Abstract
Herein, we report total syntheses of the tetramethyldihydroxanthene natural product rhodomyrtosone B and the related bis-furan β-triketone natural product rhodomyrtosone A. Nickel-(II)-catalyzed 1,4-conjugate addition of an α-alkylidene-β-dicarbonyl substrate was developed to access the congener rhodomyrtosone B, and oxygenation of the same monoalkylidene derivative followed by cyclization was employed to obtain the bis-furan natural product rhodomyrtosone A.
INTRODUCTION
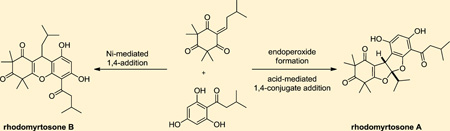
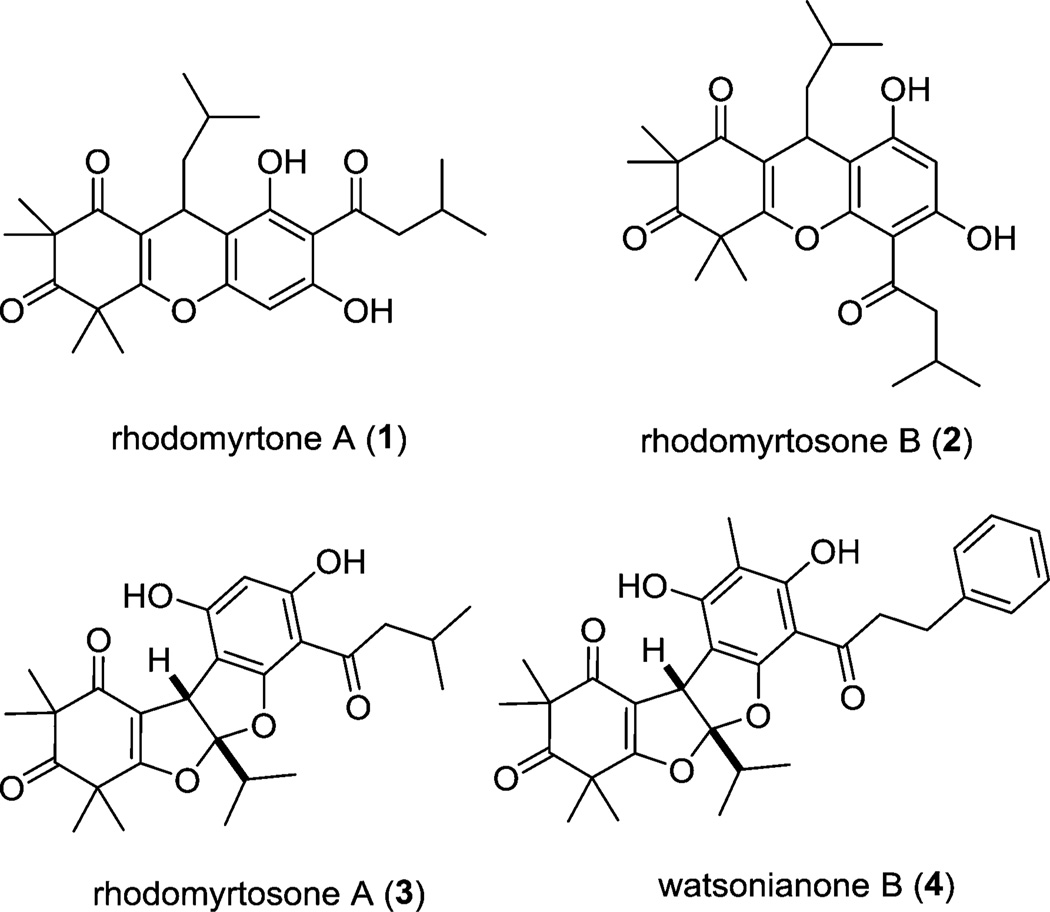
Rhodomyrtone A (1) and its isomer rhodomyrtosone B (2) are derived from the plant Rhodomyrtus Tomentosa found in Thailand (Figure 1).1,2 These natural products present interesting synthetic challenges due to the presence of a highly oxygenated β-triketone moiety fused to an acylphloroglucinol which is present in both isomers. In rhodomyrtone A (1), the ether linkage is para to the acyl group, while in rhodomyrtosone B (2), it is ortho. Rhodomyrtosone A (3) was also isolated from the Rhodomyrtus genus and possesses an intriguing bis-furan acylphloroglucinol core.2,3 Recently, the related natural product watsonianone B (4) was isolated from the plant Corymbia watsonia.4 Rhodomyrtone A (1) and rhodomyrtosone B (2) were found to be potent antibiotics against Gram-positive bacteria including Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), and several Streptococcus strains (MIC = 4 and 16 µg/mL, respectively).5 Watsonianone B (4) possesses antimalarial properties by inhibiting the growth of chloroquine sensitive (3D7) and resistant (Dd2) strains of Plasmodium falciparum displaying IC50 values of 0.44 and 0.29 µM, respectively.4 Accordingly, these interesting structures and highly relevant biological activities make rhodomyrtosones A (3) and B (2) appealing synthetic targets. The Maier laboratory6 recently achieved syntheses of compounds 1 and 2 adopting a similar strategy developed by Jauch and co-workers for the synthesis of myrtucommulone A.7 In this paper, we report a strategy involving nickel(II)-catalyzed 1,4-conjugate addition to an α-alkylidene-β-dicarbonyl substrate to selectively access rhodomyrtosone B (2) and oxygenation of the same monoalkylidene derivative to obtain the bis-furan congener rhodomyrtosone A (3).
Figure 1.
Rhodomyrtone A and related natural products.
RESULTS AND DISCUSSION
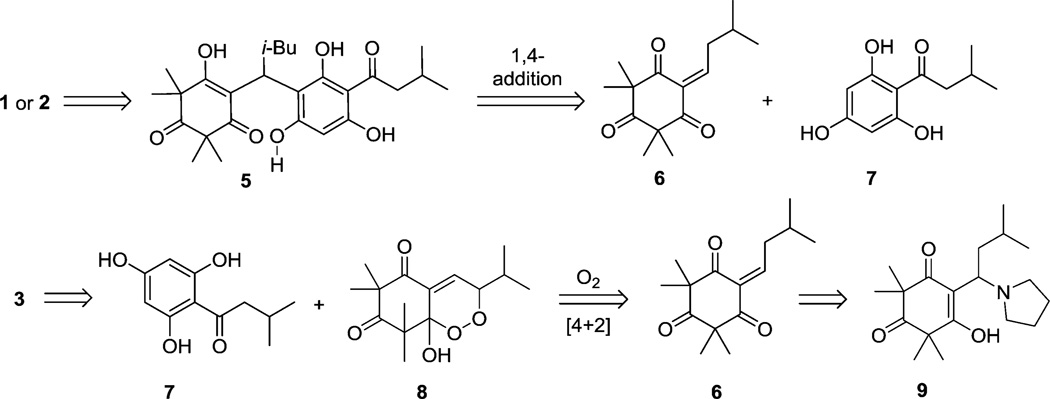
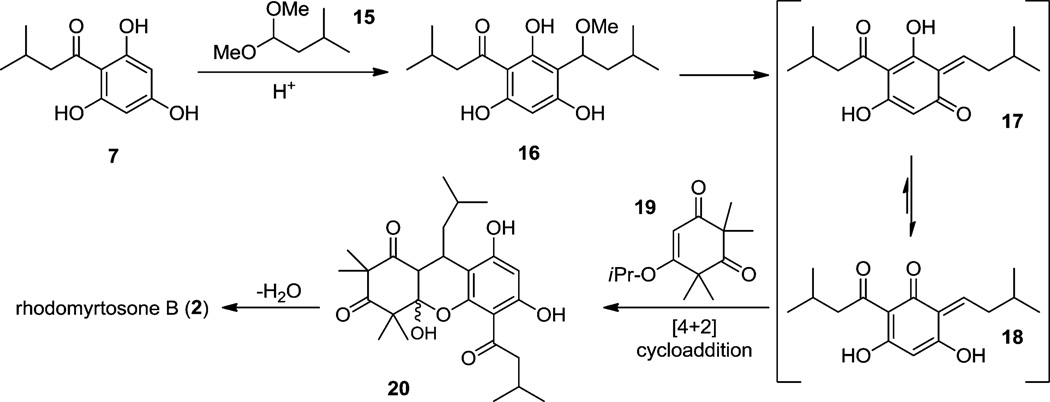
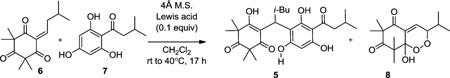
In our retrosynthetic analysis, we envisioned that rhodomyrtone A (1), rhodomyrtosone B (2), and rhodomyrtosone A (3) could be synthesized from a common starting material in a divergent manner (Figure 2). Selective dehydrative cyclizations of intermediate 5 could be used to access both rhodomyrtone A (1) and rhodomyrtosone B (2). Intermediate 5 may arise from conjugate addition of the known acylphloroglucinol 78 to monoalkylidene 6. In accordance with the proposed biosynthesis for rhodomyrtosone A,2 natural product 3 may be obtained from acylphloroglucinol 7 and endoperoxide 8 after bis-furan formation. Endoperoxide 8 may arise from [4 + 2] cycloaddition of oxygen with a dienol intermediate that may be obtained via photoenolization of monoalkylidene 6 (Figure 2).9
Figure 2.
Retrosynthetic analyses for rhodomyrtone A and rhodomyrtosone A.
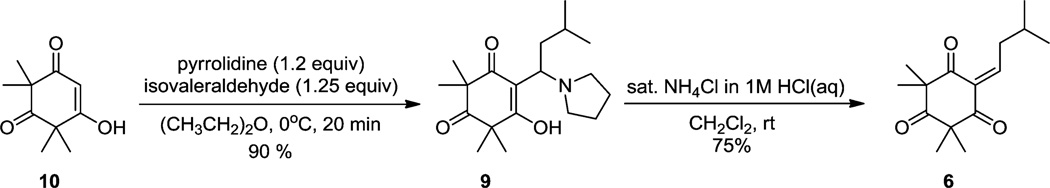
Our synthetic efforts began with the synthesis of monoalkylidene reaction partner 6 (Scheme 1). Treatment of syncarpic acid 10,6 isovaleraldehyde, and pyrrolidine (diethyl ether, 0 °C) afforded the Mannich product 9 (90%). Acid-mediated elimination of 9 cleanly afforded monoalkylidene 6 (75% yield). We next examined a range of catalysts for 1,4-conjugate addition10 of acylphloroglucinol 7 to enone 6. A reaction conducted without catalyst provided a 9% yield of adduct 5 along with a significant amount of the endoperoxide byproduct 8 (stereochemistry unassigned) (Table 1, entry 1). The latter compound may be derived from [4 + 2] cycloaddition between the dienol tautomer of 6 and triplet oxygen (Table 1, entry 1) (vide infra).11–13 In the presence of YbCl3 or FeCl3, a moderate amount of 5 was isolated, albeit in low selectivity (Table 1, entries 2 and 3). Rare-earth metal triflate catalysts were also investigated; however, these reactions resulted in low yields and selectivity (Table 1, entries 4–6).10a We also investigated metal perchlorate salts, which have previously been used in 1,4-conjugate additions.14 Cu(ClO4)2, Mg(ClO4)2, and Zn(ClO4)2 did not result in significant amounts of adduct 5 (Table 1, entries 7–9). Additionally, it was found that Pd (II) catalysis was not effective for this transformation (Table 1, entry 10). Ni(ClO4)2 proved to be an effective catalyst, affording a 4.6:1 ratio of compounds 5:8 in 74% isolated yield (Table 1, entry 11). Using a 5:1 mixture of CH2Cl2 and acetic acid as solvent led to an increased yield of the desired adduct 5 (Table 1, entry 12). Moreover, thorough degassing of solvent eliminated the production of endoperoxide 8 (Table 1, entry 13) and provided adduct 5 in 90% yield. A control screen using the same catalysts as listed in Table 1 with degassed CH2Cl2 was also conducted. Although degassing the solvent alleviated the formation of 8, the isolated yields remained low (Table 1, entries 2, 6–8) except when Ni(ClO4)2·6H2O was employed as catalyst. Based on the results highlighted in Table 1, Ni(ClO4)2·6H2O was identified as a promising catalyst for conjugate addition.15
Scheme 1.
Synthesis of a Monoalkylidene Reaction Partner
Table 1.
Evaluation of the Conjugate Addition under Lewis Acidic Conditions
 | |||
|---|---|---|---|
| entrya | Lewis acid | 5:8c | 5 (%)b |
| 1 | no catalyst | 1:2.7 | 9 |
| 2 | YbCl3 | 1.15:1 | 38 |
| 3 | FeCl3 | 1.3:1 | 36 |
| 4 | Yb(OTf)3 | 1:3.3 | 16 |
| 5 | Gd(OTf)3 | 1:2.4 | 29 |
| 6 | Lu(OTf)3 | 1:1.7 | 17 |
| 7 | Cu(ClO4)2 | 1:4.3 | 6 |
| 8 | Mg(ClO4)2 | N/A | 0 |
| 9 | Zn(ClO4)2 | N/A | 0 |
| 10 | PdCl2(PhCN)2 | N/A | 19 |
| 11 | Ni(ClO4)2·6H2O | 4.6:1 | 74 |
| 12 | Ni(ClO4)2·6H2O (7 mol %) CH2Cl2:AcOH (6:1) | 2.8:1 | 80 |
| 13d | Ni(ClO4)2·6H2O (7 mol %) CH2Cl2:AcOH (6:1) | 1:0 | 90 |
Reactions conducted with monoalkylidene 6 (1 equiv) and acylphloroglucinol 7 (1.5 equiv) in 1 mL of CH2Cl2.
Yields reported after isolation by silica gel column chromatography.
Compound 8 was the only byproduct observed after complete consumption of starting materials. Ratio was determined after isolating both products.
Solvents were thoroughly degassed using the freeze–pump–thaw method.
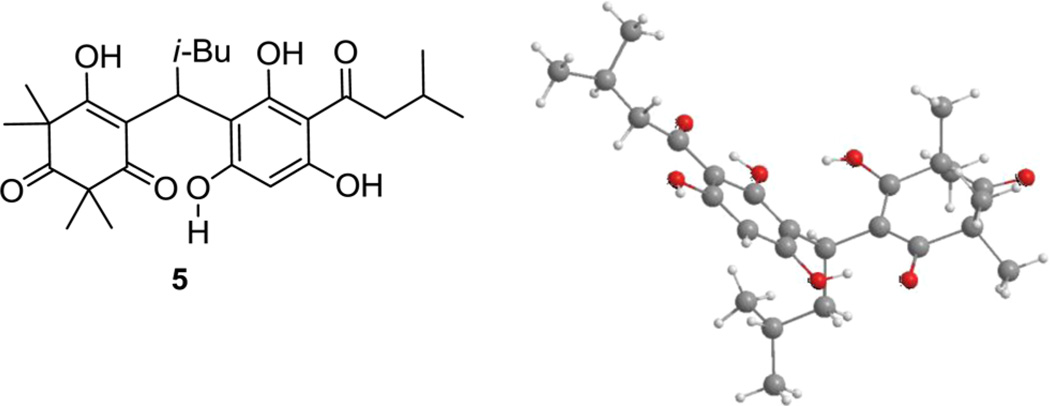
Upon characterization by 1H NMR analysis in CDCl3, we observed that the adduct 5 existed as a mixture of two atropisomers in a 2.4:1 ratio at room temperature. The tight hydrogen-bond network existing within the structure may hinder free rotation generating two rotational conformers in equilibrium at room temperature (Figure 3).16 Variable-temperature NMR studies were performed to determine the coalescence temperature for 5 that was found to be 60 °C, which corresponds to a rotational barrier of 15 kcal/mol.17 A single X-ray crystal structure analysis of compound 5 was obtained which further highlights the strong internal hydrogen-bond network between the acylphloroglucinol and vinylogous acid segments (Figure 3).
Figure 3.
X-ray crystal structure of adduct 5.
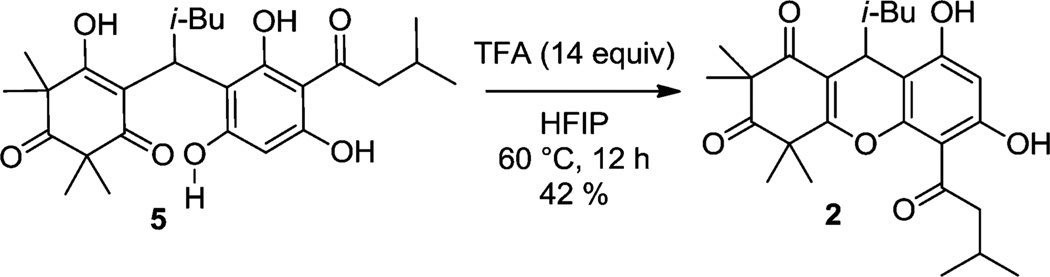
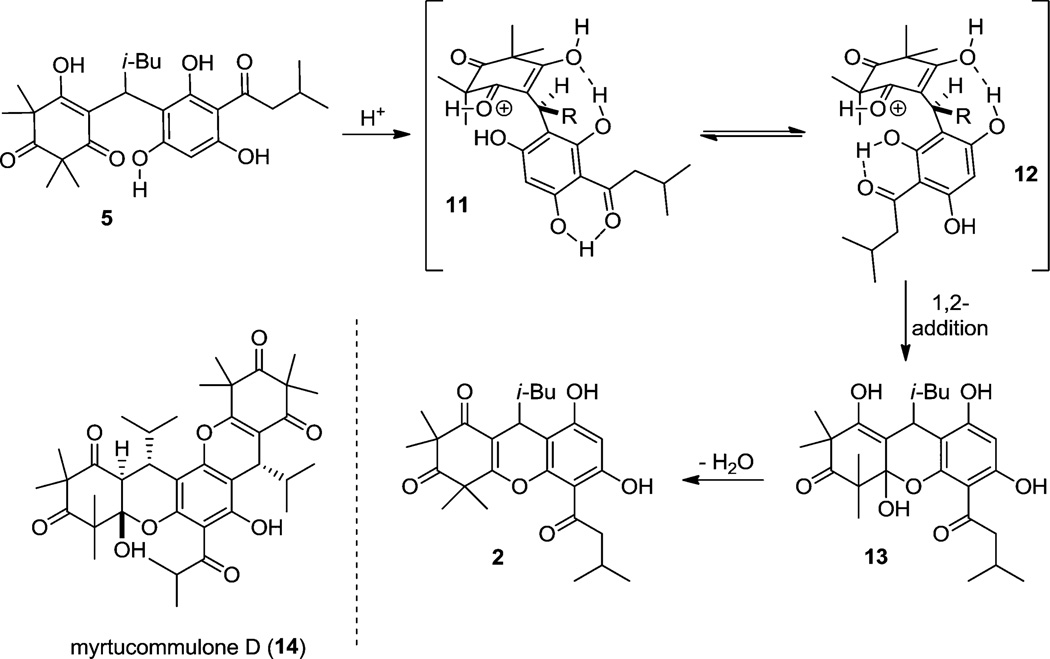
We anticipated that adduct 5 could be utilized to achieve dehydrative cyclizations to afford natural products 1 and 2 (vida supra). Under acidic conditions, we observed exclusive formation of rhodomyrtosone B 2 via dehydrative cyclization of the phenol ortho to the acyl group (Scheme 2).6 After considerable optimization, it was found that treatment of 5 in the carbocation-stabilizing protic solvent hexafluoroisopropanol (HFIP)18 with added trifluoroacetic acid (60 °C, 12 h) afforded rhodomyrtosone B (2) in 42% yield (Scheme 3). Our proposed mechanism for selective dehydrative cyclization leading to rhodomyrtosone B (2) is shown in Scheme 3. Protonation of vinylogous acid 5 leads to the vinyl oxocarbenium intermediate 11 which may exist in equilibrium with its atropisomer 12. We believe that hydrogen bonding between the ortho-phenol and the ketone within the acyl substituent renders the ortho-phenol more nucleophilic by increasing the electron density of the phenolic oxygen.19 Cyclization of 12 to hemiacetal 13 followed by dehydration affords rhodomyrtosone B (2). Hemiacetal 13 is likely a relevant intermediate in the proposed mechanism as this structural motif exists in closely related natural products including myrtucommulone D (14) (Scheme 4).20,21
Scheme 2.
Dehydrative Cyclization of 5 to 2
Scheme 3.
Model for Selective Formation of 2
Scheme 4.
Quinone Methide Annulation Strategy towards 2
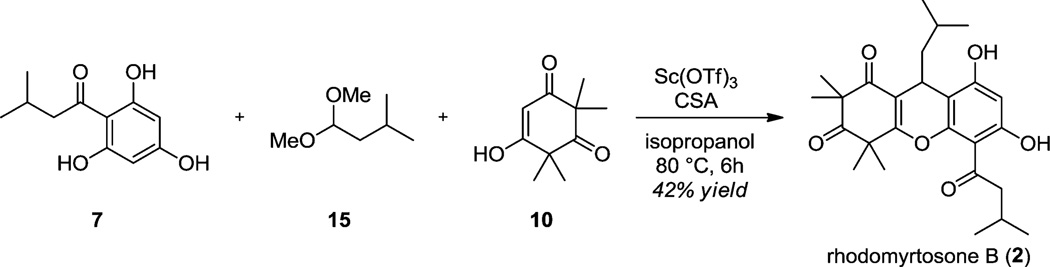
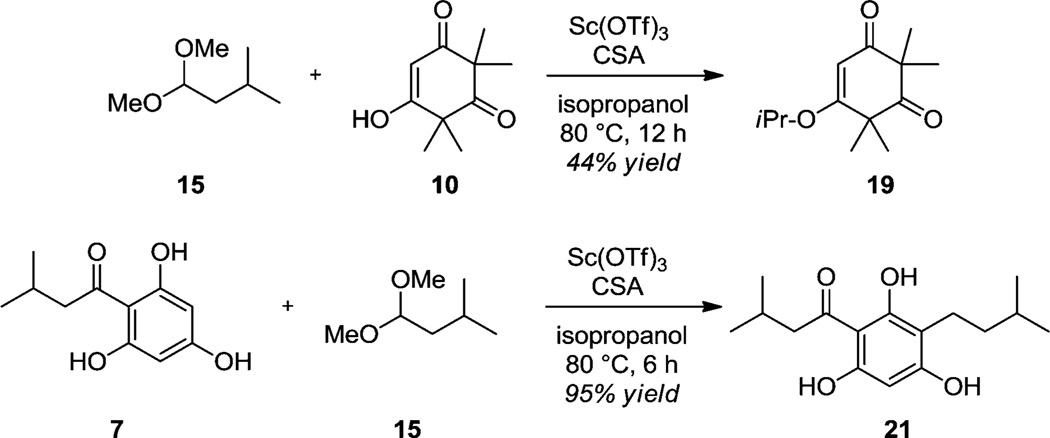
In line with recent literature describing asymmetric annulations between quinone methides and cyclic 1,3-dicarbonyls,22,23 we also explored an alternative three-component coupling approach toward the synthesis of rhodomyrtosone B (2) (Scheme 4). We found that treatment of a mixture of acylphloroglucinol 7, dimethyl acetal 15, and syncarpic acid 10 with 20 mol % of scandium (III) triflate and camphorsulfonic acid (CSA) in refluxing isopropanol generated the natural product 2 in a single step. While the reaction still proceeds if only a Lewis acid or a Brønsted acid was used, a modest increase in yield was observed by using both in a cooperative manner. A plausible reaction mechanism is proposed in Scheme 5. Acylphloroglucinol 7 first condenses with dimethyl acetal 15 derived from isovaleraldehyde to generate the benzylic methyl ether 16. In the presence of acid and heat, the methyl ether may be extruded to produce quinone methides 17/18. We believe quinone methide 18, likely activated by complexation with Sc(OTf)3, is a more relevant tautomeric form as its increased conjugation affords greater stability relative to 17. Quinone methide 18 then engages with vinylogous ester 19 (derived from the condensation of syncarpic acid 10 and isopropanol)24 thereby forming the tricyclic acetal 20 which subsequently loses isopropanol to afford 2. It is possible that the annulations proceed via cycloaddition; however, literature reports on quinone methide annulations also propose stepwise mechanisms.26,27
Scheme 5.
Proposed Mechanism for Formation of 2 via Quinone Methides 17/18
Alternatively, we considered that syncarpic acid (10) may be acting as the relevant nucleophile and may condense with the oxocarbenium to form monoalkylidene 6, similar to our previous approach (cf. Table 1). Using the reaction conditions shown in Scheme 4, we tested this possibility by running two control experiments: one without the acylphloroglucinol 7 and one without syncarpic acid (10) (Scheme 6). We found that in the absence of 7, syncarpic acid (10) was quickly converted to vinylogous ester 19, but minimal condensation was observed. On the other hand, in the absence of syncarpic acid (10), acylphloroglucinol 7 completely reacted with dimethyl acetal 15 to form the quinone methide (cf. 17 in Scheme 5). The quinone methide was subsequently reduced25 in the presence of Lewis acid and isopropanol, cleanly affording the alkylated phenol 21 which could be isolated in excellent yield. The latter reaction conditions represent a promising and mild reductive transformation for alkylation of electron-rich arenes. These control experiments strongly support a mechanism for the three-component coupling (cf. Scheme 4) in which quinone methide formation precedes the annulation step.
Scheme 6.
Control Experiments Probing the Mechanism of the Three-Component Coupling
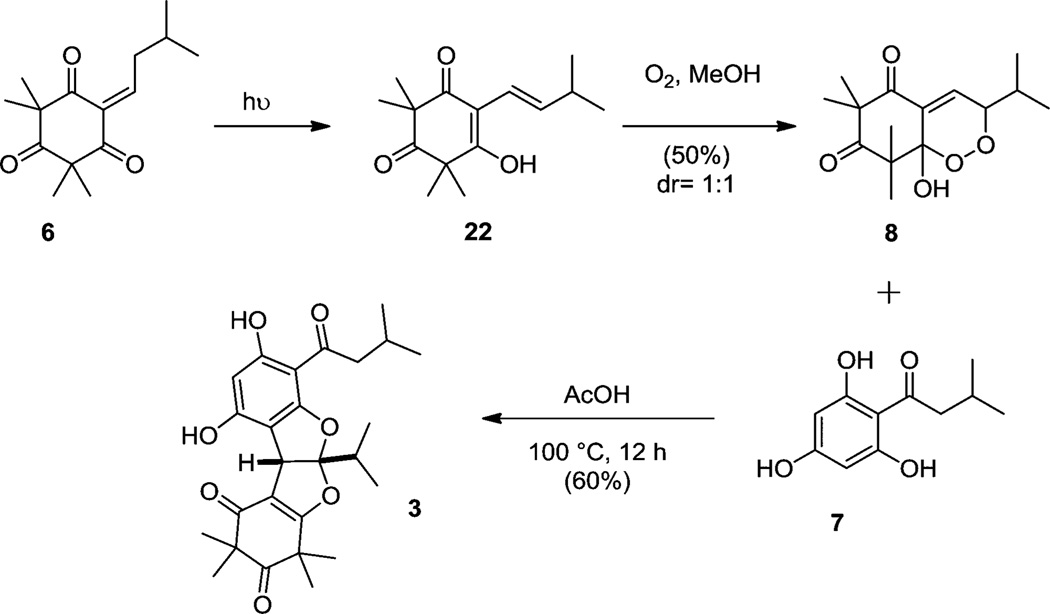
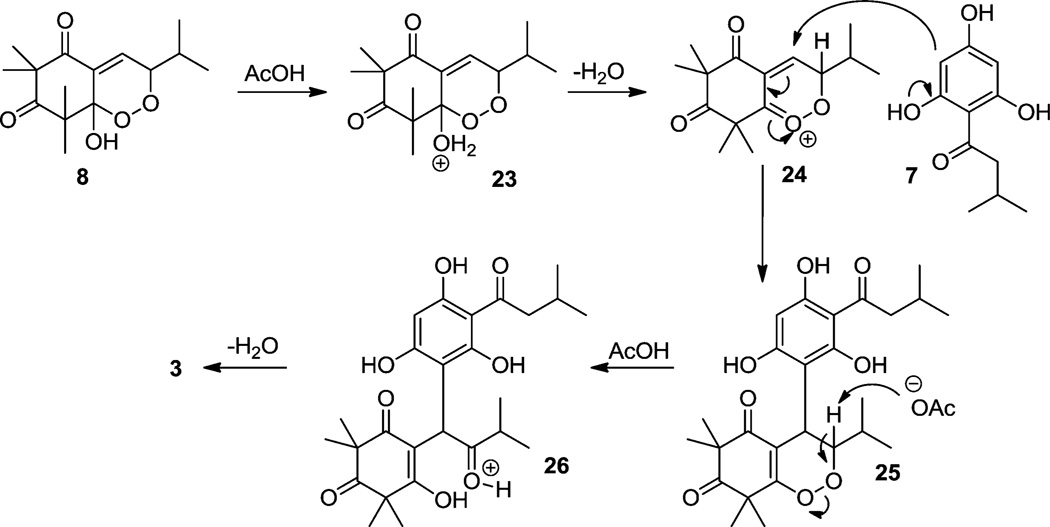
After successfully synthesizing rhodomyrtosone B (2), we next turned our attention to the synthesis of rhodomyrtosone A (3). Specifically, we wished to prepare endoperoxide 8 as the penultimate intermediate in the synthesis of 3 (Scheme 7). This was achieved using flow photochemistry26 employing mono-alkylidene 6 using oxygen-saturated methanol as solvent to provide a 50% yield of endoperoxide27 8 as a mixture of diastereomers. The stereochemistry of endoperoxide 8 was not assigned due to its labile nature and as its configuration was inconsequential for the subsequent cyclization step. Endoperoxide 8 and acylphloroglucinol 7 were subsequently thermolyzed in acetic acid (100 °C) which afforded the bis-furan rhodomyrtosone A (3) in 60% yield. A proposed mechanism for the acid-mediated formation of 3 is shown in Scheme 8. Treatment of peroxy acetal 8 with acid promotes the formation of the endocyclic peroxycarbenium ion 2428 via protonated intermediate 23. Interception of the putative peroxocarbenium intermediate 23 with acylphloroglucinol 7 may generate endoperoxide adduct 25. This peroxide may then undergo a Kornblum–DeLaMare29-type opening to 1,4-diketone 26 which can further cyclize to bis-furan 3 under acidic conditions. It should be noted that the reaction sequence may also formally be reversed in which endocyclic peroxide cleavage may occur prior to nucleophilic addition of the acylphloroglucinol.
Scheme 7.
Synthesis of 3 via Endoperoxide 8
Scheme 8.
Proposed Mechanism for the Formation of 3
CONCLUSION
In summary, we have developed a Ni (II)-mediated 1,4-addition of acylphloroglucinols to a syncarpic acid-derived alkylidene as a Michael acceptor to access the natural product rhodomyrtosone B after selective dehydrative cyclization. A three-component coupling strategy using a quinone methide intermediate was also found to be effective. In addition, oxygenation of a dienol derived from the same alkylidene scaffold permitted access to an endoperoxide intermediate which was utilized in a short synthesis of the bis-furan natural product rhodomytosone A. Further studies on the synthesis and biological properties of rhodomyrtone A and related molecules are currently in progress and will be reported in due course.
EXPERIMENTAL SECTION
General Methods
1H NMR spectra were recorded at either at 400 or 500 MHz (as noted) at ambient temperature with CDCl3 as the solvent unless otherwise stated. 13C NMR spectra were recorded either at 100.0 or 125.0 MHz (as noted) at ambient temperature with complete proton decoupling using CDCl3 as the solvent unless otherwise stated. Infrared spectra were recorded on a FT-IR spectrophotometer. High-resolution mass spectra (HRMS) was carried out with electrospray ionization (ESI) using a Q-TOF mass spectrometer. Melting points were recorded on a Mel-temp apparatus. Analytical thin-layer chromatography (TLC) was performed using 0.25 mm silica gel 60-F plates. Preparative TLC was conducted with glass backed 1000 µm silica gel 60-F plates. Flash chromatography was performed using 200–400 mesh silica gel. Photochemistry experiments were performed using a Hanovia 450 W medium pressure mercury lamp housed in quartz immersion cooler with a system circulator. Analytical LC-MS was performed on a UPLC (Ultra Performance Liquid Chromatography) with a Binary solvent manager, SQ mass spectrometer, PhotoDiode Array detector, and ELSD (Evaporative Light Scattering Detector). An Acquity UPLC BEH C18 1.7 µm column was used for analytical UPLC-MS. Yields refer to chromatographically and spectroscopically pure materials, unless otherwise stated. All reactions were carried out in flame-dried glassware under an argon atmosphere unless otherwise noted. Syncarpic acid (10)30 and acylphloroglucinol 731,32 were prepared using literature procedures.
5-Hydroxy-2,2,6,6-tetramethyl-4-(3-methyl-1-(pyrrolidin-1-yl)-butyl)cyclohex-4-ene-1,3-dione (9)
To a flask was added syncarpic acid 10, (500 mg, 3 mmol, 1 equiv) and anhydrous diethyl ether (45 mL). The resulting solution was cooled to 0 °C, and pyrrolidine (275 µL, 3.29 mmol, 1.2 equiv) was added dropwise followed by the addition of isovaleraldehyde (370 µL, 3.43 mmol, 1.25 equiv). The solution became slightly cloudy. The reaction was stirred at 0 °C until a white precipitate was formed (about 30 min). The white solid was filtered and washed with cold ether and dried in vacuo to yield 9 as a white powder (800 mg, 90%). Rf: 0.44 hexanes:EtOAc:MeOH (1:1:0.1). Mp: 165–168 °C (diethyl ether), IR (thin film): 2974.01, 1698.89, 1583.04, 1470.19, 1455.19, 1410.05, 1366.04, 1296.99, 1215.98 cm−1, 1H NMR (CDCl3, 500 MHz): δ 4.43 (d, J = 9.9 Hz, 1 H); 3.66–3.51 (ddd, J = 2.70, 5.98, 10.44, 1H); 3.07–2.88 (m, 1 H); 2.89–2.71 (m, 1H); 2.23–2.08 (m, 1H); 2.08–1.86 (m, 5H); 1.53–1.34 (m, 2H); 1.34–1.22 (m,12 H); 0.96–0.87 (m, 4 H); 0.87–0.75 (m, 3H) 13C NMR (CDCl3, 125.67 MHz): δ 216.9, 98.7, 69.4, 54.1, 48.9, 32., 25.3, 24.5, 22.6, 20.4, 17.5 ppm HRMS-ESI (m/z): [M + H]+ calculated for C19H32NO3, 322.2382; found, 322.2384
2,2,4,4-Tetramethyl-6-(3-methylbutylidene)cyclohexane-1,3,5-trione (6)
To a flask was added adduct 9 (200 mg, 0.62 mmol), and then methylene chloride was added (15 mL, 0.04 M). A 1 M solution of hydrochloric acid in water was prepared and saturated with ammonium chloride. This solution was added to the reaction mixture. The mixture was vigorously stirred at room temperature for 1h. The organic layer was washed with saturated brine (3×), and the organic fractions were dried over anhydrous sodium sulfate. Solvents were concentrated in vacuo to yield compound 6 (116 mg, 75%) as a pale yellow oil. Rf: 0.6 hexanes:EtOAc (3:1), IR (thin film): 2965.63, 1695.43, 1606.38, 1465.51, 1383.58, 1296.07, 1215.98, 1132.50, 1039.52 cm−1, 1H NMR (CDCl3, 500 MHz): δ 7.52 (t, J = 7.6 Hz, 1H), 2.61 (t, J = 7.3 Hz, 2H), 1.89 (ddt, J = 13.5, 10.8, 6.8 Hz, 1H), 1.31–1.23 (m, 12 H); 0.97 (d, J = 6.7 Hz, 6H) 13C NMR (CDCl3, 125.67 MHz): δ 208.9, 199.5, 196.4, 159.1, 133.1, 58.5, 57.9, 38.9, 35.6, 28.7, 22.5, 22.3, 21.9, HRMS-ESI (m/z): [M + H]+ calculated for C15H23O3, 251.1647; found, 251.1647
5-Hydroxy-2,2,6,6-tetramethyl-4-(3-methyl-1-(2,4,6-trihydroxy-3-(3-methylbutanoyl)phenyl)butyl)cyclohex-4-ene-1,3-dione (5)
The dichloromethane and acetic acid used in this reaction were degassed three times using the freeze–pump–thaw method. To the acyl phloroglucinol 7 (25 mg, 0.12 mmol, 1.5 equiv) was added Ni(ClO4)2·6H2O (2.9 mg, 0.0080 mmol, 0.1 equiv) and methylene chloride (2 mL) at room temperature under argon followed by addition of 4 Å MS (20 mg). Next, a solution of monoalkylidene 6 (20 mg, 0.08 mmol, 1 equiv) in dichloromethane (1 mL) was added to the reaction mixture followed by acetic acid (0.5 mL). The reaction was stirred at room temperature for 5 h and heated to 40 °C for 12 h. The reaction was quenched with water and a solution of 1 M KHSO4 at 0 °C until reaching a pH ≈ 2. The reaction mixture was extracted with CH2Cl2 and washed with saturated brine. Organic fractions were gathered and dried over anhydrous sodium sulfate. Solvents were evaporated in vacuo yielding a yellow oil. Column chromatography purification on silica gel with a gradient of CH2Cl2: MeOH (90:1 to 20:1) provided 29 mg (0.06 mmol) of compound 5 in 80% yield. Mp: 51–54 °C (hexanes, MeOH), IR (thin film): 2958.19, 2872.19, 1716.58, 1622.77, 1594.68, 1467.34, 1383.88, 1367.29, 1300.61, 1215.23, 1118.62, 754.18 cm−1, 1H NMR (CDCl3, 500 MHz): δ 0.83 (q, J = 5 Hz, 6H), 0.97 (d, J = 5 Hz, 6H), 1.23 (s, 3H), 1.31 (d, J = 5 Hz, 3H), 1.36 (d, J = 5 Hz, 3H), 1.42 (broad m, 1H), 1.47 (s, 3H), 1.75 (m, J = 10 Hz, 1H), 2.06 (m, J = 10 Hz, 1H), 2.25 (m, J = 10 Hz, 1H), 2.95 (d, J = 5 Hz, 2H), 4.34 (t, J = 10 Hz, 1H), 5.86 (s, 2/3H) and 5.92 (s, 1/3H), 10.28 (s, 2/3H) and 10.55 (s, 1/3H); 11.15 (s, 1/3H) and 11.57 (s, 2/3H), 13C NMR (CDCl3, 125.67 MHz): δ 22.5, 22.8, 23.1, 24.5, 25.5, 26.4, 27.1, 28.2, 29.6, 29.9, 38.4, 48.9, 52.4, 55.3, 98.2, 109.5, 114.9, 158.6, 176.8, 203.4, 206.4, 212.5, HRMS-ESI (m/z): [M + H]+ calculated for C26H37O7, 461.2539; found, 461.2534.
Rhodomyrtosone B (2) from Compound 5
To a solution of compound 5 (20 mg, 0.043 mmol, 1 equiv) in hexafluoroisopropanol (0.2 mL) was added trifluoroacetic acid (0.05 mL, 14 equiv) at room temperature. The reaction was stirred at 60 °C for 12 h. Then, the reaction was diluted with water and saturated brine, and the mixture was extracted with ethyl acetate (3 × 1 mL). The organic fractions were dried over anhydrous sodium sulfate, and solvents were evaporated in vacuo to yield a yellow solid. Purification using column chromatography on silica gel with a hexane:acetone gradient (12:1 to 4:1) provided 8 mg (0.018 mmol) of rhodomyrtosone B 2 (42% yield). Rf: 0.4 hexanes: acetone (3:1), Mp: 58–62 °C IR (thin film): 3359.86, 2959.21, 2925.37, 2869.81, 1719.44, 1656.43, 1625.75, 1597.94, 1504.38, 1469.46, 1431.80, 1388.20, 1368.12, 1255.28, 1161.24, 1121.96, 1041.55, 1015.89 cm−1, 1H NMR (CDCl3, 500 MHz): δ 13.46 (s, OH), 6.26 (s, OH), 4.30 (t, J = 6.1 Hz, 1H), 3.24–2.89 (m, 2H), 2.36 (dd, J = 13.2, 6.6 Hz, 1H), 1.64 (s, 3H), 1.47 (s, 3H), 1.43 (s, 3H), 1.39 (s, 3H), 1.39–1.31 (m, 4H), 1.02 (dd, J = 14.3, 6.6 Hz, 6H), 0.87 (dd, J = 8.5, 6.4 Hz, 6H), 13C NMR (CDCl3, 125.67 MHz): δ 211.7, 203.9, 198.3, 167.2, 164.2, 159.5, 153.1, 114.5, 105.9, 105.5, 100.2, 56.1, 53.4, 47.2, 46.9, 25.3, 25.0, 24.7, 24.7, 24.5, 24.4, 24.2, 23.4, 23.1, 22.9, 22.6, HRMS-ESI (m/z): [M + H]+ calculated for C26H35O6 443.2434; found, 443.2427
Rhodomyrtosone B (2) (Three-Component Reaction)
Acylphloroglucinol 7 (25 mg, 0.119 mmol), dimethyl acetal 15 (16 mg, 0.119 mmol), and syncarpic acid 10 (22 mg, 0.119 mmol) were suspended in dry isopropanol (1 mL). Camphorsulfonic acid (6 mg, 0.2 equiv) was added followed by the addition of scandium(III) triflate (12 mg, 0.2 equiv). The mixture was heated to 80 °C for 6 h and was then cooled to room temperature. The reaction was quenched with saturated sodium bicarbonate solution (3 mL) and diluted with ethyl acetate (5 mL). The organic phase was removed, and the aqueous phase was extracted with ethyl acetate (3 × 5 mL). The combined organic layers were washed with brine and dried over sodium sulfate before being concentrated under vacuum. The resulting crude oil was then purified by silica gel chromatography eluting with 10% ethyl acetate in hexanes to provide compound 2 (23 mg, 42% yield).
5-Isopropoxy-2,2,6,6-tetramethylcyclohex-4-ene-1,3-dione (19)
Syncarpic acid 10 (22 mg, 0.119 mmol) and dimethyl acetal 15 (16 mg, 0.119 mmol) were suspended in dry isopropanol (1 mL). Camphorsulfonic acid (6 mg, 0.2 equiv) was added followed by the addition of scandium(III) triflate (12 mg, 0.2 equiv). The solution was then heated to 80 °C for 12 h. The reaction mixture was then cooled to room temperature and quenched with sodium bicarbonate solution (3 mL) and diluted with ethyl acetate (5 mL). The organic phase was removed, and the aqueous phase was extracted with ethyl acetate (3 × 5 mL). The combined organic layers were washed with brine and dried over sodium sulfate before being concentrated under vacuum. The crude oil was purified by silica gel chromatography yielding compound 19 as a clear liquid (12 mg, 44% yield). IR (thin film): 2980.8, 2928.6, 1714.6, 1653.8, 1604.1, 1471.0, 1378.2, 1236.1, 1178.4, 1106.4,1042.7, 926.3 cm−1; 1H NMR (CDCl3, 500 MHz): δ 5.41 (s, 1H), 4.47 (sep, J = 6.1 Hz, 1H), 1.34–1.26 (m, 18H); 13C NMR (CDCl3, 100 MHz): δ 213.4, 199.3, 176.4, 99.6, 71.3, 55.0, 48.0, 24.9, 24.2, 21.1 ; HRMS-ESI (m/z): [M + H]+ calculated for C13H21O3; 225.1485 found, 225.1483
3-Methyl-1-(2,4,6-trihydroxy-3-isopentylphenyl)butan-1-one (21)
Acylphloroglucinol 7 (25 mg, 0.119 mmol) and dimethyl acetal 15 (16 mg, 0.119 mmol) were suspended in dry isopropanol (1 mL). Camphorsulfonic acid (6 mg, 0.2 equiv) was added followed by the addition of scandium(III) triflate (12 mg, 0.2 equiv). The mixture was heated to 80 °C for 6 h and was then cooled to room temperature. The reaction was then quenched with saturated sodium bicarbonate solution (3 mL) and diluted with ethyl acetate (5 mL). The organic phase was removed, and the aqueous phase was extracted with ethyl acetate (3 × 5 mL). The combined organic layers were washed with brine and dried over sodium sulfate before being concentrated under vacuum. The resulting crude oil was then purified on silica using 10% ethyl acetate in hexanes to provide compound 21, which was a white oil (32 mg, 95% yield). IR(thin film): 2955.8, 2560 (br), 1609.4, 1579.8, 1522.9, 1301.6, 1214.9, 1094.9 cm−1; 1H NMR (methanol-d4, 500 MHz): δ 5.90 (s, 1H), 2.94 (d, J = 6.8 Hz, 2H), 2.56–2.49 (m, 2H), 2.24 (dt, J = 13.4, 6.7 Hz, 1H), 1.57 (dt, J = 13.2, 6.6 Hz, 1 H), 1.40–1.32 (m, 2H), 0.97 (dd, J = 12.2, 6.6 Hz, 13 H); 13C NMR (MeOD, 100 MHz): δ 207.9, 166.0, 164.6, 162.1, 110.0, 106.3, 95.7, 54.7, 40.2, 30.3, 27.7, 24.1, 24.0, 22.0 ; HRMS-ESI (m/z): [M + H]+ calculated for C16H25O4; 281.1747 found, 281.1759
8a-Hydroxy-3-isopropyl-6,6,8,8-tetramethyl-8,8a-dihydrobenzo-[c][1,2]dioxine-5,7(3H,6H)-dione (8)
A 0.01 M solution of alkylidene 6 (80 mg, 0.32 mmol) was prepared in 32 mL of methanol in a flamed dried flask under argon at room temperature. A balloon of oxygen was bubbled through the solution for 30 min. The reaction flask with a balloon of oxygen bubbling through was connected to the flow setup. This setup employed Idex Health Science PFA (perfluoroalkoxyalkane) tubing (ID 0.062 in, OD 0.125 in, 500 psi max pressure) looped tightly around the Hanovia lamp which was then placed in a chiller. The reactor was 1.614 m in length, and the total volume was 3.1 mL. A recirculating chiller maintained the flow reactor at −10 °C, and a fan ensured proper air circulation in the photobox to maintain the overall temperature of the reaction mixture in the system at 23 °C. The optimal flow rate determined and used was 0.5 mL/min. After the entire solution was consumed, the system was flushed with an additional 5 mL of methanol, and the receiving flask was replaced by a waste container. Irradiation was stopped, and the entire flow setup was flushed with benzene. The solution contained in the receiving flask was collected and the solvent was evaporated. Purification using column chromatography over silica gel using a gradient of hexanes:acetone (20:1 to 10:1) provided 45 mg (50%) of endoperoxides 8 as a 1:1 mixture or diastereomers (stereochemistry unassigned).
Diastereomer 1: Rf: 0.67 hexanes:EtOAc (2:1), Mp: 115–120 °C (CH2Cl2, MeOH), IR (thin film): 3408.97, 2975.64, 1726.15, 1690.59, 1633.86, 1469.19, 1377.38, 1286.22, 1216.22, 1159.82.11, 1100.16 cm-1, 1H NMR (500 MHz, chloroform-d) δ 7.29 (d, J = 1.6 Hz, 1H), 4.74 (dd, J = 5.9, 1.6 Hz, 1H), 3.51 (s, 1H), 2.03 (dt, J = 13.4, 6.8 Hz, 1H), 1.38 (d, J = 8.1 Hz, 7H), 1.31 (s, 3H), 1.06 (dd, J = 6.8, 1.4 Hz, 7H), 1.03 (s, 3H). 13C NMR (126 MHz, cdcl3) δ 210.5, 197.5, 137.9, 134.3, 97.9, 83.5, 54.9, 51.7, 30.5, 26.6, 24.1, 20.7, 18.2, 17.9, 15.1., HRMS-ESI (m/z): [M + H]+ calculated for C15H23O5, 283.1545; found, 265.1440 [M+H–H2O]+
Diastereomer 2: Rf: 0.38 hexanes:acetone (2:1), IR (thin film): 3000.50, 2933.43, 2872.53, 1693.60, 1638.03, 1470.98, 1375.00, 1262.55, 1187.51, 1131.25, 1100.01 cm-1, 1H NMR (500 MHz, chloroform-d): δ 7.43 (d, J = 4.1 Hz, 1H), 4.13 (dd, J = 8.3, 4.1 Hz, 1H), 3.62 (s, 1H), 2.19–2.09 (m, 1H), 1.43 (s, 3H), 1.39 (d, J = 5.4 Hz, 6H), 1.33 (d, J = 3.4 Hz, 3H), 0.97 (d, J = 6.7 Hz, 6H), 13C NMR (126 MHz, CDCl3): δ 207.1, 197.9, 137.9, 97.6, 85.0, 32.2, 31.1, 30.1, 29.9, 26.8, 24.2, 21.1, 20.0, 19.2, 15.3., HRMS-ESI (m/z): [M + H]+ calculated for C15H23O5, 283.1545; found, 283.1547
Rhodomyrtosone A (3)
A solution of endoperoxides 8 (1:1 mixture of diastereomers) (6 mg, 0.021 mmol) in acetic acid 1 mL was transferred in a sealed tube and heated at 100 °C for 12 h. Then the reaction mixture was diluted with water and quenched with a saturated sodium bicarbonate solution. The pH was kept slightly acidic. The reaction mixture was extracted with ethyl acetate (3 × 5 mL), and the organic fractions were gathered and dried over anhydrous sodium sulfate. The solvents were evaporated in vacuo to yield a dark yellow oil. Purification by column chromatography over silica gel provided 6 mg (60%) of 3 as a pale yellow gum. Rf: 0.7 hexanes:EtOAc 1:1, IR (thin film): 2960.88, 2930.37, 2873.99, 1720.40, 1649.79, 122.93, 1469.93, 1420.62, 1384.14, 1305.44, 1215.98, 1171.78, 1052.58, 921.63 cm−1, 1H NMR (500 MHz, CDCl3): δ 13.27 (s, 1H), 9.79 (s, 1H), 6.11 (s, 1H), 4.49 (s, 1H), 2.96 (dd, J = 14.7, 6.6 Hz, 1H), 2.76 (dd, J = 14.7, 7.2 Hz, 1H), 2.40 (d, J = 6.8 Hz, 1H), 2.17(m, J = 6.6 Hz, 1H) 1.52 (s, 3H), 1.42 (d, J = 5.3 Hz, 6H), 1.34 (s, 3H), 1.10 (dd, J = 11.8, 6.8 Hz, 6H), 1.00 (dd, J = 10.8, 6.7 Hz, 6H), 13C NMR (126 MHz, CDCl3): δ 211.3, 203.8, 198.5, 179.8, 166.8, 159.9, 159.8, 129.5, 113.3, 104.3, 101.8, 99.7, 55.3, 51.69, 45.8, 45.1, 35.5, 26.1, 24.5, 24.3, 23.3, 22.9, 22.9, 15.9, 15.9. HRMS-ESI (m/z): [M + H]+ calculated for C26H32O7; 457.2226 found, 457.2218
Supplementary Material
ACKNOWLEDGMENTS
Financial support from the National Institutes of Health (R01-GM073855, J.A.P., Jr.) is gratefully acknowledged. Research at the Center for Molecular Discovery (BU-CMD) is supported by NIH grant GM-111625. We thank Prof. Ramesh Jasti (University of Oregon), Prof. Aaron Beeler (Boston University), Prof. Stephane Roche (Florida Atlantic University), Dr. Stephen Scully (Bind Therapeutics), and Dr. Andrew Little (AbbVie) for helpful and stimulating discussions and Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analysis.
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.5b01570.
Copies of 1H and 13C NMR spectra for all new compounds (PDF)
X-ray crystal data for compound 5 (CIF)
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Salni D, Sargent MV, Skelton BW, Soediro I, Sutisna M, White AH, Yulinah E. Aust. J. Chem. 2002;55:229–232. [Google Scholar]
- 2.Hiranrat A, Mahabusarakam W. Tetrahedron. 2008;64:11193–11197. [Google Scholar]
- 3.Hiranrat A, Mahabusarakam W, Carroll AR, Duffy S, Avery MV. J. Org. Chem. 2012;77:680–683. doi: 10.1021/jo201602y. [DOI] [PubMed] [Google Scholar]
- 4.Carroll AR, Avery VM, Duffy S, Forster PI, Guymer GP. Org. Biomol. Chem. 2013;11:453–458. doi: 10.1039/c2ob26931g. [DOI] [PubMed] [Google Scholar]
- 5.(a) Saising J, Hiranrat A, Mahabusarakam W, Ongsakul M, Voravuthikunchai SP. J. Health Sci. 2008;54:589–595. [Google Scholar]; (b) Sianglum W, Srimanote P, Wonglumsom W, Kittiniyom K, Voravuthikunchai PS. PLoS One. 2011;6:16628. doi: 10.1371/journal.pone.0016628. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Leejae S, Taylor PW, Voravuthikunchai SP. J. Med. Microbiol. 2013;62:78–85. doi: 10.1099/jmm.0.049205-0. [DOI] [PubMed] [Google Scholar]
- 6.Morkunas M, Dube L, Geotz F, Maier ME. Tetrahedron. 2013;69:8559–8563. [Google Scholar]
- 7.Muller H, Paul M, Hartmann D, Huch V, Blaesius D, Koeberle A, Werz O, Jauch J. Angew. Chem., Int. Ed. 2010;49:2045–2049. doi: 10.1002/anie.200903906. [DOI] [PubMed] [Google Scholar]
- 8.Bharate SB, Bhutani KK, Khan SI, Tekwani BL, Jacob MR, Khan IA, Singh IP. Bioorg. Med. Chem. 2006;14:1750–1760. doi: 10.1016/j.bmc.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 9.(a) Duhaime MR, Weedon AC. J. Am. Chem. Soc. 1985;107:6723–6724. [Google Scholar]; (b) Duhaime MR, Weedon AC. J. Am. Chem. Soc. 1987;109:2479–2483. [Google Scholar]; (c) Givelet C, Bernat V, Danel M, André-Barrès C, Vial H. Eur. J. Org. Chem. 2007;2007:3095–3101. [Google Scholar]; (d) Bernat V, André-Barrès C, Baltas M, Saffon N, Vial H. Tetrahedron. 2008;64:9216–9224. [Google Scholar]
- 10.(a) Kobayashi S, Sugiura M, Kitagawa H, Lam WW-L. Chem. Rev. 2002;102:2227–2302. doi: 10.1021/cr010289i. [DOI] [PubMed] [Google Scholar]; (b) Comelles J, Moreno-Mañas M, Vallribera A. Arkivoc. 2005:207–238. [Google Scholar]; (c) Dumas AM, Fillion E. Org. Lett. 2009;11:1919–1922. doi: 10.1021/ol9003959. [DOI] [PubMed] [Google Scholar]; (d) Fillion E, Dumas AM, Kuropatwa BA, Malhotra NR, Sitler TC. J. Org. Chem. 2006;71:409–412. doi: 10.1021/jo052000t. [DOI] [PubMed] [Google Scholar]
- 11.Bolte ML, Crow WD, Yoshida S. Aust. J. Chem. 1982;35:1411–1419. [Google Scholar]
- 12.(a) Givelet C, Bernat V, Danel M, André-Barrès C, Vial H. Eur. J. Org. Chem. 2007;2007:3095–3101. [Google Scholar]; (b) Bernat V, André-Barrès C, Baltas M, Saffon N, Vial H. Tetrahedron. 2008;64:9216–9224. [Google Scholar]
- 13.(a) Najjar F, Baltas M, Gorrichon L, Moreno Y, Tzedakis T, Vial H, Andre-Barres C. Eur. J. Org. Chem. 2003;17:3335–3343. [Google Scholar]; (b) Najjar F, André-Barrès C, Lauricella R, Gorrichon L, Tuccio B. Tetrahedron Lett. 2005;46:2117–2119. [Google Scholar]
- 14.Dalpozzo R, Bartroli G, Sambri L, Melchiorre P. Chem. Rev. 2010;110:3501–3551. doi: 10.1021/cr9003488. [DOI] [PubMed] [Google Scholar]
- 15.For representative examples of conjugate addition using nickel (II) perchlorate, see: Kanemasa S, Oderaotoshi Y, Wada E. J. Am. Chem. Soc. 1999;121:8675–8676. Zhuang W, Hazell RG, Jorgensen KA. Chem. Commun. 2001;14:1240–1241. Itoh K, Hasegawa M, Tanaka J, Kanemasa S. Org. Lett. 2005;7:979–981. doi: 10.1021/ol047872g.
- 16.Appendino G, Bianchi F, Minassi A, Sterner O, Ballero M, Gibbons S. J. Nat. Prod. 2002;65:334–338. doi: 10.1021/np010441b. [DOI] [PubMed] [Google Scholar]
- 17.Please see the Supporting Information for details.
- 18.Richard JP, Toteva MM, Amyes TL. Org. Lett. 2001;3:2225–2228. doi: 10.1021/ol016103j. [DOI] [PubMed] [Google Scholar]
- 19.(a) Gal J-F, Geribaldi S, Pfister-Guillouzou G, Morris DG. J. Chem. Soc., Perkin Trans. 1985;2:103–104. [Google Scholar]; (b) Anderson VE, Ruszczycky MW, Harris ME. Chem. Rev. 2006;106:3236–3251. doi: 10.1021/cr050281z. [DOI] [PubMed] [Google Scholar]
- 20.Carroll AR, Lamb J, Moni R, Guymer GP, Forster PI, Quinn RJ. J. Nat. Prod. 2008;71:1564–1568. doi: 10.1021/np800247u. [DOI] [PubMed] [Google Scholar]
- 21.Lee C-K. Tetrahedron Lett. 1999;40:7255–7259. [Google Scholar]
- 22.El-Sepelgy O, Haseloff S, Alamsetti SK, Schneider C. Angew. Chem., Int. Ed. 2014;53:7923–7927. doi: 10.1002/anie.201403573. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao C-C, Liao H-H, Rueping M. Angew. Chem., Int. Ed. 2014;53:13258–13263. doi: 10.1002/anie.201406587. [DOI] [PubMed] [Google Scholar]
- 24.Curini M, Epifano F, Genovese S. Tetrahedron Lett. 2006;47:4697–4700. [Google Scholar]
- 25.For reduction of ortho-quinone methides, see: Arjona O, Garranzo M, Mahugo J, Maroto E, Plumet J, Sáez B. Tetrahedron Lett. 1997;38:7249–7252. Laube T, Bernet A, Dahse H-M, Jacobsen ID, Seifert K. Bioorg. Med. Chem. 2009;17:1422–1427. doi: 10.1016/j.bmc.2009.01.028.
- 26.For recent reviews on flow photochemistry, see: Knowles JP, Elliott LD, Booker-Milburn KI. Beilstein J. Org. Chem. 2012;8:2025–2052. doi: 10.3762/bjoc.8.229. Gilmore K, Seeberger PH. Chem. Rec. 2014;14:410–418. doi: 10.1002/tcr.201402035. Su Y, Straathof NJW, Hessel V, Noel T. Chem. - Eur. J. 2014;20:10562–10589. doi: 10.1002/chem.201400283.
- 27.For endoperoxidation of dienols, see: Ruiz J, Tuccio B, Lauricella R, Maynadier M, Vial H, Andre-Barres C. Tetrahedron. 2013;69:6709–6720.
- 28.For an example of a cyclic peroxycarbenium ion, see: Dussault P, Liu X. Org. Lett. 1999;1:1391–1393. doi: 10.1021/ol990954y.
- 29.Kornblum N, DeLaMare H. J. Am. Chem. Soc. 1951;73:880–881. [Google Scholar]
- 30.(a) Riedl VW, Risse KH. Liebigs. Ann. Chem. 1954;585:209–218. [Google Scholar]; (b) Beaudegnies R, Edmunds A, Fraser T, Hall R, Hawkes T, Mitchell G, Schaetzer J, Wendeborn S, Wibley J. Bioorg. Med. Chem. 2009;17:4134–4152. doi: 10.1016/j.bmc.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhou K, Ludwig L, Li S-M. J. Nat. Prod. 2015;78:929–933. doi: 10.1021/np5009784. [DOI] [PubMed] [Google Scholar]
- 32.Reininger W, Hartl A. Method of acylation of phloroglucinol. 4053517. U.S. Patent. 1977 Oct 11;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.