Abstract
Background. The current standard of treatment of invasive candidiasis with echinocandins requires once-daily therapy. To improve quality of life, reduce costs, and improve outcome, we studied the pharmacokinetics (PK), efficacy, and safety of alternate dosing regimens of micafungin (MFG) for the treatment of experimental subacute disseminated candidiasis.
Methods. MFG was administered for 12 days starting 24 hours after intravenous inoculation of 1 × 103 Candida albicans blastoconidia. Study groups consisted of MFG at 1 mg/kg every 24 hours (MFG1), 2 mg/kg every 48 hours (MFG2), and 3 mg/kg every 72 hours (MFG3), and untreated controls. PK of MFG were determined on day 7 by high-performance liquid chromatography and modeled using nonparametric adaptive grid program. A 2-compartment PK model with volume of the central compartment (Vc), clearance (SCL), and the intercompartmental rate constants Kcp and Kpc was used. The fungal burden in 7 tissues was determined 312 hours after the initiation of therapy.
Results. PK of MFG were linear and the parameter means ± SD were Vc = 0.41 ± 0.18 L, Kcp = 2.80 ± 1.55/hour, Kpc = 1.71 ± 0.93/hour, and SCL = 0.16 ± 0.003 L/hour (r2 = 0.99). The area under the plasma drug concentration - time curve for MFG1, MFG2, and MFG3 was 198.7 ± 19.8, 166.3 ± 36.7, and 192.8 ± 46.2 mg × hour/L, respectively (P = .24). All treatment groups showed significant and comparable resolution of (1→3)-β-D-glucan levels and clearance of C. albicans from liver, spleen, kidney, brain, lung, vitreous humor, and vena cava in comparison to untreated controls (P ≤ .05). There were no differences in hepatic or renal function among study groups.
Conclusions. Less fractionated MFG regimens of every 48 and 72 hours are safe and as effective in experimental disseminated candidiasis as once-daily therapy in neutropenic hosts.
Keywords: micafungin, Candida albicans, disseminated candidiasis, neutropenia, serum (1→3)-β-D-glucan
Disseminated candidiasis is an important cause of healthcare-associated fungal infections in immunocompromised patients [1–4]. Echinocandins (anidulafungin, caspofungin, and micafungin) inhibit the enzyme (1→3)-β-D-glucan synthase. Echinocandins are effective for the treatment of candidemia and disseminated candidiasis, and are usually administered once daily. However, intermittent schedules may facilitate more flexible outpatient care, improve quality of life, and reduce hospital costs. We therefore studied the pharmacokinetics, efficacy, and safety of intermittent dosing schedules of micafungin for the treatment of experimental disseminated candidiasis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Animals
Female New Zealand White rabbits (weighing 2.2–3.3 kg; n = 30; Covance Research Products, Inc, Denver, Pennsylvania) were used in this study. Animals were monitored under humane care and use of standards in facilities, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and according to the guidelines of the National Research Council for the care and use of laboratory animals and under approval of an institutional Animal Care and Use Committee [5]. Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum. Atraumatic vascular access was established in each rabbit by the surgical placement of a Silastic tunneled central venous catheter as previously described [6]. The Silastic catheter permitted nontraumatic venous access for repeated blood sampling for studies of biochemical and hematological parameters, plasma pharmacokinetics, and administration of parenteral agents. Blood samples were drawn from rabbits at the initiation of immunosuppression, during the course of disseminated candidiasis, and before euthanasia. Rabbits were euthanized according to Animal Care and Use Committee–approved prespecified humane endpoints by intravenous administration of pentobarbital (65 mg/kg of body weight of pentobarbital sodium in the form of 0.5 mL beuthanasia-D special [euthanasia solution; Schering-Plough Animal Health Corp, Union, New Jersey]) at the end of the study, 312 hours after study drug initiation.
Immunosuppression and Maintenance of Neutropenia
Cytarabine (AraC; Cytosar-U, Pharmacia, Kalamazoo, Michigan) was administered intravenously for induction and maintenance of neutropenia. Profound neutropenia (a neutrophil concentration of <100 neutrophils/µL) was achieved with an initial intravenous course of 440 mg/m2 of AraC daily for 5 days before inoculation of the rabbits. A maintenance dose of 440 mg/m2 of AraC was administered at 2-day intervals during the study.
Ceftazidime (75 mg/kg intravenous twice daily; Glaxo Pharmaceuticals, Division of Glaxo Inc, Research Triangle Park, North Carolina), gentamicin (5 mg/kg intravenous every other day; Elkins-Sinn, Inc, Cherry Hill, New Jersey), and vancomycin (15 mg/kg intravenous daily; Abbott Laboratories, North Chicago, Illinois) were administered from day 4 of immunosuppression for the prevention of opportunistic bacterial infections during neutropenia. To prevent antibiotic-associated diarrhea due to Clostridium spiroforme, all rabbits received 50 mg of vancomycin per liter of drinking water. Total leukocyte counts and the percentages of neutrophils were monitored twice weekly with a Coulter Counter (Coulter Corporation, Miami, Florida) and by use of peripheral blood smears and differential counts, respectively.
Organism and Inoculation
A well-characterized clinical isolate of Candida albicans NIH-8621 (American Type Culture Collection strain MYA-1237), obtained from a neutropenic patient with autopsy-proven disseminated candidiasis, was used for the study. The minimum inhibitory concentration (MIC) for C. albicans was performed according to Clinical and Laboratory Standards Institute (CLSI) criteria [7]. The MIC of micafungin (Astellas Pharma US, Inc, Deerfield, Illinois) was 0.125 µg/mL.
For preparation of the inoculum, C. albicans isolate was subcultured from a frozen stock culture stored at −80°C on potato dextrose agar slants (K-D Medical, Inc, Columbia, Maryland) on Sabouraud dextrose agar (SGA) plates (K-D Medical, Inc), incubated at 37°C for 24 hours. Three- to 5-well–isolated colonies were sampled from freshly grown culture plates and suspended into 50 mL of Emmon's modified Sabouraud glucose broth (K-D Medical, Inc) (pH 7.0) in a 250-mL Erlenmeyer flask. The suspension was incubated in a gyratory water bath at 80 oscillations/minute at 37°C for 18 hours. The Candida suspension was then centrifuged at 1600g for 10 minutes, and washed 3 times with sterile 0.9% normal saline (K-D Medical, Inc). The concentration was adjusted by use of a hemacytometer and was confirmed by quantitative cultures of a 10-fold serial dilution. The final inoculum of 1 × 103 blastoconidia (suspended in a 5-mL volume of 0.9% sterile normal saline) was slowly administered to each rabbit via the indwelling Silastic central venous catheter on day 6 of immunosuppression to establish the model of subacute disseminated candidiasis [8]. The inoculum size was confirmed by plating serial dilutions onto SGA plates.
Antifungal Compound, Treatment Groups, and Pharmacokinetic Design
Micafungin powder was reconstituted and diluted further in sterile 0.9% normal saline to achieve the desired concentration of 1 mg/mL. Treatment groups consisted of untreated control animals (n = 6) and animals treated with micafungin (n = 24) at dosages of 1 mg/kg every 24 hours (n = 8), 2 mg/kg every 48 hours (n = 8), and 3 mg/kg every 72 hours (n = 8) administered intravenously. Therapy was initiated 24 hours postinoculation, and continued for 12 days.
Assessment of In Vivo Antifungal Efficacy
Antifungal activity in the model of disseminated candidiasis was determined by quantitative clearance of C. albicans from tissues. Representative sections of liver, spleen, kidney, lung, cerebrum, and anterior vena cava were weighed, and each tissue sample was then homogenized (Stomacher 80; Tekmar Corp, Cincinnati, Ohio) in sterile reinforced polyethylene bags (Tekmar Corp) with sterile 0.9% saline for 30 seconds.
Antifungal activity in treatment of Candida infection of the eyes was also assessed postmortem. The globes of the eyes were carefully dissected using aseptic technique. The removed globe was transferred to a sterile Petri dish (Falcon, Becton Dickinson Labware, Becton Dickinson and Co, Franklin Lakes, New Jersey). The sclera was incised with sharp scissors at the posterior pole, and 0.3–0.4 mL of vitreous humor was slowly aspirated into a sterile tuberculin syringe (Tyco Healthcare Group LP, Mansfield, Massachusetts). The specimens of vitreous humor from both globes were pooled into 2-mL Sarstedt microtubes (Sarstedt Ag & Co, Numbrecht, Germany), and processed together.
Each tissue homogenate or vitreous humor specimen was serially diluted 10−1 to 10−4 in sterile 0.9% normal saline. The aliquots of 100 µL of undiluted tissue homogenate or vitreous humor and of each dilution were separately plated onto Emmon's modified SGA containing chloramphenicol and gentamicin. Culture plates were incubated at 37°C for 24 hours, after which colony-forming units (CFU) were counted and the number of CFU/g of tissue was calculated for each organ. Potential carryover of the drug was minimized by serial dilution and by streaking a small-volume (100 µL) aliquot onto a large volume of agar (1 full agar plate/100-µL aliquot). The limit of detection was ≥10 CFU/g or ≥10 CFU/mL, respectively. The culture-negative plates were counted as 0 CFU/g or 0 CFU/mL. Data were graphed as the mean of log10 (CFU/g or CFU/mL) ± standard error of the mean (SEM).
(1→3)-β-D-Glucan Levels
Antifungal efficacy also was assessed by (1→3)-β-D-glucan levels in sequential serum samples. Blood from each rabbit infected with C. albicans was collected every day, and (1→3)-β-D-glucan levels were determined according to the manufacturer's specifications. In brief, aliquots of 5 µL of serum were added to duplicate wells of a 96-well microtiter plate and pretreated for 10 minutes at 37°C with an alkaline reagent (20 µL; 0.125 M potassium hydroxide/0.6 M potassium chloride). Then, an aliquot of 25 µL of the standards (100 to 6.25 pg/mL pure pachyman, a linear β-glucan) was added to each well. An aliquot of 100 µL of Fungitell reagent (lyophilized (1→3)-β-D-glucan–specific Limulus amebocyte lysates) was reconstituted with 2.8 mL of glucan-free reagent grade water (RGW), followed by 2.8 mL of pyrosol reconstitution buffer (2 M Tris hydrochloric acid, pH 7.4), and 100 µL of this mixture was added to each sample. The plate was monitored at 405 nm (with 490 nm background subtraction) for 40 minutes at 37°C in a Bio-Tek ELx808 automated microplate reader (Bio-Tek Instruments, Inc, Winooski, Vermont) equipped with KC4 software (Bio-Tek Instruments, Inc). The mean rate of optical density change was determined for each well, and (1→3)-β-D-glucan concentration was determined by comparison to a standard curve. When absorbance was outside the range of the standard curve, the serum samples were serially diluted in RGW and tested again. The serum value of ≥80 pg/mL was considered positive. The correlation coefficient of the standard curve was r ≥ 0.992 (range, 0.980–0.998).
Pharmacokinetic Studies
The plasma pharmacokinetics of micafungin were investigated in 6 infected rabbits in each dosage group using minimal plasma sampling and determined on postinoculation day 7 (144 hours after drug initiation), when the drug was administered to all groups. Micafungin was administered as a steady intravenous bolus over 4 minutes. Blood samples were collected into heparinized syringes before administration of drug at 0 (baseline) and then at the maximum concentration of drug (Cmax), 0.07, 0.25, 0.5, 2, 4, 6, 8, and 24 hours after the drug administration. Plasma was immediately separated by centrifugation at 400g, and samples were stored in 2-mL Sarstedt microtubes at −80°C prior to analysis.
Micafungin was extracted from plasma using solid phase extraction with C8 Bond Elut cartridges (100 mg, 1 mL) (Varian, Palo Alto, California) and Vac-Elut vacuum manifold (Analytichem International, Harbor City, California). Cartridges were conditioned by completely draining 500 µL of high-performance liquid chromatography (HPLC)–grade acetonitrile and then half-draining 1 mL of acetonitrile-ammonium acetate (phosphate buffer, pH 4.0) (10:90 vol/vol). An aliquot of 300 µL of plasma was applied to the half-filled cartridge, together with 25 µL of the internal standard (50 mg/L anidulafungin; Vicuron Pharmaceuticals Inc, King of Prussia, Pennsylvania). Acetonitrile-ammonium acetate (10:90 vol/vol) (pH 4.0) was then added to completely fill the cartridge (300 µL), and the sample was drained slowly under vacuum.
After drainage, C8 cartridges were washed with 1 mL of acetonitrile-ammonium acetate (10:90 vol/vol), and dried under the vacuum. The cartridges remained at high vacuum for 30 seconds to dry. Subsequently, 1 mL of 100% chloroform (Mallinckrodt, Phillipsburg, New Jersey) was added, and the cartridges were drained and thoroughly dried under vacuum for 1 minute. Micafungin was eluted with 1 mL of acetonitrile-ammonium acetate (70:30 vol/vol) (50 mM, pH 4.0), briefly followed by full vacuum pressure to get the draining started, then vacuum pressure was reduced to allow material to drain slowly into 12- × 75-mm disposable culture test tubes. After all the eluent in the cartridge was pulled through into culture test tubes, vacuum was applied briefly (approximately 5 seconds) to deplete any remaining eluent remaining in the guide needles attached to the vacuum. The culture tubes containing eluent were then evaporated under nitrogen using a Zymark Turbo Vap LV evaporator (American Laboratory Trading LLC, Niantic, Connecticut), at 40°C for 90 minutes or until dry.
Dried eluent was reconstituted in 150 µL of methanol-ammonium acetate (50:50 vol/vol; 50 mM, pH 4.0; methanol was from J.T. Baker, Phillipsburg, New Jersey) in culture test tube, vortexing the tube for 1 minute. The reconstituted eluent was transferred to Eppendorf tubes and centrifuged for 5 minutes at 2600g before being transferred into a microvial insert for HPLC injection and placed in the autosampler at 9°C.
Concentrations of micafungin were determined using a reversed-phase HPLC (Waters 2695 separation module). Acetonitrile-ammonium acetate (50:50, vol/vol; 50 mM, pH 4.0) was used as the mobile phase, with an isocratic flow rate of 0.5 mL/minute. A C8 analytical column (150 × 4.6 mm, 5 µm) (Alltech Inertsil; Alltech Associates, Deerfield, Illinois) maintained at 50°C was preceded by a C8 column guard (7.5 × 4.6mm, 5 µm) (Alltech Inertsil). The injection volume was 75µL. Micafungin and the internal standard, anidulafungin, were detected using ultraviolet light (wavelength of 271 nm) and eluted between 7.6 and 8.1 and between 12.7 and 13.1 minutes, respectively. Quantitation was based on the ratio of peak area of micafungin relative to that of the internal standard.
Standard curves that encompassed the expected experimental range of micafungin concentrations were constructed in their respective matrix. The lower limit of quantification was ≤0.075 mg/L. The coefficient of determination was r2 ≥ 0.996. The intra- and interday coefficients of variation were both <14%.
The pharmacokinetic data from individual rabbits were modeled using a population methodology by means of the NPAG (nonparametric adaptive grid) program with adaptive γ [9]. An open 2-compartment model with zero-order time-delimited input and first-order elimination from the central compartment was used. The data were weighted by the inverse of the estimated variance of the drug assay. Models were discriminated on the basis of the log-likelihood value, mean weighted error, bias-adjusted weighted mean squared error, and a visual inspection of the regression of observed-vs-predicted values obtained after the Bayesian step. The entire data set (ie, 1, 2, 3 mg/kg groups) was co-modeled. The Bayesian estimates of the PK parameters for each individual rabbit were determined by using the “population of one” utility within NPAG. For each dosage group, these Bayesian estimates were collected and the average obtained. The total area under the plasma drug concentration - time curve (AUC0–312h) at steady state was calculated for each rabbit by integration, and compared for rabbits within the 1, 2, 3 mg/kg dosage groups using analysis of variance (ANOVA).
Safety
Chemical determinations of creatinine, urea nitrogen, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and potassium in serum were tested in the penultimate sample drawn from each rabbit.
Statistical Analysis
Comparisons between groups were performed using the Kruskal–Wallis test (nonparametric ANOVA) or Mann–Whitney U test, as appropriate. A 2-tailed P value ≤.05 was considered to be statistically significant. Values are expressed as mean and SEMs. Statistical comparisons of pharmacokinetic parameters across dosage cohorts were made using ANOVA.
RESULTS
Antifungal Therapy
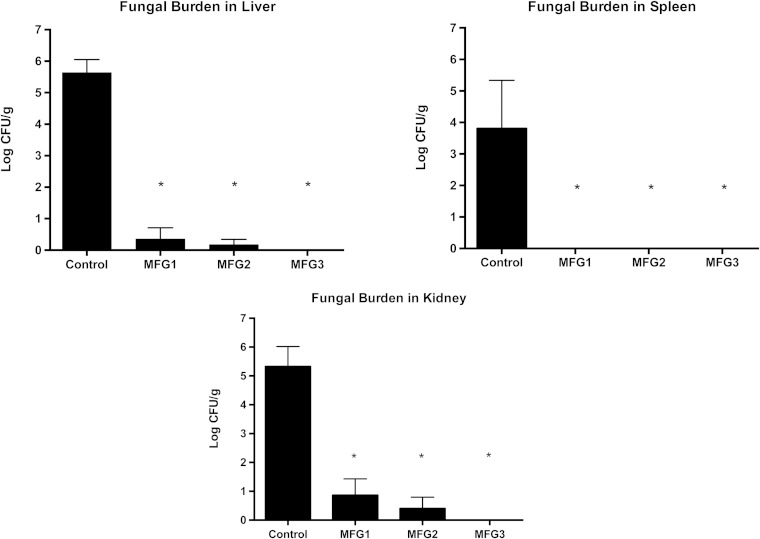
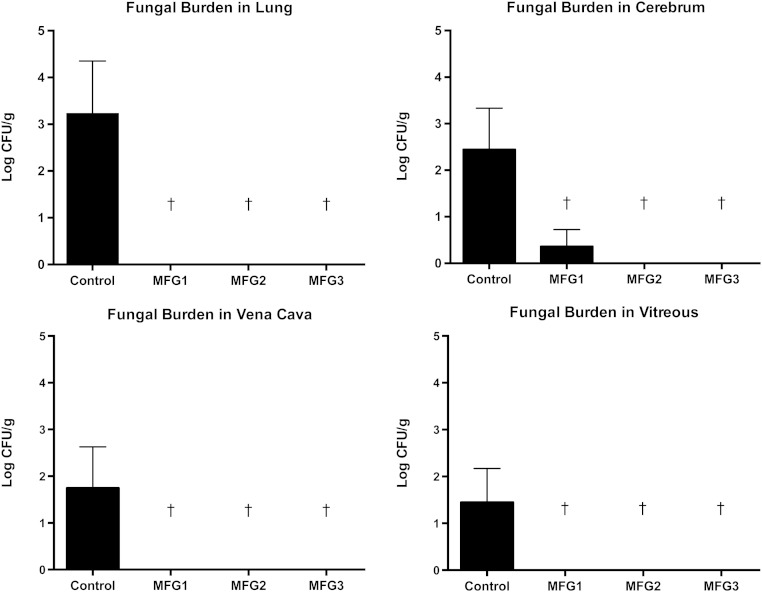
All treatment groups showed significant and comparable clearance of C. albicans from liver, spleen, and kidney in comparison to untreated controls (P < .01; Figure 1). There were no significant differences between treatment groups. There also was a significant decrease and comparable clearance of C. albicans across the dosage groups of mean fungal burden in lung tissue, cerebrum, vena cava, and vitreous humor in micafungin-treated rabbits compared with untreated controls (P ≤ .05; Figure 2). There were no significant differences between treatment groups receiving micafungin.
Figure 1.
All treatment groups showed significant and comparable clearance of Candida albicans across the micafungin dosage groups of 1 mg/kg every 24 hours (MFG1), 2 mg/kg every 48 hours (MFG2), and 3 mg/kg every 72 hours (MFG3) from liver, spleen, and kidney and in comparison to untreated control rabbits (*P < .01). Abbreviation: CFU, colony-forming units.
Figure 2.
All treatment groups showed significant and comparable clearance of Candida albicans across the micafungin dosage groups of 1 mg/kg every 24 hours (MFG1), 2 mg/kg every 48 hours (MFG2), and 3 mg/kg every 72 hours (MFG3) from lung tissue, cerebrum, vena cava, and vitreous humor and in comparison to untreated control rabbits (†P < .05). Abbreviation: CFU, colony-forming units.
(1→3)-β-D-Glucan Levels
Levels of (1→3)-β-D-glucan in serum collected from micafungin-treated and untreated control rabbits are displayed in Figure 3. A significant increase of the mean concentration of (1→3)-β-D-glucan was observed in serum of untreated control rabbits, whereas serum (1→3)-β-D-glucan levels in micafungin-treated rabbits across all dosage groups remained <80 pg/mL through the entire study (P < .001). The (1→3)-β-D-glucan levels in serum obtained from all rabbits before inoculation with C. albicans ranged from 12 pg/mL to 18 pg/mL. After inoculation of C. albicans, the serum (1→3)-β-D-glucan levels in untreated rabbits reached their highest levels of 1593 ± 570 pg/mL (range, 409 to >969 pg/mL) on day 14 postinoculation. The serum (1→3)-β-D-glucan levels in micafungin-treated rabbits ranged from 12 pg/mL to 46 pg/mL through the entire study, and from 17 pg/mL to 20 pg/mL on postinoculation day 14.
Figure 3.
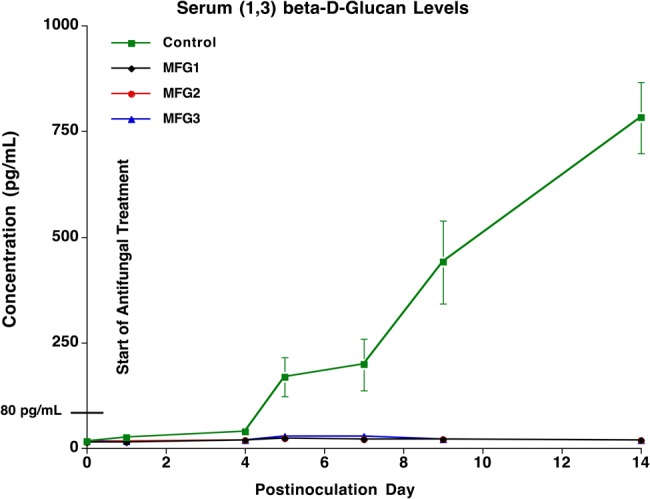
Serial levels of (1→3)-β-D-glucan in serum collected from micafungin-treated and untreated control (UC) rabbits. A significant increase of the mean concentrations of (1→3)-β-D-glucan was observed in serum of UC rabbits, whereas the concentrations of (1→3)-β-D-glucan in serum of micafungin-treated rabbits across all dosage groups remained <80 pg/mL through the entire study (P < .001). Abbreviations: MFG1, micafungin 1 mg/kg every 24 hours; MFG2, micafungin 2 mg/kg every 48 hours; MFG3, micafungin 3 mg/kg every 72 hours.
Pharmacokinetics of Micafungin
Micafungin exhibited linear plasma pharmacokinetics over the dose range used in the study. The fit of the pharmacokinetic model to the data was excellent, with a coefficient of determination of 0.95 after the Bayesian step. The estimates for the means and standard deviations of the pharmacokinetic model parameters are summarized in Table 1, which include volume of central compartment, first-order rate constants connecting the central and peripheral compartments, and clearance from central compartment. The fit of the PK model to the data was highly acceptable, with an r2 of 0.99 (P < .01).
Table 1.
Estimates of the Means and Standard Deviations for Each Parameter From the Population Pharmacokinetic Models of Micafungin in Rabbits
| Treatment Regimen of Micafungin |
Vc, L | Kcp, h−1 | Kpc, h−1 | SCL, L/h | AUC0–312h, mg × h/L |
|---|---|---|---|---|---|
| 1 mg/kg | 0.435 (0.065) | 0.552 (0.124) | 0.396 (0.036) | 0.118 (0.024) | 198.7 (19.8) |
| 2 mg/kg | 0.450 (0.098) | 0.664 (0.230) | 0.350 (0.210) | 0.115 (0.018) | 166.3 (36.7) |
| 3 mg/kg | 0.533 (0.126) | 0.584 (0.182) | 0.514 (0.144) | 0.134 (0.024) | 192.8 (46.2) |
Data are presented as mean (standard deviation).
Abbreviations: AUC0–312h, the area under the plasma drug concentration - time curve; Kcp and Kpc, intercompartmental rate constants; SCL, clearance from central compartment; Vc, volume of central compartment.
The mean AUC0–312h values over the 13 days of dosing were similar for the 3 dosage groups (Figures 4 and 5). The mean AUC0–312h in the 1 mg/kg dosage group after 12 administered dosages was 198.7 mg × hour/L (±19.8). Mean AUC0–312h in the 2 mg/kg dosage group after 6 administered dosages was 166.3 mg × hour/L (±36.7). Mean AUC0–312h in the 3 mg/kg dosage group after 4 administered dosages is 192.8 mg × hour/L (±46.2) (P = .235; ANOVA).
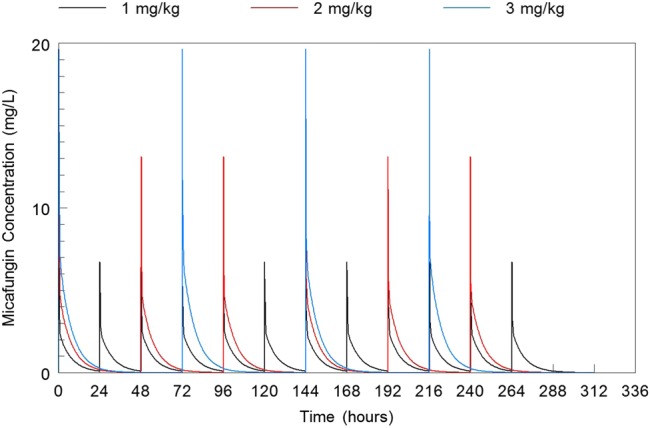
Figure 4.
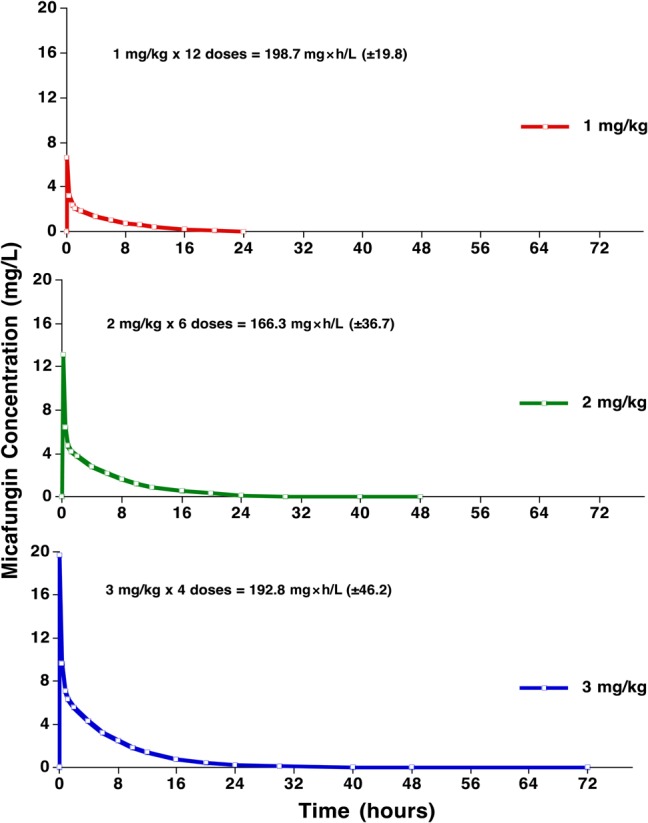
Plasma concentration-time curves of the 3 dosages of 1 mg/kg, 2 mg/kg, and 3 mg/kg micafungin administered intravenously on day 7.
Figure 5.
The mean AUC 0–312h values over the 13 days of dosing were similar for the 3 micafungin dosage groups of 1 mg/kg every 24 hours, 2 mg/kg every 48 hours, and 3 mg/kg every 72 hours administered intravenously. Abbreviation: AUC0–312h, the area under the plasma drug concentration - time curve.
Safety
Micafungin-treated rabbits had no significant increases in levels of serum creatinine, serum urea nitrogen, or serum hepatic aminotransferases, and no decreases in serum potassium (Table 2). There were no differences in renal or hepatic safety parameters in micafungin-treated rabbits compared with untreated controls and across dosage groups.
Table 2.
Effects of Micafungin on Renal and Hepatic Safety Parameters in Persistently Neutropenic Rabbits With Subacute Disseminated Candidiasis
| Treatment Group | Serum Creatinine, mg/dL |
Serum Urea Nitrogen, mg/dL |
Serum ALT, U/L | Serum AST, U/L | Serum Potassium, mmol/L |
|---|---|---|---|---|---|
| Controls (n = 6) | 1.02 ± 0.12 | 18.50 ± 1.43 | 24.17 ± 6.04 | 35.83 ± 13.11 | 4.00 ± 0.17 |
| MFG1 (n = 6) | 0.92 ± 0.10 | 22.83 ± 5.13 | 21.33 ± 4.95 | 23.33 ± 7.98 | 4.05 ± 0.18 |
| MFG2 (n = 6) | 0.85 ± 0.03 | 19.50 ± 1.56 | 13.17 ± 1.08 | 15.17 ± 0.98 | 3.72 ± 0.65 |
| MFG3 (n = 6) | 0.96 ± 0.06 | 19.83 ± 1.88 | 15.00 ± 1.79 | 14.67 ± 2.86 | 4.56 ± 0.11 |
All values are expressed as mean ± standard error of the mean.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; MFG1, micafungin 1 mg/kg every 24 hours; MFG2, micafungin 2 mg/kg every 48 hours; MFG3, micafungin 3 mg/kg every 72 hours.
DISCUSSION
These data demonstrate that less fractionated micafungin regimens of as long as once every 3 days are as effective and as safe as a once-daily regimen against experimental disseminated candidiasis in persistently neutropenic hosts. These findings provide a pharmacokinetic and pharmacodynamic foundation for clinical studies to investigate intermittent dosing strategies.
While standard treatment of invasive candidiasis with micafungin requires once-daily therapy, longer dosing intervals of several days may allow for ambulatory management, earlier discharge, reduced costs, and improved quality of life. Reduced access to intravenous lines also may reduce healthcare-associated catheter-related bloodstream infections.
The comparable outcomes on tissue clearance at multiple sites of 1 mg/kg every 24 hours, 2 mg/kg every 48 hours, and 3 mg/kg every 72 hours is compatible with the known concentration-dependent pharmacodynamics of echinocandins where either the peak:MIC or AUC:MIC ratio is the relevant dynamically linked variable [10, 11].
The role of micafungin in treatment of central nervous system (CNS) candidiasis merits further discussion. As a large N-acyl–substituted cyclic hexapeptide, micafungin does not penetrate significantly through an intact blood–brain barrier. However, our studies of micafungin in treatment of CNS candidiasis demonstrated efficacy and penetration through a disrupted blood brain barrier. As reported by Hope et al in 2008 in the rabbit model of experimental hematogenous Candida meningoencephalitis (HCME), micafungin penetrated most CNS compartments, associated with an AUC of dosage with the highest concentrations of micafungin found in the meninges and choroid [12]. The antifungal efficacy of micafungin followed a sigmoidal Cmax response relationship with an approximate 99% reduction of C. albicans in cerebral tissue corresponding to a dosage of 8 mg/kg and an AUC:MIC ratio of approximately 10 000. These data predicted the dosage of 10 mg/kg/day of micafungin for treatment of infants with candidemia potentially complicated by HCME [13, 14].
These studies also provide insight into the pharmacodynamics of micafungin in the treatment of Candida endophthalmitis. A recent case report noted inadequate vitreal concentrations of systemically administered caspofungin in treatment of Candida endophthalmitis [15]. Our data from series of studies of Candida endophthalmitis demonstrate that C. albicans can be eradicated from the vitreous by micafungin at 2 mg/kg in persistently neutropenic rabbits. These findings were confirmed in the current experiments with a finding of comparable activity at 1 mg/kg, also in persistently neutropenic rabbits. By comparison, the nonneutropenic rabbit model of HCME showed incomplete reduction of C. albicans in the vitreous at dosages ≥16 mg/kg/day [16], suggesting that the inflammatory vitreal opacities may impair diffusion of the echinocandin. These data clearly demonstrate a dose-response relationship of micafungin in treatment of Candida endophthalmitis. Similar findings of dose dependency and the need for higher-than-standard dosages were observed by Livermore et al for anidulafungin [17].
The linear kinetics of micafungin in our study permitted a predictable AUC that was dose-proportional, such that the sum of the AUCs of the less fractionated dosing regimen are similar to that of the daily regimen. This property of dose proportionality may predict safety as well as toxicity. The data in this study indicate that higher single dosages were not associated with hepatic or renal toxicity. Assuming that safety may be toxicokinetically linked to total exposure, then toxicity should be no greater in the 3 dosage regimens than that of the daily dosage of 1 mg/kg.
All echinocandins have a potential for infusion-related toxicity based on histamine release [18]; however, such events are uncommon. Despite the extensive global use of echinocandins, the number of well-documented cases of histamine-associated infusion-related toxicity is small and may be related more to relatively rapid infusion than to dosage. Within multiple, carefully monitored phase 1–2 clinical trials of the safety, tolerability, and pharmacokinetics of micafungin, anidulafungin, and caspofungin [13, 14, 19–21], only 1 episode of histamine-based infusion-related reaction was observed [14]. That patient, who was enrolled in the low-dosage cohort, was a 16-year-old male, who experienced moderate facial erythema and rash at the start of anidulafungin infusion that were not accompanied by additional adverse events of wheezing or hypotension. The symptoms observed with this patient were reminiscent of “red man syndrome,” due to vancomycin-mediated systemic histamine release. The erythema and rash resolved within 1.5 hours after interruption of the study drug and administration of diphenhydramine. Subsequent resumption and 10 other infusions of anidulafungin occurred without recurrence of these adverse events in this patient. A review of literature reveals a paucity of well-characterized cases of infusion-related toxicity. One patient was reported to have developed flash pulmonary edema with a rapidly infused standard dose of anidulafungin [22].
The safety of high doses of 300 mg vs 150 mg was evaluated by Yamazaki et al in a retrospective study of adult patients with hematological malignancies [23]. These investigators found no significant differences in overall adverse events or in hepatobiliary adverse events. The safety of repeated doses of 300 mg was also demonstrated in an adult patient with CNS aspergillosis [24]. These findings lay the foundation for further exploration of once-weekly dosages for the treatment of invasive candidiasis.
Future pharmacokinetic and pharmacodynamic laboratory animal studies in the persistently neutropenic rabbit model of disseminated candidiasis would study the efficacy, safety, and tolerability of once-weekly micafungin in dosages of 7 mg/kg/day for 2 weeks for treatment and prevention against several medically important Candida species. The rabbit model's pharmacokinetics, intravenous route of administration, profound persistent neutropenia, presence of vascular catheter, duration of therapy, and distribution of infected tissues sites strongly correlates with those conditions in profoundly neutropenic patients. These data demonstrate that less fractionated micafungin regimens are safe and as effective as daily dosage of 1 mg/kg, and provide an experimental foundation for clinical trials to investigate alternate dosing strategies.
Notes
Acknowledgments. We thank Margaret P. Cotton and Johanna E. Hughes for their laboratory technical support. T. J. W. is a Scholar of the Henry Schueler Foundation and a Scholar of Pediatric Infectious Diseases of the Sharp Family Foundation, and receives support from the Save Our Sick Kids Foundation.
Supplement sponsorship. This article appears as part of the supplement “Advances and New Directions for Echinocandins,” sponsored by Astellas Pharma Global Development, Inc.
Financial support. This work was supported in part by the Intramural Research Program of the National Cancer Institute, US National Institutes of Health.
Potential conflicts of interest. W. W. H. has received support from Gilead, Astellas Pharma, Pfizer, and F2G. T. J. W. has received research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, Pfizer, and Cubist; and has served as consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gamaletsou MN, Walsh TJ, Zaoutis T et al. . A prospective, cohort, multicenter study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect 2014; 20:O50–7. [DOI] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005; 41:1232–9. [DOI] [PubMed] [Google Scholar]
- 3.Sipsas NV, Lewis RE, Tarrand J et al. . Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009; 115:4745–52. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis DP, Marr KA, Park BJ et al. . Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 5.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academy Press, 2011. [Google Scholar]
- 6.Walsh TJ, Bacher J, Pizzo PA. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci 1988; 38:467–71. [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3. 3rd ed. Wayne, PA: CLSI, 2008. [Google Scholar]
- 8.Walsh TJ, Aoki S, Mechinaud F et al. . Effects of preventive, early, and late antifungal chemotherapy with fluconazole in different granulocytopenic models of experimental disseminated candidiasis. J Infect Dis 1990; 161:755–60. [DOI] [PubMed] [Google Scholar]
- 9.Leary R, Jelliffe R, Schumitzky A, van Guilder M. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models. In: Proceedings of the 14th Institute of Electrical and Electronics Engineers Computer Society, Bethesda, MD, 2001:389–94. [Google Scholar]
- 10.Gumbo T, Drusano GL, Liu W et al. . Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother 2007; 51:968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andes D, Diekema DJ, Pfaller MA et al. . In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 2008; 52:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope WW, Mickiene D, Petraitis V et al. . The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 2008; 197:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibel NL, Schwartz C, Arrieta A et al. . Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother 2005; 49:3317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin DK, Driscoll T, Seibel NL et al. . Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother 2006; 50:632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier GM, Nork TM, Prince R, Andes D. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis 2005; 41:e27–8. [DOI] [PubMed] [Google Scholar]
- 16.Petraitiene R, Petraitis V, Hope WW et al. . Cerebrospinal fluid and plasma (1→3)-β-D-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 2008; 52:4121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore JL, Felton TW, Abbott J et al. . Pharmacokinetics and pharmacodynamics of anidulafungin for experimental Candida endophthalmitis: insights into the utility of echinocandins for treatment of a potentially sight-threatening infection. Antimicrob Agents Chemother 2013; 57:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary JD, Schwartz M, Rogers PD, de Mestral J, Chapman SW. Effects of amphotericin B and caspofungin on histamine expression. Pharmacotherapy 2003; 23:966–73. [DOI] [PubMed] [Google Scholar]
- 19.Walsh TJ, Adamson PC, Seibel NL et al. . Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother 2005; 49:4536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PB, Walsh TJ, Hope W et al. . Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 2009; 28:412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin DK Jr, Smith PB, Arrieta A et al. . Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther 2010; 87:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindahl CB, Wilson JW. Flash pulmonary oedema during anidulafungin administration. Clin Pharm Ther 2012; 37:491–3. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki S, Nakamura F, Yoshimi A, Ichikawa M, Nannya Y, Kurokawa M. Safety of high-dose micafungin for patients with hematological diseases. Leuk Lymphoma 2014; 55:2572–6. [DOI] [PubMed] [Google Scholar]
- 24.Okugawa S, Ota Y, Tatsuno K, Tsukada K, Kishino S, Koike K. A case of invasive central nervous system aspergillosis treated with micafungin with monitoring of micafungin concentrations in the cerebrospinal fluid. Scand J Infect Dis 2007; 39:344–6. [DOI] [PubMed] [Google Scholar]