Abstract
STUDY QUESTION
Are there any associations of dietary patterns with semen quality, reproductive hormone levels, and testicular volume, as markers of testicular function?
SUMMARY ANSWER
These results suggest that traditional Mediterranean diets may have a positive impact on male reproductive potential.
WHAT IS KNOWN ALREADY
The Mediterranean diet has been related to lower risk of multiple chronic diseases, but its effects on reproduction potential are unclear.
STUDY DESIGN, SIZE, DURATION
Cross-sectional sample of 215 male university students recruited from October 2010 to November 2011 in Murcia Region (Spain).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Two hundred and nine healthy men aged 18–23 years were finally included in this analysis. Diet was assessed using a validated food frequency questionnaire, and dietary patterns were identified by factor analysis. Linear regression was used to analyze the relation between diet patterns with semen quality parameters, reproductive hormone levels and testicular volume adjusting for potential confounders.
MAIN RESULTS AND THE ROLE OF CHANCE
We identified two dietary patterns: a Mediterranean (characterized by high intakes of vegetables, fruits and seafood) and a Western pattern (characterized by high intakes of processed meats, French fries and snacks). The Mediterranean pattern was positively associated with total sperm count (P, trend = 0.04). The Western pattern was positively related to the percentage of morphologically normal sperm (P, trend = 0.008). We found an inverse association between adherence to the Western pattern and sperm concentration among overweight or obese men (P, trend = 0.04).
LIMITATIONS, REASONS FOR CAUTION
As with all cross-sectional studies, causal inference is limited. However, participants were blinded to the study outcomes thus reducing the potential influenced their report of diet. Although we adjusted for a large number of known and suspected confounders, we cannot exclude the possibility of residual confounding or chance findings.
WIDER IMPLICATIONS OF THE FINDINGS
This study was carried out on healthy and young men, so it is difficult to predict whether and how the observed differences in semen quality translate into reproductive success for men in couples trying to conceive. These results suggest that traditional Mediterranean diets may have a positive impact on male reproductive potential.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, Grant No. 08808/PI/08, Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS), Grant No. PI10/00985, and grant P30 DK46200 from the National Institutes of Health. The authors have no competing interests to declare.
Keywords: Mediterranean pattern, Western pattern, diet, male reproductive health, semen quality, male reproductive hormones
Introduction
Western nations, including Spain, have seen a decline in diet quality over the past decades; total calories, added sugars, refined carbohydrates, fats and meat consumption have increased substantially (Briefel and Johnson, 2004; Varela-Moreiras et al., 2010; Córdoba-Caro et al., 2012). These changes have coincided with a downward trend in semen quality in the West (Auger et al., 1995; Jørgensen et al., 2002, 2001; Swan et al., 2003; Iwamoto et al., 2006) and in Spain in particular (Mendiola et al., 2013). A meta-analysis of 101 studies of semen quality showed a large annual decline in sperm concentration in European men (2.3%) and a smaller decline in USA (0.8%) between 1934 and 1996 (Swan et al., 2000). In southern Spain, Mendiola and coworkers studied semen quality of comparable populations of healthy young men in 2001 and 2011. The 20-year interval from the oldest to the youngest man (from 1974 to 1993) was associated with a decrease in the total sperm count of 66 million (38%) or in average 1.9% (1.8, 2.0) per year (Mendiola et al., 2013).
Traditional Mediterranean diets have been shown to confer multiple health benefits (Estruch et al., 2013; Sofi et al., 2014; Pimenta et al., 2015). However, the reproductive benefits of the Mediterranean diet are less clear. In cross-sectional studies, healthier dietary patterns have been related to lower sperm DNA damage among subfertile couples in the Netherlands (Vujkovic et al., 2009) and higher sperm motility among healthy men in the USA (Gaskins et al., 2012). In an observational prospective study, a Mediterranean dietary pattern has been associated with higher pregnancy rates among infertility patients in the Netherlands (Vujkovic et al., 2010) and lower risk of infertility among Spanish women in a case–control study (Toledo et al., 2011). Most of the evidence suggesting a positive influence of the Mediterranean diet on testicular function has been conducted outside the Mediterranean region, and studies are still scarce. In addition, it is unknown whether the Mediterranean or healthier dietary patterns are related to reproductive hormones among healthy men. To address these questions, we evaluated the associations of dietary patterns with semen quality, reproductive hormone levels and testicular volume among young healthy men from Spain.
Material and Methods
Study population
The Murcia Young Men's Study (MYMS) was a cross-sectional study aimed at studying the influence of environmental and lifestyle factors on semen quality and reproductive hormone levels. The study rationale and design have been previously described in detail (Chavarro et al., 2011; Mínguez-Alarcón et al., 2012; Mendiola et al., 2013). Briefly, 215 young male university students between 18 and 23 years of age were recruited in university campuses across southern Spain. Men underwent a physical examination, completed dietary, lifestyle and personal history questionnaires, and provided semen and blood samples between October 2010 and November 2011. Inclusion criteria were to be university students, born in Spain after 31 December 1987 and able to contact their mother and ask her to complete a questionnaire. Men received a €50 gift card for their participation.
A total of 240 students contacted the study staff. Of these, 17 subjects were ineligible. Of the remaining 223 men (92.9%), 215 (89.6%) completed a study visit and agreed to participate in the study. We further excluded 6 men who reported implausible total caloric intake (>5000 kcal/day), leaving the final analytical sample thus comprised 209 men (87.1%) for the current analysis. The Research Ethics Committee of the University of Murcia approved this study (No. 495/2010, approved 14 May 2010). Written informed consent was obtained from all subjects.
Physical examination
Height and weight were measured using a wall stadiometer and digital scale, respectively (Tanita SC 330-S, London, UK), and body mass index (BMI) was calculated. The presence of varicocele was assessed during physical examination, and it was classified as no varicocele, only detected during Valsalva procedure, palpable or visible; other scrotal abnormalities were also assessed. Testicular volume was measured using a Prader orchidometer (Andrology Australia, Clayton, Victoria, Australia). All physical examinations were completed by the same investigator (J.M.) to minimize variability in study measures.
Dietary assessment
We used a validated (Vioque and Gonzalez, 1991; Vioque, 1995; Vioque et al., 2013, 2007) 101-food item semi-quantitative food frequency questionnaire (FFQ) to assess the usual diet (available at: http://bibliodieta.umh.es/files/2011/07/CFA101.pdf). Men were asked to report how often, on average, they had consumed each food item over the past year. The questionnaire offered nine options for frequency of consumption for each food, ranging from never or less than once a month to six or more times a day. Nutrient values for each food were obtained from the US Department of Agriculture and supplemented with Spanish sources (Palma et al., 2008; U.S. Department of Agriculture, 2010). The reproducibility and validity of this FFQ are comparable with other widely used FFQs (Willett et al., 1985; Block et al., 1990; Ocké et al., 1997; Subar et al., 2001; Willett, 2012). The mean correlation coefficients between nutrient intakes estimated using prospectively collected diet records and those estimated with the FFQ were 0.47 for validity and 0.40 for reproducibility (Vioque, 1995). This FFQ also showed satisfactory biochemical validity when compared with the plasma levels of carotenoids and vitamin C (Vioque et al., 2013, 2007).
Semen analysis
Men were asked to abstain from ejaculation for at least 48 h before sample collection, but they were not excluded if they failed to follow this instruction (n = 30). Abstinence time was recorded as the time between current and previous ejaculations as reported by the study subject. Men collected semen samples by masturbation at the clinic; no lubricants were used. Ejaculate volume was estimated by specimen weight, assuming a semen density of 1.0 g/ml. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). Spermatozoa were classified as either motile or immotile according to the World Health Organization (WHO, 2010) criteria, and the percentage of motile sperm [progressive (P) + non-progressive (NP)] was calculated. Smears for morphology were prepared, air dried, fixed, Papanicolaou stained and assessed using strict criteria (Menkveld et al., 1990). Total sperm count (volume × sperm concentration) was also calculated. The same specialist biologist carried out all the semen analyses. An external quality control on semen samples was carried out throughout the study period in collaboration with the University of Copenhagen's Department of Growth and Reproduction. No systematic differences in the results were identified. The mean inter-examiner coefficient of variation was 4.0%, ranging between 1.7 and 7.1%.
Reproductive hormones measurement
Blood samples were drawn from participants' cubital veins on the same time of the day of semen sample collection and centrifuged; the serum was separated, stored and frozen at −80°C. Serum samples were then shipped to Copenhagen, Denmark, on dry ice and stored at −20°C until hormone analysis was performed at Rigshospitalet. The methods have been described previously (Asklund et al., 2007). Briefly, hormone assessments were performed simultaneously to reduce intralaboratory variations. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer, Skovlund, Denmark). Intra- and inter-assay variations were <5% in each of the three assays. Serum testosterone (T) levels were determined using a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and inter-assay variations of <8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica, CA) with an intra-assay variation of <8% and an inter-assay variation of <13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd, Bicester, UK) with intra- and inter-assay variations of 13 and 18%, respectively. Free testosterone was calculated using the equation of Vermeulen et al. (1999) assuming a fixed albumin of 43.8 g/l.
Statistical analyses
Dietary patterns were identified from the reported intakes of individual foods using principal components analysis. The 101 food items in the FFQ were reclassified into 40 predefined food groups (Hu et al., 2000). Then, principal components analysis with orthogonal transformation was conducted in order to obtain uncorrelated factors (dietary patterns) with simpler structures. In order to determine the number of factors to retain, we considered the amount of variance explained by the factor, the scree plot (a plot of all the eigenvalues for the derived factors in descending order) and the interpretability of the factors. Using these criteria, we retained the first two factors (dietary patterns), which explained 18% of the variance, and the factor loadings that were >0.2 for each dietary pattern are presented in Table I. The substantive meanings of the rotated factors were considered in conjunction with the above empirical criteria, and the derived factors were labeled on the basis of our interpretation of the data and prior literature. For every subject, we calculated factor scores on each of the two retained factors by adding up the frequency of consumption multiplied by factor loadings across all food items. Thus, each participant was given a score for the two identified patterns according to their food consumption, and each participant appears in results for both dietary patterns.
Table I.
Dietary patterns identified by using principal components analysis and their food group componentsa (n = 209).
| Food groupa | Mediterraneanb | Westernb |
|---|---|---|
| Other vegetables | 0.76 | |
| Tomatoes | 0.67 | |
| Leafy green vegetables | 0.62 | |
| Dark yellow vegetables | 0.62 | |
| Fish and seafood | 0.59 | |
| Cruciferous vegetables | 0.58 | |
| Fruit | 0.50 | |
| Tea | 0.42 | 0.28 |
| Legumes | 0.41 | |
| Soups | 0.40 | |
| Potatoes | 0.35 | 0.20 |
| Coffee | 0.33 | |
| Garlic | 0.33 | |
| Eggs | 0.32 | |
| Poultry | 0.31 | |
| Olive oil | 0.30 | 0.25 |
| Low-fat dairy | 0.30 | |
| Nuts | 0.29 | 0.37 |
| Wine | 0.27 | |
| Whole grains | 0.24 | |
| Olives | 0.21 | 0.33 |
| Processed meat | 0.53 | |
| French chips | 0.50 | |
| Snacks | 0.50 | |
| Sweets | 0.50 | |
| Refined grains | 0.48 | |
| Pizza | 0.44 | |
| Butter | 0.43 | |
| Fruit juices | 0.37 | |
| High-fat dairy | 0.36 | |
| Condiments | 0.36 | |
| Other oils | 0.33 | |
| Mayonnaise | 0.29 | |
| Margarine | 0.28 | |
| Beer | 0.20 | |
| Variance explained (%) | 10.99 | 7.46 |
aLiquor, organ and red meats, high- and low-energy drinks were not included in the table because the loading factors were ≤|0.2| for both dietary patterns.
bPrincipal component analysis was used as an extraction method in which the factor loading of a food group represents the contribution of that food group to the factor identified.
Because the semen parameters, nutrient intakes and the men's characteristics had a skewed distribution, the median, 5th and 95th percentiles were used to describe those variables. Semen volume, sperm concentration, total sperm count, percentage of morphologically normal sperm, FSH and E2 concentrations were transformed using the natural log (ln) to improve normality. The average volume of the left and the right testes was used to represent testicular volume for each man. Men were divided into quartiles of each dietary pattern for the main analysis. Men with the lowest intake of each pattern category were considered as the reference group. To test for associations of baseline characteristics across quartiles of intake, Kruskal–Wallis tests were used for continuous variables and χ2 tests for categorical variables. Linear regression was used to examine the association of each dietary pattern with semen quality indicators, reproductive hormone levels and testicular volume. Tests for linear trend were performed using the median values of factor scores categories in each quartile as a continuous variable and reproductive outcomes as the response variable. The presence of non-linear associations was tested modeling dietary patterns as a continuous variable and modeling them as a linear and quadratic term. We used analysis of covariance (ANCOVA) to calculate multivariable adjusted semen quality, reproductive hormone levels and testicular volumes for each quartile by relevant covariates. Regression coefficients for outcomes that were log-transformed for analysis were exponentiated (‘back-transformed’) to allow the presentation of adjusted means in the scale variables were measured. Confounding was assessed using a hybrid method that combines previous knowledge using directed acyclic graphs (DAGs) (Weng et al., 2009) and a statistical method on change in point estimated in which the potential covariate was not retained in final models, if it resulted in a change in the β-coefficient of <10%. As potential confounders, we included factors related to male reproductive health in this and previous studies, factors associated with dietary patterns and markers of testicular function in this study, regardless of whether they had been previously described as predictors of male reproductive health. Using these criteria, final models included terms for BMI (kg/m2), smoking (current smoker versus not current smoker) and total energy intake (kcal/day). In keeping with the literature, abstinence time (h) was included into the models, even though it did not meet the inclusion criteria. Prior to analyses was decided that, models for sperm motility would include a term for time between sample production and time to semen analysis to account for the well-described and rapid decline in motility following ejaculation. Similarly, in models for reproductive hormone levels, a term was included for time of day when the blood was drawn to account for circadian variation in reproductive hormones levels.
To investigate possible interaction effects of BMI and between dietary patterns, all analyses were repeated by adding multiplicative terms (Mediterranean pattern × BMI, Western pattern × BMI, Mediterranean pattern × Western pattern) to regression models.
We considered that an association was present when a statistically significant linear trend across quartiles of intake was present. All tests were two tailed, and the level of statistical significance was set at 0.05. Statistical analyses were performed with the IBM Statistical Package for the Social Sciences 19.0 (IBM Corporation, Armonk, NY, USA).
Results
Study participants were young [median (5th, 95th percentiles) = 20.4 (18.1, 22.8) years], all except three participants were Caucasian (98%) and the median BMI was 23.7 (19.4, 30.0) kg/m2. Almost one-third (32%) of the participants were current smokers. All participants considered themselves to be in good general health, and 24.9% of subjects defined it as excellent. In total, 4 men (1.9%) had a history of cryptorchidism, and 32 (15.3%) had a varicocele in the left testis detected during the physical examination. The median (5th, 95th percentiles) values for semen analysis parameters were 43.3 × 106/ml (8.8, 130.2 × 106/ml) for sperm concentration, 120.2 × 106 (17.4, 401.0 × 106) for total sperm count, 9.0% (2.5, 23.0%) for morphologically normal sperm and 57.1% (38.8, 74.2%) for sperm motility (P + NP). Median abstinence time was 71.0 h (39.0, 136.5).
Two dietary patterns were identified in this study population (Table I): a Mediterranean pattern characterized by high intakes of low-fat dairy, eggs, poultry, fish, tomatoes, vegetables, legumes, fruit, whole grains, wine, coffee, soups and garlic and a Western pattern characterized by high consumption of vegetable oils, high-fat dairy, processed meat, refined grains, French chips, snacks, pizza, margarine, butter, sweets, beer, fruit juices, mayonnaise and condiments. High intakes of tea, potatoes, nuts, olives and olive oil were present in both patterns. Men with higher adherence to the Mediterranean pattern had also higher intakes of total calories, protein, antioxidant vitamins and carotenoids, and lower intake of trans fatty acids (Table II).
Table II.
Characteristics of participants in the Murcia Young Men's Study in the lower and upper quartiles (Q1, Q4) of consumption of each dietary pattern. Values presented are median (5th, 95th percentiles) unless otherwise indicated.
| Mediterranean pattern |
Western pattern |
||||||
|---|---|---|---|---|---|---|---|
| All men | Q1 (n = 53) | Q4 (n = 52) | P-valuea | Q1 (n = 52) | Q4 (n = 52) | P-valuea | |
| Age (years) | 20.4 (18.1, 22.8) | 20.8 (18.1, 23.0) | 20.3 (11.7, 23.2) | 0.80 | 20.8 (17.8, 23.1) | 20.3 (18.1, 22.7) | 0.43 |
| BMI (kg/m2) | 23.7 (19.4, 30.0) | 22.9 (19.0, 29.9) | 23.9 (19.5, 29.8) | 0.49 | 24.1 (19.4, 31.0) | 23.1 (19.0, 29.6) | 0.27 |
| Obese men, n (%) | 68 (32.5) | 15 (28.3) | 18 (34.6) | 0.49 | 19 (36.5) | 14 (26.9) | 0.37 |
| Current smoker, n (%) | 66 (31.9) | 18 (34.0) | 15 (28.8) | 0.86 | 11 (21.2) | 17 (32.7) | 0.14 |
| Excellent general health, n (%) | 52 (24.9) | 11 (20.8) | 15 (24.9) | 0.21 | 19 (36.5) | 6 (11.5) | 0.07 |
| Abstinence time (h) | 71 (39, 137) | 74 (39, 124) | 72 (31, 201) | 0.60 | 72 (31, 145) | 72 (45, 198) | 0.28 |
| Time to start analyses (min) | 35 (25, 60) | 30 (24, 63) | 40 (25, 57) | 0.007 | 35 (18, 60) | 38 (25, 62) | 0.36 |
| Time to blood sampling (min) | 245 (43, 340) | 230 (56, 355) | 240 (37, 337) | 0.41 | 228 (37, 334) | 240 (45, 337) | 0.70 |
| Varicocele, n (%) | 32 (15.3) | 8 (15.1) | 7 (13.4) | 0.54 | 5 (9.6) | 11 (21.2) | 0.38 |
| History of cryptorchidism n, (%) | 4 (1.9) | 1 (1.9) | 1 (1.9) | 0.88 | 1 (1.9) | 1 (1.9) | 0.25 |
| Semen volume (ml) | 3.0 (1.02, 6.50) | 3.0 (1.04, 6.30) | 3.0 (1.05, 6.27) | 0.12 | 3.24 (0.69, 6.89) | 2.97 (1.06, 6.63) | 0.36 |
| Sperm concentration (millions/ml) | 43.3 (8.74, 130) | 42.4 (8.29, 136) | 56.7 (6.65, 109) | 0.83 | 51.1 (8.25, 118) | 41.0 (2.96, 148) | 0.26 |
| Total sperm count (106) | 120.2 (17.4, 401) | 126 (14.7, 441) | 145 (17.8, 486) | 0.59 | 153 (19.9, 396) | 106 (19.7, 301) | 0.26 |
| Morphologically normal sperm (%) | 9.0 (2.5, 23.0) | 10.0 (3.0, 21.0) | 9.0 (2.0, 23.4) | 0.67 | 8.0 (2.55, 23.4) | 11.0 (2.0, 25.38) | 0.63 |
| Total motile sperm (P + NP) (%) | 57.1 (38.8, 74.2) | 59.0 (24.7, 74.8) | 54.8 (39.2, 73.3) | 0.78 | 58.0 (41.4, 75.4) | 56.1 (36.7, 75.6) | 0.64 |
| Testicular volume (ml) | 21.0 (15.0, 26.0) | 21.0 (15.5, 25.3) | 21.3 (17.3, 26.0) | 0.21 | 21.3 (14.8, 25.7) | 20.8 (15.7, 26.4) | 0.91 |
| Follicle-stimulating hormone (FSH) levels (IU/l) | 2.21 (0.92, 5.37) | 2.04 (0.88, 4.89) | 2.34 (0.89, 5.20) | 0.38 | 2.18 (0.99, 5.52) | 2.16 (0.88, 5.64) | 0.50 |
| Luteinizing hormone (LH) levels (IU/l) | 3.98 (1.95, 7.16) | 3.90 (1.62, 7.24) | 4.21 (1.93, 7.17) | 0.97 | 4.54 (1.85, 7.29) | 4.11 (1.70, 7.38) | 0.16 |
| Inhibin B levels (pg/ml) | 193 (101, 337) | 219 (98.7, 363) | 189 (99.0, 340) | 0.06 | 196 (108, 336) | 176 (91.9, 332) | 0.67 |
| Total testosterone (nmol/l) | 21.2 (11.5, 34.2) | 21.2 (11.6, 37.5) | 21.4 (12.1, 33.8) | 0.26 | 19.3 (12.0, 36.9) | 20.0 (8.90, 32.8) | 0.22 |
| Free testosterone (ng/dl) | 13.5 (7.7, 23.7) | 13.6 (8.34, 24.6) | 14.0 (7.84, 23.9) | 0.17 | 13.3 (7.9, 24.5) | 13.40 (6.20, 23.17) | 0.32 |
| Sex hormone binding globulin (SHBG) (nmol/l) | 30.0 (16.0, 54.5) | 31.0 (14.7, 54.6) | 28.0 (13.9, 51.1) | 0.89 | 31.5 (14.7, 56.4) | 27.5 (12.9, 48.1) | 0.28 |
| Estradiol (E2) (pmol/l) | 76.0 (48.5, 117) | 70.0 (48.7, 117) | 80.0 (49.9, 129) | 0.58 | 76.0 (43.8, 123) | 75.0 (45.4, 131) | 0.75 |
| Calories (kcal/day) | 2278 (1292, 3840) | 1934 (1084, 3377) | 2933 (1928, 4466) | <0.0001 | 1801 (1077, 3129) | 3199 (2023, 4466) | <0.00011 |
| Carbohydrate intake (g/day) | 230 (168, 302) | 228 (158, 306) | 225 (162, 306) | 0.97 | 226 (130, 310) | 230 (178, 312) | 0.54 |
| Protein intake (g/day) | 105 (74, 138) | 101 (67, 122) | 112 (83, 149) | 0.001 | 114 (78, 160) | 97 (73, 125) | <0.0001 |
| Total fat intake (g/day) | 92 (69, 117) | 94 (69, 120) | 90 (63, 117) | 0.44 | 89 (60, 124) | 95 (68, 116) | 0.32 |
| Saturated fat intake (g/day) | 30 (20, 42) | 31 (20, 45) | 26 (17, 41) | 0.001 | 29 (16, 45) | 30 (20, 43) | 0.99 |
| Monounsaturated fat intake (g/day) | 40 (29, 55) | 40 (27, 52) | 40 (28, 58) | 0.58 | 39 (25, 57) | 42 (28, 55) | 0.14 |
| Polyunsaturated fat intake (g/day) | 15 (11, 21) | 14 (10, 24) | 15 (10, 22) | 0.66 | 14 (10, 21) | 15 (10, 25) | 0.11 |
| Trans fatty acid intake (g/day) | 1.7 (0.6, 2.8) | 2.2 (0.9, 3.1) | 1.4 (0.5, 2.5) | <0.0001 | 1.3 (0.5, 2.7) | 2.1(0.8, 3.0) | <0.0001 |
| Vitamin C intake (mg/day) | 113 (49, 217) | 82 (28, 221) | 142 (70, 273) | <0.0001 | 129 (46, 273) | 111 (51, 215) | 0.17 |
| Lycopene intake (µg/day) | 3939 (1209, 9054) | 2659 (714, 8978) | 5319 (1975, 9627) | <0.0001 | 3886 (262, 8665) | 4189(1429, 8871) | 0.95 |
| β-Carotene intake (µg/day) | 2465 (697, 7495) | 1403 (414, 3375) | 4562 (1872, 10 015) | <0.0001 | 3048 (547, 8423) | 2298 (627, 7284) | 0.65 |
| Cryptoxanthin intake (mcg/day) | 277 (68, 663) | 193 (17, 683) | 345 (88, 811) | <0.0001 | 294 (16, 835) | 269 (68, 684) | 0.40 |
| Alcohol intake (g/day) | 6.8 (0, 24.9) | 7.4 (0, 38.9) | 5.2 (0.3, 23.6) | 0.46 | 5.2 (0, 22.6) | 6.0 (0, 19.6) | 0.19 |
| Caffeine intake (mg/day) | 77 (8, 398) | 82 (8, 330) | 99 (6, 399) | 0.08 | 81 (7, 331) | 80 (11, 396) | 0.80 |
aFor continuous variables, Kruskal–Wallis tests were used to test for associations between the levels of diet score. For categorical variables, χ2 tests were used to test the associations between the levels of diet score.
Men with greater adherence to the Western pattern had a higher total caloric intake, higher trans fatty acid intake and lower protein intake. As expected, the Mediterranean and Western dietary pattern scores were independent of each other (r = 0.001; P = 1.00).
No associations were observed between Western or Mediterranean patterns and semen parameters in unadjusted models (Supplementary data, Table SI). However, there was a positive association between the Mediterranean pattern and total sperm count (Table III). In the multivariable adjusted models, the total sperm count of men in the top quartile of Mediterranean dietary pattern consumption was 51.5% (1.9–125.4%), higher than that of men in the bottom quartile, and there was a statistically significant linear trend for increasing total sperm count with increasing adherence to these pattern (P, trend = 0.04). On the other hand, there was a strong positive association between the Western pattern and the percent of morphologically normal sperm. Relative to men in the lowest quartile of Western pattern consumption, the adjusted differences [95% confidence interval (CI)] in the percent of morphologically normal sperm for men in the 2nd, 3rd and 4th quartiles were 16.6% (8.5–48.4%), 33.6% (2.3–74.3%) and 58.1% (14.3–118.5%), respectively. This positive association remained among lean men (BMI <25 kg/m2) but was not statistically significant among overweight or obese men (BMI ≥25 kg/m2) (Supplementary data, Table SII; P, interaction = 0.12). No other associations were observed between these two dietary patterns and semen quality indicators (Table III). Similarly, neither the Mediterranean nor the Western pattern was associated with changes in reproductive hormone levels in unadjusted or adjusted models (Table IV and Supplementary data, Table SIII). The adherence to Mediterranean dietary pattern and testicular volume were unrelated in multivariate models adjusted by BMI (kg/m2), smoking (current smoker versus not current smoker), abstinence time (hours) and total calorie intake (kcal/day). The adjusted means (95% CI) for testicular volume (ml) were 21.3 (20.3, 22.3), 20.6 (19.6, 21.5), 22.0 (21.0, 22.9) and 21.7 (20.7, 22.7) from the lowest to the highest quartile categories, respectively (P, trend = 0.29). Likewise, we did not find statistically significant relationship between Western dietary pattern and testicular volume (P, trend = 0.29).
Table III.
Multivariate adjusted associations of the Mediterranean and Western patterns with semen quality parameters in the Murcia Young Men's Study (n = 209) according to quartiles (Q1–4) of consumption of the two dietary patterns.
| Mediterranean pattern |
Western pattern |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted means (95% CI) | Q1 (n = 53) | Q2 (n = 51) | Q3 (n = 53) | Q4 (n = 52) | P, trend | Q1 (n = 52) | Q2 (n = 53) | Q3 (n = 52) | Q4 (n = 52) | P, trend |
| Semen volume (ml) | ||||||||||
| Energy adjusteda | 2.52 (2.12, 3.01) | 2.51 (2.11, 2.98) | 3.23 (2.74, 3.82) | 2.98 (2.48, 3.59) | 0.19 | 3.04 (2.50, 3.69) | 2.51 (2.10, 2.99) | 2.99 (2.52, 3.55) | 2.70 (2.19, 3.33) | 0.64 |
| Adjustedb | 2.50 (2.09, 2.98) | 2.59 (2.17, 3.08) | 3.17 (2.67, 3.76) | 2.97 (2.47, 3.58) | 0.13 | 3.08 (2.54, 3.74) | 2.46 (2.07, 2.94) | 3.17 (2.66, 3.78) | 2.68 (2.16, 3.31) | 0.61 |
| Sperm concentration (millions/ml) | ||||||||||
| Energy adjusted | 35.3 (26.8, 48.7) | 37.3 (28.6, 48.7) | 33.7 (25.6, 43.7) | 44.2 (33.2, 58.8) | 0.32 | 40.0 (29.5, 54.0) | 38.4 (29.2, 54.0) | 38.4 (29.2, 48.6) | 34.2 (24.7, 47.5) | 0.54 |
| Adjusted | 35.7 (27.0, 47.1) | 36.8 (28.0, 48.4) | 33.8 (25.8, 44.1) | 44.2 (33.1, 59.0) | 0.34 | 40.9 (30.2, 55.2) | 38.2 (29.1, 50.2) | 37.4 (28.4, 49.3) | 36.5 (26.2, 50.9) | 0.57 |
| Total sperm count (millions) | ||||||||||
| Energy adjusted | 92.9 (71.8, 120) | 104 (81.2, 134) | 109 (85.4, 139) | 140 (107, 185)* | 0.04 | 127 (95.4, 169) | 99.3 (76.8, 128) | 121 (94.3, 156) | 96.9 (71.1, 132) | 0.35 |
| Adjusted | 91.9 (71.3, 118) | 109 (84.9, 140) | 107 (84.1, 137) | 138 (105, 180)* | 0.04 | 128 (97.1, 170) | 95.3 (74.0, 123) | 132 (102, 171) | 95.3 (69.9, 129) | 0.34 |
| Morphologically normal sperm (%) | ||||||||||
| Energy adjusted | 9.13 (7.60, 10.9) | 9.08 (7.60, 10.8) | 8.16 (6.89, 9.69) | 8.34 (6.90, 10.1) | 0.79 | 7.14 (5.88, 8.69) | 8.37 (7.01, 9.99) | 9.08 (7.64, 10.8) | 10.4 (8.40, 13.0)* | 0.03 |
| Adjusted | 9.25 (7.71, 11.1) | 9.38 (7.85, 11.2) | 8.27 (6.96, 9.84) | 8.17 (6.77, 9.87) | 0.28 | 7.06 (5.82, 8.54) | 8.22 (6.92, 9.79) | 9.42 (7.92, 11.2)* | 11.2 (8.98, 13.8)* | 0.008 |
| Total motile sperm (progressive + non-progressive; WHO Grades A–C) (%) | ||||||||||
| Energy adjusted | 56.4 (53.4, 59.4) | 57.8 (54.9, 60.8) | 56.9 (54.0, 59.7) | 56.3 (53.1, 59.4) | 0.44 | 57.2 (54.0, 60.5) | 57.7 (54.7, 60.7) | 55.7 (52.8, 58.6) | 56.7 (53.1, 60.3) | 0.78 |
| Adjusted | 56.2 (53.3, 59.2) | 58.8 (55.8, 61.8) | 57.2 (54.1, 59.9) | 56.1 (52.9, 59.2) | 0.68 | 57.3 (54.1, 60.5) | 57.6 (54.6, 60.5) | 55.2 (52.2, 58.1) | 57.1 (53.5, 60.8) | 0.60 |
Models for sperm motility are further adjusted for time to start semen analysis (minutes).
aAdjusted for total calorie intake (kcal/day).
bFurther adjusted for BMI (kg/m2), smoking (current smoker versus not current smoker) and ejaculation abstinence time (hours).
*LSD post hoc analyses P < 0.05, (Q1 = reference category).
Table IV.
Multivariate adjusted associations of the Mediterranean and the Western patterns with testicular volume and reproductive hormones in Murcia Young Men's Study by quartiles (Q1–4) of consumption of the two dietary patterns (n = 209).
| Mediterranean pattern |
Western pattern |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted means (95% CI) | Q1 (n = 53) | Q2 (n = 51) | Q3 (n = 53) | Q4 (n = 52) | P, trend | Q1 (n = 52) | Q2 (n = 53) | Q3 (n = 52) | Q4 (n = 52) | P, trend |
| Testicular volume (ml) | ||||||||||
| Energy adjusteda | 21.3 (20.4, 22.3) | 20.6 (19.7, 21.6) | 21.9 (21.0, 22.8) | 21.7 (20.7, 22.6) | 0.34 | 21.7 (20.6, 22.7) | 21.5 (20.6, 22.5) | 21.6 (20.6, 22.5) | 20.7 (19.6, 21.9) | 0.29 |
| Adjustedb | 21.3 (20.3, 22.3) | 20.6 (19.6, 21.5) | 22.0 (21.0, 22.9) | 21.7 (20.7, 22.7) | 0.29 | 21.7 (20.6, 22.7) | 21.7 (20.7, 22.6) | 21.5 (20.6, 22.5) | 20.8 (19.7, 22.0) | 0.29 |
| Follicle-stimulating hormone (FSH) levels (IU/l) | ||||||||||
| Energy adjusted | 2.18 (1.86, 2.55) | 2.58 (2.22, 3.01) | 2.20 (1.89, 2.55 | 2.31 (1.96, 2.73) | 0.99 | 2.17 (1.83, 2.58) | 2.14 (1.93, 2.51) | 2.54 (2.18, 2.96) | 2.41 (2.00, 2.92) | 0.39 |
| Adjusted | 2.17 (1.85, 2.54) | 2.58 (2.21, 3.01) | 2.16 (1.85, 2.51) | 2.34 (1.98, 2.75) | 0.93 | 2.18 (1.83, 2.59) | 2.11 (1.80, 2.46) | 2.57 (2.21, 2.99) | 2.38 (1.97, 2.87) | 0.45 |
| Luteinizing hormone (LH) levels (IU/l) | ||||||||||
| Energy adjusted | 4.15 (3.67, 4.64) | 4.30 (3.83, 4.77) | 4.24 (3.78, 4.70) | 4.23 (3.73, 4.74) | 0.90 | 4.18 (3.65, 4.70) | 3.78 (3.31, 4.26) | 4.56 (4.10, 5.02) | 4.41 (3.84, 4.98) | 0.49 |
| Adjusted | 4.11 (3.62, 4.60) | 4.33 (3.84, 4.81) | 4.21 (3.73, 4.68) | 4.29 (3.78, 4.80) | 0.75 | 4.24 (3.70, 4.77) | 3.77 (3.28, 4.25) | 4.53 (4.06, 5.00) | 4.39 (3.81, 4.98) | 0.51 |
| Inhibin B levels (pg/ml) | ||||||||||
| Energy adjusted | 224 (202, 247) | 192 (171, 213)* | 187 (166, 208)* | 205 (182, 228) | 0.41 | 205 (181, 229) | 197 (175, 220) | 212 (190, 234) | 194 (168, 221) | 0.66 |
| Adjusted | 222 (201, 244) | 192 (171, 214) | 190 (169, 210) | 205 (182, 227) | 0.46 | 205 (181, 229) | 200 (178, 221) | 212 (191, 233) | 193 (167, 219) | 0.64 |
| Total testosterone levels (nmol/l) | ||||||||||
| Energy adjusted | 22.1 (20.1, 24.1) | 20.3 (18.4, 22.2) | 22.7 (20.8, 24.6) | 22.2 (20.1, 24.2) | 0.57 | 21.9 (19.8, 24.1) | 22.3 (20.4, 24.3) | 23.2 (21.3, 25.1) | 19.8 (17.4, 22.1) | 0.24 |
| Adjusted | 21.6 (19.7, 23.5) | 20.4 (18.5, 22.2) | 22.7 (20.8, 24.5) | 22.5 (20.5, 24.5) | 0.28 | 22.2 (20.2, 24.3) | 22.4 (20.5, 24.2) | 23.1 (21.3, 24.9) | 19.3 (17.0, 21.5) | 0.09 |
| Free testosterone levels (ng/dl) | ||||||||||
| Energy adjusted | 14.4 (13.0, 15.8) | 13.1 (11.8, 14.4) | 14.8 (13.5, 16.5) | 14.7 (13.3, 16.1) | 0.41 | 14.1 (12.6, 15.6) | 14.4 (13.0, 15.8) | 15.2 (13.8, 16.5) | 13.3 (11.7, 14.9) | 0.53 |
| Adjusted | 14.3 (12.9, 15.6) | 13.1 (11.7, 14.4) | 14.9 (13.6, 16.2) | 14.7 (13.3, 16.1) | 0.32 | 14.2 (12.7, 16.0) | 14.5 (13.1, 15.8) | 15.01 (13.70, 16.33) | 13.2 (11.5, 14.8 | 0.42 |
| Sex hormone binding globulin (SHBG) levels (nmol/l) | ||||||||||
| Energy adjusted | 31.7 (28.4, 35.1) | 30.8 (27.5, 34.0) | 31.8 (28.6, 35.0) | 31.6 (28.1, 35.0) | 0.94 | 31.9 (28.3, 35.6) | 32.4 (29.1, 35.8) | 32.5 (29.2, 36.7) | 29.0 (25.0, 33.0) | 0.33 |
| Adjusted | 30.5 (27.5, 33.5) | 31.3 (28.4, 34.3) | 31.3 (28.38, 34.2) | 32.4 (29.3, 35.5) | 0.43 | 32.5 (29.3, 35.8) | 32.2 (29.3, 35.2) | 32.7 (29.9, 35.6) | 27.9 (24.3, 31.5) | 0.09 |
| Estradiol (E2) levels (pmol/l) | ||||||||||
| Energy adjusted | 76.1 (70.2, 82.5) | 73.8 (68.2, 79.8) | 76.9 (71.3, 83.1) | 76.0 (69.9, 82.7) | 0.85 | 77.3 (70.7, 84.4) | 79.0 (72.9, 85.5) | 76.4 (70.7, 82.5) | 70.5 (64.1, 77.6) | 0.18 |
| Adjusted | 76.1 (70.1, 82.5) | 73.5 (67.8, 79.6) | 77.6 (71.6, 83.9) | 75.7 (69.6, 82.4) | 0.86 | 77.0 (70.5, 84.2) | 79.3 (73.1, 86.0) | 76.2 (70.4, 82.4) | 70.5 (63.9, 77.6) | 0.19 |
aAdjusted for total calorie intake (kcal/day).
bFurther adjusted for BMI (kg/m2), smoking (current smoker versus not current smoker) and time to blood sampling (min).
*LSD post hoc analyses P < 0.05, (Q1 = reference category).
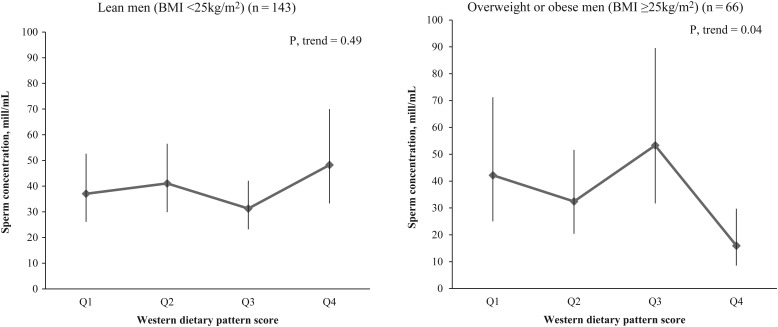
BMI modified the association between the Western dietary pattern and sperm concentration (P, interaction = 0.014). The Western pattern was inversely related to sperm concentration among overweight or obese men but not among lean men (Fig. 1). A similar trend was found in the relation between the Western pattern and total sperm count (P, interaction = 0.15) (Supplementary data, Table SII). There was no evidence of significant heterogeneity of the associations between Western dietary pattern and other semen quality parameters or reproductive hormones by BMI (P, interactions >0.10). Similarly, no significant associations modified by BMI were found with Mediterranean dietary pattern and all the outcomes. And finally, intake of Mediterranean dietary pattern did not modify the relations between the intake of Western dietary pattern and semen quality parameters or reproductive hormones, and vice versa (P, interactions >0.10).
Figure 1.
Mean (95% CI) of sperm concentration according to Western pattern by body mass index (BMI) level. Models are adjusted by smoking (current smoker versus not current smoker), ejaculation abstinence time (hours), total calorie intake (kcal/day) and BMI (kg/m2).
Discussion
We identified two dietary patterns among 209 young healthy men from Spain: a Mediterranean, characterized by high intakes of low-fat dairy, eggs, poultry, fish, tomatoes, vegetables, legumes, fruit, whole grains, wine, coffee, soups and garlic and a Western pattern characterized by high consumption of vegetable oils, high-fat dairy, processed meat, refined grains, French chips, snacks, pizza, margarine, butter, sweets, beer, fruit juices, mayonnaise and condiments. The Mediterranean pattern was positively associated with total sperm count, while the Western pattern was positively related to the percentage of morphologically normal sperm. Furthermore, the Western pattern was inversely related to sperm concentration among overweight or obese men but not among lean men. Neither pattern was related to reproductive hormone levels or testicular volume. These findings provide further evidence that diet may be an important modifiable determinant of male reproductive potential. More specifically, these results suggest that traditional Mediterranean diets may have positive impacts on sperm production in addition to their well-described benefits for the prevention of chronic diseases (Estruch et al., 2013; Sofi et al., 2014; Pimenta et al., 2015).
The positive association between the Mediterranean dietary pattern and total sperm count is consistent with the previous findings from this study. We have previously reported that intake of antioxidant vitamins and carotenoids, whose intake is increased with increasing adherence to the Mediterranean pattern, is related to higher sperm counts among these young healthy men in this cross-sectional study (Mínguez-Alarcón et al., 2012). In addition, others have previously found similar dietary patterns to be related to better semen quality, although not always to sperm counts specifically. Higher intakes of fruit and cereals were positively related to sperm motility and concentration in an observational study among 250 subfertile men presenting to a fertility clinic and undergoing ICSI cycles (Braga et al., 2012). In a case–control study, high intakes of fruit and vegetables were related to lower risk of asthenozoospermia attending infertility clinics in Iran (Eslamian et al., 2012). Also, in a comparable study population from USA of young, healthy and unselected men, higher adherence to a ‘Prudent’ pattern bearing close resemblance to the Mediterranean pattern was associated with higher progressive motility (Gaskins et al., 2012). Similarly, Health Conscious pattern consumption (characterized by high intake of fruits, vegetables, whole grains and fish) was associated with lower sperm DNA damage among subfertile men of couples undergoing IVF/ICSI in the Netherlands (Vujkovic et al., 2009).
While there is no consistency across studies with regard to the semen quality indicator with which this or similar diet patterns appear to be beneficial (counts versus motility), the apparent benefit of these diets may be due to the high intake of antioxidants and carotenoids resulting from the foods highly consumed in the Mediterranean and similar patterns. Sperm membranes are very sensitive to oxidation by reactive oxygen species (ROS). Greater adherence to patterns like the Mediterranean pattern and similar patterns described in other populations results in greater intake of antioxidants and carotenoids, which in turn have been related to better semen quality (Eskenazi et al., 2005; Mendiola et al., 2010; Mínguez-Alarcón et al., 2012; Zareba et al., 2013) most likely by preventing oxidative damage caused by ROS (Agarwal and Sekhon, 2010). Otherwise, a recent study found consumption of fruits and vegetables with high levels of pesticide residues was associated with a lower total sperm count and a lower percentage of morphologically normal sperm (Chiu et al., 2015). This study analyzes prospectively 338 semen samples provided by 155 male partners in subfertile couples, utilizing a novel approach to examine these associations (Levine and Swan, 2015). The highest levels of pesticide residues were related with the highest scores of the ‘Prudent’ pattern. There were no associations between total intake of fruits and vegetables, and semen quality parameters showing dietary pesticide exposure could weaken the potential benefit of healthy diets.
We also found an inverse relation between the Western pattern and sperm concentration, albeit restricted to overweight or obese men. This result is also consistent with the existing literature. Although others have not reported a similar interaction, previous studies have found that foods included in the Western pattern, particularly intake of red meats (as a source of trans fatty acids), have been inversely related with sperm parameters (Mendiola et al., 2009; Afeiche et al., 2014). Afeiche et al. (2014) found that processed red meat intake was inversely associated with total sperm count in a study population comparable with ours (189 young healthy US men aged between 18 and 22 years). Also, intake of dairy and meat processed products was higher in men with poor semen quality (cases) compared with normozoospermic men (controls) (Mendiola et al., 2009). Higher intake of trans fat, which increases with greater adherence to this pattern and has been previously related to lower sperm counts in this and other populations (Attaman et al., 2012; Chavarro et al., 2014, 2011), may underlie this relation. Although intake of trans fats was comparable with that observed in the general Spanish population (Spanish Food Safety and Nutrition Agency, 2011), this kind of fat still exists in commercial Spanish food since, unlike the USA, there is no Spanish legislation to restrict it.
Unexpectedly, we also found that greater adherence to the Western pattern was positively related to normal sperm morphology. Previous work on this relation is scarce and inconsistent. Gaskins et al. (2012) found no associations between the Western dietary pattern and semen quality indicators among young healthy men in the USA. Similarly, Vujkovic et al. (2009) found that a Traditional Dutch dietary pattern, characterized by high intakes of potatoes, meat products and whole grains, was unrelated to sperm morphology in men undergoing IVF/ICSI treatment. The lack of consistency with our results and the lack of a previously described biological mechanism suggesting a positive influence on spermatogenesis of foods in this pattern or their nutritional correlates suggest that the observed relation may represent a chance finding. Further work in other study populations could clarify this issue.
This study is not without limitations. First, as is true for all cross-sectional studies, causal inference is limited. However, participants were young men with untested fertility who were blinded to the study outcomes reducing the possibility that knowledge of their reproductive potential influenced their report of diet. A related weakness of this study design is that not all of the outcomes investigated here may be equally suitable for investigation in a cross-sectional study of young healthy men with untested fertility. Specifically, since the validated dietary assessment referred to diet over the last year, there may be few concerns for semen quality as an outcome given that the spermatogenic cycle typically lasts ∼70 days in the testis and a total of ∼90 days including maturation in the epididymis. On the other hand, the significance of associations with testicular volume is more difficult to evaluate in part because little is known about how much this outcome could be influenced by environmental factors, like diet, among sexually mature men. While others have documented seasonal changes in testicular volume among sexually mature non-human primates (Hamada et al., 2005) and changes in testicular volume in response to surgical procedures in adult men (Akbulut et al., 2003), whether and to what extent to testicular volume could change in response to modifiable environmental factors remains an open question. Second, only one semen sample was obtained per man. Nonetheless, some studies have shown that one sample is enough to assess semen quality in epidemiological studies (Carlsen et al., 2005; Stokes-Riner et al., 2007). Likewise, a single sample can be used to classify men's reproductive hormones (Vermeulen and Verdonck, 1992). Third, although we cannot exclude the possibility of unmeasured confounding, we adjusted for a large number of known and suspected confounders. It is not possible to predict whether and how the observed differences in semen quality translate into reproductive success for men in couples trying to conceive. However, previous works relating higher adherence to the Mediterranean dietary pattern to lower risk of difficulty conceiving among Spanish women (Toledo et al., 2011) and to higher biochemical pregnancy rates among Dutch women undergoing IVF/ICSI (Vujkovic et al., 2010) suggest the possibility of greater reproductive success resulting from higher adherence of men to these patterns. Since neither of these studies adjusted for the male partner's adherence to the Mediterranean pattern and diet is known to be positively related within couples (Lioret et al., 2012), it is possible that the apparent benefit of the Mediterranean diet attributed to female intakes in these studies may actually represent, at least partly, a benefit in the male partners instead. Strengths of the study include the use of a previously validated FFQ for use in Spanish populations (Vioque and Gonzalez, 1991; Vioque, 1995; Vioque et al., 2013, 2007) and sample size that was similar to the usual size of published semen quality studies.
In summary, we found a positive association between greater adherence to the Mediterranean dietary pattern and total sperm count and an inverse association between adherence to the Western pattern and sperm concentration among obese men. In addition, there was a positive association between a Western dietary pattern and sperm morphology. The associations of the Mediterranean and Western patterns with sperm count and concentration, respectively, are consistent with previous work on diet and semen quality. The association between the Western pattern and sperm morphology, on the other hand, may represent a chance finding. Additional work is necessary to refute or confirm these findings and to evaluate whether the observed differences in semen quality translate into differences fertility rates among couples trying to become pregnant.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
A.M.T.C., J.M., and N.J. were involved in study conception and study design. J.M. was involved in study execution and acquisition of data. L.M.A, J.E.C, J.J.L.E. and E.M.N.M. contributed to data analysis and interpretation. A.C.T., L.M.A. and J.E.C. drafted the manuscript. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
Funding
This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, Grant No. 08808/PI/08, Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS), Grant No. PI10/00985, and grant P30 DK46200 from the National Institutes of Health.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge C. Ruiz, E. Belmonte, F. Mas and all the Quirón Dexeus Murcia clinic staff for their assistance in data collection, and the young men of the study for their participation. We also thank L. Sarabia and G. Vivero for semen analyses, K. Ruiz and E. Estrella for database management and J. Vioque for dietary assessment.
References
- Afeiche MC, Williams PL, Gaskins AJ, Mendiola J, Jørgensen N, Swan SH, Chavarro JE. Meat intake and reproductive parameters among young men. Epidemiol 2014;25:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil 2010;13:217–225. [DOI] [PubMed] [Google Scholar]
- Akbulut G, Serteser M, Yucel A, Degirmenci B, Yilmaz S, Polat C, San O, Dilek ON. Can laparoscopic hernia repair alter function and volume of testis? Randomized clinical trial. Surg Laparosc Endosc Percutan Tech 2003;13:377–381. [DOI] [PubMed] [Google Scholar]
- Asklund C, Jørgensen N, Skakkebaek NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Hum Reprod 2007;22:2639–2646. [DOI] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 1995;332:281–285. [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- Braga DP, de AF, Halpern G, Figueira R, de CS, Setti AS, Iaconelli A, Borges E. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil. Steril 2012;97:53–59. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 2004;24:401–431. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Swan SH, Petersen JH, Skakkebæk NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod 2005;20:942–949. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, Campos H, Hauser R. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril 2011;95:1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Mínguez-Alarcón L, Mendiola J, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod 2014;29:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 2015;30:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba-Caro LG, Luego Pérez LM, García Preciado V. Nutritional adequacy of students of compulsory secondary education in Badajoz [in Spanish]. Nutr Hosp 2012;27:1065–1071. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod 2005;20:1006–1012. [DOI] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod 2012;27:3328–3336. [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Martínez-González MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013;369:676–677. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 2012;27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Suzuki J, Ohkura S, Hayakawa S. Changes in testicular and nipple volume related to age and seasonality in Japanese macaques (Macaca fuscata), especially in the pre- and post-pubertal periods. Primates 2005;46:33–45. [DOI] [PubMed] [Google Scholar]
- Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000;72:912–921. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Nozawa S, Yoshiike M, Hoshino T, Baba K, Matsushita T, Tanaka SN, Naka M, Skakkebaek NE, Jørgensen N. Semen quality of 324 fertile Japanese men. Hum Reprod 2006;21:760–765. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, Andersen AN, Auger J, Cawood EH, Horte A et al. Regional differences in semen quality in Europe. Hum Reprod 2001;16:1012–1019. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK et al. East-west gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199–2208. [DOI] [PubMed] [Google Scholar]
- Levine H, Swan SH. Is dietary pesticide exposure related to semen quality? Positive evidence from men attending a fertility clinic. Hum Reprod 2015;30:1287–1289. [DOI] [PubMed] [Google Scholar]
- Lioret S, McNaughton SA, Crawford D, Spence AC, Hesketh K, Campbell KJ. Parents’ dietary patterns are significantly correlated: findings from the Melbourne Infant Feeding Activity and Nutrition Trial Program. Br J Nutr 2012;108:518–526. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case-control study. Fertil Steril 2009;91:812–818. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Vioque J, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. A low intake of antioxidant nutrients is associated with poor semen quality in patients attending fertility clinics. Fertil Steril 2010;93:1128–1133. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Mínguez-Alarcón L, Sarabia-Cos L, López-Espín JJ, Vivero-Salmerón G, Ruiz-Ruiz KJ, Fernández MF, Olea N, Swan SH et al. Sperm counts may have declined in young university students in Southern Spain. Andrology 2013;1:408–413. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586–592. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Sarabia-Cos L, Vivero-Salmerón G, Vioque J, Navarrete-Muñoz EM, Torres-Cantero AM. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod 2012;27:2807–2814. [DOI] [PubMed] [Google Scholar]
- Ocké MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, van Staveren WA, Kromhout D. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol 1997;26(Suppl 1):S49–S58. [DOI] [PubMed] [Google Scholar]
- Palma I, Farran P, Cervera P. Tablas de composición de Alimentos por medidas caseras de consumo habitual en España: CESNID [in Spanish], 4th edn Barcelona, Spain: McGraw Hill, 2008. [Google Scholar]
- Pimenta AM, Toledo E, Rodriguez-Diez MC, Gea A, Lopez-Iracheta R, Shivappa N, Hébert JR, Martinez-Gonzalez MA. Dietary indexes, food patterns and incidence of metabolic syndrome in a Mediterranean cohort: The SUN project. Clin Nutr 2015;34:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17:2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanish Food Safety and Nutrition Agency. 2011. Available at: http://aesan.msssi.gob.es/ (20 November 2011, date last accessed).
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH. One semen sample or 2? Insights from a study of fertile men. J Androl 2007;28:638–643. [DOI] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001;154:1089–1099. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW, Study For Future Families Research Group. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect 2003;111:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E, Lopez-del Burgo C, Ruiz-Zambrana A, Donazar M, Navarro-Blasco I, Martínez-González MA, de Irala J. Dietary patterns and difficulty conceiving: a nested case-control study. Fertil Steril 2011;96:1149–1153. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service 2010 National Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory, 2010. Available at: http://www.ars.usda.gov/ba/bhnrc/ndl (20 November 2011, date last accessed). [Google Scholar]
- Varela-Moreiras G, Avila JM, Cuadrado C, del Pozo S, Ruiz E, Moreiras O. Evaluation of food consumption and dietary patterns in Spain by the Food Consumption Survey: updated information. Eur J Clin Nutr 2010;64(Suppl 3):S37–S43. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab 1992;74:939–942. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Vioque J. Validez de la evaluación de la ingesta dietética [in Spanish]. In: Serra-Majem L, Aranceta J, Mataix J (eds). Nutrición Y Salud Pública. Métodos, Bases Científicas Y Aplicaciones, 1st edn Barcelona, Spain: Masson, 1995. [Google Scholar]
- Vioque J, Gonzalez L. Validity of a food frequency questionnaire (preliminary results). Europ J Cancer Prev 1991;1:19–20. [Google Scholar]
- Vioque J, Weinbrenner T, Asensio L, Castelló A, Young IS, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr 2007;97:977–986. [DOI] [PubMed] [Google Scholar]
- Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, Ramón R, Ballester F, Murcia M, Rebagliato M et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EP, Steegers-Theunissen RPM. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod 2009;24:1304–1312. [DOI] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EAP, Steegers-Theunissen RPM. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril 2010;94:2096–2101. [DOI] [PubMed] [Google Scholar]
- Weng HY, Hsueh YH, Messam LLM, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 2009;169:1182–1190. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology, 3rd edn Oxford, New York: Oxford University Press, 2012. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn Geneva, Switzerland: WHO Press, 2010. [Google Scholar]
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jørgensen N, Mendiola J, Swan SH, Chavarro JE. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril 2013;100:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.