Abstract
Expression of interleukin-6 (IL-6) upon acute inflammatory stress is significantly augmented by aging in adipose tissue, a major source of this cytokine. In the present study, we examined the mechanism of age-dependent IL-6 overproduction using visceral white adipose tissue from C57BL/6 mice. Upon treatment with lipopolysaccharide (LPS) in vitro, IL-6 was produced by adipose tissue explants, and secreted levels were significantly higher in cultures from aged (24 months) mice compared to young (4 months). Interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα), two inducers of IL-6, were mainly produced by the lungs and spleen rather than adipose tissue in mice after LPS injection. Treatment of adipose explants with physiological levels of IL-1β induced significant age-dependent secretion of IL-6, while treatment with TNFα had little effect, demonstrating an augmented response of adipose tissues to IL-1β in the aged. In vitro experiments utilizing a neutralizing antibody against IL-1β and in vivo experiments utilizing IL-1-receptor-1 deficient mice, confirmed that IL-6 overproduction in the aged is regulated by autocrine/paracrine action of IL-1β which specifically occurs in aged adipose tissues. These findings indicate an elevated inflammatory potential of adipose tissue in the aged and a unique IL-1β-mediated mechanism for IL-6 overproduction, which may impact age-associated vulnerability to acute inflammatory diseases such as sepsis.
Key Words: Aging, Adipose tissue, IL-6, IL-1β, Inflammation, Sepsis
Aging is associated with increased basal levels of interleukin-6 (IL-6), an inflammatory cytokine which is usually very low under normal conditions (1–5). Gene expression, as well as tissue and circulating levels of IL-6, are also dramatically increased by inflammatory stimuli and these induced levels are significantly augmented by aging (6–10). This is of particular clinical importance during sepsis, an acute systemic inflammatory condition caused by infection. Sepsis is the 10th leading cause of death in patients aged 65 and over, and both incidence and mortality increase progressively with advancing age (10). Sepsis is also a frequent complication in patients with pneumonia, appendicitis, acute pancreatitis, urinary tract infection, and severe burn (10).
We have previously shown that augmented production of IL-6 in the aged is associated with high mortality during experimental animal models of sepsis, acute pancreatitis, and endotoxemia (6,8–10). We also demonstrated that of all the major tissues in the body, visceral white adipose tissue expresses the highest level of IL-6 mRNA in both young and aged mice during systemic inflammation induced by injection with gram-negative bacterial endotoxin lipopolysaccharide (LPS), with the level of expression being more than double in the aged (11,12). We and others have also shown that aged IL-6−/− mice are more resistant to LPS compared to age-matched control mice, demonstrating that overproduction of IL-6 is detrimental in the aged (11,13). While IL-6 is a heavily studied cytokine, the mechanism for this age-associated increase in production of IL-6 from adipose tissue under acute inflammatory stress is not clear. Specifically, it is unclear whether augmented IL-6 expression in adipose tissue is due to a physiological change in the adipose tissue itself or rather a result of increased systemic inflammation in the aged environment.
Furthermore, whether age-associated IL-6 upregulation in adipose tissue is due to a direct action of LPS or mediated by such early inflammatory cytokines as interleukin 1 beta (IL-1β) or tumor necrosis factor alpha (TNFα) remains unknown. Direct action of LPS on adipose tissue is likely as multiple adipose tissue cell types including both adipocytes and preadipocytes, in addition to inflammatory cells, are known to constitutively express toll-like receptor 4 [TLR4 (14–16)], the main receptor which recognizes LPS and mediates its signal transduction including downstream activation of NFκB (17,18). IL-1β and TNFα are cytokines, released early in the inflammatory response, which share a wide range of biological activities including neutrophil activation and bacterial clearance (19). IL-1β and/or TNFα have been shown to induce the expression of IL-6 in various tissues and cell types including cardiac myocytes and vascular cells (20), osteoblasts (21), airway smooth muscle cells (22), lung epithelial cells (23), and subcutaneous adipocytes (24). TNFα has also been shown to compensate for IL-1β during bacterial infection (25) or after LPS administration (26).
In this study, an in vitro explant culture system was used to study the mechanism of IL-6 overexpression in visceral white adipose tissue after inflammatory stimulation with LPS, used to mimic the effects of gram-negative bacterial sepsis. The key feature of using this explant culture system, as opposed to traditional isolated cell culture, is that the integrity of the tissue is maintained which preserves autocrine and paracrine interactions among the different cell types.
Methods
Animals
Young (4-month old) and aged (24-month old) male C57BL/6 mice were obtained from a colony at the National Institute on Aging. Male IL-1 receptor 1 knockout mice (IL-1r1−/−, B6.129S7-Il1r1tm1Imx/J) and wild-type control mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained for at least 7 days in an environment under controlled temperature (21–23°C), humidity (30%–70%), and lighting (14 hours light/10 hours dark) with free access to water and chow (Rodent Diet No. 2500, LabDiet, St. Louis, MO). All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Explant Culture of Adipose Tissues
Mice were anesthetized with 2% isoflurane in air, the inferior vena cava cut, and the entire vasculature perfused with sterile physiological saline through the cardiac ventricles. Epididymal adipose tissues were harvested from mice after perfusion, weighed, and divided into 50mg pieces. Each 50mg piece was then further cut into approximately 5–10mg pieces and the pieces (50mg total) were placed in a single well of a six-well tissue culture plate with 5mL Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals), penicillin (100 units/mL), streptomycin (100 μg/mL; Life Technologies, Grand Island, NY), and insulin (10 μg/mL; Sigma–Aldrich, St. Louis, MO). Cultures were incubated at 37°C in an atmosphere of 5% CO2/air. Thirty minutes after the start of culture, adipose tissues were treated with either bacterial endotoxin LPS derived from Pseudomonas aeruginosa (Sigma–Aldrich, St. Louis, MO), recombinant TNFα, or recombinant IL-1β (R&D Systems, Minneapolis, MN) and the medium was sampled at the designated time points after treatment. In selected experiments, a neutralizing polyclonal antibody against IL-1β (R&D Systems) was added to the cultures. All treatments and samplings were performed on a slide warmer set to 37°C to maintain cultures at a constant temperature. Medium samples were stored at −80°C for later analysis. After the final sampling, adipose tissue pieces were collected and stored at −80°C for later analysis of DNA content.
DNA Content Analysis
DNA was extracted from the adipose tissue fragments by overnight digestion at 42°C with proteinase K (100 μg/mL) in buffer containing 10mM Tris-HCl, 10mM EDTA, 0.005% SDS. DNA concentration of each sample was determined using Quant-iT™ PicoGreen® dsDNA Reagent and Kit (Life Technologies) using the protocol recommended by the manufacturer.
In Vivo LPS Model of Systemic Inflammation
Acute systemic inflammation was induced by intraperitoneal injection with the same LPS used for above explant cultures. LPS was dissolved in physiologic saline and administered intraperitoneally with a dose of 2.5mg/kg. For time-course analysis of plasma cytokines, 30 μL of blood was drawn sequentially from the tail vein of mice 0, 1.5, 3, and 6 hours after LPS injection. For analysis of mRNA expression, mice were anesthetized with 2% isoflurane in air 1.5 hours after LPS injection, the entire vasculature perfused as described earlier, and whole tissues harvested and flash frozen in liquid nitrogen.
Plasma and Culture Medium Cytokine Analysis
Plasma samples were obtained by centrifugation of blood at 2,000g for 10 minutes. Plasma and medium were analyzed by enzyme-linked immunosorbent assay (ELISA, Thermo Scientific, Rockford, IL) for quantification of TNFα, IL-1β, and IL-6 levels using the protocol recommended by the manufacturer. According to the manufacturer, the detection limits of these assays are 9, 3, and 7 pg/mL for TNFα, IL-1β, and IL-6, respectively.
RNA Isolation and Northern Blot Analysis
Total RNA was isolated from tissues by a method similar to our previously described protocols (11,20). Twenty micrograms of RNA from each tissue was electrophoretically fractionated through 1.2% agarose gels containing 3% formaldehyde buffered in 20mM MOPS and 1mM EDTA at pH 7.4. The RNA was transferred to Zeta-Probe GT nylon membranes (Bio-Rad Laboratories, Hercules, CA) overnight and fixed by ultraviolet cross-linking. Radiolabeled IL-1β, TNFα, and 18S probes were prepared from mouse cDNA using DECAprime II Random Primed DNA Labeling Kit and NucAway Spin Columns (Ambion, Austin, TX). Hybridization and washing were performed at 65°C. Membranes were exposed to Blue Lite Autorad Film (ISC Bio Express, Kaysville, UT) in the presence of an intensifying screen at −80°C.
Statistical Analysis
Data were analyzed by one- or two-way analysis of variance when appropriate using SigmaPlot Statistical Software, Version 11.0 (Systat Software, Chicago, IL). Tukey test or Holm-Sidak method were used for multiple comparisons. A p value less than .05 was considered statistically significant.
Results
Adipose Tissues From Aged Mice Produce More IL-6 Than Adipose Tissues From Young Mice After LPS Stimulation, In Vitro
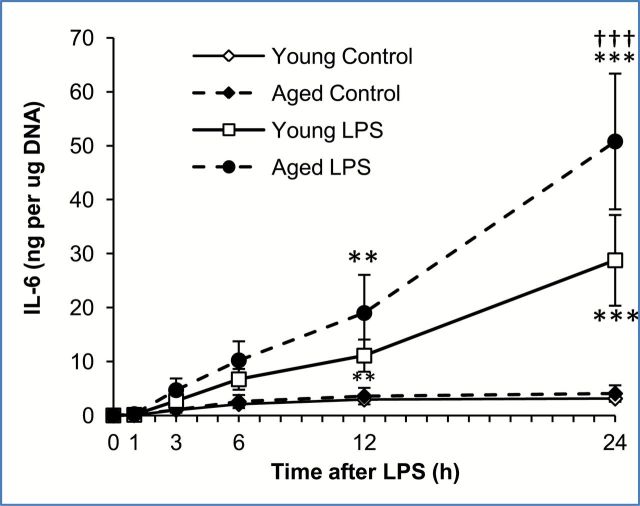
To examine whether adipose tissues from aged mice produce more IL-6 than adipose tissues from young mice, equal sized pieces of epididymal adipose tissues were harvested from young (4-month old, average body weight 31.1±2.05g) and aged (24-month old, average body weight 34.9±1.42g) mice and treated with LPS (10 μg/mL) in vitro. Control cultures received the same volume of vehicle (PBS) which did not stimulate cytokine secretion. A clear difference in response to LPS was visible between adipose tissues from young and aged mice (Figure 1). IL-6 secretion was detectable as early as 3 hours after LPS treatment in adipose tissues from both young and aged mice and reached statistically significant induction 12 and 24 hours later. A significant age-associated difference in the level of IL-6 was observed 24 hours after LPS treatment (p < .001). Approximately 50ng of IL-6 per μg of adipose tissue DNA was secreted into the medium by LPS-treated adipose tissues from aged mice over a 24-hour period, while approximately 25ng of IL-6 per μg of DNA was secreted into the medium by LPS-treated adipose tissues from young mice. When given a lower dose of LPS (1 μg/mL), a similar trend was observed, approximately 25ng of IL-6 per μg of adipose tissue DNA was secreted by adipose tissues from aged mice, while less than half that amount (10ng IL-6 per μg of adipose tissue DNA) was secreted by adipose tissue from young mice (data not shown). A higher dose of LPS (100 μg/mL) produced a saturating effect that was similar to results using the 10 μg/mL dose. These data clearly indicate that adipose-derived IL-6 production occurs independent of systemic inflammation and that, given the same dose of LPS, adipose tissues from aged mice produce and secrete significantly more IL-6 than an equal amount of adipose tissues from young mice. These results suggest that aging alters the nature of the adipose tissue causing it to be more responsive to LPS.
Figure 1. Age-associated increase in interleukin-6 (IL-6) release from mouse white adipose tissues after LPS treatment, in vitro. Explant cultures of adipose tissue from young and aged mice were treated with lipopolysaccharide (LPS; 10 μg/mL) in vitro and the media sampled at multiple timepoints for analysis of IL-6 by ELISA. IL-6 levels were adjusted for adipose tissue DNA content. Data are expressed as the mean ± standard deviation, n = 3 per age and timepoint. This experiment was reproduced three additional times with different mice, and similar results were obtained in each experiment. * indicates a statistically significant change as compared to the 0 hour timepoint of the same age group. † indicates a statistically significant difference between young and aged at the same timepoint. Two or three symbols signify p < .01, or .001, respectively.
IL-1β and TNFα Are Early Cytokines Produced During the Inflammatory Response, In Vivo
IL-1β and TNFα are pro-inflammatory cytokines produced in the early phase of systemic inflammation and in turn induce a variety of other inflammatory mediators including IL-6 (20). To determine the circulating levels and kinetics of IL-6, IL-1β, and TNFα, young and aged mice (n = 5 each) were injected with LPS (2.5mg/kg, i.p.) and blood was collected consecutively from the tail vein at the given timepoints for plasma analysis by ELISA. As shown in Figure 2, both IL-1β and TNFα levels in the plasma peaked 1.5 hours after LPS injection, while IL-6 levels in the plasma of both young and aged mice peaked 3 hours after LPS injection. A significant age-associated difference in the concentration of these cytokines was observed for TNFα at 1.5 (p < .001) and 3 hours (p < .001) after LPS injection and for IL-6 at 3 and 6 hours after LPS injection (p < .001 and p < .001, respectively). The concentration of IL-1β in the plasma was not significantly different in the two age groups although the average concentration was higher in the aged. These data confirm that IL-1β and TNFα are systemically induced earlier than IL-6 and are induced to a higher degree in aged mice suggesting that increased induction of these early cytokines may be responsible for age-associated overproduction of IL-6.
Figure 2.
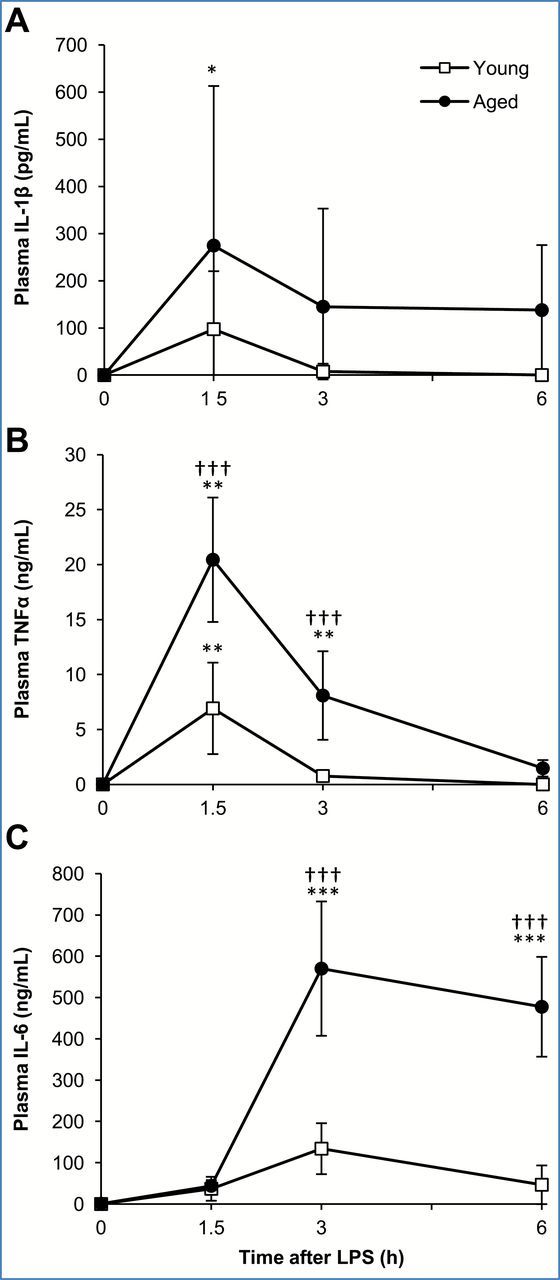
Interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα) are early cytokines released during lipopolysaccharide (LPS)-induced systemic inflammation, in vivo. Plasma samples were obtained from the tail vein of young and aged mice 0, 1.5, 3, and 6 hours after LPS (2.5mg/kg) injection. Plasma levels of (A) IL-1β, (B) TNFα, and (C) interleukin-6 (IL-6). Data are expressed as the mean ± standard deviation, n = 5 per age and timepoint. * indicates a statistically significant change as compared to the 0 hour timepoint of the same age group. † indicates a statistically significant difference between young and aged at the same timepoint. One, two, or three symbols signify p<.05, .01, or .001, respectively.
IL-1β and TNFα Are Produced Mostly by the Lungs and Spleen During Inflammatory Stress
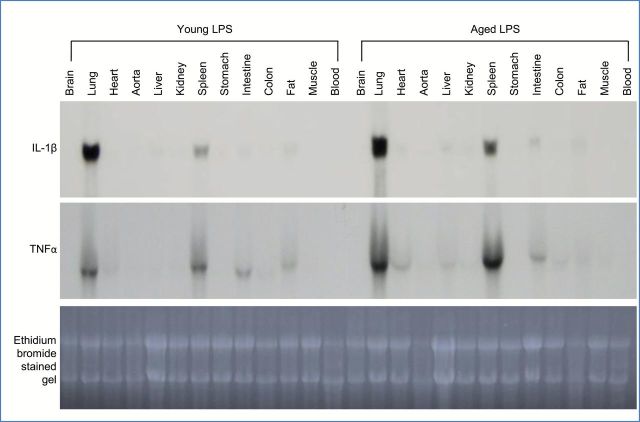
To determine which organs produce IL-1β and TNFα during the early phase of systemic inflammation, mRNA levels of these cytokines were examined in multiple tissues that were harvested from young and aged mice 1.5 hours after LPS injection. IL-1β and TNFα were strongly expressed in the lungs and modestly in the spleen of both young and aged mice with higher levels observed in the aged (Figure 3). Minimal levels of IL-1β and TNFα were detected in other tissues including adipose tissue (fat). This data indicates that adipose tissue is not a major source of IL-1β or TNFα during LPS-mediated inflammation and suggests an important role for the lung and/or spleen as major producers of these cytokines during the early phase of systemic inflammation.
Figure 3.
Lung and spleen are the major tissue sources of lipopolysaccharide (LPS)-mediated interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNFα) expression. Systemic inflammation was induced in young (4 months old) and aged (24 months old) C57BL/6 male mice by LPS injection (2.5mg/kg, i.p.), and mice were sacrificed 1.5 hours later. RNA was isolated from various tissues and analyzed by Northern blotting. Each lane represents pooled mRNA samples (20 µg per lane) from three mice.
IL-1β, But Not TNFα, Significantly Mediates IL-6 Production From Adipose Tissue, In Vitro
To test whether IL-1β and TNFα induce IL-6 production by adipose tissue, explant cultures of adipose tissue from young mice were treated with IL-1β or TNFα (1 or 10ng/mL) and the media sampled 0, 1, 3, 6, and 12 hours later for analysis by ELISA (Figure 4A). IL-1β induced significantly higher levels of IL-6 from adipose tissue compared to TNFα. A dose of 1ng/mL IL-1β resulted in the production of approximately 16ng of IL-6 per μg of adipose tissue DNA over a 12-hour period. The same dose of TNFα (1ng/mL) did not induce any detectable levels of IL-6. When a higher dose of TNFα (10ng/mL) was applied to adipose tissues in culture, a modest amount of IL-6 (approximately 4ng per μg of adipose tissue DNA) was produced (data not shown). A high dose of IL-1β (10ng/mL) produced IL-6 levels similar to those produced when given 1ng/mL IL-1β (data not shown) indicating a saturating effect. Combined treatment with TNFα and IL-1β did not induce IL-6 at higher levels than for treatment with IL-1β alone, eliminating a possible synergistic effect by the two cytokines (data not shown). These results suggest that the early cytokine, IL-1β, rather than TNFα, is likely to play an important role in IL-6 production and secretion from the adipose tissue.
Figure 4.
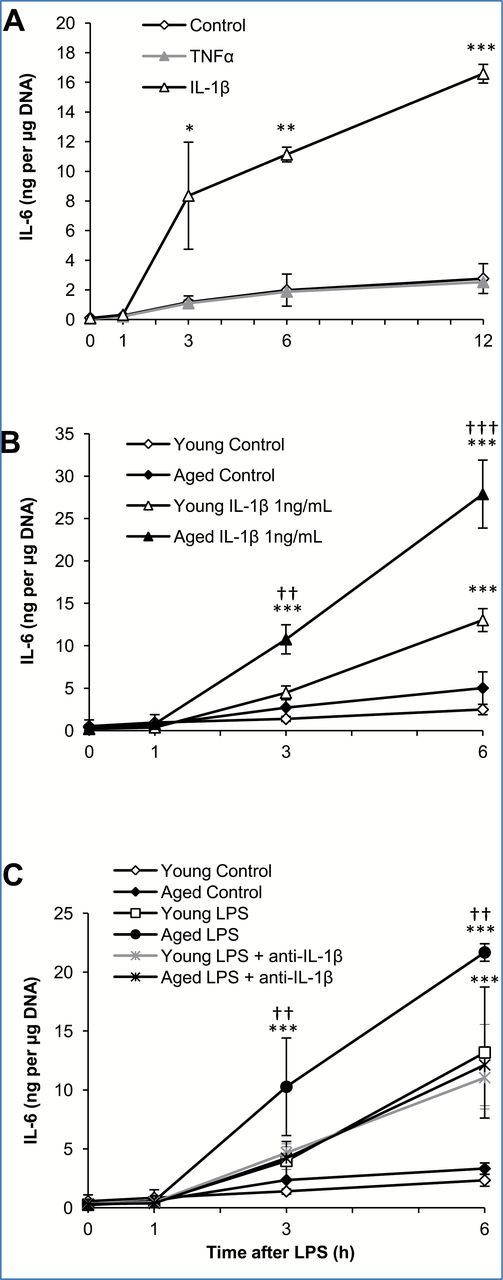
Interleukin 1 beta (IL-1β), but not tumor necrosis factor alpha (TNFα), mediates interleukin-6 (IL-6) overproduction from adipose tissue of aged mice. (A) Explant cultures of adipose tissue from young mice were treated with TNFα or IL-1β (1ng/mL) in vitro and the media sampled at indicated time points for analysis of IL-6 by ELISA. (B) Explant cultures of adipose tissue from young and aged mice were treated with IL-1β (1ng/mL) in vitro and the media sampled for analysis of IL-6 by ELISA. (C) Explant cultures of adipose tissue from young and aged mice were treated with lipopolysaccharide (LPS; 10 µg/mL) and a neutralizing antibody against IL-1β in vitro and the media sampled for analysis of IL-6 by ELISA. IL-6 levels were adjusted for adipose tissue DNA content. Data are expressed as the mean ± standard deviation, n = 3 per age and timepoint. * indicates a statistically significant change as compared to the 0 hour timepoint of the same group. † indicates a statistically significant difference between young and aged at the same timepoint. One, two, or three symbols signify p < .05, .01, or .001, respectively.
IL-1β-Induced IL-6 Production Is Higher in Adipose Tissues From Aged Mice Compared to Young Mice
Subsequently, adipose tissues from young and aged mice were compared for their responsiveness to IL-1β; adipose tissues from aged mice produced significantly more IL-6 than adipose tissues from young mice after stimulation with IL-1β (Figure 4B). Six hours after the addition of IL-1β (1ng/mL), 25–30ng IL-6 per μg adipose tissue DNA was present in the medium of adipose tissue cultures from aged mice, while only 10–15ng IL-6 per μg adipose tissue DNA was present in the medium of adipose tissue cultures from young mice. Additionally, in response to a 10 times lower dose of IL-1β (0.1ng/mL), adipose tissues from aged mice still produced significant amounts of IL-6 (approximately 20–25ng IL-6 per μg adipose tissue DNA); this amount still far exceeds the IL-6 levels produced by the addition of 100-times more TNFα (data not shown). These results suggest an important role for IL-1β in the age-associated overproduction of IL-6 from adipose tissue.
LPS-Induced IL-6 Production Is Mediated by Paracrine/Autocrine Action of IL-1β in Adipose Tissues From Aged, But Not Young Mice
To further investigate the role of IL-1β on LPS-induced overproduction of IL-6 in adipose tissues from the aged, the effects of an anti-IL-1β neutralizing antibody on IL-6 production was examined. Explant cultures of adipose tissue harvested from young and aged mice were treated with LPS alone (10 μg/mL) or LPS plus a neutralizing antibody against IL-1β (10ng/mL), and the medium collected 1, 3, and 6 hours later for analysis of IL-6 by ELISA (Figure 4C). Control cultures received the same volume of vehicle (PBS). In response to LPS, as shown previously in Figure 1, adipose tissues from aged mice secreted more IL-6 than adipose tissues from young mice (22ng vs 13ng per μg DNA at 6 hours, respectively). When the neutralizing antibody against IL-1β was added to the explant cultures in combination with LPS, the amount of IL-6 secreted by aged adipose tissues was significantly reduced. IL-6 levels in the medium from young adipose tissues did not change by addition of the neutralizing antibody. This data indicates that adipose-derived IL-1β mediates age-associated overproduction of IL-6 in the adipose tissue; however such IL-1β does not seem to play a role in IL-6 production from young adipose tissues. To eliminate the possibility that an insufficient amount of neutralizing antibody was added, adipose tissues from aged mice were subsequently treated with LPS plus a 10-times higher dose of neutralizing antibody against IL-1β (100ng/mL), which did not reduce IL-6 levels any further than was shown for 10ng/mL (data not shown).
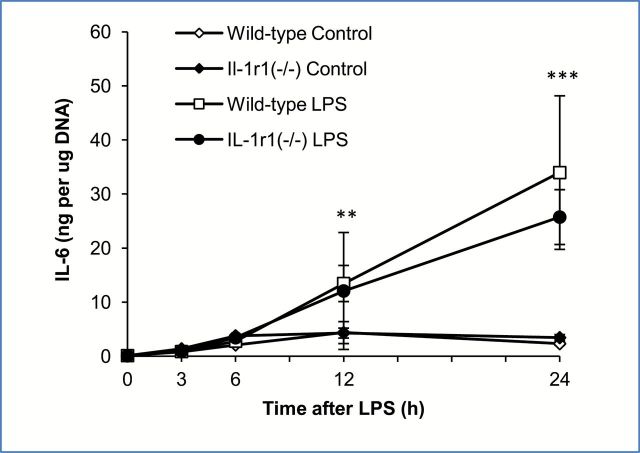
To further support the suggestion that autocrine/paracrine IL-1β is not involved in the upregulation of IL-6 from adipose tissues of young mice, LPS-induced IL-6 production was compared in adipose tissues from young IL-1 receptor 1 null mutant mice (IL-1r1(−/−)) and age and sex-matched wild-type control mice. These mice display a normal acute phase response to systemic LPS challenge, but fail to respond to IL-1 because the IL-1 signaling receptor has been knocked out (27). Explant cultures of adipose tissue harvested from young wild-type and young IL-1r1(−/−) mice were treated with LPS (10 μg/mL) and the medium sampled 3, 6, 12, and 24 hours later and analyzed for IL-6 expression by ELISA. Both wild-type and knockout mice responded to LPS similarly; no significant difference in the levels of IL-6 following LPS treatment was observed (Figure 5). This data indicates that the absence of IL-1β signaling did not impact the ability of young adipose tissues to produce IL-6; thus IL-1β is not involved in IL-6 upregulation in adipose tissue of young mice.
Figure 5.
Interleukin 1 beta (IL-1β) does not play a role in the lipopolysaccharide (LPS)-mediated induction of interleukin-6 (IL-6) in adipose tissue from young mice. Explant cultures of adipose tissue from young wild-type and young IL-1r1(−/−) mice were treated with LPS (10 μg/mL) in vitro and the media sampled for analysis of IL-6 by ELISA. Data are expressed as the mean ± standard deviation, n = 3 for each group. * indicates a statistically significant change as compared to the 0 hour timepoint of the same group. Two or three symbols signify p < .01, or .001, respectively.
Discussion
The major findings of this study include: (a) IL-6 is secreted from visceral white adipose tissue directly in response to LPS and adipose tissues from aged mice secrete significantly more IL-6 than adipose tissues from young mice; (b) unlike IL-6, expression of IL-1β and TNFα in adipose tissue is relatively low compared to other organs, and exogenous IL-1β, rather than TNFα, is responsible for production and secretion of IL-6 from adipose tissue; and (c) despite low endogenous levels in adipose tissue, autocrine/paracrine action of IL-1β mediates age-associated overproduction of IL-6 which does not occur in the young. Collectively, these findings indicate that the mechanism of IL-6 production in adipose tissue differs by aging and that the pro-inflammatory nature of aged adipose tissue is not simply a result of increased adiposity or increased inflammatory signals from the aged environment.
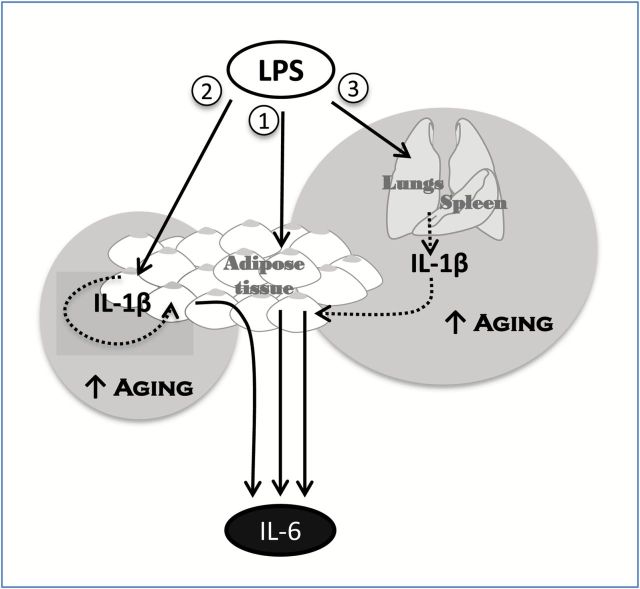
From these findings, we propose three mechanisms for the LPS-mediated expression of IL-6 from adipose tissue (Figure 6). Mechanism 1: LPS directly stimulates adipose tissue to secrete IL-6; the amount of IL-6 secreted by this mechanism does not differ by aging. Although a direct effect of LPS on IL-6 production is likely as many cells within adipose tissue express TLR4, other cytokines may play intermediate roles in this mechanism. Nevertheless, regardless of the factors involved, it appears that they do not influence age-associated overproduction of IL-6. Mechanism 2: LPS stimulates adipose tissue to produce IL-1β, which then acts in an autocrine and/or paracrine fashion to induce the production and secretion of IL-6 from adipose tissue; this mechanism is unique to adipose tissue in aged mice and absent in young mice. IL-1β secretion from adipose tissue was not confirmed by our in vitro studies; however, we recently reported that adipose tissues from aged mice expresses significantly more IL-1β than adipose tissues from young mice in response to LPS treatment in vivo (12), suggesting that IL-1β produced by adipose tissues acts within the tissue without being secreted. Mechanism 3: IL-1β is synthesized largely by the lungs and spleen and such secretion of this protein acts as an endocrine signal to adipose tissue resulting in the production and secretion of IL-6 which occurs in significantly higher amounts from aged adipose tissue. During LPS-mediated inflammation in vivo, IL-1β is produced more highly in the lungs from aged compared to young mice (28,29) and higher levels of IL-1β are known to be circulating in aged mice (9,28). However, when adipose tissues from young and aged mice were treated with the same concentration of IL-1β, in vitro, adipose tissue from the aged still produced more IL-6 than that from young, indicating that aging increases the sensitivity of adipose tissue to IL-1β. We previously reported that the microvasculature from the aged is more sensitive to inflammatory mediators present in the circulation of patients with sepsis (30). Our current findings extend this notion to adipose tissue.
Figure 6.
Three mechanisms of lipopolysaccharide (LPS)-mediated interleukin-6 (IL-6) expression in adipose tissue. (1) LPS directly stimulates IL-6 production by adipose tissue. (2) LPS directly stimulates interleukin 1 beta (IL-1β) production by adipose tissue which acts in an autocrine/paracrine manner to stimulate IL-6 production. (3) LPS stimulates IL-1β production in the lung and/or spleen which is subsequently released into the circulation and induces IL-6 production by the adipose tissue. Age-associated overproduction of IL-6 by adipose tissue is likely mediated by mechanisms (2) and (3) but not (1).
Presumed changes in adiposity with age may be deemed accountable for increased adipose derived cytokines; however, despite increased body weight of aged mice in this study, the weight of the epididymal fat pads were not significantly different between young and aged mice. Furthermore, although aged mice are significantly heavier than young mice, imaging studies have shown that percentage of total body fat, and abdominal fat volume are not significantly different (31). Unpublished data from our laboratory also show that average adipocyte size is not significantly different between the ages of mice used in this study, although size is more variable among aged mice. While this study did not examine the effects of adipose aging in obese animals, other studies have reported that aging exacerbates obesity induced chronic systemic inflammation (5,32).
Another cytokine of interest with respect to systemic inflammation and adipose tissue is TNFα. Despite many reports that TNFα is produced and secreted by adipocytes in vitro (33), the extent to which it is actually secreted by adipocytes into the circulation remains a matter of debate (34). We were not able to detect TNFα in the medium after treatment of mouse visceral adipose tissue explants with LPS; however, we previously reported induction of TNFα mRNA in adipose tissue in vivo after LPS injection (12). These results are supported by other studies which show that human subcutaneous adipose tissue from normal weight subjects expresses but does not release TNFα, in vivo (35) or in vitro (36). One study did find an extremely low amount of TNFα (0.098 pg/mL/mg) released into the medium of human adipose tissue explant cultures from morbidly obese patients after stimulation with 1 μg/mL LPS (37). Studies reporting TNFα expression in adipose tissue indicate that it is the stromal vascular cells which are responsible for secretion of TNFα and these studies are mainly performed on adipose tissue from obese subjects (38,39). As recent research has indicated that varied mechanisms exist to govern age-related versus obesity-induced adipose tissue inflammation (11,40–42), it is plausible that TNFα plays an important role in inflammatory and metabolic processes of adipose tissue in the obese state but plays a rather insignificant role in adipose tissue of healthy weight individuals.
Sepsis can be caused by either gram-positive bacteria or gram-negative bacteria. Gram-negative bacteria-induced sepsis is known to stimulate a significantly greater inflammatory response as compared to that caused by gram-positive bacteria (43). Thus, the use of LPS, a major cell wall component of gram-negative bacteria, for sepsis research may not be relevant to address inflammatory responses caused by gram-positive bacteria, but is useful to study excessive inflammatory mechanisms. We previously reported that the inflammatory response induced by LPS in adipose tissue in vivo is significantly greater in aged animals as compared to young (11,12); thus in the present study, we used LPS in vitro to study this mechanism.
An obvious limitation of this study is that we did not identify which cell types within the adipose tissue produce and/or secrete IL-6, IL-1β, or TNFα. Although this may have been possible using traditional cell separation techniques, evidence suggests that the standard procedure for isolation of primary adipose cells induces the transcription and secretion of multiple inflammatory mediators and generates changes in global gene expression which are indicative of an inflammatory insult (44). This complication was avoided in the current study by using explant culture; however, the downside is that only limited conclusions can be drawn regarding specific cell regulation. Multiple other studies have reported that cytokine release from adipose tissue is primarily due to nonfat cells (12,42,45–48). Nevertheless, studying adipose tissues in explant culture is an essential step in understanding the biology and pathophysiology of this organ as the technique preserves the autocrine and paracrine signals within the tissue, maintains the existing cross-talk among various cell types and avoids artificial activation (49). Regardless of the cell type, the adipose tissue as a whole is a major inflammatory organ which contributes to the systemic response during an inflammatory insult (11). The issue of which cell type contributes most towards the inflammatory nature of adipose tissue is still unclear and has only recently begun to be investigated. Future studies in this area must be addressed to completely understand the inflammatory nature of adipose tissue and how it reflects systemically. Another limitation of this study is that we cannot determine whether IL-1β is an autocrine or paracrine mediator of IL-6. Identifying specific cell types within the adipose tissue which express these cytokines would be the necessary next step to further answer these questions.
In conclusion, our results demonstrate that the inflammatory potential of adipose tissue is exaggerated by aging and that this phenomenon occurs independent of fat mass. Our previous studies have shown adipose tissue to be a major source of IL-6 during acute inflammatory stress which contributes to increased age-dependent mortality. The present results provide evidence that overproduction of IL-6 in adipose tissue of the aged is mediated by a combination of increased sensitivity to IL-1β derived within the adipose tissue and from the lungs/spleen. These results support the notion that alterations in adipose tissue physiology contribute to exaggerated inflammation with age. This may be of particular clinical importance in diseases of acute systemic inflammation, such as sepsis, where elderly patients tend to be more vulnerable.
Funding
This work was supported by National Institute on Aging/National Institutes of Health (RO1 AG025908 and R01 AG039732 to H.S. and R36 AG038547 to M.E.S.).
Acknowledgments
This study was presented in part during the Biological Sciences Poster Session at the 62nd Annual Meeting of the Gerontological Society of America and received the George Sacher Student Award for the best student poster presentation (M.E.S.). The authors would like to thank Ms. Donna Gilbreath of the Markey Cancer Center Research Communications Office for illustrative assistance.
References
- 1. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. [DOI] [PubMed] [Google Scholar]
- 2. Ramanathan R, Kohli A, Ingaramo MC, et al. Serum chitotriosidase, a putative marker of chronically activated macrophages, increases with normal aging. J Gerontol A Biol Sci Med Sci. 2013;68:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saito H, Papaconstantinou J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J Biol Chem. 2001;276:29307–29312. [DOI] [PubMed] [Google Scholar]
- 7. McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011;66:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okamura D, Starr ME, Lee EY, Stromberg AJ, Evers BM, Saito H. Age-dependent vulnerability to experimental acute pancreatitis is associated with increased systemic inflammation and thrombosis. Aging Cell. 2012;11:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. [DOI] [PubMed] [Google Scholar]
- 10. Starr ME, Saito H. Sepsis in old age: review of human and animal studies. Aging Dis. 2014;5:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Starr ME, Hu Y, Stromberg AJ, et al. Gene expression profile of mouse white adipose tissue during inflammatory stress: age-dependent upregulation of major procoagulant factors. Aging Cell. 2013;12:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–585. [DOI] [PubMed] [Google Scholar]
- 14. Vitseva OI, Tanriverdi K, Tchkonia TT, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring). 2008;16:932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255–24263. [DOI] [PubMed] [Google Scholar]
- 16. Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors—a mini-review. Gerontology. 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 18. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. [DOI] [PubMed] [Google Scholar]
- 19. Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol. 2001;166:4042–4048. [DOI] [PubMed] [Google Scholar]
- 20. Saito H, Patterson C, Hu Z, et al. Expression and self-regulatory function of cardiac interleukin-6 during endotoxemia. Am J Physiol Heart Circ Physiol. 2000;279:H2241–H2248. [DOI] [PubMed] [Google Scholar]
- 21. Webb SJ, McPherson JR, Pahan K, Koka S. Regulation of TNF-alpha-induced IL-6 production in MG-63 human osteoblast-like cells. J Dent Res. 2002;81:17–22. [DOI] [PubMed] [Google Scholar]
- 22. Ammit AJ, Lazaar AL, Irani C, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–474. [DOI] [PubMed] [Google Scholar]
- 23. Eda H, Burnette BL, Shimada H, Hope HR, Monahan JB. Interleukin-1β-induced interleukin-6 production in A549 cells is mediated by both phosphatidylinositol 3-kinase and interleukin-1 receptor-associated kinase-4. Cell Biol Int. 2011;35:355–358. [DOI] [PubMed] [Google Scholar]
- 24. Flower L, Gray R, Pinkney J, Mohamed-Ali V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21:32–37. [DOI] [PubMed] [Google Scholar]
- 25. Tanabe M, Matsumoto T, Shibuya K, et al. Compensatory response of IL-1 gene knockout mice after pulmonary infection with Klebsiella pneumoniae . J Med Microbiol. 2005;54(Pt 1):7–13. [DOI] [PubMed] [Google Scholar]
- 26. Bluthé RM, Layé S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- 27. Glaccum MB, Stocking KL, Charrier K, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 28. Chang CK, LoCicero J., III Overexpressed nuclear factor kappaB correlates with enhanced expression of interleukin-1beta and inducible nitric oxide synthase in aged murine lungs to endotoxic stress. Ann Thorac Surg. 2004;77:1222–1227. [DOI] [PubMed] [Google Scholar]
- 29. Gomez CR, Hirano S, Cutro BT, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. [DOI] [PubMed] [Google Scholar]
- 30. Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemmeryckx B, Loeckx D, Dresselaers T, Himmelreich U, Hoylaerts MF, Lijnen HR. Age-associated adaptations in murine adipose tissues. Endocr J. 2010;57:925–930. [DOI] [PubMed] [Google Scholar]
- 32. Tucsek Z, Toth P, Sosnowska D, et al. Obesity in aging exacerbates blood–brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. [DOI] [PubMed] [Google Scholar]
- 35. Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. [DOI] [PubMed] [Google Scholar]
- 36. Sopasakis VR, Nagaev I, Smith U. Cytokine release from adipose tissue of nonobese individuals. Int J Obes (Lond). 2005;29:1144–1147. [DOI] [PubMed] [Google Scholar]
- 37. Hoch M, Eberle AN, Peterli R, et al. LPS induces interleukin-6 and interleukin-8 but not tumor necrosis factor-alpha in human adipocytes. Cytokine. 2008;41:29–37. [DOI] [PubMed] [Google Scholar]
- 38. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int J Obes Relat Metab Disord. 2004;28:616–622. [DOI] [PubMed] [Google Scholar]
- 40. Jerschow E, Atzmon G, Fishman S, et al. Aging increases macrophage accumulation and inflammatory cytokines expression in the adipose tissue independent of obesity. Diabetes. 2007;56:A323. [Google Scholar]
- 41. Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. [DOI] [PubMed] [Google Scholar]
- 42. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abe R, Oda S, Sadahiro T, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. 2010;14:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J Biol Chem. 2003;278:47585–47593. [DOI] [PubMed] [Google Scholar]
- 45. Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297:E999–E1003. [DOI] [PubMed] [Google Scholar]
- 46. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–712. [DOI] [PubMed] [Google Scholar]
- 47. Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. [DOI] [PubMed] [Google Scholar]
- 49. Thalmann S, Juge-Aubry CE, Meier CA. Explant cultures of white adipose tissue. In: Methods in Molecular Biology. 2nd ed. Totowa, NJ: Humana Press; 2008:195–199. [DOI] [PubMed] [Google Scholar]