2217/cns.13.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Bell EH, Mischel P, Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr Pharm Des. 2014;20:2619–2626. doi: 10.2174/13816128113199990486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009b;2:ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al. An LXR agonist promotes GBM cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty AH, Shimano H, Yahagi N, Amemiya-Kudo M, Perrey S, Yoshikawa T, Osuga J, Okazaki H, Tamura Y, Iizuka Y, et al. Sterol regulatory element-binding protein-1 is regulated by glucose at the transcriptional level. The Journal of biological chemistry. 2000;275:31069–31077. doi: 10.1074/jbc.M003335200. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer research. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends in endocrinology and metabolism: TEM. 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, Shinohara T, Kawazoe Y, Sato S, Asakura K, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chemistry & biology. 2009;16:882–892. doi: 10.1016/j.chembiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Kerry R, Aviram M, Hayek T. High glucose concentration increases macrophage cholesterol biosynthesis in diabetes through activation of the sterol regulatory element binding protein 1 (SREBP1): inhibitory effect of insulin. J Cardiovasc Pharmacol. 2008;52:324–332. doi: 10.1097/FJC.0b013e3181879d98. [DOI] [PubMed] [Google Scholar]
- Li C, Yang W, Zhang J, Zheng X, Yao Y, Tu K, Liu Q. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. Int J Mol Sci. 2014;15:7124–7138. doi: 10.3390/ijms15057124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell metabolism. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohturfft A, Brown MS, Goldstein JL. Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing domain. The Journal of biological chemistry. 1998;273:17243–17250. doi: 10.1074/jbc.273.27.17243. [DOI] [PubMed] [Google Scholar]
- Nohturfft A, Yabe D, Goldstein JL, Brown MS, Espenshade PJ. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102:315–323. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- Nohturfft A, Zhang SC. Coordination of lipid metabolism in membrane biogenesis. Annual review of cell and developmental biology. 2009;25:539–566. doi: 10.1146/annurev.cellbio.24.110707.175344. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RB, DeBose-Boyd R, Goldstein JL, Brown MS. Failure to cleave sterol regulatory element-binding proteins (SREBPs) causes cholesterol auxotrophy in Chinese hamster ovary cells with genetic absence of SREBP cleavage-activating protein. The Journal of biological chemistry. 1999;274:28549–28556. doi: 10.1074/jbc.274.40.28549. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes & development. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru P, Williams TM, Chakravarti A, Guo D. Tumor metabolism of malignant gliomas. Cancers (Basel) 2013;5:1469–1484. doi: 10.3390/cancers5041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai J, Nohturfft A, Goldstein JL, Brown MS. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. The Journal of biological chemistry. 1998;273:5785–5793. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, Song BL. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell metabolism. 2011;13:44–56. doi: 10.1016/j.cmet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Wojton J, Chu Z, Mathsyaraja H, Meisen WH, Denton N, Kwon CH, Chow LM, Palascak M, Franco R, Bourdeau T, et al. Systemic delivery of SapC-DOPS has antiangiogenic and antitumor effects against glioblastoma. Mol Ther. 2013;21:1517–1525. doi: 10.1038/mt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell metabolism. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.