Abstract
Background/Aims
All agree that informed consent is a process, but past research has focused content analyses post-consent or in one conversation in the consent series. Our aim was to identify and describe the content of different types of consent conversations.
Methods
We conducted a secondary analysis of 38 adult oncology phase 1 consent conversations, which were audio-recorded, transcribed, coded, and qualitatively analyzed for type and content.
Results
Four types of consent conversations were identified: 1) priming; 2) patient-centered options; 3) trial-centered; and 4) decision made. The analysis provided a robust description of the content discussed in each type of conversation. Two themes, supportive care and prognosis, were rarely mentioned. Four themes clustered in the patient-centered (type 2) conversations: affirmation of honesty, comfort, progression and offer of supportive care.
Conclusion
We identified and described four types of consent conversations. Our novel findings include 1) four different types of conversations with one – priming – not mentioned before; 2) a change of focus from describing the content of one consent conversation to describing the content of different types. These in-depth descriptions provide the foundation for future research to determine if the four types of conversations occur in sequence, thus describing the structure of the consent process and providing the basis for coaching interventions to alert physicians to the appropriate content for each type of conversation. A switch from a focus on one conversation to the types of conversations in the process may better align the consent conversations with the iterative process of shared-decision making.
Keywords: Phase 1 trials, informed consent process, ethics, oncology, physician-patient communication
Introduction
Phase 1 oncology research can be difficult to explain to patients. The primary endpoints of phase 1 clinical trials are safety and dose, not efficacy, and they offer little chance of therapeutic benefit.1 Nonetheless, patients often fail to distinguish research procedures from standard therapy.2,3–5 Many patients do not consider alternatives,6 exhibit unrealistic optimism7 and may overestimate their likelihood of benefit,8, 9 though their reported estimates of benefit may not be intended as factual statements4, 10 and may not be related to lack of knowledge.11 Evidence of patient misunderstanding of aspects of these trials demonstrates the need for improvement of phase one consent communication.
Although all agree that obtaining consent for phase one trials is a process that occurs over multiple conversations, emphasis in the literature and in regulatory review has been on informational elements of consent, focusing on one conversation in the series and thus not contributing to our knowledge of the process. Brown focuses on the conversation nominated by the oncologist so the point in the process is not known,12 Jenkins on the conversation in which a decision was made,13 and Kass on the “options” discussion.14 Most studies about the ethical issues in phase I research target patients who had already consented for participation and do not provide any information on the consent conversations themselves. For example, Daugherty et al.8 and Agrawal et al.,6 who report that the major goal of enrolling in phase I research is to combat one’s cancer with alternatives not seriously considered, and Weinfurt et al.,11 who demonstrate that high expectations of benefit may be expressions of optimism, not fact, all interviewed patients after the completion of the consent process but before treatment. A lack of focus on the types of conversations in the process of consent in previous literature is particularly concerning given the current emphasis on shared decision-making, which at its core is an iterative process over time. The patient, with the guidance and input from the physician, gathers information, considers alternatives, aligns the options with his or her values and preferences, and then, with the physician, makes a decision.15, 16 In order to improve phase 1 consent and better align it with shared decision-making, the types of conversations in the process, not just the content of one conversation, must be described.
There have been three studies17–19 that have recognized phase 1 consent as a process. Each of these studies tested a pre-specified consent process with a series of three or four consent conversations. In these studies, the focus was again on what content was communicated: Rodenhuis listed seven key content areas, Tomamichel six, and Willems five. Another study by Jenkins listed eight key content areas to be covered in the conversation in which a decision about trial participation was made. We have grouped the key content areas in similar categories, summarized in Table 1. Once again, all but Tomamichel interviewed the patient after the patient either consented or refused and therefore do not reveal information on the process. Tomamichel analyzed only the second conversation.
Table 1.
Necessary Content Identified in the Literature
| Code | Jenkins | Tomamichel | Rodenhuis |
|---|---|---|---|
| Is research | Used in animals and a few humans* | Experimental – first in humans | |
| Prognosis | Prognosis | ||
| Treatment options | Other treatment or care options | Lack of proven treatments | |
| Aims | Major aims | Objective is to further therapy progress | |
| Benefit unknown | Probable lack of therapeutic benefits | Anti-tumor effect unknown/unlikely* | Effect on tumor uncertain. Cure improbable |
| Extra Procedures | Extra blood draws, frequent visits | Extra Research Procedures | |
| Unknown AEs | Unknown adverse effects | Limited knowledge of side effects* | Side effects expected, may be serious |
| Voluntary | Voluntary | Voluntary* | |
| Withdraw | Can withdraw | Can withdraw* | Can withdraw |
| Questions | Consent given and questions answered |
Mentioned by Willems
Having completed two studies during which phase 1 consent conversations were audio-recorded and transcribed, we undertook a qualitative secondary analysis of the combined set of transcripts. Since in each study the physician alerted our team whenever he or she expected to mention a phase 1 trial, our aim in this secondary analysis was to describe the types of conversations in our data set. Our “catch as catch can” methodology provided insight into the varying types of consent conversations in an academic phase 1 practice and can therefore be the first preliminary step in designing future interventions to address the deficits in communication in phase 1 research.4, 6–9, 20
Patients and methods
Study population
The phase 1 conversations were conducted with cancer patients and providers, 4 hematologists, 11 oncologists and one nurse practitioner, at a single academic institution with an active phase 1 program. All patients and providers spoke English. IRB approval was obtained.
Methods
The conversations were originally audio-recorded to collect data for two completed studies, one to determine the clarity of the description of research biopsies2 (18 conversations) and a second to test the feasibility of introducing a patient preference tool into phase 1 consent conversations (20 conversations).21 The only difference between the two cohorts (research biopsy or preference tool) was that in the latter, the patient was approached before the conversation and given a preference tool to complete, which he or she then gave to the provider, who then usually briefly discussed it. However, in both studies the majority of the discussion centered on aspects of phase I trials. We did not analyze mentions of patient preferences, since one study introduced and targeted them.
For both studies, providers were asked to contact the ethics researcher whenever they anticipated that a phase 1 trial would be mentioned. There was no attempt to target any particular consent conversation in the consent process. Thus, some patients had had prior extensive conversations about phase 1 trials, others had been briefly introduced and others were naïve. With written consent of providers and verbal permission from patients, the ethics team member attended the patient/provider conference, audio-taped and transcribed it verbatim. The observer was instructed to be non-intrusive so as not to influence the conversation, a method used successfully in other studies. 22 The observer noted the participants in the discussion by family or provider role and specific behaviors that occurred during the conversation (i.e. signing the consent) in order to supplement and to ensure the accuracy of the transcription.
Analysis
Content
A semantic content analysis method 23 was used to systematically extract meaning from the transcribed interviews, using a multi-level coding strategy. Transcripts were inductively coded with attention to two areas: 1) content areas discussed by the provider and patient and 2) behaviors such as ‘handing the informed consent document to the patient.’ The behaviors section relied heavily on the observer notes. Family and friends participated in the discussions, when present, but the analysis focused on the provider-patient discussion and interactions.
LW coded 10 interviews for inductive content and created a code book, which was finalized in consultation with RP. Using the codebook, transcripts were inductively coded by LW and MDD. All discrepancies were discussed and resolved among the coders and RP.
Subsequently, the apriori codes based on published frameworks (Table 1) were compared to the inductive codes to determine if the inductive process produced all of the codes deemed to be ethically necessary in prior literature. All codes were then grouped into themes by RP, which were reviewed by LW. Simple frequencies of the number of conversations in which a code was discussed were calculated.
Types of conversations
LW reviewed all the transcripts, not attending to specific content but to the major focus of each conversation.24 RP reviewed LW’s identification of three major foci. LW and MDD then categorized each conversation into one of the three foci, until they reached consensus. Two research assistants then independently categorized all the transcripts by focus as a quality check. RP resolved disputes between the two research assistants. The result was agreement between the original categorization and the quality check.
Results
We observed and audio-taped 38 healthcare provider-patient conversations between 15 providers and 38 patients, 18 from study 1 and 20 from study 2. Ten of the conversations discussed hematological phase 1 trials, conducted by four providers, and 28 of the conversations discussed solid tumor phase 1 trials, conducted by 11 providers. Whether the patient was new to the provider or an established patient and whether the patient was alone or accompanied are recorded in Table 2.
Table 2.
Discussion Participants
| Discussion conducted by | Total n=38(%) | Type 1 n=4(%) | Type 2 n=22(%) | Type 3 n=9(%) | Type 4 n=3(%) |
|---|---|---|---|---|---|
| Physician (MD) w/new patients | 17(45) | 4 (100) | 8 (36) | 4 (45) | 1 (33) |
| Physician (MD) w/established patients | 18(47) | 14 (64) | 3 (33) | 1 (33) | |
| Nurse Practitioner (NP) w/new patients | 2(5) | 2 (22) | |||
| Nurse Practitioner (NP) w/established patients | 1(3) | 1 (33) | |||
| Was the patient accompanied? | |||||
| Patient alone | 7(18) | 1 (25) | 4(18) | 1(11) | 1(33) |
| Patient w/family or friends | 31(82) | 3 (75) | 18(82) | 8(89) | 2(67) |
Twenty three codes were inductively identified. An additional code, discussing patient preferences, was also identified, but since it may have been an artifact of the intervention design of the parent trial that tested a patient preference tool, we do not report that code here. Four codes occurred in almost all of the conversations: Review of disease status, options, introduction to phase 1 trials, and logistics. Two codes were rarely discussed: offers of supportive care and prognosis.
Our inductive coding included all the apriori codes. We grouped the 23 codes into five themes: medical review; options and recommendations; clinical trial information; consent content; and communication behaviors. Table 3 lists the codes grouped into themes, with the inductive codes that corresponded to apriori codes shaded grey.
Table 3.
Frequencies of Codes. The inductive codes that correspond to apriori codes (abstracted from the literature) are shaded in grey.
| Codes | Frequency of conversations (%) |
|---|---|
| Medical Review | |
| Review of disease status | 34 (89) |
| Statement of progression (disease has progressed) | 19 (50) |
Statement of 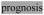
|
10 (26) |
| Options and Recommendations | |
| Options | 33 (87) |
| Offer of supportive care | 9 (23) |
| Physician recommends phase 1 trial | 19 (50) |
| Clinical Trial Information | |
| Introduction to clinical trials | 21 (55) |
| Introduction to phase one trials | 37 (97) |
| Logistics of trial participation | 33 (87) |
| Resources available for trial participation | 26 (68) |
| Informed Consent document given | 23 (61) |
| Consent Content | |
| Is Research | 30 (79) |
| Aims | 33 (87) |
| Benefit Unknown | 27 (71) |
| Unknown AEs | 22 (58) |
| Extra Procedures | 29 (76) |
| Is Voluntary | 30 (79) |
| Can Withdraw | 8 (21) |
| Communication Behaviors | |
| Healthcare Professional (HP) gives time to think | 26 (68) |
| HP asks for questions | 25 (66) |
| HP checks patient understanding | 9 (23) |
| HP assures will be honest | 18 (47) |
| HP comforts patient | 6 (16) |
The inductive codes that correspond to apriori codes (abstracted from the literature) are shaded in grey
Foci of consent conversations and their content
The 38 conversations divided into the three foci: patient-centered, trial-centered and decision discussion. We therefore grouped the conversations into three types, each type with a different focus. Some of the patient-centered conversations occurred early in care before the patient was eligible for a phase 1 trial, whereas the others presented the option of a phase I trial for immediate consideration. We therefore separated the early discussions into their own type, resulting in four types of consent conversations: priming (type 1); patient-centered options (type 2); trial-centered (type 3); and decision discussion (type 4). Codes stratified to each type are presented in Table 4.
Table 4.
Codes by Conversation Type
| Code* | Priming (N= 4) | Patient Centered (N= 22) | Trial Centered (N= 9) | Decision Discussion (N= 3) |
|---|---|---|---|---|
| Medical Review | ||||
| Review of disease status | 4 (100) | 21 (95) | 6 (67) | 3 (100) |
| Statement of progression | 0 | 17 (77) | 2 (22) | 0 |
| Prognosis | 0 | 8(36) | 1 (11) | 1(33) |
| Options | ||||
| Discussion of Options | 4(100) | 22(100) | 6 (67) | 1 (33) |
| Offer of supportive care | 0 | 8(36) | 1 (11) | 0 |
| Clinical Trial Education | ||||
| Introduction to clinical trials | 2 (50) | 13 (59) | 5 (56) | 1 (33) |
| Introduction to phase one trials | 3 (75) | 22 (100) | 9 (100) | 3 (100) |
| Logistics of trial participation | 1 (25) | 20 (91) | 9 (100) | 3 (100) |
| Resources available | 1 (25) | 14 (64) | 8 (89) | 2 (67) |
| Consent Content | ||||
| Is research | 2 (50) | 17 (77) | 8 (89) | 3 (100) |
| Aims | 2 (50) | 19 (86) | 9 (100) | 3 (100) |
| Benefit Unknown | 2 (50) | 14 (64) | 8 (89) | 3 (100) |
| Unknown AEs | 2 (50) | 11 (50) | 8 (89) | 1 (33) |
| Extra Procedures. | 1 (25) | 17 (77) | 8 (89) | 3 (100) |
| Is Voluntary | 1 (25) | 18 (82) | 9 (100) | 2 (67) |
| Can Withdraw | 0 | 2 (9) | 4 (44) | 2 (67) |
| Consenter Behaviors | ||||
| Healthcare professional(HP gives time to think | 2 (50) | 16 (73) | 6 (67) | 1 (33) |
| HP asks for questions | 2 (50) | 15 (68) | 6 (67) | 3 (100) |
| HP checks patient understanding | 1 (25) | 1 (5) | 6 (67) | 1 (33) |
| IC document given | 1 (25) | 14 (64) | 5 (56) | 3 (100) |
| Physician Behaviors | ||||
| MD recommends phase 1 trial | 2 (50) | 9 (41) | 4 (44) | 0 |
| MD assures will be honest | 1(5) | 15 (68) | 2 (22) | 0 |
| MD comforts patient | 0 | 5 (23) | 1 (11) | 0 |
Type 1: The priming discussion (4 conversations)
The priming discussions occurred notably early in the continuum of care, when patients had not exhausted standard care options nor were currently candidates for phase 1 trials. In these conversations, the physicians initiated discussions with patients about phase 1 trials in order to make them aware of potential future options. Conversations focused on the patients’ disease status (100%) and treatment options (100%). While most introduced the concept of clinical trials (50%) and/or phase 1 trials (75%), few discussed procedures, logistics, or resources (25%). The emphasis in priming discussions was on the potential future use of experimental agents with explanations by the physician that standard of care options were still available.
Type 2: The patient-centered options discussion (22 conversations)
The main focus of 22 of the conversations was the patient and his or her options for treatment and research. In patient-centered discussions, the patient had reached a crucial junction in treatment and had the option to enroll in a phase 1 trial. Four codes occurred more frequently in this type than other types: affirmations of physician honesty, expressions of comfort, discussion of progression and offers of supportive care.
The conversations followed a similar trajectory. Physicians often prefaced these discussions by noting that it was important to be honest about the patients’ current medical situation (68%). The physician then reported bad news: the cancer had progressed (77%), showing that current therapy was not working. The physician continued by presenting new therapy options (100%), including a phase 1 trial, standard care therapies and supportive care. This type contained almost all (8/9; 89%) of the offers of supportive care.
The physician next conducted an in-depth discussion of phase 1 trials (100%), including the logistics of the specific trial (91%). Resources, including phone numbers of research coordinators and physicians (64%) were discussed and informed consent documents (64%) were given to the patient. In the majority of conversations (73%), the physician encouraged the patient to take time to think.
We distinguished two types of options discussions: one in which the physician made a recommendation and an open-ended one in which the physician did not. Physicians recommended phase 1 participation in 9 (41%) conversations, only 2 of which were end-stage situations in which the only alternative therapies offered were ones that the patient had either already used without success or were unapproved, determined in consultation with the Director of the Phase 1 Unit. In the remaining 13 (59%) conversations, no recommendation was made; six of these were end-stage.
When a recommendation was made, the physician sometimes explained that the having other options was just the reason the recommendation was made. The physician could recommend one option and then if it proved unsuccessful, they could try the other option(s).
When a recommendation was not made, the physicians offered multiple options, including a phase 1 trial. No option was presented as more favorable than the others.
Type 3: The trial-centered discussion (9 discussions)
All of the type 3 discussions introduced phase 1 trials and included most of the consent content [is research (89%); aims (100%); benefit unknown (89%); unknown AEs (89%); extra procedures (89%); and is voluntary (100%)]. All described the logistics of trial participation, and most reviewed current medical status (67%), discussed options (67%), and resources available (89%), but overlooked statements of honesty (22%) and discussions of disease progression (22%), which are more characteristic of type 2 conversations. The informed consent document was usually given (56%) to the patient with checks of understanding, asking for questions and time to think (67%). The consent was not signed in these conversations, and no final decision was made.
Type 4: The decision discussion (3 discussions)
All of the decision discussions began with a review of disease status, introduction to phase 1 trials including that it was research, aims, benefit unknown, and extra procedures and trial logistics. The provider then asked for questions in all conversations. At this point, a decision was made, and the informed consent was signed.
In these conversations, the providers did not bring up disease progression, supportive care or recommendations, and infrequently (33%) discussed options.
Discussion
Although most recognize that consent is a process, with each conversation aimed at a different goal, few incorporate these different conversations into their consent research. By asking oncologists and nurse practitioners to alert us whenever a phase 1 study would be mentioned to a patient, we were able to observe the conversations that typically take place and to identify four types of conversations, including a new type – the priming discussion - not discussed in previous literature.13, 17, 18 Most past studies analyzed only one type of conversation. Tomamichel assessed the conversation discussing the phase 1 trial, most akin to our type 3, the trial-centered type. Kass assessed the “options” discussion, most akin to our patient-centered options conversation. Jenkins assessed the decision-making conversation, our type 4. Our description of the four types and their content should assist in contextualizing prior and future research by helping to identify which type of conversation is analyzed. Identification of the type of conversation will be especially important since the goal of each is entirely different and both the focus and content will vary depending on the type.
This secondary analysis of transcripts coupled with observer notes also allowed us to provide a robust description of the content of each type of consent conversation. We noticed a clustering of codes in the Type 2 discussion: affirmations of physician honesty, expressions of comfort, discussion of progression and offers of supportive care. This type of conversation not only presented the patient with options, but also focused on being with the patient after giving the ‘bad news’ of progression, thus our nomenclature ‘patient-centered.’
Discussion of supportive care was left out of the majority of conversations across all types, while therapy options were included in most, a discovery similar to previous studies.25 These findings suggest that some physicians are not including supportive care as a treatment alternative when discussing options. As a result, many patients may not be aware of this option. In some cases, physicians may not have addressed supportive care because the patients had not exhausted all therapy options; however, studies show there is benefit to introducing supportive care as an option earlier in therapy so that patients are more receptive to the therapy later in care.26
As found before,13, 27 discussion of prognosis was not included in most conversations. This lack may be due to the fact that the physicians reviewed disease status and made statements of disease progression – discussed frequently in type 2 conversations - in lieu of addressing the probable course and outcome of the disease. Whether a thorough discussion of progression, without explicit description of prognosis, is sufficient deserves further investigation. Prognosis may have been brought up in earlier appointments. This would be the case especially in situations of an established relationship between physician and patient.
Similarly to the findings of Brown et al., we identified explicit recommendations, most notably in type 2 conversations. Unexpectedly, however, in the nine conversations in which the physician recommended a phase 1 trial, there was a suggestion that the physician more frequently recommended participation when alternative approved therapy existed (7/9 (78%) v. 2/9 (22%)). The qualitative analysis provided one explanation: physicians who had an approved therapy available may have been more inclined to recommend explicitly that their patients enroll in the phase 1 trial, because they had an approved therapy available if the trial was not beneficial. Phase 1 trials thus become one more option for patients to consider, not a last ditch effort, an important divergence from the traditional way of considering phase 1 research. Given the importance of the physician recommendation,28, 29 particularly during life threatening illnesses,30–33 more research is needed to determine if this finding is corroborated and, if so, why it happens.
This study had several limitations. First, the conversations were originally recorded for other studies, one regarding research biopsies; the other a patient preference tool. While discussions of the prior study topics took up only small portions of the taped conversations, we do not know how the topics may have influenced the conversation overall. Second, we did not follow individual patients throughout the continuum of care but rather naturalistically observed phase 1 discussions. Therefore, we do not know what proportion of individuals participated in all the types, nor the sequence of the types in practice. Third, only a few conversations in the data set fell under each conversation type, especially types 1 and 4, so we cannot be as confident of their descriptions. Fourth, we describe the experience at an academic center with active phase 1 research and so cannot generalize our results to other settings, though many phase 1 trials are conducted in this kind of setting. Finally, the provider had to plan to mention a phase 1 trial in order for the ethics team to be called. We therefore do not have data on spontaneous discussions.
Future research directions and implications
Our descriptions of the types of conversations have practical implications. The next very necessary step in this line of research is to follow individual patients through the continuum of care to determine whether the types occur in sequence: first priming, second patient-centered options, third trial-centered and fourth decision-making. If they do occur in sequence, we will be able to develop recommendations regarding essential elements of each component of the process and coaching interventions that emphasize that process. During the options phase, for example, the physician should focus on alternatives, their risks and benefits, and how each alternative aligns with patient values. During the trial-centered phase, the physician should focus on the details of the trial and the burdens and potential benefits entailed by the particular trial. The precise components of an ideal process will require further refinement, but the paradigm shift that these data support of focusing on the different types of conversations and coaching physicians to tailor discussions to the purpose of each type - either the patient’s options in one type or the trial’s benefit and burdens in a subsequent type - may at last make headway against the persistent problem of patient misunderstanding of clinical trials.4, 6–9 More importantly, attention to consent as a process over time with multiple types of conversation may make possible aligning consent practices with stages of shared decision-making. Shared decision-making is an iterative process that necessarily occurs over time. Focusing on the types of conversations in the process and synching the types with crucial steps in the shared decision-making process could increase the opportunity for patient values and preferences to be incorporated in the final shared decision.
Conclusion
This description of the types of phase 1 consent conversations has several important findings. Four types of consent conversations were identified, including one new type – the priming discussion. A robust description of the content of each type was provided, lacking in previous descriptions. Several codes clustered in the patient-centered options discussion, such as professions of physician honesty and comfort, expanding this type beyond a mere options discussion to a patient-centered discussion. We also confirmed the underrepresentation of discussions of supportive care and prognosis. Further research needs to determine if discussion of disease progression, commonly done, is sufficient if prognosis is not discussed. The identification of the consent types and their content can assist future researchers in designing interventions to correct the communication problems identified in the phase 1 setting. These results can also serve as the foundation for future research to discover if the types occur in sequence. And identification of the types can direct development of a physician coaching intervention, so that the information presented is tailored to the type and purpose of the conversation.
Acknowledgments
Research Support: This work was supported by a grant from the National Cancer Institute (NCI P01 CA 116676 to Fadlo Khuri).
The authors wish to acknowledge our funding by U.S. Department of Health and Human Services-National Institutes of Health-National Cancer Institute P01 CA 116676. We also wish to thank the NCI P01 investigators Fadlo Khuri, Taofeek Owonikoko and Suresh Ramalingam for their participation as subjects, as well as Kristopher A. Hendershot and Neal Dickert for their careful reviews of the manuscript and Minisha Lohani and Travis Deal for their help with the categorization of types of conversation.
Footnotes
Conflict of interest statement: None declared.
References
- 1.Eisenhauer EA, O’Dwyer PJ, Christian M, et al. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 2.Pentz RD, Harvey RD, White M, et al. Research biopsies in phase I studies: views and perspectives of participants and investigators. IRB. 2012;34:1–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum PS, Roth LH, Lidz CW, et al. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep. 1987;17:20–24. [PubMed] [Google Scholar]
- 4.Pentz RD, White M, Harvey RD, et al. Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer. 2012;118:4571–4578. doi: 10.1002/cncr.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson GE, Churchill LR, Davis AM, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS Med. 2007;4:e324. doi: 10.1371/journal.pmed.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal M, Grady C, Fairclough DL, et al. Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol. 2006;24:4479–4484. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 7.Jansen LA, Appelbaum PS, Klein WM, et al. Unrealistic optimism in early-phase oncology trials. IRB. 2011;33:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13:1062–1072. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty CK, Banik DM, Janish L, et al. Quantitative analysis of ethical issues in phase I trials: a survey interview of 144 advanced cancer patients. IRB. 2000;22:6–14. [PubMed] [Google Scholar]
- 10.Weinfurt KP, Sulmasy DP, Schulman KA, et al. Patient expectations of benefit from phase I clinical trials: linguistic considerations in diagnosing a therapeutic misconception. Theor Med Bioeth. 2003;24:329–344. doi: 10.1023/a:1026072409595. [DOI] [PubMed] [Google Scholar]
- 11.Weinfurt KP, Castel LD, Li Y, et al. The correlation between patient characteristics and expectations of benefit from Phase I clinical trials. Cancer. 2003;98:166–175. doi: 10.1002/cncr.11483. [DOI] [PubMed] [Google Scholar]
- 12.Brown R, Bylund CL, Siminoff LA, et al. Seeking informed consent to Phase I cancer clinical trials: identifying oncologists’ communication strategies. Psychooncology. 2011;20:361–368. doi: 10.1002/pon.1748. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins V, Solis-Trapala I, Langridge C, et al. What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol. 2011;29:61–68. doi: 10.1200/JCO.2010.30.0814. [DOI] [PubMed] [Google Scholar]
- 14.Kass N, Taylor H, Fogarty L, et al. Purpose and benefits of early phase cancer trials: what do oncologists say? What do patients hear? J Empir Res Hum Res Ethics. 2008;3:57–68. doi: 10.1525/jer.2008.3.3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 16.Alston C, Berger ZD, Brownlee S, et al. Discussion Paper. Institute of Medicine; Washington, DC: 2014. Shared decision-making strategies for best care: patient decision aids. [Google Scholar]
- 17.Rodenhuis S, van den Heuvel WJ, Annyas AA, et al. Patient motivation and informed consent in a phase I study of an anticancer agent. Eur J Cancer Clin Oncol. 1984;20:457–462. doi: 10.1016/0277-5379(84)90229-3. [DOI] [PubMed] [Google Scholar]
- 18.Tomamichel M, Sessa C, Herzig S, et al. Informed consent for phase I studies: evaluation of quantity and quality of information provided to patients. Ann Oncol. 1995;6:363–369. doi: 10.1093/oxfordjournals.annonc.a059185. [DOI] [PubMed] [Google Scholar]
- 19.Willems Y, Sessa C. Informing patients about phase I trials--how should it be done? Acta Oncol. 1989;28:106–107. doi: 10.3109/02841868909111191. [DOI] [PubMed] [Google Scholar]
- 20.Kass NE, Sugarman J, Medley AM, et al. An intervention to improve cancer patients’ understanding of early-phase clinical trials. IRB. 2009;31:1–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Pentz RD, Hendershot KA, Wall L, et al. Development and testing of a tool to assess patient preferences for phase I clinical trial participation. Psychooncology. 2015;24:835–838. doi: 10.1002/pon.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodish E, Eder M, Noll RB, et al. Communication of randomization in childhood leukemia trials. JAMA. 2004;291:470–475. doi: 10.1001/jama.291.4.470. [DOI] [PubMed] [Google Scholar]
- 23.Krippendorf K. Content analysis: An introduction to its methodology. Thousand Oaks, California: Sage; 2004. [Google Scholar]
- 24.Cohen MZ, Kahn D, Steeves R. Hermeneutic phenomenological research: a practical guide for nurse researchers. Thousand Oaks, CA: Sage; 2000. [Google Scholar]
- 25.Miller VA, Cousino M, Leek AC, et al. Hope and persuasion by physicians during informed consent. J Clin Oncol. 2014;32:3229–3235. doi: 10.1200/JCO.2014.55.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty CKN, Roter D, Larson S, et al. A study of physician investigator (PI) disclosure of alternatives of care and prognostic information to advanced cancer patients (ACP) enrolling in phase I trials. J Clin Oncol. 2009;27:abstract 6508. [Google Scholar]
- 28.Cox K. Informed consent and decision-making: patients’ experiences of the process of recruitment to phases I and II anti-cancer drug trials. Patient Educ Couns. 2002;46:31–38. doi: 10.1016/s0738-3991(01)00147-1. [DOI] [PubMed] [Google Scholar]
- 29.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 30.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45:941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 31.Siminoff LA, Fetting JH. Factors affecting treatment decisions for a life-threatening illness: the case of medical treatment of breast cancer. Soc Sci Med. 1991;32:813–818. doi: 10.1016/0277-9536(91)90307-x. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer MH, Krantz DS, Wichman A, et al. The impact of disease severity on the informed consent process in clinical research. Am J Med. 1996;100:261–268. doi: 10.1016/S0002-9343(97)89483-1. [DOI] [PubMed] [Google Scholar]
- 33.Beaver K, Luker KA, Owens RG, et al. Treatment decision-making in women newly diagnosed with breast cancer. Cancer Nurs. 1996;19:8–19. doi: 10.1097/00002820-199602000-00002. [DOI] [PubMed] [Google Scholar]


