Abstract
STUDY QUESTION
Are significant abnormalities of CatSper function present in IVF patients with normal sperm concentration and motility and if so what is their functional significance for fertilization success?
SUMMARY ANSWER
Sperm with a near absence of CatSper current failed to respond to activation of CatSper by progesterone and there was fertilization failure at IVF.
WHAT IS KNOWN ALREADY
In human spermatozoa, Ca2+ influx induced by progesterone is mediated by CatSper, a sperm-specific Ca2+ channel. A suboptimal Ca2+ influx is significantly associated with, and more prevalent in, men with abnormal semen parameters, and is associated with reduced fertilizing capacity. However, abnormalities in CatSper current can only be assessed directly using electrophysiology. There is only one report of a CatSper-deficient man who showed no progesterone potentiated CatSper current. A CatSper 2 genetic abnormality was present but there was no information on the [Ca2+]i response to CatSper activation by progesterone. Additionally, the semen samples had indicating significant abnormalities (oligoasthenoteratozoospermia) multiple suboptimal functional responses in the spermatozoon. As such it cannot be concluded that impaired CatSper function alone causes infertility or that CatSper blockade is a potential safe target for contraception.
STUDY DESIGN, SIZE, DURATION
Spermatozoa were obtained from donors and subfertile IVF patients attending a hospital assisted reproductive techniques clinic between January 2013 and December 2014. In total 134 IVF patients, 28 normozoospermic donors and 10 patients recalled due to a history of failed/low fertilization at IVF took part in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Samples were primarily screened using the Ca2+ influx induced by progesterone and, if cell number was sufficient, samples were also assessed by hyperactivation and penetration into viscous media. A defective Ca2+ response to progesterone was defined using the 99% confidence interval from the distribution of response amplitudes in normozoospermic donors. Samples showing a defective Ca2+ response were further examined in order to characterize the potential CatSper abnormalities. In men where there was a consistent and robust failure of calcium signalling, a direct assessment of CatSper function was performed using electrophysiology (patch clamping), and a blood sample was obtained for genetic analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 101/102 (99%) IVF patients and 22/23 (96%) donors exhibited a normal Ca2+ response. The mean (±SD) normalized peak response did not differ between donors and IVF patients (2.57 ± 0.68 [n = 34 ejaculates from 23 different donors] versus 2.66 ± 0.68 [n = 102 IVF patients], P = 0.63). In recall patients, 9/10 (90%) showed a normal Ca2+ response. Three men were initially identified with a defective Ca2+ influx. However, only one (Patient 1) had a defective response in repeat semen samples. Electrophysiology experiments on sperm from Patient 1 showed a near absence of CatSper current and exon screening demonstrated no mutations in the coding regions of the CatSper complex. There was no increase in penetration of viscous media when the spermatozoa were stimulated with progesterone and importantly there was failed fertilization at IVF.
LIMITATIONS, REASONS FOR CAUTION
A key limitation relates to working with a specific functional parameter (Ca2+ influx induced by progesterone) in fresh sperm samples from donors and patients that have limited viability. Therefore, for practical, technical and logistical reasons, some men (∼22% of IVF patients) could not be screened. As such the incidence of significant Ca2+ abnormalities induced by progesterone may be higher than the ∼1% observed here. Additionally, we used a strict definition of a defective Ca2+ influx such that only substantial abnormalities were selected for further study. Furthermore, electrophysiology was only performed on one patient with a robust and repeatable defective calcium response. This man had negligible CatSper current but more subtle abnormalities (e.g. currents present but significantly smaller) may have been present in men with either normal or below normal Ca2+ influx.
WIDER IMPLICATIONS OF THE FINDINGS
These data add significantly to the understanding of the role of CatSper in human sperm function and its impact on male fertility. Remarkably, these findings provide the first direct evidence that CatSper is a suitable and specific target for human male contraception.
STUDY FUNDING/COMPETING INTEREST(S)
Initial funding was from NHS Tayside, Infertility Research Trust, TENOVUS, Chief Scientist Office NRS Fellowship, the Wellcome Trust, University of Abertay. The majority of the data were obtained using funding from a MRC project grant (# 4190). The authors declare that there is no conflict of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: CatSper, male fertility, sperm motility, ion channels, calcium stores, electrophysiology, failed fertilization, contraception, unexplained infertility, sperm dysfunction
Introduction
Whilst sperm dysfunction is the single most common cause of infertility (Hull et al., 1985; Irvine, 1998), currently there is no drug a man can take or that can be added to his sperm in vitro to improve fertility (Tardif et al., 2014). A fundamental problem with developing new therapies has been the limited understanding of the physiological workings of the normal and dysfunctional spermatozoon (Barratt, 2010; Aitken and Henkel, 2011; Barratt et al., 2011; Aitken and Nixon, 2013).
However, during the last 8 years major progress in our understanding has been achieved through application of the patch clamp technique (Kirichok et al., 2006). This has included the characterization of the proton (HV1) and chloride channels (Lishko et al., 2010; Orta et al., 2012) and the identification and characterization of the potassium conductance underlying the regulation of sperm membrane potential (Santi et al., 2010; Mannowetz et al., 2013; Brenker et al., 2014; Mansell et al., 2014). Ca2+ signalling is particularly important in the regulation of sperm function (Publicover et al., 2007) and potentially the most significant outcome has been the characterization of the sperm-specific Ca2+ channel CatSper (Ren et al., 2001). CatSper-null sperm show no detectable Ca2+ current, defective calcium signalling, ATP depletion and abnormalities in motility (including hyperactivation; HA). CatSper knockout mice are consequently infertile (Carlson et al., 2003; Jin et al., 2007).
In humans, the importance of CatSper was emphasized by the demonstration that the rapid influx of calcium into the spermatozoon upon interaction with progesterone (and other substances such as prostaglandins) is via CatSper (Lishko et al., 2011; Strünker et al., 2011; Brenker et al., 2012). Patch clamp studies have revealed that CatSper is weakly voltage sensitive and progesterone strongly potentiates CatSper currents while shifting the channel half activation voltage to a less depolarized potential (Lishko et al., 2011; Strünker et al., 2011). This progesterone induced, CatSper-mediated Ca2+ influx stimulates the acrosome reaction, either directly and/or by ‘priming’ the cell and also regulates motility (Alasmari et al., 2013b; Tamburrino et al., 2014). Several clinical studies have shown that suboptimal [Ca2+]i signalling in response to progesterone is associated with oligoasthenoteratozoospermia, and with reduced fertilization at IVF (Falsetti et al., 1993; Shimizu et al., 1993; Oehninger et al., 1994; Krausz et al., 1995). The occurrence of poor [Ca2+]i responses to agonists of CatSper and to mobilization of stored Ca2+ is related to IVF fertilization rates (Alasmari et al., 2013a). Release of stored Ca2+ is more potent than CatSper activation in inducing HA and secondary release of stored Ca2+, downstream of CatSper activation, is likely to be required for HA (Alasmari et al., 2013a). In summary, the clinical data indicate that defects in [Ca2+]i signalling do occur and that these affect IVF fertilizing capacity.
Significant functional differences between the spermatozoa of mice and men, including regulation of motility by Ca2+-signalling (Alasmari et al., 2013b), are such that findings cannot simply be applied to humans. Equivalent human studies rely upon the identification of ‘natural knockouts’, subfertile men with specific lesions of key genes. However, case reports of CatSper genetic abnormalities are rare. Recently, Smith et al. (2013) showed that sperm from a CatSper 2 deficient man had no progesterone potentiated CatSper current; consistent with the idea that CatSper is the primary channel responsible for Ca2+ influx. However, there was no information on the Ca2+ influx induced by progesterone. Further, the semen samples in this study had multiple abnormalities suggesting defects at additional loci and multiple suboptimal functional responses in the spermatozoon (Avidan et al., 2003; Zhang et al., 2007; Avenarius et al., 2009; Bhilawadikar et al., 2013). Though important, these studies do not establish either that the specific cause of infertility in these men was failure of CatSper or that inactivation of CatSper is sufficient to prevent/reduce fertilization in humans. Furthermore, they raise the possibility that loss of CatSper function affects spermatogenesis in humans, which would greatly reduce the potential suitability of CatSper as a male contraceptive target (Ren et al., 2001).
Therefore, the primary objective of this study was to examine whether significant abnormalities of CatSper function can be identified in IVF patients and assess their functional significance.
Materials and Methods
Experimental design
Abnormalities in CatSper current can only directly be assessed using electrophysiology; however, this is a low-throughput technique. Therefore, to identify men with potential CatSper abnormalities for electrophysiology, we devised a combination of screening methods to examine calcium signalling. The primary assay was the Ca2+ influx induced by progesterone followed by penetration into viscous media and HA (Alasmari et al., 2013a,b). In total 134 IVF patients, 28 donors and 10 patients recalled due to a history of low/failed fertilization at IVF took part in the study. The focus was examination of Ca2+ influx but samples were allocated to the other assays if cell number was sufficient. Samples showing a defective Ca2+ influx response were further examined in order to characterize the potential CatSper abnormalities. Direct assessment of CatSper was performed using electrophysiology where a consistent and robust failure of calcium signalling (i.e. repeatable in more than one semen sample) was found (Supplementary Fig. S1).
Patient and donor numbers
Of the 134 IVF patients, 102 were screened for calcium response to progesterone, 66 for 4-aminopyridine (4-AP)-induced HA and 16 for penetration of viscous medium. A total of 111 of these patients fertilized at IVF (average 78.6 ± 22% (1 SD)), while 9 patients had total failed fertilization, 8 had less than 4 oocytes retrieved and for 6 there were no fertilization data available.
Of the 28 donors, 23 provided one or more samples (34 ejaculates in total) for the Ca2+ response to progesterone, 35 ejaculates for the 4-AP-induced HA assay and 18 ejaculates for the viscous media penetration test.
All of the 10 recall patients were screened for Ca2+ response to progesterone and one was screened for 4-AP-induced HA and viscous medium penetration.
Ethical approval and subjects
Written informed consent was obtained from three groups: (i) patients (subfertile men who underwent assisted reproduction techniques in Ninewells Hospital, Dundee, Scotland); (ii) sperm donors (selected with no known fertility problems and normal semen parameters) (Cooper et al., 2010); (iii) patients with a history of failed/low fertilization at IVF. Men were recruited in accordance with the Human Fertilisation and Embryology Authority Code of Practice (version 8) under local ethical approval (13/ES/0091) from the Tayside Committee of Medical Research Ethics B.
Semen samples
Samples from donors and patients were used as previously described (Tardif et al., 2014). Patients were selected for IVF according to clinical indications and semen quality: e.g. normal sperm concentration and motility (Cooper et al., 2010) and ∼ 1 × 106 progressively motile cells post-preparation. The surplus of the clinical sample used in the IVF treatment process was assessed for intracellular Ca2+ and where possible HA and penetration into viscous media.
Media and preparation
Commercially available Quinn's Advantage Fertilization Medium (HTF) plus 5% human serum albumin (HSA) and Quinn's Sperm Washing Medium (SW) were used to prepare and capacitate sperm. Non-capacitating HEPES-buffered media (NCM) lacking in both albumin and bicarbonate (4.69 mM KCl, 2.04 mM CaCl2, 0.2 mM MgSO4, 97.8 mM NaCl, 2.78 mM D-Glucose, 0.33 mM Na pyruvate, 21.4 mM lactic acid and 21 mM HEPES, ∼280 mOsm/kg H2O, adjusted to pH 7.4 using 10 M NaOH) was used for sample re-suspension in the FLUOstar.
Spermatozoa were isolated by density gradient centrifugation (DGC) using Percoll/PureSperm diluted with SW for donors/patients respectively. After centrifugation (300g, 20 min), the supernatant was discarded and pellet was washed (500g for 10 min), resuspended in HTF, and incubated for a minimum of 2 h at 37°C in 5% CO2.
Agonists and reagents
Ca2+ signalling was manipulated using progesterone (Lishko et al., 2011; Alasmari et al., 2013a) and 4-AP) (Bedu-Addo et al., 2008). Reagents were purchased from Sigma Aldrich unless otherwise stated. 4-AP and progesterone were dissolved in distilled water and ethanol, respectively. Fura-2 acetoxymethyl ester (Fura-2/AM) (Molecular Probes, Invitrogen, Oregon, USA) was dissolved in dimethylsulphoxide (final concentration of 1 µM).
Measurement of intracellular Ca2+
Approximately 4 million cells were prepared and assessed as previously described using a FLUOstar microplate reader (BMG Labtech Offenburg, Germany) (Alasmari et al., 2013a) with minor modifications encompassing use of 0.05% Pluronic acid F127 and manganese chloride (9.1 mM). Progesterone-induced increments in the ratio of emission intensities (at 340 and 380 excitation) were used to quantify changes in [Ca2+]i concentration (Alasmari et al., 2013a,b). After adjustment for background fluorescence normalization was achieved by dividing each ratio value at time point × (Rx) by the mean fluorescence ratio value taken from hundreds of basal fluorescence ratio readings (Rbasal).
Assessment of HA and motility
A Hamilton Thorne CEROS computer aided sperm analysis (CASA) machine (Beverley, MA, USA) was used to assess motility for semen and prepared sperm samples (as described in Supplementary Table SI) (Alasmari et al., 2013a,b). Sperm were treated with 4-AP at a final concentration of 2 mM, and the percentage of hyperactivated cells was assessed (Mortimer et al., 1998).
Penetration into viscous media
This was performed as previously described (Ivic et al., 2002; Alasmari et al., 2013b). Methylcellulose solution was created using a 1% w/v methylcellulose in HTF media supplemented with 5% HSA. Methylcellulose solution was introduced into capillary tubes by capillary action (Vitrotubes™, Rectangle Capillaries 0.4 mm × 400 mm, VitroCom, New Jersey, USA). Prepared sperm were adjusted to ∼10–20 × 10−6/ml before 3.6 µM progesterone/vehicle were added to the aliquots. Following 1 h incubation at 37°C and 5% CO2, cell numbers at 1 and 2 cm were normalized to values from parallel, untreated controls (C/C, V/C and P/C, where C = number of cells under control conditions, V = under vehicle controls and P = after treatment with progesterone).
Definition of failed HA and Ca2+ responses among donors and sub-fertile patients
A failed HA response was recorded when agonist stimulation did not induce a significant change in the percentage of hyperactivated cells compared with control (basal) level (assessed by paired two-tailed Student's t-test, P < 0.05). To define a defective Ca2+ response to progesterone, the 99% confidence interval was determined from the distribution of response amplitudes in 34 ejaculates from 23 normozoospermic donors: <0.41 and >4.85 delta response upon addition of progesterone.
Fertilization rate at IVF
Oocytes were considered normally fertilized when two pronuclei (2PN) and two polar bodies were observed. In IVF, the fertilization rate was calculated from the number of oocytes normally fertilized divided by the total number of inseminated oocytes. The fertilization rate was calculated where four or more mature oocytes (metaphase II) were present. Low fertilization was defined where <30% of four or more metaphase II oocytes were normally fertilized.
Sperm preparation for electrophysiology
Sperm were prepared, for electrophysiology using a swim-up procedure into HTF as previously described (Lishko et al., 2013; Mansell et al., 2014). The cells were re-suspended in capacitating media and maintained at 37°C for 4 h (5% CO2). Capacitated cells were transferred into petri dishes at room temperature containing standard bath solution in order to allow cells to adhere to glass coverslips. Coverslips were transferred into a perfusion chamber and perfused with standard bath solution until whole-cell configuration was achieved. The biophysical properties of individual sperm were recorded under whole-cell conditions (Lishko et al., 2011; Mansell et al., 2014) using borosilicate glass pipettes (10–15 MOhms) filled with Cs+-based divalent free solution. Gigaohm seals were formed on cytoplasmic droplets or on the midpiece region. Break in was achieved via light suction and 1 ms voltage pulses (450–610 mV). Flagellar beating was observed in all cells selected. In order to record CatSper, currents were recorded in Cs+-based divalent free extracellular solution evoked by a depolarizing ramp protocol imposed (−80 to 80 mV) over 2 s and membrane potential (Vm) was held at 0 mV between test pulses.
Experimental solutions
All concentrations are in mM. Synthetic human tubular fluid (HTF): NaCl, 97.8; KCl, 4.69; MgSO4; 0.2; CaCl2, 2.04; HEPES, 21; Glucose, 2.78; Na-lactate 21.4; Na-pyruvate, 0.33; pH adjusted to 7.4 with NaOH. Capacitating medium: NaCl, 135; KCl, 5, MgSO4, CaCl2, 2; HEPES, 20; Glucose, 5; Lactic acid, 10; Na-Pyruvate, 1; NaHCO3, 25; fetal bovine serum, 20%; pH adjusted to 7.4 with NaOH. Standard bath solution: NaCl, 135; KCl, 5; CaCl2, 2; MgSO4,1; HEPES, 20; Glucose, 5; Na Pyruvate, 1; Na lactate, 10; pH adjusted to 7.4 with NaOH which brought [Na] to 154 mM. Non-selective cation currents flowing via spermatozoon cation channels (CatSper) were quantified using pipette (Cs-methanesulphonate, 130; HEPES, 40; Tris–HCl, 1; EGTA, 3; EDTA, 2 mM, pH adjusted to 7.4 with CsOH) and bath (Cs-methanesulphonate, 140; HEPES, 40; EGTA, 3; pH adjusted to 7.4 with CsOH) solutions devoid of Ca2+ and Mg2+ that contained Cs+ as the principal cation.
Genetic screening for mutations in CatSper Complex loci
Blood samples were obtained from Patient 1 and processed for Exome sequencing (Centrillion Biosciences, Palo Alto, USA). Samples were selected using Agilent SureSelect Human all Exon v5-51Mb kit and yielded 1.83 Gbase, giving a raw coverage of 36X. Coverage for the CatSper Complex loci (CatSper 1-4, CatSperB, CatSperG and CatSperD) was verified. Sequences were mapped to human genome and sequences overlapping the loci of interest (Supplementary Table SII) were extracted and realigned using Geneious version 8.1.4 (http://www.geneious.com) and polymorphisms identified and manually verified.
Statistical analysis
Normality of data was assessed according to the Shapiro–Wilk test. Statistical comparisons for the viscous penetration test and induced HA were made using paired two-tailed Student's t-tests or the analysis of variance if the data were either originally normally distributed or normalized after transformation by arcsin or square root. To define a cut-off value for a failed Ca2+ response, the data were log transformed and cut-off values were calculated based on mean and SD (Alasmari et al., 2013a). Unpaired t-tests were performed on currents obtained from whole-cell patch clamp. P < 0.05 was considered significant. The statistical package Prism GraphPad (La Jolla, CA, USA) was used.
Results
In total, 134 IVF patients, 28 donors and 10 patients recalled for assessment due to failed/low fertilization at IVF were included in the study. Patients and donors were screened using one or more of the three techniques depending on sample quality: Ca2+ response, HA and the viscous media assay.
Screening of IVF patients and donors for Ca2+ influx induced by progesterone
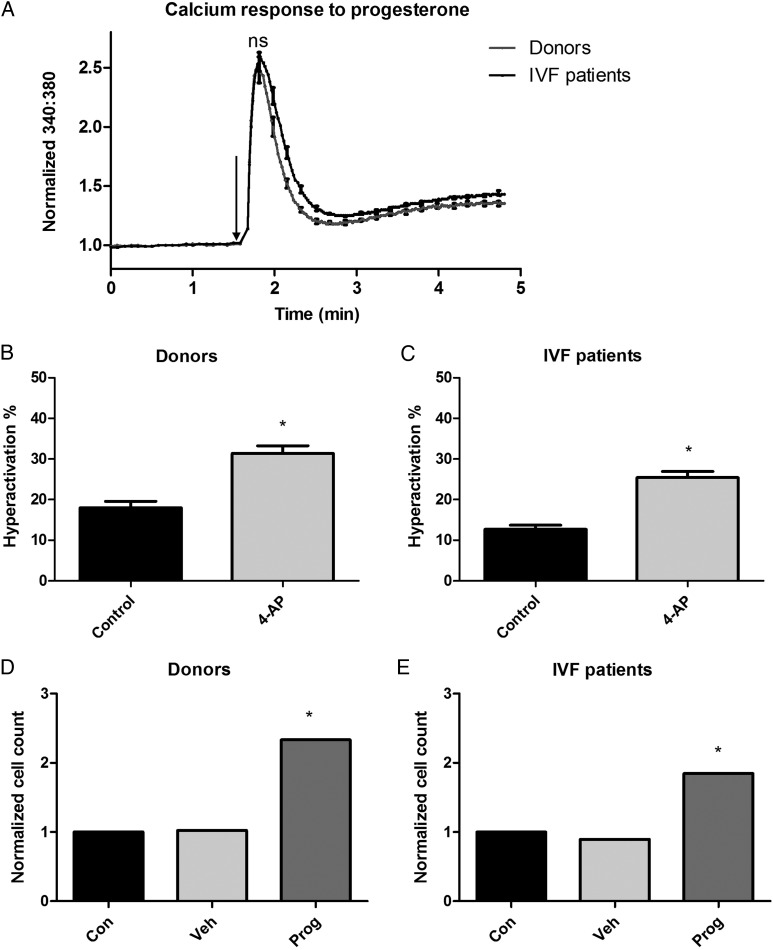
The majority of IVF patients (101/102; 99%) and donors (22/23; 95.7%) exhibited a biphasic [Ca2+]i response as previously described (Alasmari et al, 2013a). The [Ca2+]i transient amplitude was similar in cells from donors and patients attending for IVF treatment (Fig. 1A; donors = 2.57 ± 0.68 [n = 34 ejaculates from 23 different donors], IVF patients = 2.66 ± 0.68 [n = 102 patients], P = 0.63) but the plateau phase of the [Ca2+]i response was greater in IVF patients (P = 0.003). In total, 9/10 patients with previous low/failed fertilization at IVF showed a normal Ca2+ response.
Figure 1.
Calcium response to progesterone, hyperactivation in response to 4-AP and penetration into viscous media for spermatozoa from donors and IVF patients. (A) Peak calcium response to progesterone (progesterone addition indicated by the arrow) was not significantly different between donors and IVF patients (donor n = 34 ejaculates from 23 different donors, IVF n = 102), however, the plateau phase response (mean of the last 12 s of recording) was (donor versus IVF peak calcium response to progesterone, P = 0.003, Student's unpaired t-test). (B and C) 4-aminopyridine (4-AP) significantly increased hyperactivation (HA) in spermatozoa from both donors and IVF patients (P < 0.001 versus control, Student's paired t-test). HA assay donor n = 35, IVF n = 66. (D and E) Penetration into viscous media was higher after stimulation with progesterone for both donors and IVF patients (progesterone versus control, donor n = 18, P < 0.05; IVF n = 16, P < 0.05, one-way analysis of variance). Asterisk denotes statistical significance at the P < 0.05 level.
Screening of IVF patients for HA
IVF patient samples had significantly lower basal HA than donors (Fig. 1B and C, P < 0.05, IVF n = 66, donor n = 35). Treatment with 2 mM 4-AP robustly and significantly increased HA for both IVF patients and donors (P < 0.001).
Screening of IVF patients in the viscous media assay
Sperm from donors and IVF patients (15/18 donors [83%], 13/16 IVF patients [81%]) responded to progesterone with an increase in cell numbers at 1 cm (P < 0.05, Fig. 1D and E).
Identification of defective Ca2+ influx induced by progesterone
Three individuals had spermatozoa that were unable to respond to progesterone. One was from the group of 10 patients recalled due to a previous low/failed fertilization at IVF (Patient 1), one was a patient undergoing IVF treatment (Patient 2) and one was a donor (Donor 1). In each case, diagnostic semen analysis was normal (Supplementary Table SI).
Patient 1
Patient 1 had one previous cycle of IVF that resulted in a total fertilization failure for all nine oocytes retrieved. Consequently he was recalled for further analysis and provided a sample for research. He had a subsequent cycle of ICSI where 5/6 oocytes fertilized (83.3% fertilization rate). Female age was 39 years with an anti-Mullerian hormone (AMH) of 10 pmol/l. Semen analysis was normal (concentration of 66 × 106/ml and motility of 60%, Supplementary Table SI).
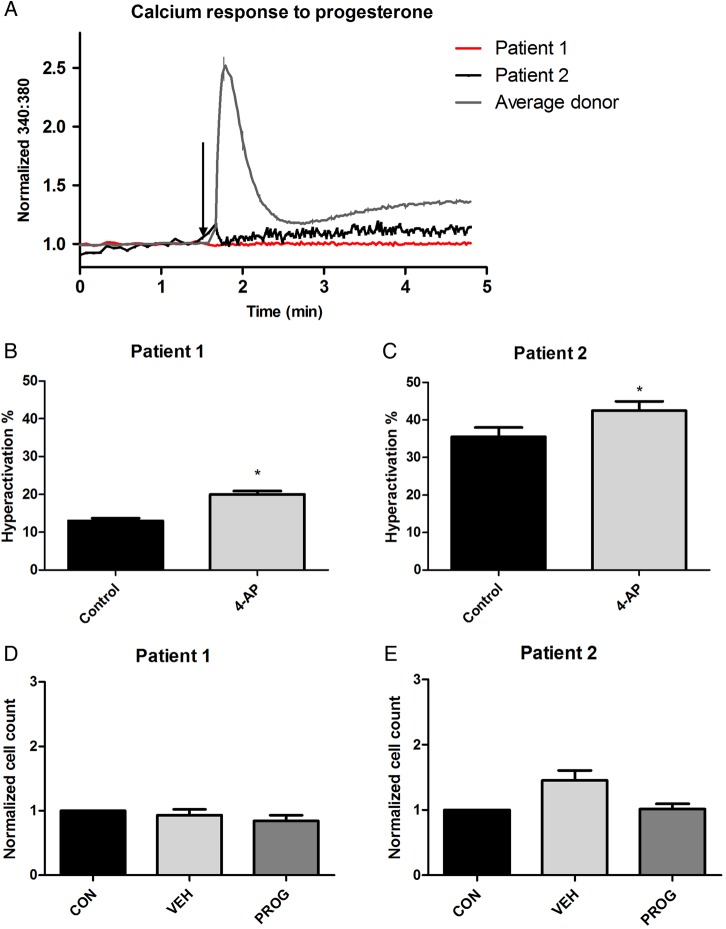
In the first sample, the spermatozoa were unable to produce a Ca2+ influx induced by progesterone (Fig. 2A), though donor sperm tested at the same time and under identical conditions (positive control) gave a normal biphasic response. Furthermore, there was an abnormal response to progesterone in the viscous media assay (Fig. 2D). Stimulation of capacitated cells with 4-AP induced a significant increase in HA (P < 0.001, Fig. 2B).
Figure 2.
Calcium response to progesterone, hyperactivation in response to 4-AP and penetration into viscous media for spermatozoa from Patients 1 and 2. (A) The spermatozoa from Patient 1 and 2 showed no significant calcium response to progesterone (compared with representative average donor trace, as seen in Fig. 1A). Patient 1 trace shows an average of four different aliquots from two ejaculates separated by 7 weeks; Patient 2 trace shows analysis of one sample on the day of IVF treatment. (B and C) 4-AP induced an increase in HA in the spermatozoa from Patient 1 (P < 0.001) and Patient 2 (4-AP versus control, P = 0.01, Student's paired t-test). (D and E) Spermatozoa from Patients 1 and 2 showed no significant enhancement in penetration into viscous media when stimulated with progesterone.
Another sample submitted 7 weeks later showed the same result (Fig. 2A shows the average trace from four assays, two aliquots from the initial sample and two from second) and thus the cells were studied using patch clamp electrophysiology (see below).
Patient 2
On the day of IVF treatment, prepared spermatozoa had a concentration of 20 × 106/ml with a total motility of 87% (Supplementary Table SI). However, as with Patient 1, exposure to progesterone elicited no [Ca2+]i response or stimulation in the viscous media penetration test (Fig. 2A and E). Additionally, similarly to Patient 1, the spermatozoa were able to significantly hyperactivate when exposed to 2 mM 4-AP (P = 0.01) (Fig. 2C). All nine oocytes inseminated by IVF failed to fertilize. Female age was 24 years, with an AMH of 8 pmol/l.
Patient 2 returned for ICSI treatment 7 months later. Three out of five oocytes normally fertilized. Analysis of this sample showed that treatment with progesterone induced both a [Ca2+]i signal and a significant response in the viscous media penetration test (data not shown). Therefore, Patient 2 was not recalled for further analysis for electrophysiology.
Donor 1
Donor 1 was a healthy young male who had never contributed to a pregnancy. Two semen analyses showed an average sperm concentration of 109 × 106/ml with a total motility of 69%. In the initial sample, the sperm were unable to produce a [Ca2+]i response to progesterone (Supplementary Fig. S2A) and were not able to respond to progesterone in the viscous media assay (Supplementary Fig. S2D): an outcome similar to that of Patient 1 and the first sample provided by Patient 2. However, the spermatozoa were able to produce a significant HA response when treated with 4-AP (Supplementary Fig. S2B, P = 0.0112). Donor 1 produced another sample 1 week after the initial sample. Spermatozoa from the second sample showed a clear [Ca2+]i response when stimulated with progesterone (Supplementary Fig. S2A) and significant HA when stimulated with 4-AP (Supplementary Fig. S2C), but failed to respond to progesterone in the viscous media assay (Supplementary Fig. S2E). Therefore, Donor 1 was not recalled for further study for electrophysiology.
Electrophysiology
CatSper has typically been characterized under monovalent conditions (Lishko et al., 2011; Smith et al., 2013); therefore Cs-based divalent free solutions were used to examine CatSper channel function. CatSper currents (ICatSper) were robust in sperm from donors with a maximum outward current of 204.6 ± 17.7 pA/pF (Fig. 3). In contrast, ICatSper was essentially absent in sperm from Patient 1 with the maximum current (4.0 ± 0.4 pA/pF) being significantly lower (P < 0.01).
Figure 3.
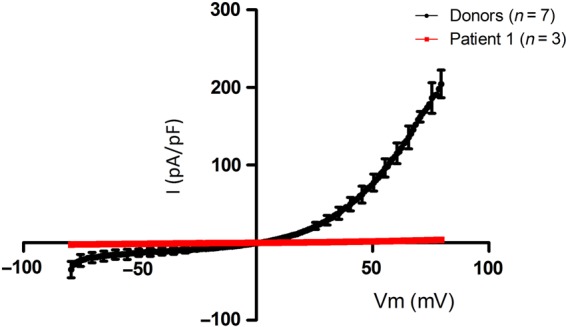
Whole-cell patch clamp recordings from spermatozoa from Donors and Patient 1. CatSper currents are absent from spermatozoa from Patient 1. Current–voltage relationship recorded under Cs-based divalent free conditions from spermatozoa from Patient 1 (n = 3). Capacitated donor data are also shown (n = 7). Current is measured in picoamps per picofarad, to normalize for variation in capacitance between cells. Error bars represent standard error of the mean (SEM).
Genetic analysis
In mice all seven members of the CatSper Complex (CatSperα(1-4), CatSperβ, γ & δ) are required for functional expression. Assuming a similar requirement in human would suggest that a genetic lesion in any single gene could result in the absence of CatSper conductance. Exome sequence for Patient 1 was analysed for the presence of single nucleotide polymorphism variants and in/dels. This analysis revealed no evidence for loss of function mutations in any of the CatSper Complex loci (Supplementary Table SII). We further analysed a set of genes which, based on mouse genetics, would lead to fertilization failure without affecting spermatogenesis. Again no loss of function mutation was observed (Supplementary Table SII).
Discussion
In previous studies genetic analysis has identified mutations in the genes coding for proteins of the CatSper channel. In humans, these abnormalities occur in association with other phenotypic abnormalities (Avidan et al., 2003; Avenarius et al., 2009; Hildebrand et al., 2010; Jaiswal et al., 2014). Analysis of CatSper currents has been undertaken in only one of these cases (Smith et al., 2013) where there was a 70 kb deletion which spans four loci, CATSPER2, Stereocilin, CKMT1 and KIAA0377 (Avidan et al., 2003). Deletion of these additional loci may explain the observation that, in addition to failure of CatSper currents, semen from this man was grossly abnormal thus making it unclear if the spermatogenesis defect was due to a loss of CATSPER2 or one of the other 3 loci. In the current study we have screened for functional deficiencies consistent with putative defects of CatSper function in men with normal semen parameters attending for IVF treatment. Our data support the conclusion that loss of CatSper conductance is rare, may occur independently of abnormalities associated with spermatogenesis and, importantly, has functional significance.
The data described here and previously (Alasmari et al., 2013a) show that, on average, sperm from IVF patients responded similarly to donors. There was no significant difference between the groups for (i) Ca2+ responses to activation of CatSper or (ii) performance in penetration in the viscous media assay (artificial mucus) under control conditions and when stimulated with progesterone. However, samples from three of the men studied (two IVF patients and one donor) combined normal semen parameters with striking CatSper functional abnormalities. Motility parameters, including HA, were normal (and stable for up to 6 h) as was penetration of the sperm into viscous media in the absence of progesterone. However, these sperm failed to respond to activation of CatSper by progesterone (Lishko et al., 2011; Strünker et al., 2011) either with elevation of [Ca2+]i or a functional response in the viscous media penetration test, which is via activation of CatSper (Alasmari et al., 2013b). Samples from two of these men were also used for IVF and both showed complete failure to fertilize. In contrast, only 14/111 patient samples that had a normal progesterone-induced [Ca2+]i response (including 9 who previously achieved low/failed fertilization at IVF) failed to fertilize. This is a clear significant difference (P < 5*10−4; Chi square contingency test) strongly supporting the concept that functional failure of CatSper (progesterone induced [Ca2+]i) is sufficient to compromise fertility of human sperm, although such defects are rare. Furthermore, the ‘stable’ loss of CatSper function (see below) was not associated with poor semen characteristics. This is in contrast to the mouse phenotype and suggests that the roles of the CatSper complex in normal physiology differ between mouse and human.
Two of the men whose sperm failed to respond to progesterone (Patient 2 and Donor 1) provided further samples (interval of 1 week for Donor 1 and 7 months for Patient 2) that gave [Ca2+]i responses upon stimulation with progesterone (e.g. Supplementary Fig. S2c). The assumption is that these men, therefore, have no CatSper genetic lesions. However, sperm from Patient 1 consistently failed to respond to stimulation with progesterone in two samples, and electrophysiological study confirmed the absence of CatSper currents. This suggests the presence of a consistent functional lesion of CatSper in Patient 1 that was aetiologically distinct to that seen in Patient 2 and Donor 1. The most obvious candidate is a genetic alteration. However, no candidate mutations in the CatSper complexes were observed.
Previous analysis of the impact of CatSper loss has been in patients with deletions in multiple loci. This resulted in diverse defects in the spermatozoa raising the question of whether CatSper function is required for normal spermatogenesis (Avidan et al., 2003; Smith et al., 2013). In contrast, our data suggest that spermatogenesis is normal in men where CatSper function cannot be detected in ejaculated spermatozoa. Together with the observation that loss of CatSper activity may be transient (Patient 2 and Donor 1), this further emphasizes the potential of CatSper interruption as a feasible target for contraception.
The absence of any overt genetic abnormality in Patient 1 together with the transient phenotype in Patient 2 and Donor 1 suggests a defect(s) in testicular and/or epididymal sperm maturation and/or potential processing in the mature cell. However, although impaired localization and/or assembly of the CatSper complex is one explanation for loss of function, detailed proteomic studies combined with high quality co-localization and imaging (Chung et al., 2014) would be required to address this.
In this study, and that of Alasmari, the number of men whose partners had IVF and thus had normal semen profiles (concentration and motility) yet defective Ca2+ influx induced by progesterone was small (1% and 0%) respectively. Similarly, in the 10 patients with a history of fertilization failure/low fertilization at IVF only one showed a defective [Ca2+]i response (Patient 1). Although these findings indicate that CatSper defects are not a common cause of fertilization failure/reduced success, there are caveats to this conclusion. Firstly, since samples from 22% of IVF patients could not be screened (due to practical, technical and/or logistical reasons) the incidence of significant abnormalities might be underestimated. Secondly, the strict definition of a defective Ca2+ influx used is such that only substantial abnormal responses were selected for further study. Electrophysiological analysis was performed on Patient 1 with a clear and consistent defect and the sperm from this man showed negligible CatSper current. However, more subtle abnormalities of CatSper function, for example significantly reduced but not absent current, may occur and have some biological significance.
Our studies also shed light on the link between the CatSper complex and the regulation of HA. We demonstrated that 4-AP enhanced HA in sperm from Patient 1, who consistently showed failure of progesterone-induced [Ca2+]i elevation and was null for CatSper current. The progesterone-insensitive sample from Patient 2 also responded to 4-AP with an increase in HA. This is consistent with a model for regulation of HA in which the Ca2+ stored at the sperm neck is pivotal to HA induction and CatSper-mediated Ca2+ influx in the flagellum acts as a trigger for mobilization of this store (Alasmari et al., 2013a,b). Mobilization of stored Ca2+ in mouse sperm null for CatSper has also been shown to induce HA (Marquez et al., 2007). Intriguingly, this suggests that pharmacological mobilization of stored Ca2+ can bypass failure due to impaired function or regulation of CatSper. Further investigations into the role of calcium response to progesterone and CatSper function, and their relationship to fertilization rate in IVF, should include the use of single-cell Ca2+ imaging. This would enable a high resolution investigation into the diverse cellular responses between individuals, and could provide further insight into Ca2+ signalling dysfunction.
We conclude that, in IVF patients, a total lack of CatSper current is rare, functionally significant and does not necessarily involve mutation in the CatSper gene complex.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
H.L.W. performed the initial screening on all of the IVF patients, recalling patients and research donors. W.A. performed initial screening and identification of initial recall patients. S.M.d.S., S.M. and H.L.W. were involved in the recruiting, recalling and consenting of patients and donors. S.M. and S.G.B. performed the patch clamping experiments. S.G.B. and S.M. performed the detailed analysis of the electrophysiological data. K.A.S., P.V.L. and M.R.M. were responsible for the genetic analysis. S.M.W., S.J.P., S.G.B. and C.L.R.B. were involved in the design of the study and obtained funding for the experiments. The initial, interim and final manuscript was drafted by C.L.R.B., H.L.W., S.G.B. and S.J.P. All authors contributed to the construction, writing and editing of the manuscript. All authors approved the final manuscript for submission.
Funding
The initial funding for the project was supported by grants from NHS Tayside, Infertility Research Trust, TENOVUS, Chief Scientist Office NRS Fellowship (S.M.d.S.), the Wellcome Trust, University of Abertay (sabbatical for S.G.B.). P.V.L. was supported by NIH grant R01GM111802 (NIGMS) and Alfred P. Sloan Award. The majority of the data were obtained using funding from a MRC project grant (#4190). Funding to pay the Open Access publication charges for this article was provided by MRC.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
Work in the authors' laboratories is funded by The Welcome Trust, TENOVUS (Scotland), University of Dundee, MRC, Ministry of Higher Education-Kingdom of Saudi Arabia (PhD studentship to W.A.), Dundee Drug Discovery Unit, NHS Tayside, Scottish Enterprise. The authors are very grateful to all members of the Assisted Conception Unit at Ninewells Hospital for their invaluable assistance in obtaining donor and patient samples for research and training purposes, in particular the embryologists (Kath, Ellen, Philip, Sylwia, Anne and Mandy) and nurses. We are also grateful to all the patients and donors who took part in this study. The authors acknowledge other members of the laboratory for their continual helpful advice and comments, and Evelyn Barratt for assisting with the recruitment of patients and donors. We also acknowledge the statistical support of Professor Steve Hubbard, Professor C De Jonge for reading and commenting on the manuscript, Timo Strunker for advice regarding FLUOstar and, Yuriy Kirichok from UCSF for his invaluable assistance in developing the patch clamping in Dundee.
References
- Aitken RJ, Henkel RR. Sperm cell biology: current perspectives and future prospects. Asian J Androl 2011;13:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 2013;19:785–793. [DOI] [PubMed] [Google Scholar]
- Alasmari W, Barratt CL, Publicover SJ, Whalley KM, Foster E, Kay V, Martins da Silva S, Oxenham SK. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod 2013a;28:866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Ramalho-Santos J, Kirkman-Brown J, Michelangeli F, Publicover S et al. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J Biol Chem 2013b;288:6248–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius M, Hildebrand M, Zhang Y, Meyer N, Smith L, Kahrizi K, Najmabadi H, Smith R. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet 2009;84:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet 2003;11:497–502. [DOI] [PubMed] [Google Scholar]
- Barratt CL. Male infertility joins the translational medicine revolution. Sperm DNA: from basic science to clinical reality. Mol Hum Reprod 2010;16:1–2. [DOI] [PubMed] [Google Scholar]
- Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl 2011;13:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedu-Addo K, Costello S, Harper C, Machado-Oliveira G, Lefievre L, Ford C, Barratt C, Publicover S. Mobilisation of stored calcium in the neck region of human sperm—a mechanism for. Int J Dev Biol 2008;52:615–626. [DOI] [PubMed] [Google Scholar]
- Bhilawadikar R, Zaveri K, Mukadam L, Naik S, Kamble K, Modi D, Hinduja I. Levels of Tektin 2 and CatSper 2 in normozoospermic and oligoasthenozoospermic men and its association with motility, fertilization rate, embryo quality and pregnancy rate. J Assist Reprod Genet 2013;30:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strünker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 2012;31:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Zhou Y, Muller A, Echeverry FA, Trotschel C, Poetsch A, Xia XM, Bonigk W, Lingle CJ, Kaupp UB et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. ELife 2014;3:e01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in. Proc Natl Acad Sci USA 2003;100:14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014;157:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Falsetti C, Baldi E, Krausz C, Casano R, Failli P, Forti G. Decreased responsiveness to progesterone of spermatozoa in oligozoospermic patients. J Androl 1993;14:17–22. [PubMed] [Google Scholar]
- Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, Auer J, Serres C, Kahrizi K, Najmabadi H, Beckmann JS et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet 2010;18:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. BMJ (Clinical research ed) 1985;291:1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod 1998;13 Suppl 1:33–44. [DOI] [PubMed] [Google Scholar]
- Ivic A, Onyeaka H, Girling A, Brewis IA, Ola B, Hammadieh N, Papaioannou S, Barratt CL. Critical evaluation of methylcellulose as an alternative medium in sperm migration tests. Hum Reprod 2002;17:143–149. [DOI] [PubMed] [Google Scholar]
- Jaiswal D, Singh V, Dwivedi US, Trivedi S, Singh K. Chromosome microarray analysis: a case report of infertile brothers with CATSPER gene deletion. Gene 2014;542:263–265. [DOI] [PubMed] [Google Scholar]
- Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility. Biol Reprod 2007;77:37–44. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006;439:737–740. [DOI] [PubMed] [Google Scholar]
- Krausz C, Bonaccorsi L, Luconi M, Fuzzi B, Criscuoli L, Pellegrini S, Forti G, Baldi E. Intracellular calcium increase and acrosome reaction in response to progesterone in human spermatozoa are correlated with in-vitro fertilization. Hum Reprod 1995;10:120–124. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 2010;140:327–337. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011;471:387–391. [DOI] [PubMed] [Google Scholar]
- Lishko P, Clapham DE, Navarro B, Kirichok Y. Sperm patch-clamp. Methods Enzymol 2013;525:59–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannowetz N, Naidoo NM, Choo SA, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. ELife 2013;2:e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell SA, Publicover SJ, Barratt CL, Wilson SM. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod 2014;20:392–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol 2007;303:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod 1998;13:2139–2146. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Blackmore P, Morshedi M, Sueldo C, Acosta AA, Alexander NJ. Defective calcium influx and acrosome reaction (spontaneous and progesterone-induced) in spermatozoa of infertile men with severe teratozoospermia. Fertil Steril 1994;61:349–354. [DOI] [PubMed] [Google Scholar]
- Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol 2012;590:2659–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm—making the most of what you've got. Nat Cell Biol 2007;9:235–242. [DOI] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature 2001;413:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 2010;584:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Nord EP, Bronson RA. Progesterone-evoked increases in sperm [Ca2+]i correlate with the egg penetrating ability of sperm from fertile but not infertile men. Fertil Steril 1993;60:526–532. [DOI] [PubMed] [Google Scholar]
- Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci USA 2013;110:6823–6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011;471:382–386. [DOI] [PubMed] [Google Scholar]
- Tamburrino L, Marchiani S, Minetti F, Forti G, Muratori M, Baldi E. The CatSper calcium channel in human sperm: relation with motility and involvement in progesterone-induced acrosome reaction. Hum Reprod 2014;29:418–428. [DOI] [PubMed] [Google Scholar]
- Tardif S, Madamidola OA, Brown SG, Frame L, Lefièvre L, Wyatt PG, Barratt CLR, Martins Da Silva SJ. Clinically relevant enhancement of human sperm motility using compounds with reported phosphodiesterase inhibitor activity. Hum Reprod 2014;29:2123–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Malekpour M, Al-Madani N, Kahrizi K, Zanganeh M, Mohseni M, Mojahedi F, Daneshi A, Najmabadi H, Smith RJH. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J Med Genet 2007;44:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.