Highlights
-
•
Cation Exchange Resins have been the mainstream treatment for chronic hyperkalemia.
-
•
In 1987 the first case series of uremic patients with colonic perforations associated with the use of sodium polystyrene sulfonate was reported.
-
•
The pathologic damage of Cation Exchange Resin in gastrointestinal tract goes from mucosal edema, ulcers, pseudomembranes, and the most severe transmural necrosis.
-
•
Surgeons must avoid therapies with intestinal osmotic challenge implication in patients presenting gastrointestinal adverse reactions derived from Cation Exchange Resins.
Keywords: Cation Exchange Resin, Colonic necrosis, Calcium polystyrene sulfonate
Abstract
Introduction
Since 1961 the use of Cation Exchange Resins has been the mainstream treatment for chronic hyperkalemia. For the past 25 years different kind of complications derived from its clinical use have been recognized, being the colonic necrosis the most feared and lethal of all.
Presentation of case
We report a case of a 72-year-old patient with chronic kidney disease, treated with calcium polystyrene sulfonate for hyperkalemia treatment who presented in the emergency department with constipation treated with hypertonic cathartics. With clinical deterioration 48 h later progressed with colonic necrosis requiring urgent laparotomy, sigmoidectomy and open abdomen management with subsequent rectal stump perforation and dead. The histopathology finding: calcium polystyrene sulfonate embedded in the mucosa, consistent with the cause of perforation.
Discussion
Lillemoe reported the first case series of five uremic patients with colonic perforation associated with the use of SPS in sorbitol in 1987 and in 2009 the FDA removed from the market the SPS containing 70% of sorbitol.
The pathophysiologic change of CER goes from mucosal edema, ulcers, pseudomembranes, and the most severe case transmural necrosis.
Up to present day, some authors have questioned the use of CER in the setting of lowering serum potassium. Despite its worldwide use in hyperkalemia settings, multiple studies have not demonstrated a significant potassium excretion by CER.
Conclusion
Despite the low incidence of colonic complication and lethal colonic necrosis associated with the CER clinical use, the general surgeon needs a high index of suspicion when dealing with patients treated with CER and abdominal pain.
1. Introduction
Hyperkalemia is a well known complication in patient with chronic kidney disease, its life threating if unrecognized and untreated, this pathophysiological entity is encounter by internists, intensivists, nephrologists an emergency department physicians [1].
Since 1961 the use of Cation Exchange Resins (CER) has been the mainstream of chronic hyperkalemia treatment [2]. For the past 25 years different kind of complications derived from its clinical use have been recognized, being the intestinal obstruction and colonic necrosis the most feared and lethal of all. In this setting, the general and acute care surgeons joined the specialists listed before in the emergency management of these patients. We present a review of CER along with the clinical case of a 74-year-old woman with chronic kidney disease treated with calcium polystyrene sulfonate (CPS) (Novefazol Probiomed, S.A de C.V. México) seen at emergency room with uremia and abdominal pain.
2. Case report
A 72-year-old-woman was admitted to the emergency department for uremic syndrome, hemodynamic instability and chronic abdominal pain associated with constipation for 2 weeks. She had a history of nephrectomy and chronic renal failure without replacement therapy of renal function and hypertension. She was treated with telmisartan–hydrochlorothiazide, amlodipine and furosemide.
Three weeks before the current admission she was on treatment for hyperkalemia with calcium polystyrene sulfonate PO 29.92 g daily.
At the emergency department she was treated with polyethylene glycol powder for oral solution 4 l at conventional dilution, and rectal enemas with buffered sodium phosphate solution for constipation treatment.
She was admitted to the ICU with metabolic acidosis and uremia exacerbation requiring hemodialysis in the next 48 h.
One day after the admission to the ICU the abdominal distension augmented. Abdominal CT shows free intraperitoneal air consistent with colonic perforation (Fig. 1).
Fig. 1.
Abdominal CT scan obtain in the second day of hospital stay in the ICU showing free intraperitoneal air (white arrow) consistent with colonic perforation.
The patient went to exploratory laparotomy where a perforation at the sigmoid colon was found (Fig. 2). A Hartmann procedure was performed and open abdomen protocol was initiated with Open Abdomen Negative Pressure Therapy with ABThera (KCI) in the need for a “second look” surgery. Forty-eight hours later, the rectal stump was found necrotic and a rectal resection on the superior third of rectum was perfomed.
Fig. 2.
Sigmoid colon. Transmural necrosis (white arrow).
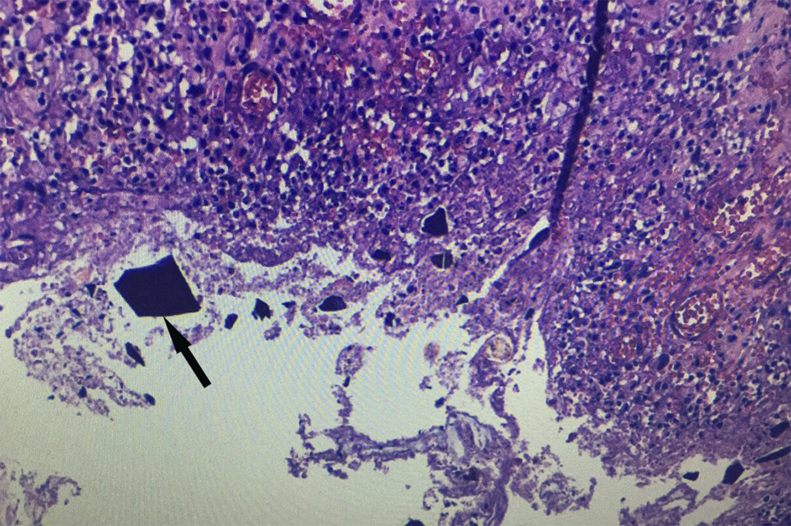
Microscopically examination revealed areas of transmural necrosis in sigmoid colon and rectal stump of heterogeneous distribution and acute peritonitis with small basophilic angulated crystals embedded in the mucosa (Fig. 3). There was no evidence of recent vascular thrombosis. The final diagnosis was colonic necrosis due to CPS associated with hypertonic cathartic use.
Fig. 3.
Sigmoidectomy specimen stained with Haematoxylin–Eosin. Small basophilic angulated crystals (black arrow) embedded in mucosa consisted with calcium polystyrene sulfonate related colonic perforation.
3. Discussion
The CER was first synthesized in 1935 and in late 1950s became available in the open market. Its hypokalemic properties were recognized until 1961, when its clinical use was first described. In 1975 it gained the US federal approval for hyperkalemia treatment [2]. The first CER used was the sodium polystyrene sulfonate (SPS). The complications derived from the sodium overload in patients with chronic kidney disease (CKD) led to the use of alternative salts containing Aluminum and Calcium instead.
Until today, there exists only two therapeutic approaches approved by FDA to eliminate potassium from the body besides hemodialysis: Diuretics and CER [3]. In an era where chronic hemodialysis was not available, different therapies were sought to deal with such a complex problem. The use of laxatives and cathartics in order to achieve potassium excretion is an historical example. Phenolphthalein and sorbitol were the main compounds used with such purpose. The combination of CER and sorbitol had its origins based on these grounds [4]. In alleviating constipation secondary to CER use, its combination with sorbitol gain popularity in 1997 [5].
In 1987 Lillemoe et al. first reported that sorbitol enema leads to intestinal complications, being the most severe the colonic necrosis. Their data suggested that the addition of sorbitol is an important factor for mucosal toxicity. In patients with concomitant use of kayexalate and sorbitol, only 0.27–1.8% developed intestinal injuries in the following 1–7 days [6]. In 2009 the FDA removed from the market the SPS containing 70% of sorbitol and a introduce black-box warning in 33% SPS in sorbitol solution pointing that is associated with colonic necrosis and serious gastrointestinal adverse reactions [7].
CER is currently administered orally or as an enema, acting in the large intestine by extracting and collecting potassium in the stools, so it can be eliminated, although the exact mechanism for this is still under debate [8]. Some authors believe that CER exerts its action directly in the ileal and colonic lumen binding potassium to the stools which is eliminated from the body. As an additional effect, CER can bind calcium ions resulting in fecal impaction and bowel obstruction. Other authors suggest a more complex mechanism: In the acid milieu of the stomach, CER’s cations are released from the resin binding hydrogen ions. In the large intestine, hydrogen ions are exchanged for potassium ions, which are eliminated in the stool [9]. From the second theory one could argue against enema administration, as CER require their transformation in the foregut before they can exchange potassium effectively in the mid and hindgut [10]. When SPS is given orally (30 g) or rectal (60 g), its hypokalemic effect and timing are variable and can take up to 10 h to achieve the desire hypokalemic effect. Most cases of colonic necrosis has been reported from hours to days after administration of SPS with doses ranging from 20 g to 60 g daily [11].
Sorbitol is the most used and FDA approved cathartic in conjunction with CER mixed in a 33% solutiol. It is metabolized by colonic bacteria into short-chain fatty acids. If the concentration of these acids exceeds the patient's absorption capacity, there is an osmotic entrance of fluid into the gastrointestinal lumen producing osmotic ischemia [12]. Hutchins’s work demonstrated that inoculated tissue with sodium polystyrene sulfonate leads to the development of an acute inflammatory reaction within 24 h, the production of inflammatory cytokines and prostaglandins may lead to further impairment in local hemodynamic mechanisms leading to vascular injury and subsequent mucosal injury [13].
The gastrointestinal adverse effects including colonic perforation have been documented in both type of resins sodium and calcium polystyrene sulfonate and both in the setting with sorbitol or alone [14]. The pathophysiologic change of mucosa exposed to CER ranges from mucosal edema, ulcers, pseudomembranes, and the most severe case transmural necrosis. CER can be seen in histopatological specimens; crystals detected microscopically can be diagnostic in colonic perforation of unknown origin. SPS crystals on Hematoxylin–Eosin stain, appear as basophilic polygonal crystals; they stain magenta on periodic acid–Schiff and with acid-fast stain [15]. CPS crystal appearance do not differ from its sodium counterpart [16].
At the beginning SPS was only associated with intestinal complications in the setting of patients with multiple comorbidities, such as advance renal disease, renal transplant patients, postsurgical patients and hypovolemic in which the intestinal transit was diminished and the time in which the resin and the intestinal mucosa contact is prolonged and activation of renin-angiotensin system cause vasospasm of the mesenteric vessels leading to a non obstructive mesenteric isquemia and intestinal necrosis. The true mechanism remains unclear [17]. sorbitol-free SPS still carry risk [18].
4. Conclusion
SPS has been extensively used for the treating hyperkalemia without the imprimatur of a randomized clinical trial regarding its efficacy and safety, SPS (oral or enema) either alone or with other therapy. It is often administered in the style of a cookbook recipe for all the degree of hyperkalemia.
Despite the low incidence of colonic complication and lethal colonic necrosis associated with the CER clinical use, the general surgeon needs a high index of suspicion when dealing with patients treated with CER and abdominal pain in a emergency care setting. Currently there is no data derived from prospective randomized clinical trials that could guide us in the setting of patients presenting constipated or perforated as a complication of the CER use, but our understanding of the pathophysiology has grown gradually as we have recognized the importance of this issue. No other cathartics besides sorbitol has been evaluated in conjunction with CER, but we should use basic clinical reasoning in order to treat such patients. The first thing that we should do is to stop the CER administration and start hemodynamic and metabolic resuscitation, correcting hydroelectolyte disturbances specifically to prevent gastrointestinal hypoperfusion that could lead to transmural necrosis. The use of other osmotic cathartics should also be avoided. In the setting of free perforation to abdomino-pelvic cavity, the surgeon must seek the removal of CER crystals from the peritoneum and the use of on-table colonic lavage could be used to resolve the crystal impaction. The creation of a primary anastomosis is ill-adviced and the need for surgical reexploration is a must as new intestinal perforations could arise from CER intraluminal or peritoneal remains.
As there is no other treatment for ambulatory hyperkalemia treatment, the surgeon needs a high index of suspicion for detecting and effectively treat the gastrointestinal complications due CER clinical use. New compounds for hyperkalemia treatment are being developed such as patiromer (formerly called RLY5016) and sodium zirconium cyclosilicate (ZS-9); none of them are currently approve by de FDA [19], [20].
Conflicts of interest
Nothing to declare.
Funding for your research
Nothing to declare.
Ethical approval
Nothing to declare.
Author contribution
Maria Rita Rodríguez Luna: Study design, data collection, writing.
Enrique Fernandez Rivera: Writing.
Joaquin E. Guarneros Zarate: Study design, data collection, writing.
Jorge Tuemer Izaguirre: Data collection, writing.
Roberto Hernandez Méndez: Data collection.
Guarantor
Maria Rita Rodríguez Luna.
Enrique Fernandez Rivera.
Joaquin E. Guarneros Zarate, Jorge Tuemer Izaguirre.
Roberto Hernandez Méndez.
References
- 1.Shingarev R., Allon M. A physiologic-based approach to the treatment of acute hyperkalemia. Am. J. Kidney Dis. 2010;56(3):578–584. doi: 10.1053/j.ajkd.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGowan C.E., Saha S., Chu G., Resnick M.B., Moss S.F. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South. Med. J. 2009;102(5):493–497. doi: 10.1097/SMJ.0b013e31819e8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen T., Ondrik D., Zhufyak O., To W., He S. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. J. Am. Acad. Physician Assist. 2015;28(3):41–45. doi: 10.1097/01.JAA.0000458856.92020.1e. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01720610-201503000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Kamel K.S., Schreiber M. Asking the question again: are cation exchange resins effective for the treatment of hyperkalemia? Nephrol. Dial. Transplant. 2012;27:4294–4297. doi: 10.1093/ndt/gfs293. [DOI] [PubMed] [Google Scholar]
- 5.Coogan P.F., Rosenberg L., Palmer J.R. Phenolphthalein laxatives and risk of cancer. J. Natl. Cancer Inst. 2000;92(23):1943–1944. doi: 10.1093/jnci/92.23.1943. [DOI] [PubMed] [Google Scholar]
- 6.K.D. Lillemoe, J.L. Romolo, S.R Hamilton, L.R. Pennington, J.F. Burdick, G.M. Williams. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. 1987 [cited 2015 July 4]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3824154. [PubMed]
- 7.Kayexalate - FDA prescribing information, side effects and uses. [cited 2015 July 18]. Available from: http://www.drugs.com/pro/kayexalate.html.
- 8.Albeldawi M., Gaur V., Weber L. Kayexalate-induced colonic ulcer. Gastroenterol. Rep. 2014;2:23510–23610. doi: 10.1093/gastro/gou011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charmot D. Non-systemic drugs: a critical review. Curr. Pharm. Des. 2012;18:1434–1445. doi: 10.2174/138161212799504858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandal S., Karachiwala H., Noviasky J., Wang D., Elliott W.C., Lehmann D.F. To bind or to let loose: effectiveness of sodium polystyrene sulfonate in decreasing serum potassium. Int. J. Nephrol. 2012;2012 doi: 10.1155/2012/940320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsey P.B., Chen S., Lauwers G.Y. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 37-2003. A 79-year-old man with coronary artery disease, peripheral vascular disease, end-stage renal disease, and abdominal pain and distention. N. Engl. J. Med. 2003;349(22):2147–2155. doi: 10.1056/NEJMcpc030031. [DOI] [PubMed] [Google Scholar]
- 12.Geboes K., De Hertogh G.D., Ectors N. Drug-induced pathology in the large intestine. Curr. Diagn. Pathol. 2006;12(May (4)):239–247. http://www.sciencedirect.com/science/article/pii/S096860530600055X. [Google Scholar]
- 13.Chou Y.H., Wang H.Y., Hsieh M.S. Colonic necrosis in a young patient receiving oral kayexalate in sorbitol: case report and literature review. Kaohsiung J. Med. Sci. 2011;27:155–158. doi: 10.1016/j.kjms.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi N., Nomura Y., Meda T., Iida M., Ohtsuka A., Naba K. Development of colonic perforation during calcium polystyrene sulfonate administration: a case report. Case Rep. Med. 2013;2013:102614. doi: 10.1155/2013/102614. http://www.ncbi.nlm.nih.gov/pubmed/24391670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatelain D., Brevet M., Manaouil D., Yzet T., Regimbeau J.M., Sevestre H. Rectal stenosis caused by foreign body reaction to sodium polystyrene sulfonate crystals (Kayexalate) Ann. Diagn. Pathol. 2007;11(3):217–219. doi: 10.1016/j.anndiagpath.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Kao C.-C., Tsai Y.-C., Chiang W.-C., Mao T.-L., Kao T.-W. Ileum and colon perforation following peritoneal dialysis-related peritonitis and high-dose calcium polystyrene sulfonate. J. Formos. Med. Assoc. 2013;(7):7–9. doi: 10.1016/j.jfma.2013.02.006. http://linkinghub.elsevier.com/retrieve/pii/S0929664613000910. [DOI] [PubMed] [Google Scholar]
- 17.Watson M.A., Baker T.P., Nguyen A. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am. J. Kidney Dis. 2012;60(3):409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Harel Z., Harel S., Shah P.S., Wald R., Perl J., Bell C.M. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am. J. Med. 2013;126(July (3)):e9–e24. doi: 10.1016/j.amjmed.2012.08.016. http://www.amjmed.com/article/S0002934312008698/fulltext. [DOI] [PubMed] [Google Scholar]
- 19.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N. Engl. J. Med. 2015;372(3):222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 20.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]