Abstract
STUDY QUESTION
How well can a single baseline ultrasound assessment of fibroid burden (presence or absence of fibroids and size of largest, if present) predict future probability of having a major uterine procedure?
SUMMARY ANSWER
During an 8-year follow-up period, the risk of having a major uterine procedure was 2% for those without fibroids and increased with fibroid size for those with fibroids, reaching 47% for those with fibroids ≥4 cm in diameter at baseline.
WHAT IS KNOWN ALREADY
Uterine fibroids are a leading indication for hysterectomy. However, when fibroids are found, there are few available data to help clinicians advise patients about disease progression.
STUDY DESIGN, SIZE, DURATION
Women who were 35–49 years old were randomly selected from the membership of a large urban health plan; 80% of those determined to be eligible were enrolled and screened with ultrasound for fibroids ≥0.5 cm in diameter. African-American and white premenopausal participants who responded to at least one follow-up interview (N = 964, 85% of those eligible) constituted the study cohort. During follow-up (5822 person-years), participants self-reported any major uterine procedure (67% hysterectomies). Life-table analyses and Cox regression (with censoring for menopause) were used to estimate the risk of having a uterine procedure for women with no fibroids, small (<2 cm in diameter), medium (2–3.9 cm), and large fibroids (≥4 cm). Differences between African-American and white women, importance of a clinical diagnosis of fibroids prior to study enrollment, and the impact of submucosal fibroids on risk were investigated.
PARTICIPANTS/MATERIALS, SETTING, METHODS
There was a greater loss to follow-up for African-Americans than whites (19 versus 11%). For those with follow-up data, 64% had fibroids at baseline, 33% of whom had had a prior diagnosis. Of those with fibroids, 27% had small fibroids (<2 cm in diameter), 46% had medium (largest fibroid 2–3.9 cm in diameter), and 27% had large fibroids (largest ≥4 cm in diameter). Twenty-one percent had at least one submucosal fibroid.
MAIN RESULTS AND THE ROLE OF CHANCE
Major uterine procedures were reported by 115 women during follow-up. The estimated risk of having a procedure in any given year of follow-up for those with fibroids compared with those without fibroids increased markedly with fibroid-size category (from 4-fold, confidence interval (CI) (1.4–11.1) for the small fibroids to 10-fold, CI (4.4–24.8) for the medium fibroids, to 27-fold, CI (11.5–65.2) for the large fibroids). This influence of fibroid size on risk did not differ between African-Americans and whites (P-value for interaction = 0.88). Once fibroid size at enrollment was accounted for, having a prior diagnosis at the time of ultrasound screening was not predictive of having a procedure. Exclusion of women with a submucosal fibroid had little influence on the results. The 8-year risk of a procedure based on lifetable analyses was 2% for women with no fibroids, 8, 23, and 47%, respectively, for women who had small, medium or large fibroids at enrollment. Given the strong association of fibroid size with subsequent risk of a procedure, these findings are unlikely to be due to chance.
LIMITATIONS, REASONS FOR CAUTION
Despite a large sample size, the number of women having procedures during follow-up was relatively small. Thus, covariates such as BMI, which were not important in our analyses, may have associations that were too small to detect with our sample size. Another limitation is that the medical procedures were self-reported. However, we attempted to retrieve medical records when participants agreed, and 77% of the total procedures reported were verified. Our findings are likely to be generalizable to other African-American and white premenopausal women in their late 30s and 40s, but other ethnic groups have not been studied.
WIDER IMPLICATIONS OF THE FINDINGS
Though further studies are needed to confirm and extend the results, our findings provide an initial estimate of disease progression that will be helpful to clinicians and their patients.
STUDY FUNDING/COMPETING INTEREST(S)
Funding came from the Intramural Research Program of the National Institute of Environmental Health Sciences and the Office of Research on Minority Health, National Institutes of Health, Health and Human Services (IRB #OH95-E-N048). The authors have no conflicts of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: uterine leiomyoma, ultrasonography, disease progression, hysterectomy, heath disparity
Introduction
Uterine leiomyomata (fibroids) are hormonally dependent benign tumors that develop during reproductive years in the majority of women (Stewart, 2001; Baird et al., 2003). Many women are asymptomatic, but others experience heavy vaginal bleeding, pelvic pain and/or reproductive problems (Stewart, 2001; Divakar, 2008). There is marked racial/ethnic disparity in the development of fibroids, with disease onset estimated to be 10–15 years earlier for African-Americans than for whites (Laughlin et al., 2010b). Fibroids are a leading indication for hysterectomy (Vessey et al., 1992; Farquhar and Steiner, 2002; Marret et al., 2012). African-Americans have a particularly high rate of hysterectomy for fibroids, 20% by age of menopause compared with 7% in whites (Myers et al., 2001). The cost of this condition in the USA is estimated at $6–34 billion annually (Cardozo et al., 2012).
Clinical diagnosis of fibroids can occur at all stages of disease development from small, incidentally identified tumors to large symptomatic tumors (Divakar, 2008; Laughlin and Stewart, 2011). Without an approved pharmacologic treatment that limits fibroid growth and delays symptoms, there has been no impetus for early diagnosis. Initial treatment is usually focused on watchful waiting or symptom reduction with analgesics or hormonal contraceptives. In most cases the fibroids themselves are treated only if symptoms are severe. The primary options for women with severe symptoms are major uterine procedures including uterine artery embolization, MRI-guided focused ultrasound, and surgery (Segars et al., 2014). Hysterectomy has long been the mainstay treatment in the USA (Laughlin and Stewart, 2011).
Little is known about disease progression (Jacoby et al., 2014), though this would be helpful for counseling women. Some women have a major uterine procedure soon after initial clinical diagnosis (Hartmann et al., 2006), while others never have such treatment. Of the five longitudinal studies of fibroid growth (Ichimura et al., 1998; Tsuda et al., 1998; DeWaay et al., 2002; Peddada et al., 2008; Mavrelos et al., 2010), all were short-term and all reported that the individual tumor growth rates were highly variable. The largest study (n = 262 fibroids followed with MRI for up to a year) found a median growth of 9% per 6 months, but rates varied from 89% shrinkage to 138% growth per 6 months (Peddada et al., 2008). Data from the same sample of tumors showed that rapid growth often came in spurts and was rarely sustained over time (Baird et al., 2011). Thus, long-term growth might be quite different from the short-term growth that has been studied. Almost nothing is known about symptom development in relation to fibroid growth, though data indicate that women with large fibroids are more likely to have symptoms than those with small fibroids (Stewart, 2001; DeWaay et al., 2002; Wegienka et al., 2003; Dragomir et al., 2010).
The National Institute of Environmental Health Sciences Uterine Fibroid Study used ultrasound to screen a large, randomly selected sample of premenopausal African-American and white women for fibroids. Study participants were then followed for up to 8 years to assess the occurrence of major uterine procedures. We report the association between the single baseline ultrasound assessment for presence of fibroids and tumor size (if present) and the subsequent occurrence of a major uterine procedure.
Materials and Methods
Study design
The Uterine Fibroid Study (UFS) is a prospective cohort study. Women, age 35–49 years, were randomly selected from the membership list of a large urban health plan and contacted about the study. Approximately 80% of those found to be eligible were enrolled, and response was similar for African-Americans and whites (Baird et al., 2003). At enrollment, which occurred in 1996–1999, premenopausal participants attended a clinic visit to give blood, have measurements taken (weight, blood pressure, waist and hip circumference), and have ultrasound screening for fibroids (unless a recent sonogram report was available from a recent clinical examination). Study participants were followed for up to 8 years with interviews to assess subsequent medical procedures (interview 1 in 2001–2002, interview 2 in 2004).
Participants
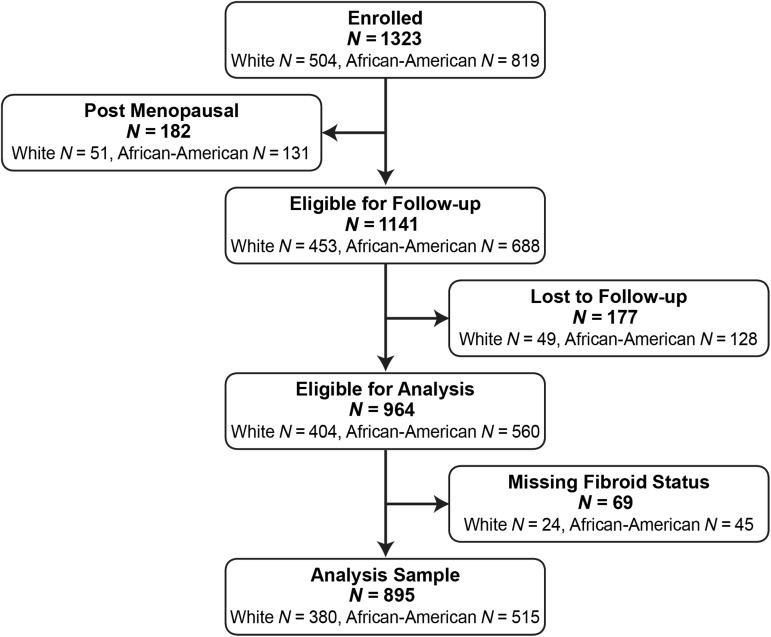
The initial study population consisted of 1430 women of whom 1323 were African-American or white women. Fourteen percent of these (n = 182) were post-menopausal at enrollment (mostly surgically menopausal) and were not followed prospectively. Eighty-five percent of the 1141 premenopausal African-American and white women eligible for follow-up completed either the first follow-up, the second follow-up, or both (N = 964, Fig. 1). Those lost to follow-up were not substantially different from the participants of the same ethnic group not lost to follow-up based on comparisons of descriptive variables such as age, education, body mass index, smoking habits, gravidity or parity. However, a larger percentage of African-Americans were lost to follow-up than whites (18.6 versus 10.8%). At enrollment, fibroid status could not be determined for 8% of African-Americans and 6% of whites (they did not complete the baseline ultrasound and no other data were available). These women were also excluded from this analysis (final analysis sample = 895).
Figure 1.
Flow chart showing the sample of National Institute of Environmental Health Sciences Uterine Fibroid Study participants available for this analysis.
Fibroid status assessment
Pelvic ultrasound examinations were used to assess participant's fibroid status regardless of whether or not the participant had had a prior clinical diagnosis of fibroids. For those who had recently had a pelvic ultrasound examination (16%), the radiology records from that examination were used to assess fibroid status. If the prior examination showed no fibroids and was done within 2 years, it was used; a sonogram showing fibroids was used if it had been done within 5 years. The study ultrasound for the remainder included both a transabdominal and transvaginal ultrasound examination. The abdominal portion was used to evaluate any fibroid that arose from the upper uterus that would not be readily seen with the transvaginal approach alone. Ultrasound examinations were conducted by experienced sonographers that were certified by the American Registry of Diagnostic Medical Sonographers and trained on the study protocol. They were under the direct supervision of a radiologist with fellowship training in ultrasound (author M.C.H.). The examinations were performed on ultrasound units ATL HDI 9, Acuson 128 XP, and Diasonics DRF 400 using transabdominal (3.5–5.0 mHz) and transvaginal (5.0–7.0 mHz) ultrasound probes. The uterus and fibroids of ≥0.5 cm were identified. Size of the uterus (volume calculated from three perpendicular diameters using the ellipsoid formula) and size of the largest fibroid (largest diameter) were measured. Any submucosal fibroids were noted. Submucosal was defined as adjacent to the endometrium with no visible normal myometrium between endometrium and fibroid. Any questionable sonograms were reviewed by a single radiologist (M.C.H.). We relied on self-report of fibroid size for a small subset of the sample who reported a previous fibroid diagnosis, but did not complete the study ultrasound (n = 28). Exclusion of these women did not change our findings. We categorized size of largest fibroid (no fibroids, largest fibroid <2 cm, 2–<4 cm, ≥4 cm).
Assessment of major uterine procedures during follow-up
Follow-up telephone interviews were conducted first in 2001–2002 and again in 2004. We gathered self-reported information on major uterine procedures (excluding pregnancy procedures). Women were asked specifically about hysterectomy, myomectomy, hysteroscopic resection, uterine artery embolization, endometrial ablation and ‘other’. The only ‘other’ procedure reported was thermal balloon ablation of the endometrium. We were able to collect medical records and confirm 88 of the 115 procedures identified. Six participants refused at the follow-up interview to release their medical records, 11 agreed but never returned the signed medical release form, and for 10 the medical provider failed to return the relevant information.
Other enrollment and follow-up data
Baseline interviews asked about demographic information (age, race/ethnicity and education), height, weight history, a full reproductive history, and lifestyle information including smoking and alcohol drinking. Baseline weight was measured at the clinic visit. At each follow-up, interviews asked about information on menstrual characteristics, time of last menstrual period, menopausal symptoms, weight, any pregnancies since last interview, and updated lifestyle information. All interviews were conducted by interviewers who were intensively trained on basic interview procedures and the study interviews in particular. Interviewers completed mock interviews that were monitored and approved before they collected data from participants, and occasional ongoing interviews were monitored. The staff for each of the three interviews numbered 10, 15, and 12 interviewers for baseline, follow-up 1, and follow-up 2, respectively, with several interviewers from baseline continuing with follow-up interviews.
Statistical analysis
Analyses were initially conducted for African-Americans and whites separately. We used discrete proportional hazards models to evaluate the association between fibroid size at enrollment and risk of future major uterine procedures. Time since enrollment was used as the longitudinal variable, and data were censored at menopause or end of follow-up. The strength of association was estimated with a hazard ratio, which estimates risk of a subsequent procedure for women in each baseline fibroid-size category relative to women with no fibroids at baseline. The potential confounders were age at baseline (enrollment), body mass index (BMI) and recent births, which might lead to fibroid regression (Laughlin et al., 2010a,b, 2011). BMI and births were entered as time-dependent variables using both baseline and follow-up data. BMI was categorized as shown in Table I. For births that occurred during follow-up, time since birth was coded as continuous in years starting with ‘0’ for the year of the birth. Years prior to any follow-up birth were coded as ‘9’. Thus, the time-dependent 8-year coding for a participant who had one birth during the third year of follow-up would be 9,9,0,1,2,3,4,5. Those not having a birth during follow-up were coded as ‘9’ throughout their follow-up years. Adjusting for age changed at least one of the hazard ratios by at least 10% and was the only potential confounder maintained in the model. We tested for ethnic differences in the association between fibroid size and the probability of a procedure by combining data for African-Americans and whites and including interaction terms for ethnicity by category of fibroid size. Because the associations were similar for African-Americans and whites (no interaction) we report the hazard ratios for the combined data set, controlling for ethnicity.
Table I.
Descriptive characteristics at enrollment, sample from National Institute of Environmental Health Sciences Uterine Fibroid Study.
| Whites 380 |
African-Americans 515 |
|||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Year of enrollment | ||||
| 1996 | 72 | (19) | 96 | (19) |
| 1997 | 160 | (42) | 224 | (44) |
| 1998 | 148 | (39) | 193 | (37) |
| 1999 | 0 | (0) | 2 | (<1) |
| Age at baseline (years) | ||||
| 35–37 | 77 | (20) | 109 | (21) |
| 38–40 | 76 | (20) | 116 | (23) |
| 41–43 | 78 | (21) | 113 | (22) |
| 44–46 | 82 | (21) | 109 | (21) |
| 47–50 | 67 | (18) | 68 | (13) |
| Education | ||||
| <High school | 0 | (0) | 9 | (2) |
| High school | 11 | (3) | 91 | (18) |
| Some college/no degree | 30 | (8) | 240 | (46) |
| College degree | 64 | (17) | 68 | (13) |
| College plus additional training | 62 | (16) | 45 | (9) |
| Post graduate degree | 212 | (56) | 60 | (12) |
| Missing | 1 | – | 2 | – |
| BMI (kg/m2) | ||||
| <25 | 222 | (58) | 133 | (26) |
| 25–29 | 91 | (24) | 154 | (30) |
| 30<35 | 33 | (9) | 104 | (20) |
| ≥35 | 34 | (9) | 124 | (24) |
| Smoking | ||||
| Never | 223 | (59) | 250 | (49) |
| Past | 131 | (34) | 115 | (22) |
| Current | ||||
| <10 cigarettes per day | 9 | (2) | 73 | (14) |
| 10–20 cigarettes per day | 10 | (3) | 37 | (7) |
| ≥20 cigarettes per day | 7 | (2) | 40 | (8) |
| Gravidity | ||||
| 0 | 152 | (40) | 46 | (9) |
| ≥1 | 228 | (60) | 469 | (91) |
| Parity | ||||
| 0 | 223 | (59) | 110 | (21) |
| 1 | 50 | (13) | 117 | (23) |
| ≥2 | 107 | (28) | 288 | (56) |
| Fibroids diagnosed prior to enrollment | ||||
| Yes | 61 | (16) | 234 | (45) |
| No | 319 | (84) | 281 | (55) |
| Fibroids at baselinea | ||||
| Yes | 189 | (50) | 383 | (74) |
| No | 191 | (50) | 132 | (26) |
| Fibroid size (diameter of largest fibroid) | ||||
| No fibroids | 191 | (50) | 132 | (26) |
| <2 cm | 66 | (17) | 89 | (17) |
| 2–3.99 cm | 80 | (21) | 184 | (36) |
| ≥4 cm | 43 | (11) | 110 | (21) |
| Fibroid type | ||||
| No fibroids | 191 | (51) | 132 | (27) |
| Submucosal | 37 | (10) | 81 | (17) |
| No submucosal | 144 | (39) | 274 | (56) |
| Missing fibroid type | 8 | – | 28 | – |
aIncludes newly detected and previously diagnosed.
Absolute cumulative risks of a procedure across follow-up time for separate fibroid size categories were calculated using life-table analyses. Women were followed in the life table until the first reported date of either a uterine procedure, menopause (date of last menstrual period plus 1 year), or end of follow-up (date of last follow-up interview).
Two secondary analyses were conducted. First we investigated whether women with a diagnosis of fibroids prior to study enrollment were more likely to have a procedure even after baseline fibroid size was taken into account, i.e. the impact of including a variable for prior diagnosis in the hazard model. Secondly, we investigated the influence of submucosal fibroids by re-running the primary hazard model after excluding women with at least one submucosal fibroid.
Ethical approval
The research was approved by the NIEHS and George Washington University Human Subjects Review Boards, and participants gave informed consent.
Results
The age distributions of white (N = 380) and African-American (N = 515) study participants were very similar (Table I). Most had more than a high school education, but white women tended to have more education and lower BMI than African-Americans. Only about 40% of whites had given birth, but most African-Americans were parous. About half of white women had fibroids, and about 20% of those had large fibroids (≥4 cm diameter). Nearly three-quarters of African-Americans had fibroids, and nearly a third of those had large tumors. The majority of whites with fibroids (68%) were new cases (fibroids were found at the baseline ultrasound, and there had been no previous diagnosis), while the majority of African-Americans with fibroids had been previously diagnosed (61%) (Table I).
Women were followed for up to 8 years (5822 person-years of follow-up, whites = 2502, African-Americans = 3320). Of the 895 women, 800 participated in both follow-ups, while 57 participated in only the first follow-up and 38 participated in only the second follow-up. Twenty-four percent of white women and 20% of African-American women went through natural menopause during follow-up. Follow-up time after menopause was not considered in analyses because fibroids often begin to shrink after menopause. A total of 115 women reported major uterine procedures during the follow-up (29 white and 86 African-American women). The majority of the procedures were hysterectomies and myomectomies (Table II). The symptoms that led to having a major uterine procedure were mostly bleeding and pelvic pain (Table III).
Table II.
Major uterine procedures during 8 years of follow-up, National Institute of Environmental Health Sciences Uterine Fibroid Study.
| Whites |
African-Americans |
|||
|---|---|---|---|---|
| Total | N = 29 | (%) | N = 86 | (%) |
| Hysterectomy | 13 | (45) | 64 | (74) |
| Myomectomy | 9 | (31) | 4 | (5) |
| Hysteroscopic resection | 5 | (17) | 7 | (8) |
| Uterine artery embolization | 0 | (0) | 9 | (11) |
| Endometrial ablation | 2 | (7) | 1 | (1) |
| Thermal balloon | 0 | (0) | 1 | (1) |
Table III.
Reported symptoms that led to having a major uterine procedure during 8 years of follow-up, National Institute of Environmental Health Sciences Uterine Fibroid Study.
| Symptom | Number of times reporteda |
|---|---|
| Bleeding | 72 |
| Pelvic pain | 50 |
| Back Pain | 8 |
| Urinary incontinence or urinary frequency | 6 |
| Infertility | 4 |
| Constipation/diarrhea | 2 |
| Leg pain | 1 |
| Don't know | 1 |
| Other, non-fibroid relatedb | 7 |
aWomen can report multiple symptoms. Six women were missing data on symptoms, and four reported that they did not have symptoms.
bIncludes items such as endometriosis, prolapse and cervical dysplasia.
Only 6 of the 323 women without fibroids at enrollment went on to have a major uterine procedure during their follow-up (2 African-American women and 4 white women). All were hysterectomies, five for endometriosis or prolapse, and one for both adenomyosis and fibroids.
The hazard ratios for having a procedure during follow-up increased dramatically with the fibroid-size category in both African-Americans and whites. When we combined African-Americans and whites to test for ethnic differences in the association between fibroid size at enrollment and risk of a uterine procedure during follow-up, we found no evidence for ethnic differences (P = 0.88). In the combined data the hazard ratio for a uterine procedure, adjusted for age and ethnicity (aHR), rose dramatically with size of the largest fibroid (aHR = 4.0, 95% CI = 1.4–11.1 for <2 cm, aHR = 10.4, 95% CI = 4.4–24.8 for 2–<4 cm, and aHR = 27.4, 95% CI = 11.5–65.2 for ≥4 cm) (Table IV).
Table IV.
Crude and adjusted hazard ratios (HR) for having a major uterine procedure associated with fibroid size at enrollment (diameter of largest fibroid), National Institute of Environmental Health Sciences Uterine Fibroid Study 8-year follow-up (N = 895).
| Fibroid size | N | HR | 95% CIb | aHRa | 95% CIb |
|---|---|---|---|---|---|
| No fibroids | 323 | 1.0 | – | 1.0 | – |
| <2 cm | 155 | 3.9 | 1.4–10.6 | 4.0 | 1.4–11.1 |
| 2–3.99 cm | 264 | 10.4 | 4.4–24.5 | 10.4 | 4.4–24.8 |
| ≥4 cm | 153 | 26.7 | 11.6–62.4 | 27.4 | 11.5–65.2 |
aModels adjusted for age and ethnicity.
bCI, confidence interval.
In secondary analyses we considered whether women who had a clinical diagnosis of fibroids prior to study enrollment (52% of those with fibroids), were more likely to have a procedure during follow-up than those whose fibroids were first identified at the study ultrasound. When ‘prior diagnosis’ was included in the hazard model along with the categories for fibroid size, it was not significantly related to having a major uterine procedure during follow-up (aHR = 1.4, 95% CI = 0.9–2.2), and the aHRs for each fibroid-size category were not substantially different from the estimates from the model without adjustment for prior diagnosis. When we investigated the impact of submucosal fibroids by excluding the 118 women who had at least one such fibroid, the fibroid-size-specific hazard ratios did not change substantially (aHR = 2.9 for small, 10.4 for medium, and 31.6 for large fibroids) (Supplementary Table SI). Nor was having a submucosal fibroid a significant predictor of subsequent procedures when it was included as a variable in a hazard model that included fibroid size (P = 0.59).
Our life-table analysis to calculate the fibroid-size-specific cumulative risk of a procedure over the up to 8-year follow-up is shown in Fig. 2. For those without fibroids at baseline, the cumulative probability of a procedure at 8 years was 2%; it was 8, 23 and 47% for those with small, medium and large fibroids, respectively. For the latter group, the cumulative risk of a procedure increased rapidly from the start of follow-up, reaching over 10% during the first year, 20% by the end of 2 years, and 30% by the end of 4 years. The cumulative risk for those with medium fibroids exceeded 10% only after 4 years of follow-up. Those with small fibroids still had only about a 5% cumulative risk after 4 years.
Figure 2.
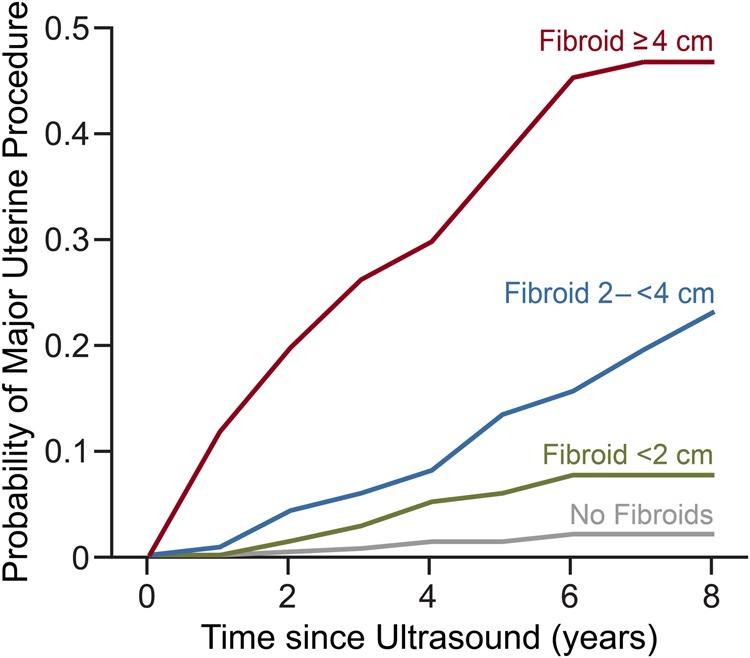
Cumulative probability of having a major uterine procedure for women with increasing fibroid size (categories based on diameter of largest fibroid), National Institute of Environmental Health Sciences Uterine Fibroid Study.
Discussion
We followed premenopausal women ages 35–49 years who had been randomly selected at baseline from a large health plan. The 8-year probability of having a major uterine procedure was highly dependent on ultrasound assessed fibroid status at baseline (presence or absence of fibroids and size of largest, if present). Women with no evidence of fibroids at baseline had only a 2% cumulative probability of a uterine procedure, but it rose to 47% for those who had baseline fibroids ≥ 4 cm in diameter.
Consistent with other studies (Laughlin et al., 2010b), at enrollment African-American study participants were more likely than whites to have fibroids, and their fibroids were larger (Baird et al., 2003). However, when we conducted the follow-up, the probability of a major uterine procedure during follow-up was similar for African-Americans and whites once baseline fibroid size was taken into account. A previous study that reported higher rates of hysterectomy for blacks with fibroids compared with whites with fibroids (Bower et al., 2009) did not take fibroid size into account.
Our study has several strengths. No previous study has assessed fibroid status with ultrasound data on fibroid size in a randomly selected sample of women and then followed them to determine the risk of a major uterine procedure. Initial response rates were high, thus limiting selection bias in our findings. Our sample included both African-American and white women in sufficient numbers to evaluate each group separately. The standardized ultrasound examination reduced misclassification of presence and absence of fibroids, and provided a standardized measure of fibroid size. Our detailed questionnaire data allowed us to evaluate confounding and censor women when they became naturally menopausal during the follow-up.
Our study also has limitations. We followed women to assess their major uterine procedures, but we were not able to do periodic systematic ultrasound examinations during follow-up. Unlike a direct measure of fibroid growth, our measure of disease progression (time to major uterine procedure) will vary both with women's perceptions of symptoms and with clinical practice. Also, though our baseline sample size was relatively large, the number of women who underwent a procedure was limited, so small effects would not have been detected. For example, covariates such as BMI may have effects on the risk of a uterine procedure that were too small for our study to detect. We had too few Hispanic or Asian women to evaluate risk of a procedure for these groups.
An aspect of our design that might have led to bias is that our study participants were told their fibroid status after the baseline ultrasound. Nearly half (48%) had not been previously diagnosed. It seems possible that this knowledge could have affected their perceptions of symptoms and future decisions to have major uterine procedures. However, a previous investigation found no evidence that symptom reporting was influenced by knowledge of fibroids (Wegienka et al., 2004), and treatment is generally reserved for those with symptoms (Stein and Ascher-Walsh, 2009). Furthermore, when we evaluated the influence of having had a clinical diagnosis of fibroids before baseline, it was not important once we accounted for tumor size at baseline. Another limitation is that the uterine procedures were self-reported, but most were verified with medical records.
Perhaps the most important limitation is that we used a single, simple measure to assess fibroid status at baseline (fibroid presence or absence and size of largest, if present). Other measures of fibroid burden need to be investigated such as categories of submucosal, intramural, or subserosal as recommended by the International Federation of Gynecology and Obstetrics (FIGO) (Munro et al., 2011). We did not have sufficiently detailed fibroid data to use the full FIGO categorization, but we did consider the influence of submucosal fibroids. First, we excluded women with any submucosal fibroids. Then we reanalyzed the full data set with the dichotomous variable ‘any submucosal fibroid’ added to control for this factor. Neither including the dichotomous variable nor excluding women with submucosal fibroids changed the results in any substantial way. However, this sensitivity analysis relies upon our ultrasound measure of fibroid location, which is less accurate than the more invasive procedures of infusion sonography or hysteroscopy.
To further explore alternative ways of defining baseline fibroid burden, we have also conducted supplemental analyses using a tumor burden variable that includes baseline uterine size. This has the advantage of integrating the impact of multiple fibroids (Supplementary Table SII). This definition of fibroid burden was again highly predictive. The estimated risk of a procedure for women with the highest uterine-size category (≥250 cm3) was 45-fold higher than for the women with no fibroids at baseline. In addition, the association between this measure of fibroid burden with risk of a future major uterine procedure was similar for African-Americans and whites (P-value for interaction = 0.92).
The similar predictive power of simple measures of fibroid burden in 35–49 year-old African-American and white women may be surprising, given the higher fibroid prevalence and symptom severity in African-Americans. However, it is consistent with the idea that the African-American/white disparity in fibroid-related health issues is primarily attributable to the approximately 10-year earlier onset of this condition in African-American women, not to a difference in how fibroids impact symptomatology once the tumors are present.
Large fibroids are more likely to cause health problems than small fibroids (Stewart, 2001; Wegienka et al., 2003; Dragomir et al., 2010), but information on the natural history of fibroid development is limited (Ichimura et al., 1998; Tsuda et al., 1998; DeWaay et al., 2002; Peddada et al., 2008; Mavrelos et al., 2010). The data on short-term fibroid growth indicate that individual fibroids grow at different rates, and sporadic growth spurts are common (Peddada et al., 2008; Baird et al., 2011). Thus, it would not have been surprising to find that data from a single ultrasound would have little predictive benefit. Instead, the single ultrasound assessment of fibroid size was highly predictive of future uterine procedures. It is likely that several of the women who had no fibroids at enrollment developed them during follow-up, but they remained at low cumulative risk of a procedure. This suggests that it is rare for fibroids to cause major problems during the first few years of their development. Likewise, women with small fibroids at baseline were at low cumulative risk. This may indicate that, despite variation in growth rates, it usually takes many years for small fibroids to grow to a size that lead to major uterine procedures. A careful, long-term study of fibroid growth with periodic assessment of fibroid size is needed.
Medical management of fibroids may be at the cusp of transition. There is a current effort to find pharmacological treatments that shrink fibroids and reduce symptoms (Sabry and Al-Hendy, 2012). Ulipristal acetate, a selective progesterone receptor modulator, is one such treatment that has been approved for pre-surgery use in Europe and Canada (Croxtall, 2012; Donnez et al., 2012; Talaulikar and Manyonda, 2012). Recent studies of up to four 3-month treatment intervals have demonstrated that ulipristal acetate may effectively manage fibroid symptoms over time; many study participants opted to forego surgery (Galliano, 2015). Our estimates of time without a treatment procedure in women in different fibroid-size categories could assist in developing efficient study designs for further investigations of non-invasive treatment strategies.
In conclusion, we found that the 8-year cumulative probability of having a major uterine procedure for 35–49 year-old women varied substantially by baseline fibroid burden, as measured in a single ultrasound examination. There was a similar increase in risk for African-American and white women, and the data are likely to be generalizable to other women of these ethnic groups in their late 30s and 40s. Though further studies are needed to confirm and extend our findings, our data on disease progression provide initial estimates that will be very helpful for counseling women.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
All authors interpreted the data, assisted with manuscript drafts, and approved the final version to be published. D.D.B. conceived the study design, acquired the data, drafted the initial manuscript, and is the guarantor for the study. T.M.S. created datasets and analysis variables, and analyzed the data. D.L.S. provided statistical consultation for design and implementation of the data analyses. M.C.H. oversaw the ultrasound screening for fibroids. J.M.S. oversaw the clinical management of the study.
Funding
Funding came from the Intramural Research Program of the National Institute of Environmental Health Sciences and the Office of Research on Minority Health, National Institutes of Health, Health and Human Services (IRB #OH95-E-N048).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
Drs Allen Wilcox and Todd Jusko provided helpful comments on an earlier version of the manuscript. Dr Sue Edelstein prepared the figures.
References
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107. [DOI] [PubMed] [Google Scholar]
- Baird DD, Garrett TA, Laughlin SK, Davis B, Semelka RC, Peddada SD. Short-term change in growth of uterine leiomyoma: tumor growth spurts. Fertil Steril 2011;95:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health 2009;99:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211 e211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD. Ulipristal acetate: in uterine fibroids. Drugs 2012;72:1075–1085. [DOI] [PubMed] [Google Scholar]
- DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol 2002;100:3–7. [DOI] [PubMed] [Google Scholar]
- Divakar H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2008;22:643–654. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421–432. [DOI] [PubMed] [Google Scholar]
- Dragomir AD, Schroeder JC, Connolly A, Kupper LL, Cousins DS, Olshan AF, Baird DD. Uterine leiomyomata associated with self-reported stress urinary incontinence. J Womens Health (Larchmt) 2010;19:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol 2002;99:229–234. [DOI] [PubMed] [Google Scholar]
- Galliano D. Ulipristal acetate in uterine fibroids. Fertil Steril 2015;103:359–360. [DOI] [PubMed] [Google Scholar]
- Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, Stang P. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol 2006;108:930–937. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Kawamura N, Ito F, Shibata S, Minakuchi K, Tsujimura A, Umesaki N, Ogita S. Correlation between the growth of uterine leiomyomata and estrogen and progesterone receptor content in needle biopsy specimens. Fertil Steril 1998;70:967–971. [DOI] [PubMed] [Google Scholar]
- Jacoby VL, Jacoby A, Learman LA, Schembri M, Gregorich SE, Jackson R, Kuppermann M. Use of medical, surgical and complementary treatments among women with fibroids. Eur J Obstet Gynecol Reprod Biol 2014;182:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Stewart EA. Uterine leiomyomas: individualizing the approach to a heterogeneous condition. Obstet Gynecol 2011;117:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Herring AH, Savitz DA, Olshan AF, Fielding JR, Hartmann KE, Baird DD. Pregnancy-related fibroid reduction. Fertil Steril 2010a;94:2421–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 2010b;28:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Hartmann KE, Baird DD. Postpartum factors and natural fibroid regression. Am J Obstet Gynecol 2011;204:496 e491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marret H, Fritel X, Ouldamer L, Bendifallah S, Brun JL, De Jesus I, Derrien J, Giraudet G, Kahn V, Koskas M et al. Therapeutic management of uterine fibroid tumors: updated French guidelines. Eur J Obstet Gynecol Reprod Biol 2012;165:156–164. [DOI] [PubMed] [Google Scholar]
- Mavrelos D, Ben-Nagi J, Holland T, Hoo W, Naftalin J, Jurkovic D. The natural history of fibroids. Ultrasound Obstet Gynecol 2010;35:238–242. [DOI] [PubMed] [Google Scholar]
- Munro MG, Critchley HO, Fraser IS, FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril 2011;95:2204–2208, 2208 e2201–2203. [DOI] [PubMed] [Google Scholar]
- Myers E, Barber MW, Couchman GM, Datta S, Gray RN, Gustilo-Ashby T, Kolimaga JT, McCrory DC. Management of uterine fibroids (Evidence Report/Technology Assessment No. 34, contract 290-97-0014 to the Duke Evidence-based Practice Center). Rockville, MD: Agency for Healthcare Research and Quality, 2001. [PMC free article] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA 2008;105:19887–19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry M, Al-Hendy A. Innovative oral treatments of uterine leiomyoma. Obstet Gynecol Int 2012;2012:943635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, Pinn VW, Dixon D. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 2014;20:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K, Ascher-Walsh C. A comprehensive approach to the treatment of uterine leiomyomata. Mt Sinai J Med 2009;76:546–556. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet 2001;357:293–298. [DOI] [PubMed] [Google Scholar]
- Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther 2012;29:655–663. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Kawabata M, Nakamoto O, Yamamoto K. Clinical predictors in the natural history of uterine leiomyoma: preliminary study. J Ultrasound Med 1998;17:17–20. [DOI] [PubMed] [Google Scholar]
- Vessey MP, Villard-Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. Br J Obstet Gynaecol 1992;99:402–407. [DOI] [PubMed] [Google Scholar]
- Wegienka G, Baird DD, Hertz-Picciotto I, Harlow SD, Steege JF, Hill MC, Schectman JM, Hartmann KE. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol 2003;101:431–437. [DOI] [PubMed] [Google Scholar]
- Wegienka G, Baird DD, Hertz-Picciotto I, Harlow SD, Hartmann KE. Uterine leiomyomata (fibroids): are bleeding symptoms more likely to be reported after diagnosis? J Clin Epidemiol 2004;57:318–320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.