Highlights
-
•
Use of VA-ECMO systems may be encumbered by severe vascular complications.
-
•
Infections developed after femoral arterial surgery cause significant morbidity.
-
•
Coverage of vascular structures with a muscle flap can achieve site sterilization.
Keywords: Groin infection, Sartorius muscle flap, VA-ECMO
Abstract
Introduction
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO1) systems are a life-saving option in the treatment of acute respiratory distress syndrome (ARDS2), but may be encumbered by severe vascular complications in the groin.
Presentation of case
A pregnant woman was admitted with respiratory failure due to H1N1 influenza. VA-ECMO was inserted percutaneously by the intensivists and then accidentally removed by the patient after 8 days. 24 h later VA-ECMO was reinstalled with surgical denudation of femoral vessels in another department. 2 h later, due to active bleeding and signs of limb ischemia, the patient was referred to our department and emergency trombectomy and patch angioplasty with PTFE were performed. Evolution was further bad with wound infection (Pseudomonas, Proteus), which imposed large debridement, replacing the PTFE patch with 2 parallel venous patches and wound reconstruction through sartorius muscle rotation. The wound underwent negative pressure therapy for 10 days and was skin grafted. The patient recovered under systemic antibiotic and virostatic therapy.
Discussion
Major complications of using VA-ECMO devices are related to vascular access, most common bleeding at the puncture site and acute limb ischemia. In the groin, sartorius muscle flap is the most used for vascular coverage and small tissue defect reconstruction because of the ease in harvesting and low donor-site complications.
Conclusion
Although ischemic complications associated with VA-ECMO are accepted by intensivists under the slogan “leg for life”, for the repair of the femoral artery in the presence of groin infection the sartorius muscle remains an efficient solution for limb salvage.
1. Introduction
Szilagyi type III groin infections developed after femoral arterial surgery cause significant morbidity and mortality among vascular patients [1], especially if Gram negative germs are involved [2]. Local treatment of this disease is complex, including wide excision of infected tissue, vascular reconstruction, negative pressure therapy and muscle flaps coverage [3]. Covering vascular structures found in infected tissue with a muscle flap positively influence healing, due to locally increased blood flow and dead space elimination [4]. Among the many possible muscle flaps, sartorius muscle is most commonly used because it is readily available at the reconstruction site, requires minimal dissection and and is large enough to cover the defect [5].
Acute respiratory distress syndrome (ARDS) is a life-threatening medical emergency which is usually treated with mechanical ventilation in the Intensive Care Unit. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) systems are an option in the treatment of ARDS, especially in patients with persistent hypercapnia/hypoxemia refractory to traditional therapy [6]. Vascular access is achieved most commonly by percutaneous cannulation and here, lies the most common complications of this method: hemorrhage and limb ischemia [7]. Major bleeding risk is caused by the large size of cannulas, between 12 and 17 Fr. Treatment of these complications in the context of related diseases can create major difficulties both for the surgeon and the intensivist.
We present the therapeutic management of a young pregnant woman, infected with H1N1 influenza virus, hospitalized with ARDS, that suffered iatrogenic injuries caused by the implantation of a VA-ECMO system.
2. Presentation of case
A 32 years old patient, 26 weeks pregnant, was admitted to intensive care unit with malaise and intense dyspnea. Clinical examination revealed tachycardia, tachypnea and decreased bilateral vesicular sounds with disseminated rales. Astrup parameters were profoundly altered, with a PaO2/FiO2 ratio under 100. On chest radiography were present multiple alveolar opacities with a tendency to confluence, spread on both lungs. Due to the impossibility of improving respiratory parameters by mechanical ventilation, VA-ECMO system (Novalung®) installation was decided by intensivists. The catheters were placed percutaneously, the left femoral artery and right femoral vein being cannulated without incidents. RT-PCR confirmed the diagnosis of H1N1 influenza and antiviral treatment was started with oseltamivir phosphate (Tamiflu, Genentech USA, Inc.), 300 mg per day. Subsequent evolution was slowly favorable, with improvement of respiratory parameters, blood analysis and chest X-ray. At 8 days after admission the patient inadvertently suppressed the arterial catheter. Given the alteration of blood gases, 24 h later the reinstatement of the VA-ECMO system was decided, with surgical denudation of the left femoral artery and right femoral vein being done in another surgical department. Two hours postoperatively the patient presented active bleeding through the left groin wound and signs of lower limb ischemia. She was admitted acutely in our clinic and underwent emergency surgical exploration. Intraoperatively, after arterial catheter removal, a hematoma was found together with laceration of the medial wall of the deep femoral artery and no pulsation in the superficial femoral artery. Fogarty thrombectomy was performed in the superficial femoral artery followed by patch angioplasty with PTFE at the superficial and deep femoral arteries level. Replacing the VA-ECMO system was contraindicated and the wound was sutured. Postoperatively the patient showed tibial arteries pulse. Due to general altered condition, the next day the gynecologist decided to terminate the pregnancy by Caesarean section, surgery performed under orotracheal intubation. Unfortunately the newborn died the same day. Respiratory status gradually improved through mechanical ventilation with ARDS parameters and pulmonary nursing, but the groin wound began to develop, at five days postoperatively, seropurulent drainage. Intravenous antibiotic treatement with meropenem was started, 4 g per day. Bacterial culture revealed infection with Pseudomonas aeruginosa and Proteus mirabilis, both showing sensitivity to meropenem. Although local evolution was slightly favorable under antibiotic therapy and daily dressings, the continued presence of secretions imposed surgical revision of the wound. Due to the fact that femoral arteries and PTFE patch showed no signs of integration (Fig. 1), being surrounded by cellular debris and purulent secretions, femoral tripod reconstruction was decided with autologous material. For this purpose, after wide debridement and lavage of the groin wound with saline, a portion of the left internal saphenous vein in the calf was harvested and cut into two patches due to its low diameter. PTFE patch was excised together with the surrounding affected arterial wall, thus cutting the deep femoral artery at its origin. In the next stage the common and superficial femoral arteries angioplasty was performed with the two venous patches and the deep femoral artery was reimplanted (Fig. 2). Because the defect resulted after debridement could only be sutured with important tension in the wound edges, flap reconstruction was chosen. Sartorius muscle was mobilized, medialy rotated and secured with continuous suture to cover the vascular reconstruction. At the end of surgery a negative pressure wound therapy (NPWT) system (RENASYS, Smith & Nephew PLC, United Kingdom) was mounted. With antibiotic treatment and NPWT foam changed every three days, the wound granulated (Fig. 3) and was skin grafted after 10 days. The patient was discharged without major physiological disturbances and distal pulse present in both legs. The groin skin graft was perfectly integrated at three months follow-up (Fig. 4 ).
Fig. 1.
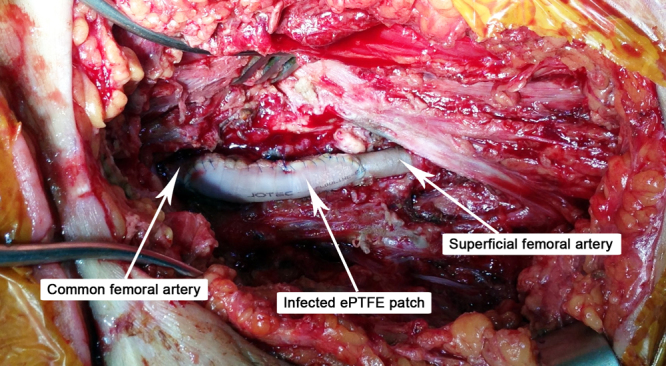
Unintegrated PTFE patch used in the first angioplasty procedure, wound debrided.
Fig. 2.
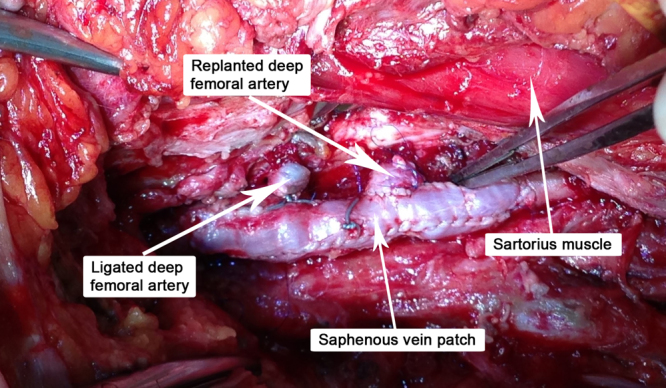
PTFE patch explanted, venous patch angioplasty performed and deep femoral artery reimplanted.
Fig. 3.
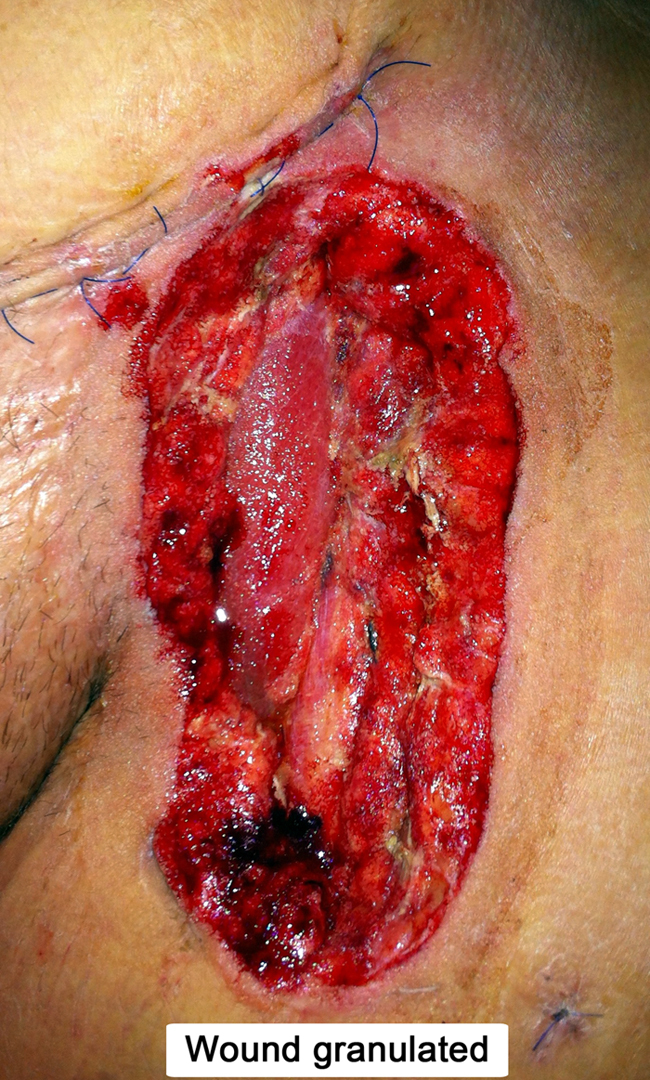
Granulation tissue after negative pressure wound therapy.
3. Discussion
Veno-arterial extracorporeal membrane oxygenation systems are a lifesaving option for patients with therapy refractory cardiac or cardiopulmonary failure [6]. Major complications of using these devices are related to vascular access, most common bleeding at the puncture site and acute limb ischemia. Substrates that cause these complications are acute embolism, thrombosis, false aneurysm, dissection/perforation/rupture of the common femoral artery and external iliac artery [8].
American National Center for Infectious Diseases defines deep incisional surgical site infection (SSI) as an infection which occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place, the infection appears to be related to the operation and involves deep soft tissues (fascial and muscle layers) of the incision. The natural evolution of an untreated SSI is toward penetration of the arterial wall or prosthetic material, causing anastomotic dehiscence and consecutive bleeding or distal embolism and septicemia in immunocompromised patients [3]. Surgical management involves removing pus, cellular debris and the entire clinically infected tissue in a first stage. In case of vascular structures involvement, whether it is native arteries or autologous or synthetic grafts, the decision on keeping them and sterilizing the site by repeated dressings or NPWT therapy or removing them and performing new reconstruction, in situ or extraanatomic, belongs only to the surgeon. This decision is based on multiple factors, most important being the involvement of the anastomosis area, infection with a gram negative bacteria, the presence of a pseudoaneurysm, history of bleeding or a graft—enteric fistula, when excision of infected material is mandatory [9]. If only a small portion of the vessel/prosthesis is affected, local sterilization can be tempted. Successes have been reported using vancomycin—loaded polymethylmethacrylate beads [10] or negative pressure wound therapy [11]. Usually, after wide excision of infected tissue, the patient remains with a major tissue defect that does not allow secondary suture after site sterilization. The options in this case are a lengthy treatment with NPWT systems [11], muscle flaps reconstruction [4], [5] or a combination of both. In the groin may be carried out a wide variety of flaps, such as sartorius muscle flap, rectus femoris flap, antero-lateral thigh flap, pedicled gracilis flap or myocutaneous flaps of rectus abdominis [3], [4], [5]. Sartorius muscle flap is the most used in case of small tissue defect requiring reconstruction because of the ease in harvesting and low donor-site complications. After the muscle flap is performed, a negative pressure wound therapy system can be used to hasten granulation, remove exudate, reduce edema and act as an effective barrier to bacterial penetration [11].
4. Conclusion
The primary “take-away” lessons from this case is that, even in vulnerable individuals receiving intensive care, a combination of therapeutic measures, including broad-spectrum antibiotics, excision of devitalized tissue, negative pressure therapy, and especially coverage of vascular structures with a muscle flap can successfully achieve site sterilization and limb salvage.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief on request.
Conflict of interest
None.
Funding
This research was funded by project “Parteneriat Interuniversitar Pentru Cresterea Calitatii si Interdisciplinaritatii Cercetarii Doctorale Medicale Prin Acordarea de Burse Doctorale—DocMed.net” POSDRU 107/1.5/S/78702.
Ethical approval
Not applicable.
Authors contribution
George V. Patrut: preparation of manuscript, literature review, consent of patient.
Claudiu Neamtu: procedure.
Mihai Ionac: procedure and review of manuscript.
Guarantor
Mihai Ionac.
Fig. 4.
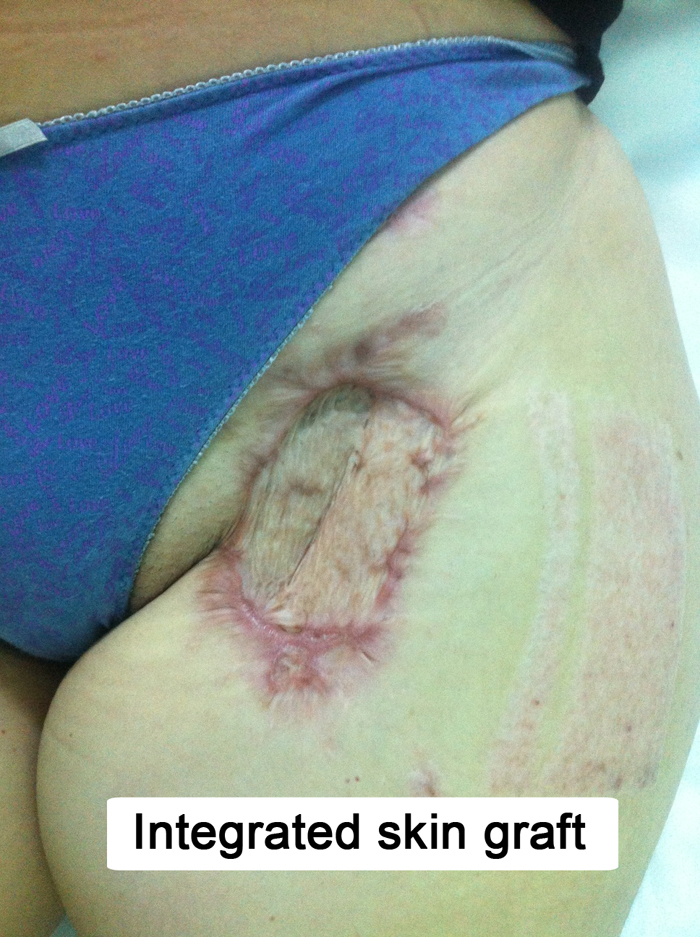
Fully integrated skin graft, at 3 months follow-up.
Footnotes
VA-ECMO: veno-arterial extracorporeal membrane oxygenation.
ARDS: acute respiratory distress syndrome.
Contributor Information
George V. Patrut, Email: georgepatrut@yahoo.com.
Claudiu Neamtu, Email: bneamtucld79@yahoo.com.
Mihai Ionac, Email: mihai.ionac@yahoo.com.
References
- 1.Kalish J.A., Farber A., Homa K., Trinidad M., Beck A., Davies M.G. Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) J. Vasc. Surg. 2014;60(November (5)):1238–1246. doi: 10.1016/j.jvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Leon L.R., Jr., Thai J., Pacanowski J.P. Gram-negative groin sepsis treated with covered stents and systemic antibiotics. Vascular. 2011;19(August (4)):226–231. doi: 10.1258/vasc.2010.cr0255. [DOI] [PubMed] [Google Scholar]
- 3.Shih P.K., Cheng H.T., Wu C.I., Chang S.C., Chen H.C., Chen H.H. Management of infected groin wounds after vascular surgery. Surg. Infect. (Larchmt) 2013;14(June (3)):325–330. doi: 10.1089/sur.2011.123. [DOI] [PubMed] [Google Scholar]
- 4.Chateau F., Duisit J., Lengelé B., Vanwijck R. Techniques for coverage of infected vascular grafts in the groin. Acta Chir. Belg. 2010;110(July–August (4)):487–491. doi: 10.1080/00015458.2010.11680662. [DOI] [PubMed] [Google Scholar]
- 5.Fischer J.P., Mirzabeigi M.N., Sieber B.A., Nelson J.A., Wu L.C., Kovach S.J. Outcome analysis of 244 consecutive flaps for managing complex groin wounds. J. Plast. Reconstr. Aesthet. Surg. 2013;66(October (10)):1396–1404. doi: 10.1016/j.bjps.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Kluge S., Braune S.A., Engel M., Nierhaus A., Frings D., Ebelt H. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012;38(October (10)):1632–1639. doi: 10.1007/s00134-012-2649-2. [DOI] [PubMed] [Google Scholar]
- 7.Ganslmeier P., Philipp A., Rupprecht L., Diez C., Arlt M., Mueller T. Percutaneous cannulation for extracorporeal life support. Thorac. Cardiovasc. Surg. 2011;59(March (2)):103–107. doi: 10.1055/s-0030-1250635. [DOI] [PubMed] [Google Scholar]
- 8.Roussel A., Al-Attar N., Khaliel F., Alkhoder S., Raffoul R., Alfayyadh F. Arterial vascular complications in peripheral extracorporeal membrane oxygenation support: a review of techniques and outcomes. Future Cardiol. 2013;9(July (4)):489–495. doi: 10.2217/fca.13.34. [DOI] [PubMed] [Google Scholar]
- 9.De Donato G., Setacci F., Galzerano G., Ruzzi U., Borrelli M.P., Mazzitelli G. Prosthesis infection: prevention and treatment. J. Cardiovasc. Surg. (Torino) 2014;55(December (6)):779–792. [PubMed] [Google Scholar]
- 10.Stone P.A., Armstrong P.A., Bandyk D.F., Brumberg R.S., Flaherty S.K., Back M.R. Use of antibiotic-loaded polymethylmethacrylate beads for the treatment of extracavitary prosthetic vascular graft infections. J. Vasc. Surg. 2006;44(October (4)):757–761. doi: 10.1016/j.jvs.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Saziye K., Afksendiyos K. The vacuum-assisted closure (V. A. C®) system for surgical site infection with involved vascular grafts. Vascular. 2015;23(April (2)):144–150. doi: 10.1177/1708538114537488. [DOI] [PubMed] [Google Scholar]


