Highlights
-
•
To the best of our knowledge, this case was, the largest HCC that had been previously reported to be resected safely.
-
•
We could avoid spontaneous tumor rupture although its risk was thought to be high.
-
•
We could control multiple intrahepatic metastases in the remnant liver with repeated TACEs after hepatectomy.
-
•
Multimodal treatment involving hepatectomy and TACE might be a good treatment strategy.
Keywords: Hepatocellular carcinoma, Multimodal treatment, Hepatectomy, Transcatheter arterial chemoembolization, Huge tumor, Intrahepatic, metastases
Abstract
Introduction
Huge hepatocellular carcinoma (HCC) possesses a potential risk for spontaneous rupture, which leads to a life-threatening complication with a high mortality rate. In addition, a large HCC is frequently accompanied by intrahepatic metastases.
Presentation of case
We describe, the case of a 74-year-old woman with a huge extrahepatically expanding HCC with multiple intrahepatic metastases who was treated by liver resection with repeated transcatheter arterial chemoembolization (TACE). To prevent tumor rupture or bleeding, we performed right hepatectomy. After the operation, TACE was applied for multiple intrahepatic metastases in the remnant liver. Furthermore, the elevated protein induced vitamin K absence (PIVKA II) level had decreased to limits within the normal range. Three months after the first TACE, computed tomography revealed several recurrences in the liver. TACE was applied for the second and third time and the tumors were well controlled.
Discussion
Although, liver resection is occasionally performed for patients with huge HCC to avoid spontaneous tumor rupture, only surgical approach might not be sufficient for such advanced HCC. To achieve long-term survival, it is necessary to control the residual intrahepatic tumors. We could control multiple intrahepatic metastases with repeated TACEs after hepatectomy.
Conclusion
Multimodal treatment involving hepatectomy and TACE might be a good treatment strategy for patients with huge HCC with multiple intrahepatic metastases if the tumors are localized in the liver without distant or peritoneal metastasis.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer-related death worldwide [1], [2], [3]. Surgical resection has been established as a standard treatment for HCC with the postoperative mortality rates of 0–6.4% and 5-year survival rates of 39–50% [1], [2], [4], [5]. Although, recent advances in imaging modalities have enabled an early detection of HCC, some patients still present with a large HCC (i.e., >10 cm). A large tumor diameter and extrahepatically expanding growth pattern are associated with spontaneous tumor rupture of HCC [6], [7]. To prevent tumor rupture, hepatectomy should be performed if liver function is maintained. Although, unfavorable, disease-free, and overall survival rates following surgical resection for large HCC may lead to the contraindication of surgical treatment, several studies have reported the efficacy and safety of liver resection for large HCC [8], [9], [10].
Large HCC is frequently accompanied by multiple intrahepatic metastases. Several authors have reported the effectiveness of hepatectomy for main tumor in combination with transcatheter arterial chemoembolization (TACE) [11], [12], [13]. Here we describe, the case of a multimodal treatment, including liver resection and TACE for a patient with a huge HCC (30 cm) with multiple intrahepatic metastases to aim for preventing tumor rupture and leading to the patient’s long-time survival.
2. Case report
2.1. Preoperative evaluation of the patient
A 74-year-old woman who complained of bilateral leg edema was admitted to a nearby hospital. The patient’s medical history was unremarkable, and she did not drink alcohol. She was negative for hepatitis B surface antigen (HBsAg), positive for anti-HBs antibody, and negative for anti-hepatitis C antibody. The patient’s serum albumin level was 3.8 mg/dl, T-bil level was 0.8 mg/dl, prothrombin time percentage was 127%, and indocyanine green retention rate at 15 min (ICGR15) was 12%. The comprehensive liver function was classified as Child’s A. Regarding biochemical analysis, alpha fetoprotein (AFP) and protein induced vitamin K absence (PIVKA II) levels were markedly elevated to 21.4 ng/ml and 108,518 MAU/ml, respectively. Computed tomography (CT) revealed a huge extrahepatically expanding HCC originating from the posterior segment of the liver to the pelvic cavity, which was 27 cm in length (Fig. 1A and B). In addition, there were multiple intrahepatic metastases in the left lobe. No peritoneal or extrahepatic metastasis was detected by CT or magnetic resonance imaging. Preoperative CT volumetry using Synapse Vincent® (Fujifilm Medical Co., Ltd., Tokyo, Japan) imaging software revealed a liver volume of 2678 ml, tumor volume of 1792 ml, functional liver volume of 886 ml, and estimated total liver volume of 1019 ml. Moreover, this analysis indicated that if right hepatectomy could be performed, the future remnant liver volume would be 652 ml (64% of the estimated total liver volume).
Fig. 1.
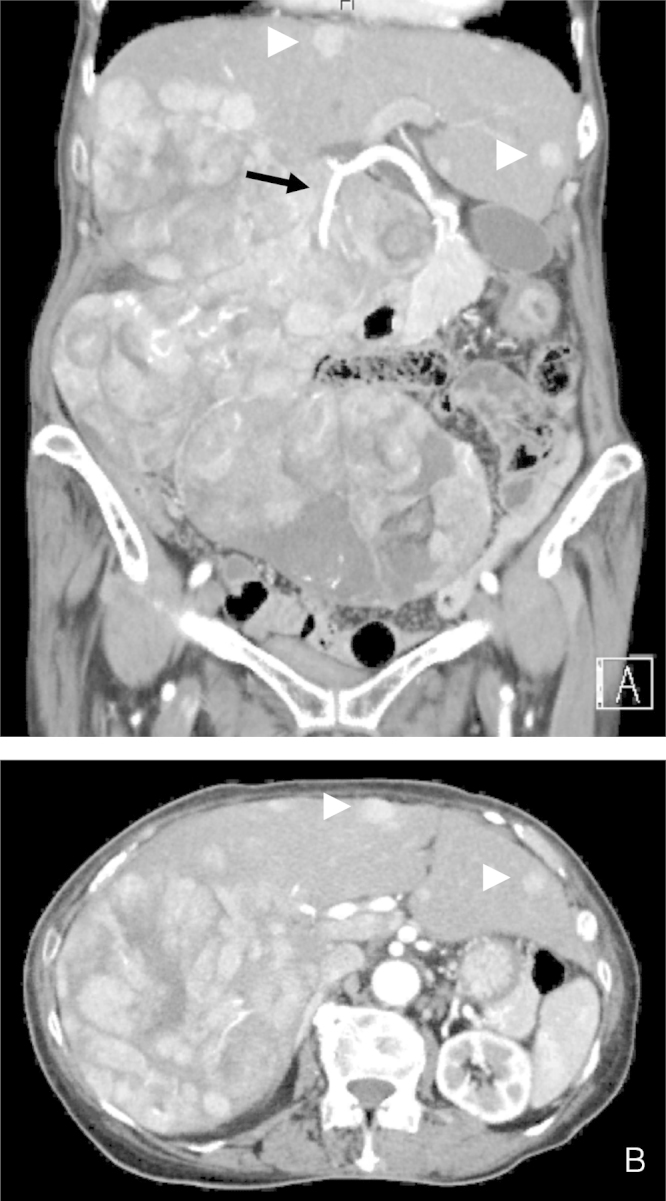
Contrast CT scan of the abdomen. (A) The CT scan of the abdomen showing a 27-cm huge tumor with extrahepatic expanding growth from the right lobe of the liver to the pelvic cavity. The posterior branch of the right hepatic artery is shown by arrows. Slight ascites was detected in the pelvic cavity. (A, B) There were multiple intrahepatic metastases in the left lobe (arrow head).
2.2. Operation
A huge HCC was found occupying the right side of the abdominal cavity from the liver to the pelvic cavity (Fig. 2). Slight ascites but no peritoneal metastasis was found in the abdominal cavity. Multiple extrahepatic collateral vessels from surrounding tissue such as the great omentum were observed. However, the tumor did not invade other organs such as the transverse colon, small intestine, diaphragm, or retroperitoneum. First, the tumor was dissected from the great omentum. Although careful attention was paid to avoid tumor rupture, oozing hemorrhage from the tumor surface continued and was controlled by the application of VIO soft-coagulation system (EREB Elecromedizin GmbH, Tuebingen, Germany) with an IO electrode™ (AMCO Inc., Tokyo, Japan) or a fibrin sealant patch (TachoSil®, Takeda, Osaka, Japan). After cholecystectomy, the right hepatic artery and the right portal vein were ligated and divided. Then, bleeding from the tumor surface remarkably decreased. Although, the complete mobilization of the right liver before parenchymal transection could not be achieved due to the huge size of the tumor, we applied the liver hanging maneuver. Liver transection was accomplished with a regular Péan clamp-crushing technique and harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH). The hemostasis of the cut surface of the liver was achieved by ligation and the application of TachoSil®. Two abdominal drains were left in place.
Fig. 2.
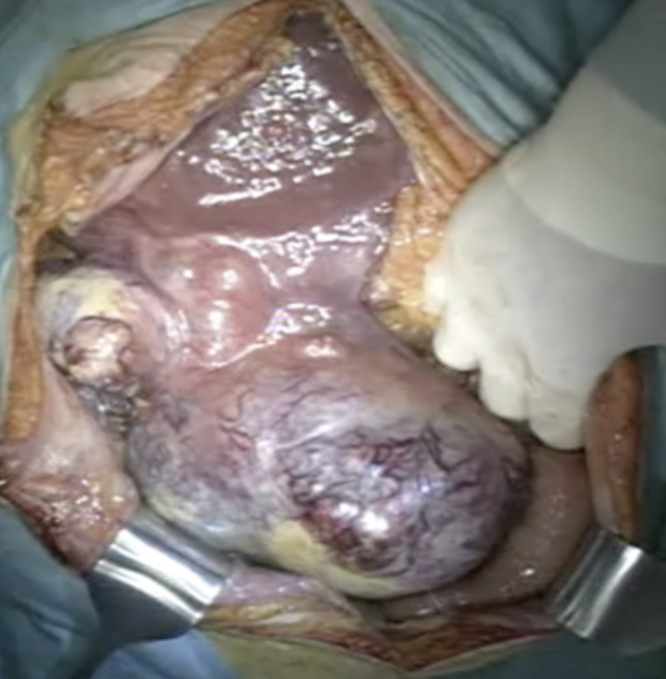
A huge tumor occupied the entire right side of the abdominal cavity from the liver to the pelvic space. Slight ascites but no peritoneal metastasis was found in the abdominal cavity.
The operative time was 309 min, and the estimated blood loss was 2825 ml with intraoperative transfusion. The resected liver volume was 3230 g and the tumor size was 30 × 20 × 9 cm (Fig. 3). Pathological examination revealed that the tumor consisted of well and moderately differentiated and some poorly differentiated HCC; the tumor margin was negative. According to the 7th edition of the UICC TNM staging system for HCC, this case was classified as pT3a pN0 pM0, Stage IIIA.
Fig. 3.
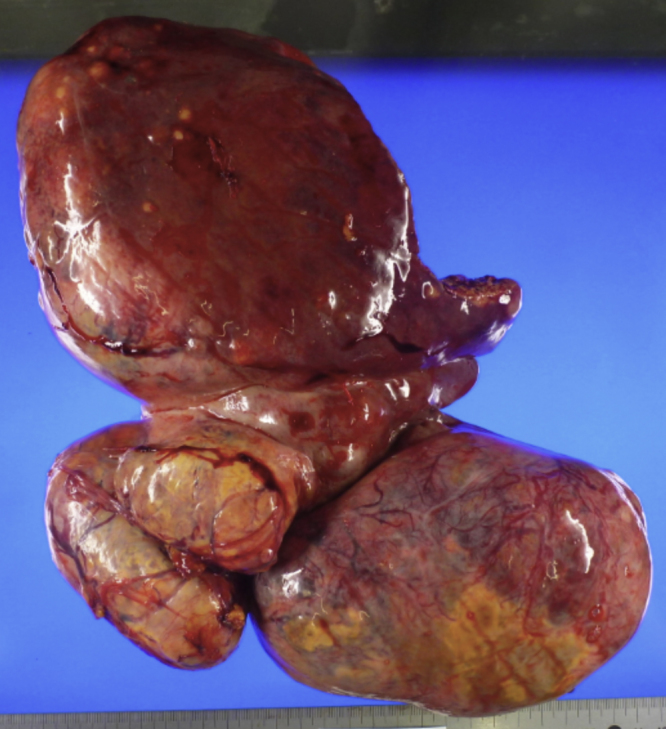
The volume of the resected liver was 3230 g, and the size of the resected tumor was 30 × 20 × 9 cm.
2.3. Postoperative course
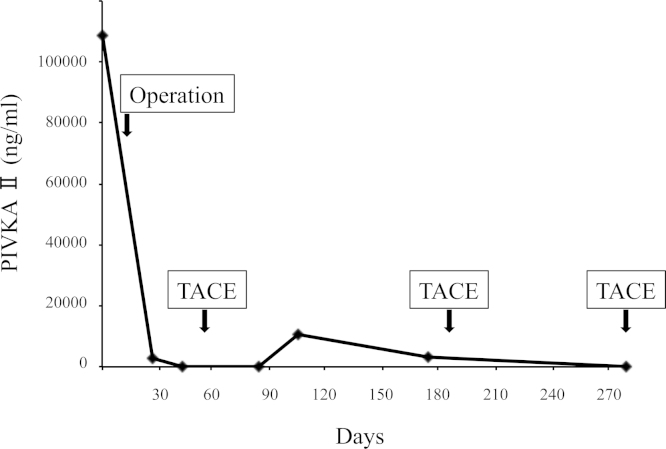
The patient developed a postoperative superficial surgical site infection, intra-abdominal abscess, and pleural effusion. She was treated with a percutaneous drainage and antibiotics. On postoperative day 60, TACE was performed to treat multiple HCCs in the remnant liver. After this multimodal therapy, the AFP and PIVKA-II levels decreased to limits within the normal range (Fig. 4).
Fig. 4.
The levels of PIVKA II during the postoperative course. After operation and the first administration of TACE, the levels of PIVKA II decreased to limits within the normal range.
One month after TACE, no new lesion was found on CT. Five months after the hepatectomy, multiple intrahepatic recurrences were detected. A second TACE was performed for the recurrent tumors. Three months after the second TACE, a third TACE was applied for recurrent tumors. One month after the third TACE, she developed pneumonia and recovered upon treatment with antibiotics. Because of her worsening performance status, TACE could not be applied again. Six months after the withdrawal of TACE her liver function gradually worsened and she died of liver failure 18 months after hepatectomy.
3. Discussion
In the present report, despite the presence of a huge HCC with multiple intrahepatic metastases, the patient was able to achieve an acceptable survival of 18 months through an aggressive multimodal therapy combining hepatectomy for the main tumor and repeated TACE for intrahepatic metastases in the remnant liver. To the best of our knowledge, this case was the largest HCC that had been previously reported.
Despite the recent improvements in imaging modalities such as multi detector row CT or contrast-enhanced magnetic resonance imaging, some patients still present with advanced-staged HCC with a large primary tumor accompanied by multiple intrahepatic metastases. However, there has been no widely accepted consensus regarding the treatment of such advanced cases of HCC. A large tumor diameter and extrahepatically expanding growth pattern are risk factors for the tumor rupture of HCC [6], [7], which is a life-threatening complication with a high mortality rate [14]. Recent publications have reported that the incidence of spontaneous tumor rupture range from 3% to 26% among patients with HCCs, and their corresponding mortality rates are notably high (25% to 100%) [15], [16]. In this setting, TACE should not be considered because of its poor prognosis for large HCC. Several authors have reported an unfavorable 5-year survival rate of 5.0–7.2% in patients who underwent TACE for HCC larger than 5 cm [17], [18]. In contrast, recent publications have reported acceptable long-term outcomes with 5-year survival rates ranging from 24.5% to 33% for patients who underwent hepatectomy for large HCC [8], [9], [10]. Although, these reports described retrospective studies, liver resection seems to be the only way to achieve long-term survival in cases of large HCC. Therefore, liver resection may be justified for patients with huge HCC to avoid spontaneous tumor rupture and to achieve long-term survival. In the present report, the patient had an extrahepatically expanding large HCC with a diameter of 30 cm, which totally occupied the right side of the abdominal cavity. It seemed that tumor rupture may occur soon, which would lead to major bleeding and death.
On the other hand, a large HCC is frequently accompanied by intrahepatic metastases. A surgical approach alone may not be sufficient for such advanced HCC, and it is necessary to control the residual intrahepatic tumors to achieve long-term survival. Several authors have reported the effectiveness of reduction surgery in combination with TACE for large HCC with multiple intrahepatic metastases [11], [12], [13]. Despite the lack of clear evidence in the treatment for such advanced HCC, multimodal treatment combining liver resection and TACE might be a good treatment strategy to achieve favorable long-term outcomes. Although our case was a far advanced HCC presenting a huge nodule of 30 cm in diameter with multiple metastases, the tumors were localized only in the liver without their invasion in the other organs or extrahepatic metastases during the treatment period.
We reported a case of advanced HCC with a huge primary tumor and multiple intrahepatic metastases. For the treatment of such patients, surgeons should keep in mind that it is necessary to prevent severe tumor rupture, which can be achieved by liver resection. Even though, the tumor is huge and extrahepatically expanding such as our case, majority of HCC has capsule and direct invasion to other organ is rare. Therefore, liver resection could be achieved safely in selected patients when sufficient remnant liver volume was preserved. Multimodal treatment involving hepatectomy and TACE might be a good treatment strategy for these patients if the tumors are localized in liver with no distant or peritoneal metastasis.
Conflicts of interest
All authors have nothing to disclose.
Funding
All authors have nothing to disclose.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of Nara Medical University.
Consent
Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Satoshi Yasuda: study concept, data collection and writing the manuscript.
Takeo Nomi: study concept, the editing and supervision of this paper.
Daisuke Hokuto, Ichiro Yamato and Shinsaku Obara: the literature research and the data extraction.
Takatsugu Yamada, Hiromichi Kanehiro and Yoshiyuki Nakajima: the editing and supervision of this paper.
Guarantor
Satoshi Yasuda and Takeo Nomi.
References
- 1.Fonseca A.L., Cha C.H. Hepatocellular carcinoma: a comprehensive overview of surgical therapy. J. Surg. Oncol. 2014;110:712–719. doi: 10.1002/jso.23673. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari N.N., Mehrabi A., Mollberg N.M., Muller S.A., Koch M., Buchler M.W., Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann. Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 3.Ng K.K., Vauthey J.N., Pawlik T.M., Lauwers G.Y., Regimbeau J.M., Belghiti J., Ikai I., Yamaoka Y., Curley S.A., Nagorney D.M., Ng I.O., Fan S.T., Poon R.T. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann. Surg. Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ishizawa T., Hasegawa K., Aoki T., Takahashi M., Inoue Y., Sano K., Imamura H., Sugawara Y., Kokudo N., Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 5.Choi S.H., Choi G.H., Kim S.U., Park J.Y., Joo D.J., Ju M.K., Kim M.S., Choi J.S., Han K.H., Kim S.I. Role of surgical resection for multiple hepatocellular carcinomas. World J. Gastroenterol. 2013;19:366–374. doi: 10.3748/wjg.v19.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki T., Kokudo N., Matsuyama Y., Izumi N., Ichida T., Kudo M., Ku Y., Sakamoto M., Nakashima O., Matsui O., Makuuchi M. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann. Surg. 2014;259:532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q., Li J., Yan J.J., Huang L., Wu M.C., Yan Y.Q. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J. Gastroenterol. 2012;18:7302–7307. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok K.T., Wang B.W., Lo G.H., Liang H.L., Liu S.I., Chou N.H., Tsai C.C., Chen I.S., Yeh M.H., Chen Y.C. Multimodality management of hepatocellular carcinoma larger than 10 cm. J. Am. Coll. Surg. 2003;197:730–738. doi: 10.1016/j.jamcollsurg.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Liau K.H., Ruo L., Shia J., Padela A., Gonen M., Jarnagin W.R., Fong Y., D’Angelica M.I., Blumgart L.H., DeMatteo R.P. Outcome of partial hepatectomy for large (>10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 10.Pandey D., Lee K.H., Wai C.T., Wagholikar G., Tan K.C. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann. Surg .Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K., Nakamura T., Kinoshita T., Konishi M., Nakagohri T., Oda T., Takahashi S., Gotohda N., Hayashi T., Nawano S. Volume reduction surgery for advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:362–366. doi: 10.1007/s00432-004-0566-7. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi H., Ushiyama T., Ishimura K., Izuishi K., Karasawa Y., Masaki T., Watanabe S., Kuriyama S., Maeta H. Significance of reduction surgery in multidisciplinary treatment of advanced hepatocellular carcinoma with multiple intrahepatic lesions. J. Surg. Oncol. 2003;82:98–103. doi: 10.1002/jso.10203. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K., Takenaka K., Kawahara N., Shimada M., Shirabe K., Itasaka H., Nishizaki T., Yanaga K., Sugimachi K. Indications for palliative reduction surgery in advanced hepatocellular carcinoma: the use of a remnant tumor index. Arch. Surg. 1997;132:120–123. doi: 10.1001/archsurg.1997.01430260018002. [DOI] [PubMed] [Google Scholar]
- 14.Hung M.C., Wu H.S., Lee Y.T., Hsu C.H., Chou D.A., Huang M.H. Intraperitoneal metastasis of hepatocellular carcinoma after spontaneous rupture: a case report. World J. Gastroenterol. 2008;14:3927–3931. doi: 10.3748/wjg.14.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y.J., Lee J.W., Park S.W., Lee J.I., Lee D.H., Kim Y.S., Cho S.G., Jeon Y.S., Lee K.Y., Ahn S.I. Survival outcome of patients with spontaneously ruptured hepatocellular carcinoma treated surgically or by transarterial embolization. World J. Gastroenterol. 2013;19:4537–4544. doi: 10.3748/wjg.v19.i28.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin H.M., Lei L.M., Zhu J., Li G.L., Min J. Risk factor analysis of perioperative mortality after ruptured bleeding in hepatocellular carcinoma. World J. Gastroenterol. 2014;20:14921–14926. doi: 10.3748/wjg.v20.i40.14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L.F., Sun H.L., Chen Y.T., Ni J.Y., Chen D., Luo J.H., Zhou J.X., Hu R.M., Tan Q.Y. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J. Gastroenterol. Hepatol. 2013;28:456–463. doi: 10.1111/jgh.12088. [DOI] [PubMed] [Google Scholar]
- 18.Guo W.J., Yu E.X., Liu L.M., Li J., Chen Z., Lin J.H., Meng Z.Q., Feng Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J. Gastroenterol. 2003;9:1697–1701. doi: 10.3748/wjg.v9.i8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]