Abstract
Multipurpose Prevention Technologies (MPTs) are new tools aimed at reducing or preventing multiple and overlapping sexual and reproductive health risks faced by women and couples around the globe. While MPTs could prove more acceptable and easier to adhere to than single-purpose prevention products, continuing high rates of HIV and unintended pregnancy remind us that these new products will need to be efficacious, acceptable and effectively used to achieve a public health impact. In this paper, we describe how a range of research methods can be applied during the pre-clinical phase of product development to inform decisions related to formulation and vehicle or product delivery mechanisms, and consider how choices in product-related characteristics may influence future demand for, delivery and use of future products. We draw on examples from the development of new single-purpose HIV and contraceptive products and then extend our discussion to the development of MPTs, including vaginal rings and injections. This article is based on a presentation at the “Product Development Workshop 2013: HIV and Multipurpose Prevention Technologies,” held in Arlington, Virginia on February 21-22, 2013. It forms part of a special supplement to Antiviral Research.
Keywords: Qualitative research, Psychometric scales, Discrete choice experiments, Conjoint analysis, MPTs
1. Introduction
Globally, women and couples face multiple and overlapping sexual and reproductive health risks (SRH). For example, an estimated 500 million cases of treatable sexually transmitted infections (STIs) occur annually, disproportionately affecting sub-Saharan African and South Asian men and women. When untreated, STIs are a leading cause of infertility and may triple the risk of HIV acquisition (World Health Organization Media Centre, 2013). Despite downward trends in new HIV infections, 34 million people are currently living with HIV, the vast majority in sub-Saharan Africa where almost 60% of prevalent HIV infections are in women (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2011). Young women aged 15–24 account for about one-quarter of all new infections, but almost one-third of new infections in sub-Saharan Africa (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012). More than two million adolescent women in this region experience unintended pregnancies each year (Guttmacher Institute and IPPF, 2010).
Multipurpose Prevention Technologies (MPTs) are new prevention tools aimed at two or more SRH risks, including treatable STIs, HIV and unintended pregnancy (Holt, 2010). At present, male and female condoms are the most common and widespread MPT. A number of other MPT approaches are currently being considered, including injectables that might be co-formulated or co-administered, vaginal and/or rectally-inserted gels, oral pills or devices including vaginal rings. One MPT currently in development is a one-month vaginal ring able to release a contraceptive progestin and an antiviral agent to reduce the risk of HIV (Holt, 2010).
MPTs, by virtue of having dual indications, could prove more acceptable and easier to adhere to than single purpose prevention products. However, the continuing high rates of HIV and unplanned pregnancy, despite widespread availability of effective and low cost prevention methods, reminds us that the relationship between development of effective technologies and public health impact is not often linear. Consequently, social and behavioral scientists have an important role to play in identifying the range of factors likely to influence individuals' willingness and ability to initiate and effectively use MPT products, thus informing the choice of product candidates for development and/or testing that best fit the needs and preferences of populations who might benefit from them (Tolley and Severy, 2006; Morrow and Ruiz, 2008).
1.1. Acceptability and adherence (A2) conceptualized
Acceptability has been conceptualized largely as a favorable “attitude” towards a product, predisposing a person to be willing to use it (see Table 1 for glossary of terms). Acceptability is influenced by a number of underlying factors – a person's perceived risk; expectations related to product effectiveness, as well as to social-behavioral challenges of using the product, including perceived ease of use, concerns about side effects, and/or impact on daily life (Tolley and Severy, 2006; Severy et al., 2005). In the context of choice, acceptability may be measured as the selection of one product or behavior over another or the continued use of the product over time. However, in the absence of an approved product, acceptability has been assessed hypothetically or as it relates to proxy products (Severy et al., 2005).
Table 1.
Glossary of terms used in this article.
| Acceptability | A favorable “attitude” towards a product, predisposing a person to be willing to use it. In the context of choice, acceptability may be measured as the selection of one product or behavior over another or the continued use of the product over time. |
| Adherence | A behavioral construct related to the extent to which a product is used as intended. Dimensions of adherence include timing of product use, dosage taken, consistency and duration of use. |
| Conjoint analysis | A statistical technique used in market research to determine how people value different features that make up an individual product or service. |
| Discrete choice experiment | A statistical technique in which individuals state their preference for one product scenario among a set of realistic scenarios in order to understand consumer choice. |
| Perceptibilty research | Research approach, with methods similar to those in sensory evaluation science, aimed at better understanding and measuring users' sensory perceptions and experiences of products in order to inform product design and formulation. |
| Psychometric scale | Sets of survey items in the form of statements that, when combined, measure a more complex construct that may not be directly observable. |
| Rheology | A science dealing with the deformation and flow of matter. |
| Qualitative Research | Research approach that favors collection of textual or other non-numeric data and aims to gather an in-depth understanding of human behavior. The approach can be especially effective in obtaining culturally specific information about the values, opinions, behaviors, and social contexts of particular populations. |
In contrast, adherence is a behavioral construct related to the extent to which a product is used as intended. Dimensions of adherence include timing of product use, dosage taken, consistency and duration of use. Adherence requirements can vary greatly by product. For example, daily use of an oral pill or vaginal gel requires the user to routinize product use and to remember to carry products when traveling or when daily routines are disrupted. Furthermore, product use would be needed even during periods when protection might not be. Unlike daily gel use, pericoital gel use would be on an “as-needed” basis, but would involve anticipating when a sexual encounter might happen or incorporating gel insertion into the sex act. Although vaginal rings may be inserted and removed by the user, adherence to vaginal ring use requires one to “do nothing” – or just leave the ring in for the intended duration. Additionally, monthly or bi-monthly vaginal rings or injections would require the user to return to a clinic within the correct time-frame for resupply.
Acceptability does not always lead to high adherence and adherence may be achieved in the absence of acceptability. Although, when users are free to choose among a range of viable options, acceptability is assumed to be a key factor driving adherence (Severy et al., 2005).
The possibility of conflating these two constructs is particularly high within the context of clinical trial research, in which participants are requested to adhere to a product of unknown efficacy, may enroll in trials for reasons unrelated to product use, or may achieve high adherence within trials without the intention for future product use, should it be found efficacious. As Morrow and Hendrix point out, the absence of an approved product with which to study “acceptability” as a phenomenon in its own right' has led the HIV prevention field to focus more on ‘adherence’, using it as a surrogate for ‘acceptability’ (Morrow and Hendrix, 2010). Indeed, much attention in recent HIV prevention research has focused on how to measure microbicide and PrEP adherence within clinical trial testing (Woodsong et al., 2013). However, it is increasingly clear that the context of product use within a clinical trial and adherence to products once approved and introduced through a country's health system may differ in important ways (Morrow and Hendrix, 2010; Woodsong et al., 2013; Tolley et al., 2013).
1.2. Continuum of A2 factors
Ultimately, efficacious, new products will not have an impact if they are not initiated and effectively used by those at risk. As shown in Fig. 1, these factors fit along a continuum, from product-related attributes to the profiles of potential/intended users as well as the service delivery and wider sociocultural contexts within which the product will be used. Therefore, research is required during early product development and clinical testing to identify and intervene upon the factors that will influence the product's eventual acceptability and use adherence.
Fig. 1.
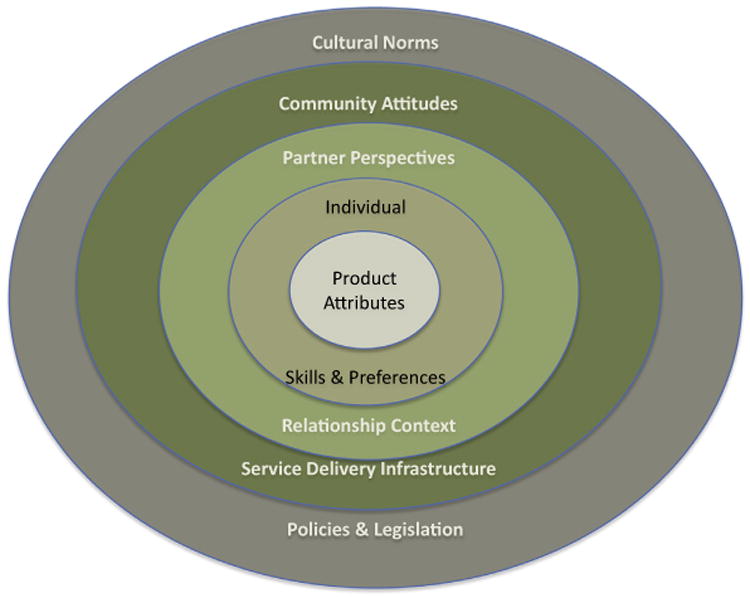
Social-ecological model of factors influencing product acceptability.
In this paper, we describe how a range of research methods can be applied during pre-clinical phases of product development to inform decisions related to formulation and vehicle or product delivery mechanisms, and consider how choices in product-related characteristics may influence future demand for, delivery and use of future products. We draw on examples from the development of new single-purpose HIV and contraceptive products and then extend our discussion to the development of MPTs, including vaginal rings and injections.
2. Methods for assessing “acceptability”
Research into product acceptability (or those factors underlying it) may employ multiple approaches – from qualitative, in-depth exploration of hypothetical or proxy product decision-making and use experiences to more structured assessments of individual preferences for sets of product attributes through conjoint analysis or discrete choice experiments. The examples presented in this paper make use of different social-behavioral science research approaches, including psychometric scales, qualitative research and structured elicitation of preferences, to integrate user perspectives into the product development process (see Table 1 glossary).
2.1. Users' sensory perceptions of physicochemical properties
Morrow and colleagues borrow from sensory evaluation science, a field with strong ties to the food and cosmetics industries, to better understand and measure users' sensory perceptions and experiences of products in order to inform product design and formulation. Her approach is based on the premise that a product's physicochemical properties and rheological performance characteristics, which affect how well a product formulation dissolves, spreads, or is absorbed and available within the tissues, are the same ones that impact the user's sensations, perceptions and experiences. Furthermore, users' sensory perceptions and experiences of these properties can be objectively evaluated, provided that the assessment tools and language are carefully crafted. By understanding how potential users' sensations and experiences relate to a diverse set of formulations, perceptibility data can be linked with data on the physicochemical properties of various formulations in order to optimize product development decisions (Morrow and Hendrix, 2010).
Morrow's perceptibility research is grounded in the development of user sensory perception and experience (USPE) scales that enable women to provide feedback on specific formulation properties of a range of vaginal gels and film. USPE scales are sets of survey items in the form of statements that, when combined, measure a more complex construct that may not be directly observable (DeVellis et al., 2003; Gamst et al., 2006).
Typically, the development and validation of psychometric scales involves both qualitative and quantitative assessments. An important first step is formative research to identify how users experience and talk about the physical characteristics of different formulations. For example, in earlier research, women were asked to describe their experiences with a range of different gel formulations after feeling the gel in their hand, inserting, walking, and using the product during simulated intercourse (Project LINK, R21/R22 MH080591). The words women used to describe their sensory perceptions of gel characteristics (for example, sensations of smoothness, stickiness or movement resulting in spreading or vaginal coating), could then be used to generate a pool of statements representing these sensory perceptions. Once a set of draft items has been generated, they are tested with a larger sample of respondents, who use the items to rate a range of gel formulations. The items can then be factor-analyzed to determine whether items expressing similar characteristics were grouped together in predictable ways and captured those elements of users' sensory perceptions and experiences that were described during qualitative assessments. A final validation step would be to determine whether women's assessment of a range of gel formulations, based on responses to the USPE scales, corresponded to the physicochemical properties of each gel.
Once validated, scales can be used to predict product choice or willingness to use, target formulations with certain USPEs to specific populations, or screen new formulations for a set of known properties that are linked to higher levels of adherence in clinical trials and presumably in post-trial use.
2.2. Feedback on the target product profile (TPP)
When conceptualizing a new product, a first step is to develop the target product profile (TPP), a schema identifying both desired and minimally acceptable targets related to the product's effectiveness, side effect profile, dosage and delivery mechanism; characteristics of the intended user population; and factors influencing other levels of the continuum shown in Fig. 1, such as the possibility of “discreet use” (relationship context); product packaging, storage conditions and cost (service delivery issues). Research assessing the social-behavioral correlates of the TPP can provide insight into the potential demand for a new product, the profile of potential users and non-users, and the relative importance of specific targeted attributes to product acceptability and adherence.
During the conceptual stage of a project to develop a longer-acting injectable contraceptive method (LAI), Tolley and colleagues conducted a substudy to examine attitudes towards LAI characteristics identified in the TPP. The parent project, funded by the Bill & Melinda Gates Foundation, aims to identify and provide proof of concept for several novel approaches to obtaining a longer acting contraceptive injectable, one that might last for six months or even longer (FHI 360, 2013). Potential LAI approaches might include increasing the dosage of an existing injectable formulation, altering the administration or injection site, or identifying drug delivery systems that could prolong the release of the drug.
One challenge to conducting the acceptability substudy was how to obtain informed feedback on specific product characteristics, given the lack of an actual prototype that could be experienced within real-life contexts. Consequently, data collection took place in Kenya and Rwanda (two countries with high levels of contraceptive injectable use but different service delivery environments) and included in-depth interviews with policymakers, providers and program managers, as well as focus group discussions with potential users who were current, former or never users of current injectable methods. The interviews and FGDs examined participants' knowledge and experiences with current injectable methods, discussed new LAI approaches, including increasing the dose or altering the formulation of existing drugs or using new drug delivery systems, and explored attitudes towards LAI characteristics identified in the TPP. At the end of each discussion, participants were asked to rank the TPP characteristics most and least important to them, using a set of laminated illustrations that had been introduced with each TPP characteristic. Targeted and minimally acceptable levels of eleven different characteristics were assessed, including an effectiveness level of 99% or lower acceptable level; administration via a single-dose prepackaged and disposable injectable versus one that might require mixing two vials prior to injection or administration via two injections; and immediate return to fertility versus a return to fertility that might take up to 18 months.
The quantifiable ranking activity highlighted points of concurrence and divergence of preferred TPP characteristics across countries and participant types. For example, while achieving a high level of effectiveness (targeted at 99%) was endorsed by all participant groups as among the most important characteristics of a new LAI, preferences for other characteristics appeared more context-dependent. A single-dose prepackaged and disposable injectable was more important to potential users than providers and more important in Rwanda than in Kenya, while having a rapid return to fertility was more important among both potential users and providers in Kenya than it was in Rwanda.
The qualitative data provided further insight into these differences. In both countries, potential users expressed strong concerns about using a LAI that required two injections, largely due to pain. They also worried about the quality and proper dosing of the injectable, if it required the provider to mix ingredients before administration. While having a prepackaged LAI was not a priority for service providers, they acknowledged that having to mix a LAI before administration could result in longer wait times and the possibility of stock-outs of a required ingredient for a two part product, unless packaged together.
Although the LAI TPP targeted an immediate return to fertility (similar to what might be experienced after discontinuing a non-hormonal contraceptive method), participants were also asked about their attitudes towards a LAI with a delayed return to fertility (up to 18 months). The concept of a contraceptive method leading to delayed fertility was familiar, and to some acceptable, though also a source of confusion. Indeed, some women and providers noted that the current injectable could lead to long delays in fertility. Women's perspectives related mostly to their fertility intentions and the role of a LAI in achieving those goals. Rwandan participants were more likely than Kenyan participants to view a long return to fertility as a benefit, enabling women to avoid future childbearing or to achieve long gaps between children. In general, long delays in fertility were more acceptable if they were predictable. Related to this point, some providers and users in both countries confused the possibility of a long contraceptive tail (period in which the drug stops having an acceptable level of effectiveness, but has not been fully eliminated from the body) with an extended “bonus” period of contraceptive effectiveness. Some participants calculated, therefore, that they could discontinue a LAI early, saving money and clinic visits, but remain protected for an additional 18 months. The potential for such misperceptions suggest the importance of client-centered information and counseling during clinical trial research and product introduction.
2.3. Assessing demand for alternative product “packages”
Finally, more structured, quantitative approaches, such as conjoint analysis or discrete choice experiments can be used to assess user preferences for a set of potential product attributes in the context of choice. Despite some underlying differences, both methods share important similarities. First, researchers must identify the attributes (i.e., effectiveness, prevention indication, and/or cost) on which to collect preference data. As with the development of psychometric scales such as the USPE scale described earlier, this preliminary stage usually involves formative, oftentimes qualitative, research or an extensive literature review. Once identified, each attribute is assigned several values, representing plausible variations in the attribute (i.e., cost choices could represent high, medium or no cost options). After the set of potential attributes and realistic range of values has been chosen, choice statements are structured in such a way that individuals are required to make trade-offs among competing sets of attributes. In most studies, the number of choice options must be limited to avoid participant fatigue (Viney et al., 2002; Louviere et al., 2010). Because the approach is quantitative and structured, larger sample sizes are feasible, enabling comparisons in preferences across different population groups. However, as with all research approaches, decisions about who to include in the sample (including whether they have tangible experience with potential product-related attributes being assessed) will affect study findings.
Both conjoint analysis and discrete choice approaches have been used to assess acceptability of new HIV prevention technologies. Lee and colleagues conducted conjoint analysis to assess consumer preferences for attributes of a new HIV vaccine among ethnically diverse populations in Los Angeles and Toronto (Lee et al., 2008). Following qualitative research to examine HIV vaccine barriers and concerns, they identified seven attributes and dichotomous values for a new vaccine. Because a full-factorial analysis of all possible combinations of vaccine scenarios (n = 128 combinations) would have been unwieldy, they employed statistical methods (fractional factorial orthogonal design) to reduce vaccine scenarios to eight, with participants rating each of the eight scenarios on a 5-point acceptability scale from “highly likely” to “highly unlikely”. Analyses of the acceptability scores enabled identification of most and least acceptable vaccine scenarios overall and by group, as well as the impact of individual vaccine attributes on acceptability. For example, they found efficacy (95% versus 50%) to have the greatest impact on acceptability across all three groups, while number of doses had a significant impact on acceptability of one group.
In South Africa, Terris-Prestholt and colleagues conducted a survey with over 1000 sexually active women to assess demand for a range of new HIV prevention methods including female condoms, microbicide gel, and diaphragms. The survey included information on socio-demographics, reproductive and sexual health histories, and two discrete choice experiments to explore how women's preferences varied by (1) product-related attributes and (2) potential distribution and promotion strategies. In the first experiment, women were presented six different scenarios, in which they chose one of three options, including the choice between two of the new prevention methods, or a third choice to select neither of the new options, but to do what they did last time they had sex. (Based on a prior response to questions about the last sexual act, this third option was either to “use no barrier method” or to “use a male condom”). Each of the three options was assigned a set of product attribute values, including HIV prevention effectiveness (0% for no barrier method, 35%, 55%, 75%, or 95% for other options), pregnancy risk reduction (0%, 35%, 55%, 75%, 95%), potential for secret use (yes/no), and price (free to 20 rand, or approximately $3 in 2005, for microbicides or female condoms and up to 80 rand for a reusable diaphragm). Analyses indicated women's general preferences between the three new HIV prevention methods (microbicides and then diaphragm was preferred over female condoms). They also identified the impact of specific attributes on method choice; for example, both HIV and pregnancy prevention effectiveness were very important to women, with HIV prevention more than twice as important as pregnancy prevention for women who considered themselves to be at high risk for HIV. Women who had used condoms were more likely to choose “neither of the two options”, whereas women who chose one of the two new prevention methods were more likely to be living with a partner or to have had problems getting a partner to use a condom. Women who reported prior problems with male condom use were also more likely to endorse “secret use” as an important product attribute (Terris-Prestholt et al., 2008).
3. Discussion
We presented several approaches to assessing acceptability and its underlying determinants (perceptibility, attitudes and understanding of product characteristics, partner dynamics, service delivery contexts) during early product development and provided examples of how this information might inform optimization of the products' physicochemical properties, identify and characterize populations most or least likely to demand new products, and consider the implications of product development decisions on further clinical testing and product introduction.
Research to inform the design of new MPTs for increased acceptability and adherence are similar to the approaches described in this paper, with some distinctions. A number of MPT approaches being considered are similar in form to existing single indication products. As such, perceptibility research that better measures the sensory perceptions and experiences of potential users, assessing their preferences among a range of formulation options, could help product formulators optimize new products for both physicochemical/rheological and acceptability/adherence characteristics.
In addition to sensory perceptions, potential users' and providers' cognitive understanding of, and attitudes towards, TPP characteristics could provide valuable information for product development, as well as successful implementation of clinical trials. Some of these characteristics are fairly straightforward. For example, as with attitudes towards the potential presentation of a LAI, acceptability of two co-administered injections versus a single larger volume one will have implications for the co-formulation versus co-administration of a MPT injectable. Low acceptability of a co-administered product could also hinder recruitment or retention of trial participants. Other characteristics are more complex to understand. For example, women (and providers) may have variable understanding about differing levels of effectiveness for the pregnancy prevention and HIV prevention indications of a MPT vaginal ring, or the impact of ring removal on pregnancy and/or HIV prevention effectiveness. Several studies of contraceptive or ARV-based rings suggest that some women wish to remove their rings during menses, for vaginal cleansing or other reasons (Montgomery et al., 2012; Nel et al., 2012). Being instructed to use a ring continuously for 30, 60, or 90 days may not be acceptable to some. Furthermore, if a woman does decide to remove her ring periodically, the timing and duration of removal will have different implications for contraceptive and HIV prevention effectiveness; for instance, removal during menses or for short durations may be more forgiving in terms of pregnancy versus HIV prevention. Identifying and dispelling any confusion about the potentially different levels of effectiveness for separate product indications, the relationship between adherence and effectiveness of each indication, or about potential side effects or other product characteristics associated with each indication could enhance acceptability and facilitate adherence during clinical trials and beyond.
Research suggests that products with multiple prevention indications (pregnancy and HIV prevention, or HIV and STI indications) are likely to have broad appeal (Terris-Prestholt et al., 2008; Morrow et al., 2007; Elias and Coggins, 2001; Woodsong and Koo, 1999). Nevertheless, potential users are also likely to value the separate indications of a MPT differently. For some women, the pregnancy indication may be of primary interest and the HIV indication a secondary advantage. For others, a key advantage may be the ability to reduce HIV risk, while negotiating use of a contraceptive product may be more difficult. Such preferences are likely to vary across geographic, sociocultural and sexual contexts. Furthermore, potential users' preferences vis-à-vis the multiple indications of a MPT may also influence their preferences for specific attributes. For example, in settings where HIV is a more stigmatizing condition than unintended pregnancy, it is possible to imagine that women who are especially interested in the HIV rather than pregnancy prevention indication of a MPT gel might prefer a product that could be used discreetly – perhaps with minimal change in vaginal wetness or the possibility of inserting many hours before a sexual event.
Finally, the demand for, delivery and use of new MPTs once introduced will be influenced by additional factors that are beyond user concerns; some warrant evaluation during the product development stage. For example, while understanding users' perspectives about new products and their specific characteristics is critical to improving the fit between the technology and use context, it is equally important to ensure that policy, programmatic and advocacy groups are “on-board” with the product. If these key stakeholders do not believe that the new method will contribute to their constituents' prevention needs, the financial, scientific and logistical efforts needed to bring the product to market may be hard to sustain. Even at this early stage, policymakers, program implementers, providers and others can provide critical insights into introduction-related barriers and facilitators, including potential service delivery channels, product-related communication needs, and pricing. However, such issues will require additional, on-going efforts as the product moves from early stage development through clinical trial testing to actual delivery.
4. Conclusions
Acceptability research has the potential to inform the product development process at various points along the continuum – from optimizing the physicochemical properties of a candidate product, its delivery vehicle and/or dosing regimens, to improving the implementation of clinical trials by identifying potential user populations, informational needs, or logistical barriers related to product use. This information may also identify the kinds of programmatic and policy issues that will require resolution, should a candidate product perform well in pre-clinical and clinical testing.
Contributor Information
Elizabeth E. Tolley, Email: btolley@fhi360.org.
Kathleen M. Morrow, Email: KMorrow@Lifespan.org.
Derek H. Owen, Email: dowen@fhi360.org.
References
- World Health Organization Media Centre. Fact Sheets. Geneva, Switzerland: 2013. Sexually Transmitted Infections (STIs) [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS World AIDS Day Report 2011. UNAIDS; Geneva, Switzerland: 2011. p. 52. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Fact Sheet. UNAIDS; New York: 2012. Adolescents, Young People and HIV; pp. 1–2. [Google Scholar]
- Guttmacher Institute IPPF. In Brief. Guttmacher Institute; New York: 2010. Facts on the Sexual and Reproductive Health Of Adolescent Women in the Developing World; pp. 1–4. [Google Scholar]
- Holt BY. Saving Lives With Multipurpose Prevention Technologies: Turning Ideas Into Solutions for Sexual and Reproductive Health. Seattle, Washington: 2010. p. 24. [Google Scholar]
- Tolley EE, Severy LJ. Integrating behavioral and social science research into microbicide clinical trials: challenges and opportunities. Am J Public Health. 2006;96(1):79–83. doi: 10.2105/AJPH.2004.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–283. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severy LJ, et al. A framework for examining the sustained acceptability of microbicides. AIDS Behav. 2005;9(1):121–131. doi: 10.1007/s10461-005-1687-y. [DOI] [PubMed] [Google Scholar]
- Morrow KM, Hendrix C. Clinical evaluation of microbicide formulations. Antiviral Res. 2010;88(Suppl 1):S40–S46. doi: 10.1016/j.antiviral.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodsong C, et al. Microbicide clinical trial adherence: insights for introduction. J Int AIDS Soc. 2013;16:18505. doi: 10.7448/IAS.16.1.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley E, et al. In: Socioeconomic and behavioral factors influencing choice, adherence and success of microbicide formulations, in drug delivery and development of anti-HIV microbicides. das Neves J, Sarmento B, editors. Pan Stanford Publishing; Singapore: 2014. [Google Scholar]
- DeVellis RF. Applied Social Research Methods Series. 2nd. Sage Publications, Inc; Thousand Oaks, California: 2003. Scale Development Theory and Applications. [Google Scholar]
- Gamst G, Meyers L, Guarino AJ, Burke HM. In: Scale development and validation, in public health research methods. Guest G, editor. Sage Publications, Inc; Thousand Oaks, CA: 2014. [Google Scholar]
- FHI 360. FHI 360 contraceptive development and family planning services. Degreeso Blog. 2013 [Google Scholar]
- Viney R, Lancsar E, Louviere J. Discrete choice experiments to measure consumer preferences for health and healthcare. Expert Rev Pharmacoecon Outcomes Res. 2002;2(4):319–326. doi: 10.1586/14737167.2.4.319. [DOI] [PubMed] [Google Scholar]
- Louviere JJ, Flynn TN, Carson RT. Discrete choice experiments are not conjoint analysis. J Choice Model. 2010;3(3):57–72. [Google Scholar]
- Lee SJ, et al. HIV vaccine acceptability among immigrant Thai residents in Los Angeles: a mixed-method approach. AIDS Care. 2008;20(10):1161–1168. doi: 10.1080/09540120701855375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris-Prestholt F, et al. International AIDS Economic Network. Cuernavaca, Mexico: 2008. Determinants of South African Women's Demand for New Barrier Methods and their Distribution: Analysis of a Discrete Choice Experiment. [Google Scholar]
- Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, Bekker L, Ganesh S, Young K, Romano J, Nel A, Woodsong C. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16(7):1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- Nel A, Kamupira M, Woodsong C, van der Straten A, Montgomery E, van Niekerk N, Nuttall J. Safety, Acceptability and Pharmacokinetic Assessment (Adherence) of Monthly Dapivirine Vaginal Rings (Ring-004) for HIV Prevention in CROI Seattle. International Partnership for Microbicides; Washington: 2012. [Google Scholar]
- Morrow KM, Fava JL, Rosen RK, Christensen AL, Vargas S, Barroso C, Vargas S, Barroso C, Christensen AL, Woodsong C, Severy L. Willingness to use microbicides is affected by the importance of product characteristics, use parameters, and protective properties. J Acquir Immune Defic Syndr. 2007;45(1):93–101. doi: 10.1097/QAI.0b013e3180415ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: where have we been? Where are we going? J Women's Health Gend Based Med. 2001;10(2):163–173. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- Woodsong C, Koo HP. Two good reasons: women's and men's perspectives on dual contraceptive use. Soc Sci Med. 1999;49(5):567–580. doi: 10.1016/s0277-9536(99)00060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


