Abstract
STUDY QUESTION
Are microRNAs (miRs) altered in the eutopic endometrium (EuE) of baboons following the induction of endometriosis?
SUMMARY ANSWER
Induction of endometriosis causes significant changes in the expression of eight miRs, including miR-451, in the baboon endometrium as early as 3 months following induction of the disease.
WHAT IS KNOWN ALREADY
Endometriosis is one of the most common gynecological disorders and causes chronic pelvic pain and infertility in women of reproductive age. Altered expression of miRs has been reported in women and has been suggested to play an important role in the pathophysiology of several gynecological disorders including endometriosis.
STUDY DESIGN, SIZE, DURATION
EuE was obtained from the same group of baboons before and 3 months after the induction of endometriosis. The altered expression of miR-451 was validated in the eutopic and ectopic endometrium of additional baboons between 3 and 15 months following disease induction. Timed endometrial biopsies from women with and without endometriosis were also used to validate the expression of miR-451.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Total RNA was extracted from EuE samples before and after the induction of endometriosis, and miRNA expression was analyzed using a 8 × 15 K miR microarray. Microarray signal data were preprocessed by AgiMiRna software, and an empirical Bayes model was used to estimate the changes. The present study focused on quantitative RT–PCR validation of the microarray data, specifically on miR-451 and its target genes in both baboons (n = 3) and women [control (n = 7) and endometriosis (n = 19)]. Descriptive and correlative analysis of miR-451 and target gene expression was conducted using in situ hybridization and immunohistochemistry, while functional analysis utilized an in vitro 3′ untranslated region (UTR) luciferase assay and overexpression of miR-451 in human endometrial and endometriotic cell lines.
MAIN RESULTS AND THE ROLE OF CHANCE
Induction of endometriosis results in the altered expression of miR-451, -141, -29c, -21, -424, -19b, -200a and -181a in the baboon endometrium. In the baboon, induction of endometriosis significantly decreased the expression of miR-451 at 3 months (P < 0.001), which was also associated with increased expression of its target gene YWHAZ (14.3.3ζ). A similar significant (P < 0.0001) decrease in miR-451 expression was observed in women with endometriosis. The 3′ UTR luciferase assay confirmed the regulation of YWHAZ expression by miR-451. Furthermore, overexpression of miR-451 in 12Z cells (immortalized human endometriotic epithelial cell line) led to the decreased expression of its target YWHAZ and this was correlated with decreased cell proliferation.
LIMITATIONS, REASONS FOR CAUTION
The study focused only on miR-451 and one of its targets, namely YWHAZ. A single miR could target number of genes and a single gene could also be regulated by number of miRs; hence, it is possible that other miRs and their regulated genes may contribute to the pathophysiology of endometriosis.
WIDER IMPLICATIONS OF THE FINDINGS
Our data suggest that the presence of ectopic lesions in baboon causes changes in EuE miR expression as early as 3 months postinduction of the disease, and some of these changes may persist throughout the course of the disease. We propose that the marked down-regulation of miR-451 in both baboons and women with endometriosis increases the expression of multiple target genes. Increased expression of one of the target genes, YWHAZ, increases proliferation, likely contributing to the pathophysiology of the disease.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD40093 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R21 HD 082453 (to A.T.F.) and R01 HD 067721 (to S.L.Y. and B.A.L.). The authors have no conflicts of interest.
Keywords: endometriosis, microRNA-451, endometrium, YWHAZ, proliferation
Introduction
Endometriosis, the presence of endometrial glands and stroma outside of the uterine cavity, is one of the most common causes of chronic pelvic pain and infertility, affecting 1 in 10 women of reproductive age (Bulun, 2009) and as many as 30–50% of patients with infertility (Eskenazi and Warner, 1997; Bulun, 2009; Giudice, 2010). Several theories have been proposed to explain the etiology of endometriosis, but the most widely accepted hypothesis is Sampson's theory of retrograde menstruation (Sampson, 1927), in which fragments of menstrual endometrium are refluxed through the Fallopian tubes into the peritoneal cavity (Giudice, 2010). Although retrograde menstruation occurs in virtually all women, endometriosis is only diagnosed in ∼10% (Bulun, 2009). Despite clear descriptions of endometriosis >100 years ago, the pathogenesis and pathophysiology of its major signs and symptoms remain poorly understood. A critical barrier to our understanding is that the disease has often been established for an average of 8–11 years prior to the time of diagnosis (Verkauf, 1987; Giudice, 2010). Since practical considerations severely limit studies of early endometriosis in humans, animal models have been used. Only Old World monkeys and apes have menstrual cycles similar to humans, and they are the only species that develop spontaneous endometriosis. Moreover, the lesions seen in baboons are histologically identical to the human disease (Hastings and Fazleabas, 2006; D'Hooghe et al., 2009). Thus, a baboon model of induced endometriosis has been developed that closely mimics human disease and allows careful study of the pathogenesis, pathophysiology and time-dependent changes (Fazleabas et al., 2002; Braundmeier and Fazleabas, 2009; D'Hooghe et al., 2009; Fazleabas, 2010).
MicroRNAs (miRs) are small, single-stranded noncoding RNA molecules ∼22 nucleotides in length that are transcribed from miRNA loci and function as repressors of gene function through mRNA cleavage and translational repression (Du and Zamore, 2005; Guo et al., 2010). Multiple studies have suggested that miRs play roles in both benign and malignant disease of the human reproductive tract, and abnormal expression levels of miRs have been observed in several reproductive tract diseases (Wang et al., 2007; Marsh et al., 2008; Zhang et al., 2008; Burney et al., 2009; Chung et al., 2009; Ohlsson Teague et al., 2009; Zhu et al., 2009; Creighton et al., 2010; Nagaraja et al., 2010; Teague et al., 2010; Hawkins et al., 2011; Santamaria and Taylor, 2014; Braza-Boils et al., 2014; Traver et al., 2014; Graham et al., 2015; Mari-Alexandre et al., 2015). Studies of miR expression (Burney et al., 2009; Filigheddu et al., 2010; Teague et al., 2010; Hawkins et al., 2011; Hull and Nisenblat, 2013; Nothnick et al., 2014; Graham et al., 2015) support the hypothesis that miRs are involved in endometriosis, but the function of aberrant miR expression in the pathogenesis of endometriosis has not been extensively investigated (Ohlsson Teague et al., 2009; Hull and Nisenblat, 2013; Okamoto et al., 2014; Cho et al., 2015).
The objective of the present study is to identify which miRs are altered during the early stage of endometriosis and how the altered miRs and their targets contribute to the biological functions related to the disease. We have identified miR species whose expression was altered after endometriosis induction in the baboon model. We have validated altered expression in baboon and human tissue, focusing on miR-451 and its predicted target gene YWHAZ, which codes for a protein, 14.3.3ζ, that is known to suppress apoptosis, and enhance cell proliferation and invasion, all of which are hallmarks of endometriosis (Niemantsverdriet et al., 2008; Neal et al., 2009; Bergamaschi and Katzenellenbogen, 2012; Chen et al., 2012; Zhang et al., 2012; Murata et al., 2014). A focus on YWHAZ as a target of miR-451 is further supported by our previous global gene expression studies demonstrating altered YWHAZ expression in the eutopic endometrium (EUE) of baboons with endometriosis as a consequence of aberrant phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling (Afshar et al., 2013). Finally, we investigated the functional consequences of miR-451 action in vitro.
Materials and Methods
Animals and induction of experimental endometriosis
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Illinois, Chicago and Michigan State University. Endometriosis was experimentally induced in female baboons (Papio anubis) by i.p. inoculation with menstrual endometrium on two consecutive menstrual cycles, as previously described (Hastings and Fazleabas, 2006; Harirchian et al., 2012; Afshar et al., 2013). This model allows us to study the progression of endometriosis by collecting EUE from these animals at various time points following the induction of disease.
Collection of baboon tissues
In the cycle prior to the induction of endometriosis, control EUE samples were obtained at laparotomy on Days 9–11 postovulation (PO), i.e. the window of implantation. Endometriosis was then induced in the same animals by i.p. inoculation of autologous menstrual tissue on two consecutive cycles. Following laparoscopic confirmation of endometriosis, EUE samples were obtained on Days 9–11 PO at 3 months postinduction of disease. At 15–16 months following the second inoculation, the animals were euthanized on Days 9–11 PO as required by the IACUC approval, which permits a maximum of four invasive surgeries, and a necropsy was carried out to obtain all of the associated reproductive tissues within the peritoneal cavity. Samples were snap-frozen in liquid nitrogen or fixed in paraformaldehyde for molecular and histological analysis (Hastings and Fazleabas, 2006; Harirchian et al., 2012; Afshar et al., 2013). The lesions used in the present study were blue/chocolate or red colored harvested at 15 months postinduction of endometriosis in baboons as described previously (Harirchian et al., 2012). Briefly, the abnormal peritoneal surface was grasped and elevated away from underlying tissues. An elliptical incision was made around the lesion with 0.5–1 cm margins. Any deeper foci that were felt to be firm or fibrotic were also excised.
Patient samples
Collection of mid-secretory phase EuE using timed endometrial biopsies was carried out with the approval of Institutional Review Board protocols from the University of North Carolina (Chapel Hill, NC, USA) and the Greenville Hospital System (Greenville, SC, USA). Written informed consent was obtained from all women whose tissues were utilized in this study. EUE was obtained from the control women (n = 7) and women with endometriosis (n = 19). The ectopic lesions were obtained from the peritoneal sidewall and rectum of the women with endometriosis during surgery. The details regarding the day of their menstrual cycle, clinical history and the American Society for Reproductive Medicine (ASRM) revised score for disease severity are provided in Supplementary Table S1. None of the women enrolled in the study were on any type of prescription medication and all were clinically healthy. Control samples were collected from two groups of women aged 18–35 years with a BMI > 29 kg/m2. In the Supplementary Table S1, women identified with an ‘N’ (N026 and N032) were from a group of paid volunteers who underwent an LH timed endometrial biopsy solely for research. Of the ‘G’ control group, three women (G173A, G212A and G214A) had no surgery, but two (G360B and G422A) had a laparoscopy for pelvic pain and were confirmed by pathological diagnosis not to have endometriosis. These control women did not have a history of more than one spontaneous pregnancy loss and were neither breastfeeding nor determined to have any uterine abnormalities, such as fibroids, at the time of collection of the tissues.
RNA isolation
For miR microarray experiments, RNA was isolated using the miRNeasy Kit from Qiagen, and RNA quality control was performed using the Agilent RNA 6000 Nano Kit and Bioanalyzer (Agilent Technologies, USA). For miR and mRNA quantitative RT–PCR (qRT–PCR) experiments, total RNA was isolated from the baboon and human endometrial tissues, endometrial epithelial cells (EECs) and endometriotic epithelial cells (12Z cells) using TRIzol reagent (Invitrogen, USA) as per the manufacturer's instructions, and RNA quality check was performed using NanoDrop 2000 (Thermo Scientific, USA).
MiR array
In the present study, we have used the human miR array (Agilent) as there is no baboon miR array available. In addition, recent studies have suggested that there is ∼90% similarity between human and baboon miR sequences (Karere et al., 2010, 2012; Cox et al., 2013) which further supports the use of the human array platform. For performing the miR microarray, RNA from the baboon EUE biopsies obtained before the induction of the disease (Control, n = 4) and 3 months postinduction of the disease in the same animals (Endometriosis, n = 4) was extracted using Qiagen® miRNeasy kit (Qiagen, USA). These RNA samples were then subjected to a quality check using the bioanalyzer thatshowed an average RNA integrity number of 7.0 ± 0.5. An 8 × 15 K miRNA microarray (Agilent Technologies, USA) was used for the miR microarray. The signal data were preprocessed by AgiMiRna, a Limma package for R enrichment. P-values were determined by moderated t-statistic using Empirical Bayes model posterior variance estimates (Smyth, 2004). The adjusted P-values were obtained after correction for multiple comparisons by the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995). All the identified miRNAs that were significantly altered as a consequence of the induction of endometriosis were above 0.05. However, seven of them were altered by 2-fold and have an average expression >64 (Table I) (Feise, 2002). Additionally, the fold-change values of miR-200a and miR-181a, relevant to endometriosis and transition into endometrial cancer (Boren et al., 2008; Gregory et al., 2008; Hiroki et al., 2010; Yang et al., 2011), respectively, are also provided in Table I. The potential mRNA targets of differentially expressed miR-451 were predicted by online database TargetScan (http://www.targetscan.org/) Human 6.2 (Lewis et al., 2005; Grimson et al., 2007; Friedman et al., 2009).
Table I.
Differentially expressed microRNAs in eutopic endometrium of baboons with and without disease.
| miR | Fold change | Average expression | P-value | Adjusted P-value |
|---|---|---|---|---|
| miR-451 | −14.94 | 163 | 3.60E−05 | 0.00875 |
| miR-141 | 5.19 | 144 | 0.0322 | 0.248 |
| miR-29c | 4.46 | 70 | 0.0088 | 0.248 |
| miR-21 | 2.49 | 1745 | 0.0138 | 0.248 |
| miR-424 | 2.43 | 500 | 0.0270 | 0.248 |
| miR-19b | 2.19 | 192 | 0.0732 | 0.292 |
| miR-200a | 1.88 | 91 | 0.0373 | 0.248 |
| miR-181a | −1.46 | 361 | 0.0678 | 0.292 |
The miRs underlined have previously been reported to be altered in women with endometriosis (Burney et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Hull and Nisenblat, 2013). The P-values were determined using empirical Bayes posterior variance estimates and adjusted P-values by the Benjamini–Hochberg procedure.
qRT–PCR for mRNA and miR
For measuring miR transcript levels, 100 ng of total RNA was reverse transcribed using a TaqMan microRNA reverse transcription kit (Applied Biosystems, USA) as per the manufacturer's protocol. Following reverse transcription, miR-specific cDNA expression of mature miR was analyzed using TaqMan miR Assays (Applied Biosystems, USA) and TaqMan universal PCR master mix (Applied Biosystems, USA). The miR expression data were normalized using expression of endogenous U6 small nuclear RNA. For analysis of miR target expression, total RNA (1000 ng) was reverse transcribed using a High-Capacity cDNA synthesis kit (Applied Biosystems, USA) according to the manufacturer's protocol. After the cDNA synthesis step, cDNA and qRT–PCR reaction was carried out for miR targets using TaqMan gene assays and TaqMan Universal PCR master mix (Applied Biosystems, USA). Ribosomal protein S17 (RPS17) was used as an endogenous control for normalizing qRT–PCR data. All quantitative real-time PCR reactions were run for 40 cycles, and fold change was calculated using ΔΔCt method (Livak and Schmittgen, 2001).
In situ hybridization
Snap-frozen eutopic endometrial tissues were sectioned to 10 µm thickness. In situ hybridization (ISH) for miR-451 was performed by modifying the method previously described (Nelson and Wilfred, 2009) and using double digoxigenin (DIG) labeled mercury locked nucleic acid (LNA) detection probes (Exiqon, Denmark) and IsHyb kit (BioChain, USA). Briefly, the sections were fixed in 4% paraformaldehyde in 1×phosphate-buffered saline (PBS, pH 7.4–7.6). Subsequently washed and incubated in prehybridation solution for 3 h at a temperature 15°C below the melting temperature (TM) of each of the mercury LNA detection probes. The hybridization mixture was applied to the slides and incubated for 12–16 h at a temperature 20°C below Tm of the probes. Following this incubation, the slides were washed with SSC (saline-sodium citrate). Subsequently, the slides were incubated with 1× blocking solution at room temperature for 1 h and incubated overnight with 1:500 diluted anti-DIG alkaline phosphatase conjugated antibody at 4°C. The next morning, the slides were then washed with 1× PBS and 1× alkaline phosphatase. Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) solution was applied and incubated for 12–16 h at room temperature in the dark. Next, slides were counterstained with methyl-green solution and rinsed with distilled water, coverslipped and analyzed under the microscope (Olympus BH-2).
Immunohistochemistry
Immunohistochemical staining for YWHAZ (Abgent, USA) and phospho-histone H3 (pH3) (Millipore, USA) was performed using formalin-fixed, paraffin-embedded endometrial samples. These sections (6 μm thick) were blocked using 10% normal goat serum in 1× PBS and then incubated overnight at 4°C with serially diluted primary antibody (YWHAZ 1:50 and pH3 1:200) in blocking buffer. Subsequently sections were incubated with biotinylated secondary antibody for 1 h at room temperature and incubated with avidin-biotinylated horse-radish peroxidase (HRP) complex for 30 min and immunoreactivity visualized by diaminobenzidine (DAB) precipitation. Images were taken under ×40 magnification using Olympus BH-2 microscope.
Digital HSCORE analysis
Digital HSCORE analysis for YWHAZ protein was performed using image analysis software (Image J 1.48, National Institutes of Health, Bethesda, Maryland, USA) as previously described (Fuhrich et al., 2013; Su et al., 2015).
Cell culture and in vitro target validation
Immortalized human ectopic endometriotic epithelial cells (12Z) were cultured using Dulbecco's modified Eagle's medium (DMEM)/F-12 (Gibco, USA) supplemented with 10% heat inactivated fetal bovine serum (HI-FBS; Gibco, USA), 1× Pen/Strep (Gibco, USA) and 1× sodium pyruvate (Gibco, USA) at 37°C temperature under 5% CO2 and 95% air (Zeitvogel et al., 2001; Banu et al., 2008). EECs (Hombach-Klonisch et al., 2005) were cultured using DMEM/Hams F-12 (Gibco, USA) supplemented with 10% HI-FBS (Gibco, USA), 1× Pen/Strep (Gibco, USA) and 1× sodium pyruvate (Gibco, USA) at 37°C temperature under 5% CO2 and 95% air. Following optimization of parameters, Lipofectamine RNAiMAX (Invitrogen, USA) was used to transfect both EEC and 12Z cells either with 5 pmol of miR-451 mimics (Ambion, USA) or with 5 pmol of nontargeting negative controls (Ambion, USA), and RNA and protein were isolated after 24 h. To check the expression of miR-451 and YWHAZ transcript, qRT–PCR was performed. YWHAZ protein levels in the same cells were also analyzed by western blot.
Western blotting
Total cellular protein was isolated from the EEC and 12Z cells using radioimmunoprecipitation assay buffer, and protein concentration was measured using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, USA). Equal amounts of protein extracts (30 µg) were subjected to electrophoresis through 10%, polyacrylamide gels containing sodium dodecyl sulphate and transferred to polyvinylidene diflouride membranes. Membranes were then blocked for 1 h in Tris-buffered saline (50 mM Tris–Cl, pH 7.6; 150 mM NaCl) supplemented with 0.5% Tween-20 and 0.5% Casein (Sigma-Aldrich, USA). Membranes were then washed and incubated with YWHAZ (Abcam, USA) primary antibodies overnight at 4°C on a rocking stage. Following the overnight incubation, membranes were washed and incubated with HRP conjugated secondary antibody for 1 h at room temperature. Immunocomplexes were visualized by enhanced chemiluminescence (GE Healthcare Life Sciences, USA) according to the manufacturer's protocol. Protein expression was normalized with β-actin (Sigma-Aldrich, USA). Data were obtained from three independent experimental setups.
3′ UTR luciferase assay
Human EEC were seeded in triplicate in 24-well plates and allowed to settle for 16 h. EEC were then co-transfected with 400 ng of pYWHAZ luciferase plasmid or mutated luciferase plasmid plus 50 ng pRL-TK renilla plasmid (Promega, USA) together with 400 ng of an expression vector for miR451 (Origene Inc., USA) or an empty control vector using Lipofectamine 2000 reagent (Invitrogen, USA). Culture medium was replaced after 6 h, and cell lysates was prepared 24 h after medium change, followed by measurement of firefly and renilla luciferase activities using the Dual Luciferase Reporter Assay Kit (Promega, USA), according to the manufacturer's protocol. Three independent experiments were performed, and data are presented as the mean ± SD.
Cell proliferation assay
The 12Z cells were seeded into 96-well plates at a density of 5000 cells per well in quadruplets. At 24, 48 and 72 h posttransfection with miR-451 or negative control, 10 µl of MTS (Promega, USA) reagent was added to each well, and the mixture was incubated for 2 h. Following incubation, optical density (OD) was measured at 495 nm wavelength. The rate of proliferation was calculated as the percentage of mean OD of the control.
Statistical analysis
Differences in miRNA and gene expression between control and endometriotic eutopic and ectopic endometrium were compared following normalization against U6 and RPS17, respectively. One-way analysis of variance was used to test the null hypothesis of group differences, followed by Tukey test. For the in vitro studies, the miR and gene expression were normalized against U6 and RPS17 followed by the Student's t-test for pairwise comparison at a 95% confidence level (P < 0.05) between negative control and miR-451 transfected cells. We used Graphpad Prism 5.0 statistical software (GraphPad Software, Inc., USA) for analyzing the data.
Results
Induction of endometriosis altered the miR expression profile in EUE of baboons
To identify which miRs are altered in EUE due to the presence of ectopic lesions, we performed a miR microarray analysis using RNA isolated from mid-secretory EUE collected before and 3 months after the induction of the disease. Our results revealed that inoculation of the autologous menstrual tissue into the peritoneal cavity, mimicking Sampson's theory of retrograde menstruation, alters the miR expression profile in the EUE as early as 3 months postinoculation. The list of the altered miRs is shown in Table I. The miRs, which are bolded and underlined in Table I, have previously been reported to be altered in women with endometriosis, further validating our baboon model (Burney et al., 2009; Ohlsson Teague et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Aghajanova and Giudice, 2011). In the present study, we have focused on miR-451 since it is significantly decreased in the EuE, and its role has only recently been studied in the context of endometriosis (Nothnick et al., 2014; Graham et al., 2015). Further, recent studies suggest that miR-451 plays a role in murine embryo implantation, validating its importance in uterine biology (Li et al., 2015). Moreover, studies in cancer tissues have reported a role for miR-451 as a tumor suppressor in multiple cancer types, where it has been shown to regulate cellular functions, including invasion, proliferation and apoptosis, all of which are the hallmarks of endometriosis (Godlewski et al., 2010a,b; Bitarte et al., 2011; Wang et al., 2011; Bergamaschi and Katzenellenbogen, 2012; Tian et al., 2012; Zhang et al., 2012).
MiR-451 expression was decreased in eutopic and ectopic endometrium of both baboon and women with endometriosis
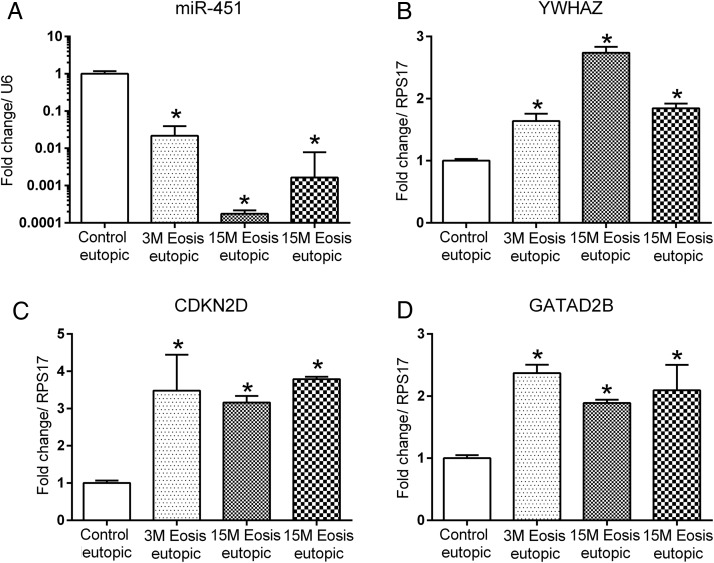
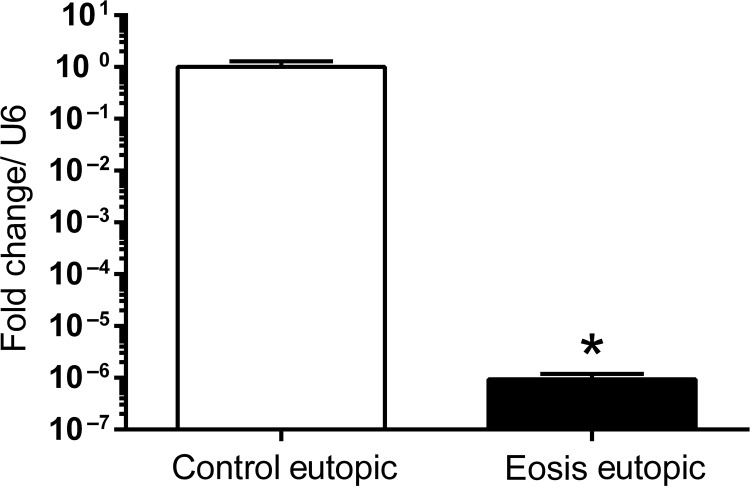
Our data suggest that miR-451 is significantly (P < 0.001) down-regulated after the induction of endometriosis in EUE collected at 3 and 15 months and in endometriotic lesions collected at 15 months (Fig. 1A). We also examined the expression pattern and localization of miR-451 during the normal menstrual cycle of disease-free baboons. The qRT–PCR analysis demonstrated a significantly higher miR-451 expression during the mid-secretory phase compared with proliferative and late secretory phase in normal baboons (Supplementary Fig. S1). We next examined miR-451 expression in mid-secretory human EUE collected from women with and without endometriosis. Similar to the findings in our baboon model, we observed a significant down-regulation of miR-451 in EuE obtained during mid-secretory phase from women with endometriosis compared with controls (Fig. 2).
Figure 1.
Quantitative RT–PCR analysis of miR-451 and its target gene expression in baboon tissues. MiR-451 (A) expression was significantly decreased in the eutopic and ectopic endometrial biopsies obtained during the mid-secretory phase from baboons (n = 3) with endometriosis compared with controls (n = 3). Increased expression of YWHAZ (B) along with CDKN2D (C) and GATAD2B (D) in eutopic and ectopic endometrium of baboons following the induction of endometriosis. Mean values with SD are shown. (*P < 0.05). M, months; Eosis, endometriosis.
Figure 2.
Quantitative RT–PCR analysis of miR-451 expression in human eutopic endometrium. MiR-451 expression was significantly decreased in the eutopic endometrium of women (n = 7) with endometriosis compared with controls (n = 19). Mean values with SD are shown. (*P < 0.05).
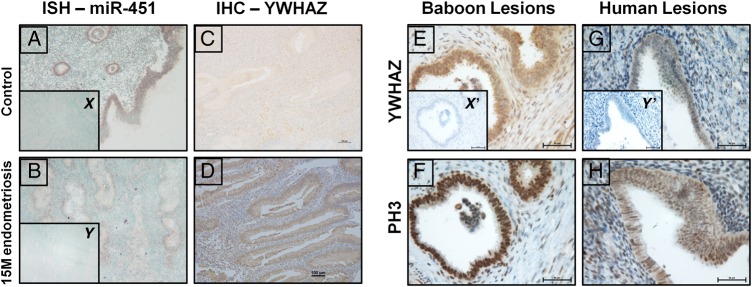
To identify the cell-specific localization of miR-451 in mid-secretory phase EUE of controls and baboons with endometriosis, we performed ISH using double DIG-labeled probes. Our ISH data show that miR-451 expression is predominantly in luminal and glandular epithelium with limited expression in stromal cells of control animals. This expression is significantly decreased in the EUE at 15 months of disease (Fig. 3A and B)
Figure 3.
Localization of miR-451 and YWHAZ in endometrial tissues from baboons with and without endometriosis. In situ hybridization analysis revealed that expression of miR-451 is higher in glandular epithelium (GE) and luminal (LE) epithelium compared with stroma in controls (A) however, miR-451 signal intensity is markedly compromised in GE, LE and stroma of eutopic endometrium of baboons with endometriosis (B). Inset figure x and y represents the nontargeting negative controls used for in situ hybridization analysis. Immunohistochemical analysis of YWHAZ revealed that in the eutopic endometrium of control baboons (C) YWHAZ expression is low in both GE and stromal cells compared with baboons with endometriosis (D). Ectopic endometrium showed YWHAZ staining in both baboon (E) and women (G) with endometriosis, and in the same section we also observed the signals for pH3 (marker for proliferation) for both (F-baboon; H-women). Inset figure x′ and y′ represents the negative controls (without primary anti-YWHAZ and anti-pH3) used for immunohistochemistry. Figure A–D (×10 magnification; Bar = 100 µm) and Figure E–G (×40 magnification; Bar = 25 µm).
Decreased miR-451 expression is associated with increased expression of its predicted targets in vivo
To predict potential targets of miR-451, we used TargetScan 6.2 software (Lewis et al., 2005; Friedman et al., 2009), resulting in a list of 20 potential targets. We investigated altered mRNA expression of three predicted miR-451 targets, YWHAZ, CDKN2D and GATAD2B. Expression of each of the three targets was up-regulated in eutopic and ectopic endometrium of baboons with endometriosis compared with controls (Fig. 1B, C and D). Further experiments were focused on the miR-451 regulation of YWHAZ expression and function, due to the reported role of YWHAZ in proliferation and anti-apoptotic activity (Wilker and Yaffe, 2004; Neal et al., 2009, 2012; Neal and Yu, 2010; Zhang et al., 2012).
Immunohistochemistry for the YWHAZ gene product (Fig. 3C and D) also revealed localization to the glandular and luminal epithelium, with a significant increase in staining in the EUE of baboons with endometriosis. Digital H-score analysis (Fuhrich et al., 2013) of YWHAZ slides also supported this observation and revealed a significant increase in YWHAZ as shown in Supplementary Fig. S3. Immunohistochemical localization also demonstrated robust expression of the YWHAZ gene product in the epithelial cells of endometriotic lesions from baboon and women (Fig. 3E and G) accompanied by strong expression of the proliferation marker pH3 (Fig. 3F and H). Taken together, these studies demonstrate expression of miR-451 and YWHAZ in the same endometrial cell types and support the inverse expression of miR-451 and YWHAZ mRNAs seen with qRT–PCR.
MiR-451 regulates the expression of YWHAZ in vitro
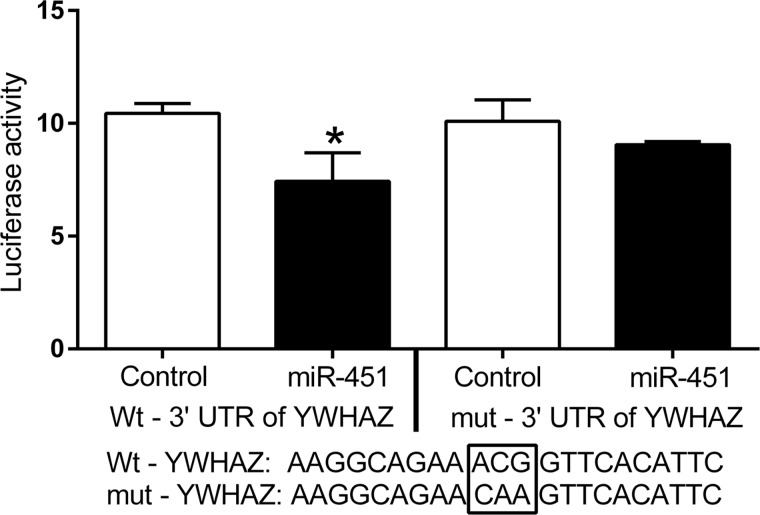
Our initial in vivo analysis had demonstrated that miR-451 was expressed in the glandular and luminal epithelial of control baboons and following the induction of endometriosis the down-regulation of miR-451 was associated with an increase in YWHAZ in these cells. Based on these observations and to investigate the mechanism underlying the inverse relation of miR-451 and YWHAZ expression, a series of in vitro experiments were performed. We chose to use the EEC cells that are representative of EECs for the 3′UTR analyses to confirm that miR-451 can directly target the YWHAZ mRNA transcript. Results show a significant (P = 0.036) decrease in firefly luciferase activity in the EEC cells co-transfected with miR-451 vector compared with the empty vector (Fig. 4). To confirm the specificity of the observed effects on 3′ UTR sequence of YWHAZ, we compared the effects of the wild-type 3′ UTR of YWHAZ with a 3′ UTR mutated (mut-YWHAZ) at the likely miRNA interaction site. Unlike the wt-YWHAZ, luciferase activity from the mut-YWHAZ plasmid did not show a significant effect of miR-451 expression (Fig. 4). Taken together, these data confirm a direct effect of miR-451 on the YWHAZ 3′ UTR.
Figure 4.
3′ untranslated region (UTR) luciferase assay results show that 3′ UTR of YWHAZ is a direct target of miR-451. The luciferase construct containing wild-type 3′ UTR of YWHAZ or mutated 3′ UTR of YWHAZ was co-transfected with either miR-451 or empty vector plasmids in endometrial epithelial cells along with renilla plasmid (internal control). The luciferase data are normalized with the renilla and presented here as mean values of three separate experiments with SD (*P < 0.05).
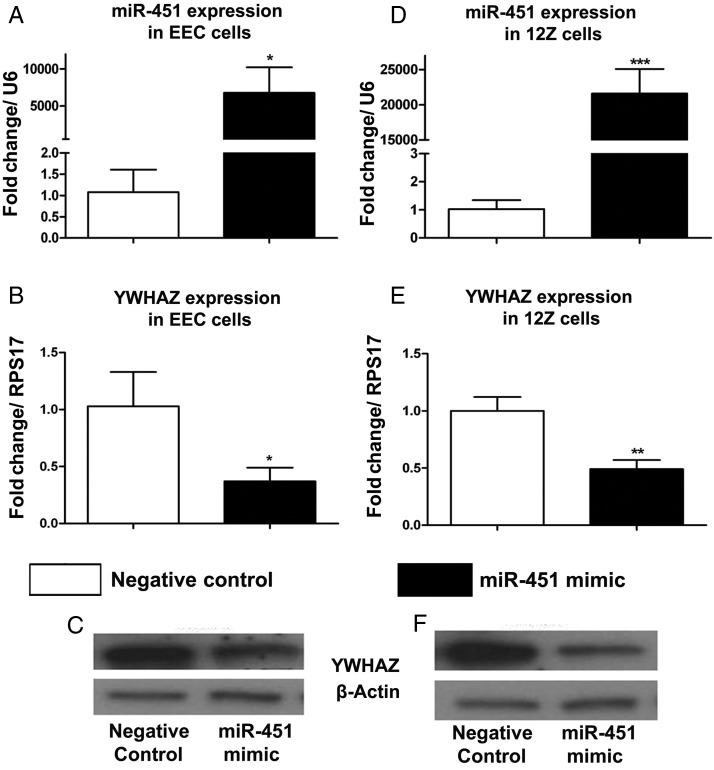
Although the above 3′ UTR luciferase data suggest that miR-451 directly targets the 3′ UTR of YWHAZ, we wanted to evaluate the effect of miR-451 overexpression on YWHAZ expression. In this experiment, we transfected both the 12Z and EEC cells with either miR-451 mimics or nontargeting negative controls and found that the endogenous YWHAZ transcript and protein expression is significantly decreased in both 12Z cells (Fig. 5A–C) and EEC cells (Fig. 5D–F) transfected with miR-451. Overall, these in vitro studies strongly support that the inverse correlation observed in vivo between miR-451 and YWHAZ expression is driven by a direct action of miR-451 on the YWHAZ transcript.
Figure 5.
qRT–PCR and western blot data suggest that overexpression of miR-451 inhibits YWHAZ expression. Effect of miR-451 mimics transfection on miR-451 (A), YWHAZ transcript (B) and YWHAZ protein (C) levels in EECs (endometrial epithelial cells). Effect of miR-451 mimics transfection on miR-451 (A), YWHAZ transcript (B) and YWHAZ protein (C) levels in 12Z cells (endometriotic epithelial cells). Mean values of three separate experiments with SD are shown. (***P < 0.001;**P < 0.001;*P < 0.05).
MiR-451 inhibits cell proliferation which is a hallmark of endometriosis
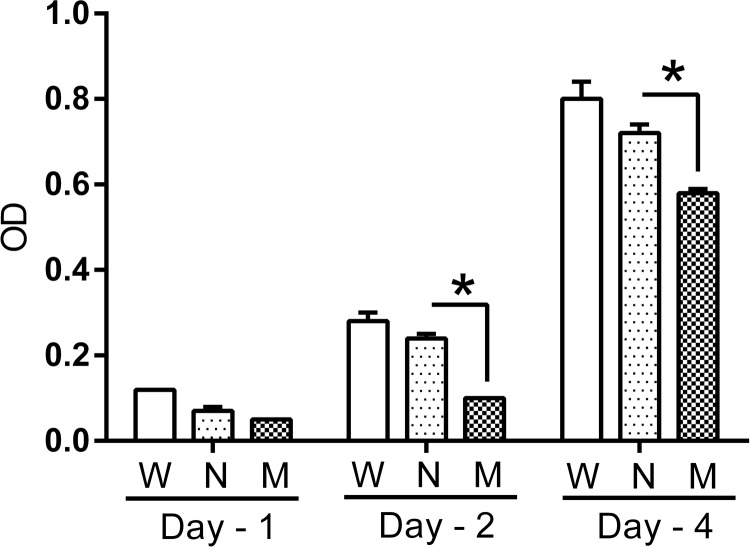
Our in vivo and in vitro data clearly demonstrated that decreased expression of miR-451 results in increased expression of YWHAZ. Immunohistochemical and Digital H-score analysis also suggested that YWHAZ increased along with a marker of proliferation, phospho-Histone H3, in eutopic and ectopic endometrial tissues from baboons with endometriosis compared with controls. We next wanted to examine the biological function of these changes in YWHAZ since it is known to increase cell proliferation (Wilker et al., 2005; Bergamaschi and Katzenellenbogen, 2012; Chen et al., 2012; Neal et al., 2012). To evaluate this effect on proliferation, we utilized 12Z cells (endometriotic epithelial cells) since they have higher proliferation rate, low miR-451 (Supplementary Fig. S2A) and higher YWHAZ (Supplementary Fig. S2B) expression compared with the EEC cells and transfected them either with miR-451 mimic or with nontargeting negative controls and performed the cell proliferation assay. 12Z cells transfected with miR-451 showed a significant (P < 0.05) decrease in their proliferation rate at both Days 2 and 4 compared with 12Z cells transfected with nontargeting negative controls (Fig. 6). This in vitro cell proliferation assay result supports the hypothesis that miR-451 can inhibit endometriotic epithelial cell proliferation, paralleling the observed in vivo increased pH3 staining and decreased miR-451 expression.
Figure 6.
MiR-451 inhibits proliferation of endometriotic epithelial cells (12Z cells). Transfection of miR-451 mimics in 12Z cells markedly reduced cell proliferation compared with the 12Z cells transfected with nontargeting negative control on Days 2 and 4 posttransfection. Mean values of three separate experiments with SD are shown. W, no treatment; N, nontargeting negative control; M, miR-451 mimic. (*P < 0.05).
Discussion
Endometriosis, the presence of endometrial glands and stroma outside of the uterine cavity, is one of the most common causes of chronic pelvic pain and infertility (Eskenazi and Warner, 1997; Bulun, 2009; Giudice, 2010). While the existence of this disease has been known for >100 years, our current knowledge of the pathogenesis of the spontaneous evolution and the pathophysiology of the related infertility remains unclear most significantly, because women have had established disease for 8–11 years at the time of clinical presentation (Verkauf, 1987; Giudice, 2010). Menstruating primates develop endometriosis, and over the past several years, it has become evident that the baboon is the most appropriate and clinically relevant model to study the pathophysiology of endometriosis (Fazleabas et al., 2002; Braundmeier and Fazleabas, 2009; D'Hooghe et al., 2009; Fazleabas, 2010).
Identification of mechanisms involved in the early pathogenesis of endometriosis is critical to the development of improved methods of diagnosis and treatment. Using our baboon model of endometriosis, we have demonstrated that the presence of ectopic endometriotic lesions alters the EUE gene signature leading to phenotypic changes (Jackson et al., 2007; Braundmeier et al., 2010; Fazleabas, 2010; Afshar et al., 2013). This notion is well supported by the observation that surgical removal of endometriotic lesions and treatment with GnRH analogs improve the pregnancy outcomes in a subset of women with endometriosis (Lessey and Young, 1997; Surrey and Schoolcraft, 2003; Littman et al., 2005). We and others have also confirmed that these phenotypic changes are also evident in baboons with disease (Braundmeier and Fazleabas, 2009; Fazleabas, 2010). The mechanisms driving these phenotypic changes which occur in both the EUE and ectopic lesions are not clear. In the present study using the baboon model, we have shown that the induction of endometriosis leads to significant changes in the expression of several miRs within 3 months, of which miR-451 was the most highly down-regulated (∼15-fold) in the mid-secretory phase compared with controls. Quantitative RT–PCR analysis confirmed that miR-451 was down-regulated in the EUE of baboon and women with endometriosis during mid-secretory phase compared with controls. The decrease in miR-451 expression leads to significant increases in the mRNA expression of its predicted targets CDKN2D, GATAD2B and YWHAZ. YWHAZ protein was also significantly increased in the glandular epithelium of eutopic and ectopic endometrium of baboons with disease where miR-451 expression was decreased. In vitro 3′ UTR luciferase assay results demonstrated a significant reduction in luciferase activity for YWHAZ in the cells transfected with the miR-451 expression vector compared with control, confirming YWHAZ as a direct target of miR-451. Also the cell proliferation assay results showed that miR-451 mimic transfected 12Z cells resulted in a significantly decreased proliferation rate compared with nontargeting negative control. Thus, our current findings suggest the possibility that an increased expression of YWHAZ mediated by miR-451 down-regulation is responsible for the increased cell proliferation and decreased apoptotic response associated with endometriosis. The decrease in miR-451 in the ectopic lesions observed in our study compared with the increase in endometriomas reported by Hawkins et al. (2011) could be attributed to the fact that our analysis was limited to the peritoneal disease. In addition, we showed that at 15 months of the disease, the ectopic tissues have a higher expression of miR-451 compared with EUE at the same time point; however, this increase was not statistically significant.
It is evident from the literature that miR-451 functions as a tumor suppressor, and its down-regulation is associated with poor prognosis in several cancers including endometrial cancer (Lawrie, 2007; Bandres et al., 2009; Cohn et al., 2010; Godlewski et al., 2010b; Wang et al., 2011; Bianchi et al., 2012). Overexpression of miR-451 significantly reduced levels of YWHAZ transcript and protein in both 12Z and EEC cells. We next evaluated the effect of miR-451 on proliferation rate using 12Z cells (endometriotic epithelial cells). 12Z cells transfected with miR-451 mimics showed a markedly decreased proliferation compared with nontargeting negative controls transfected 12Z cells. Previous studies have also reported that overexpression of miR-451 in QGY-7703, Hep3B and MCF-7 cells greatly reduces YWHAZ expression and inhibits the cell proliferation, which further supports our hypothesis that the increase in YWHAZ contributes to the increased proliferation associated with endometriosis (Bergamaschi and Katzenellenbogen, 2012; Zhang et al., 2012).
PI3K/AKT and MAPK/ERK signaling pathways are reported to be altered in women with endometriosis (Velarde et al., 2009; Yin et al., 2012; Eaton et al., 2013). Additionally, based on the baboon gene array results, our laboratory has previously reported changes in eutopic endometrial gene expression during the progression of endometriosis, and subsequent pathway analysis revealed that a number of PI3K/AKT and MAPK/ERK pathway genes are modulated including YWHAZ (Afshar et al., 2013). Other studies have reported that miR-451 inhibits the PI3K/AKT pathway in human glioma via CAB39 protein and suppresses cell proliferation (Nan et al., 2010; Tian et al., 2012). However, the pathway by which the decrease in miR-451 enhances cell proliferation in both eutopic and ectopic endometrium as a consequence of endometriosis is unclear. In the light of available literature and our previous gene array data, we hypothesize that miR-451 may increase cell proliferation as a result of aberrant PI3K/AKT or ERK/MAPK pathways (Eaton et al., 2013). An alternate mechanism by which the decrease in miR-451 leading to an increase in YWHAZ expression to inhibit apoptosis and promote proliferation is by YWHAZ binding to and stabilizing nuclear β-catenin to modulate the expression of its target genes (Chen et al., 2012). The Wnt/ β-catenin signaling pathway is aberrantly activated in both eutopic and ectopic tissues of women with endometriosis (Matsuzaki et al., 2010; Matsuzaki and Darcha, 2012), but the mechanism by which β-catenin is translocated to the nucleus and protected from degradation is unknown. We observed increased nuclear accumulation of β-catenin (data not shown), along with YWHAZ, in endometriotic lesions from women and baboons. We hypothesize that the increase in YWHAZ in endometriotic tissues stabilizes β-catenin and translocates the complex to the nucleus to activate aberrant β-catenin-dependent transcription in endometriotic tissues. Our current studies are focused on testing this proposed hypothesis and in identifying the mechanism by which the increased expression of YWHAZ regulates β-catenin function and contributes to the pathophysiology of endometriosis. Our studies reveal a crucial role for miR-451 in regulating the expression of genes involved in cell proliferation and the inhibition apoptosis, which are physiological responses associated with the pathophysiology of endometriosis in both baboons and women.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
N.R.J. designed and performed the experiments, analyzed the data and wrote the manuscript. R.W.S. performed and analyzed ISH and 3′ UTR luciferase assays. G.V.R.C. analyzed the miR array data. S.K.K. performed the miR array and analyzed it. J-W.J. contributed toward data interpretation and editing of the manuscript. B.A.L. and S.L.Y. contributed to sample collection and critical revision of the manuscript. A.T.F. contributed to the study design, interpretation of the data and assisted with writing the manuscript.
Funding
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD40093 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R21 HD 082453 (to A.T.F.) and R01 HD 067721 (to S.L.Y. and B.A.L.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Ms Plavita Sharma and Ms Samantha Bond for their help with RNA isolation and cDNA synthesis of miRs and their targets and Dr Anna Starzinski-Powitz (University of Frankfurt, Germany) for generously providing us with the 12Z cells. We would also like to appreciate the contribution of Mark Olson and Angela Houwing for baboon and human sample preparation, respectively.
References
- Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod 2013;88:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci 2011;18:229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ et al. MicroRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res 2009;15:2281–2290. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril 2008;90:972–987. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- Bergamaschi A, Katzenellenbogen BS. Tamoxifen down-regulation of miR-451 increases 14-3-3zeta and promotes breast cancer cell survival and endocrine resistance. Oncogene 2012;31:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi N, Zuccato C, Finotti A, Lampronti I, Borgatti M, Gambari R. Involvement of miRNA in erythroid differentiation. Epigenomics 2012;4:51–65. [DOI] [PubMed] [Google Scholar]
- Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011;29:1661–1671. [DOI] [PubMed] [Google Scholar]
- Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol 2008;110:206–215. [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod 2009;15:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT, Nowak RA. Extracellular matrix metalloproteinase inducer expression in the baboon endometrium: menstrual cycle and endometriosis. Reproduction 2010;140:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braza-Boils A, Mari-Alexandre J, Gilabert J, Sanchez-Izquierdo D, Espana F, Estelles A, Gilabert-Estelles J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod 2014;29:978–988. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod 2009;15:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chuang SM, Yang MF, Liao JW, Yu SL, Chen JJ. A novel function of YWHAZ/beta-catenin axis in promoting epithelial-mesenchymal transition and lung cancer metastasis. Mol Cancer Res 2012;10:1319–1331. [DOI] [PubMed] [Google Scholar]
- Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 2015;103:1252–1260 e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW et al. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer 2009;124:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn DE, Fabbri M, Valeri N, Alder H, Ivanov I, Liu CG, Croce CM, Resnick KE. Comprehensive miRNA profiling of surgically staged endometrial cancer. Am J Obstet Gynecol 2010;202:656 e651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, Nathanielsz PW, Nicolella DP, Shade RE, Voruganti S et al. Baboons as a model to study genetics and epigenetics of human disease. ILAR J 2013;54:106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Benham AL, Zhu H, Khan MF, Reid JG, Nagaraja AK, Fountain MD, Dziadek O, Han D, Ma L et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One 2010;5:e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci 2009;16:152–161. [DOI] [PubMed] [Google Scholar]
- Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development 2005;132:4645–4652. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab 2013;98:E1871–E1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997;24:235–258. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med 2010;28:75–80. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann NY Acad Sci 2002;955:308–317; discussion 340–302, 396–406. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010;2010:369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrich DG, Lessey BA, Savaris RF. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol 2013;35:210–216. [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. microRNA-451: a conditional switch controlling glioma cell proliferation and migration. Cell Cycle 2010a;9:2742–2748. [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 2010b;37:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Falcone T, Nothnick WB. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum Reprod 2015;30:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007;27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harirchian P, Gashaw I, Lipskind ST, Braundmeier AG, Hastings JM, Olson MR, Fazleabas AT. Lesion kinetics in a non-human primate model of endometriosis. Hum Reprod 2012;27:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol 2006;4 (Suppl. 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol 2011;25:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroki E, Akahira J, Suzuki F, Nagase S, Ito K, Suzuki T, Sasano H, Yaegashi N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci 2010;101:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Kehlen A, Fowler PA, Huppertz B, Jugert JF, Bischoff G, Schluter E, Buchmann J, Klonisch T. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol 2005;34:517–534. [DOI] [PubMed] [Google Scholar]
- Hull ML, Nisenblat V. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reprod Biomed Online 2013;27:515–529. [DOI] [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci 2007;14:137–150. [DOI] [PubMed] [Google Scholar]
- Karere GM, Glenn JP, VandeBerg JL, Cox LA. Identification of baboon microRNAs expressed in liver and lymphocytes. J Biomed Sci 2010;17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karere GM, Glenn JP, VandeBerg JL, Cox LA. Differential microRNA response to a high-cholesterol, high-fat diet in livers of low and high LDL-C baboons. BMC Genomics 2012;13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie CH. MicroRNAs and haematology: small molecules, big function. Br J Haematol 2007;137:503–512. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Young SL. Integrins and other cell adhesion molecules in endometrium and endometriosis. Semin Reprod Endocrinol 1997;15:291–299. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- Li Z, Jia J, Gou J, Zhao X, Yi T. MicroRNA-451 plays a role in murine embryo implantation through targeting Ankrd46, as implicated by a microarray-based analysis. Fertil Steril 2015;103:834–844 e834. [DOI] [PubMed] [Google Scholar]
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril 2005;84:1574–1578. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- Mari-Alexandre J, Garcia-Oms J, Barcelo-Molina M, Gilabert-Aguilar J, Estelles A, Braza-Boils A, Gilabert-Estelles J. microRNAs and angiogenesis in endometriosis. Thromb Res 2015;135 (Suppl. 1):S38–S40. [DOI] [PubMed] [Google Scholar]
- Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril 2008;89:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod 2012;27:712–721. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab 2010;95:3437–3445. [DOI] [PubMed] [Google Scholar]
- Murata K, Yoshitomi H, Furu M, Ishikawa M, Shibuya H, Ito H, Matsuda S. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol 2014;66:549–559. [DOI] [PubMed] [Google Scholar]
- Nagaraja AK, Creighton CJ, Yu Z, Zhu H, Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 2010;24:447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res 2010;1359:14–21. [DOI] [PubMed] [Google Scholar]
- Neal CL, Yu D. 14-3-3zeta as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets 2010;14:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan KH, Ensor J et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 2009;69:3425–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CL, Xu J, Li P, Mori S, Yang J, Neal NN, Zhou X, Wyszomierski SL, Yu D. Overexpression of 14-3-3zeta in cancer cells activates PI3 K via binding the p85 regulatory subunit. Oncogene 2012;31:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Wilfred BR. In situ hybridization is a necessary experimental complement to microRNA (miRNA) expression profiling in the human brain. Neurosci Lett 2009;466:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemantsverdriet M, Wagner K, Visser M, Backendorf C. Cellular functions of 14-3-3 zeta in apoptosis and cell adhesion emphasize its oncogenic character. Oncogene 2008;27:1315–1319. [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Graham A, Holbert J, Weiss MJ. miR-451 deficiency is associated with altered endometrial fibrinogen alpha chain expression and reduced endometriotic implant establishment in an experimental mouse model. PLoS One 2014;9:e100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 2009;23:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano Y, Kai K, Moriyama M, Narahara H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod 2014;30:632–641. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927;3:93–110 143. [PMC free article] [PubMed] [Google Scholar]
- Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertil Steril 2014;101:1545–1551. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004;3:1–25, Article3. [DOI] [PubMed] [Google Scholar]
- Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab 2015;100:E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey ES, Schoolcraft WB. Does surgical management of endometriosis within 6 months of an in vitro fertilization-embryo transfer cycle improve outcome? J Assist Reprod Genet 2003;20:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 2010;16:142–165. [DOI] [PubMed] [Google Scholar]
- Tian Y, Nan Y, Han L, Zhang A, Wang G, Jia Z, Hao J, Pu P, Zhong Y, Kang C. MicroRNA miR-451 down-regulates the PI3 K/AKT pathway through CAB39 in human glioma. Int J Oncol 2012;40:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver S, Assou S, Scalici E, Haouzi D, Al-Edani T, Belloc S, Hamamah S. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update 2014;20:905–923. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 2009;150:4701–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc 1987;74:671–675. [PubMed] [Google Scholar]
- Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 2007;46:336–347. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 2011;30:2644–2658. [DOI] [PubMed] [Google Scholar]
- Wilker E, Yaffe MB. 14-3-3 Proteins--a focus on cancer and human disease. J Mol Cell Cardiol 2004;37:633–642. [DOI] [PubMed] [Google Scholar]
- Wilker EW, Grant RA, Artim SC, Yaffe MB. A structural basis for 14-3-3sigma functional specificity. J Biol Chem 2005;280:18891–18898. [DOI] [PubMed] [Google Scholar]
- Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab 2011;22:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3 K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 2012;97:E35–E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol 2001;159:1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA 2008;105:7004–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Luo X, Ding S, Chen J, Chen T, Chen X, Zha H, Yao L, He X, Peng H. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett 2012;586:20–26. [DOI] [PubMed] [Google Scholar]
- Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies versus normal pregnancies. Am J Obstet Gynecol 2009;200:661.e661–667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.