Abstract
Background: SRC plays an important role in the pathogenesis of metastatic breast cancer (MBC). In preclinical models, paclitaxel and the oral SRC inhibitor dasatinib showed greater antitumor activity than either agent. To determine the maximum tolerated dose of this combination, we conducted a phase I study.
Patients and methods: Patients with MBC; Eastern Cooperative Oncology Group performance status of zero to one; normal hepatic, renal and marrow function were eligible. Paclitaxel 80 mg/m2 was given 3 weeks of 4. The starting dasatinib dose was 70 mg and was increased, using a standard 3 + 3 dose-escalation scheme.
Results: Fifteen patients enrolled (median age 54 years, range 35–74). No dose-limiting toxic effects (DLTs) occurred at dasatinib doses of 70–120 mg. One DLT (grade 3 fatigue) occurred in the dasatinib 150-mg cohort, which was expanded (six patients) with no further DLTs. However, due to cumulative toxic effects (rash, fatigue, diarrhea), the recommended phase II dose is dasatinib 120 mg. Of 13 assessable patients, a partial response was seen in 4 patients (31%), including 2 patients previously treated with taxanes; all received ≥120 mg dasatinib. An additional five patients (29%) had stable disease.
Conclusion: In combination with weekly paclitaxel, the recommended phase II dose of dasatinib is 120 mg daily and preliminary activity has been seen in patients with MBC.
Keywords: dasatinib, dose-limiting toxicity, metastatic breast cancer, paclitaxel, phase I
introduction
Metastatic breast cancer (MBC) is an important public health concern, accounting for >40 000 deaths in the United States in 2009 [1]. Although MBC is a chemosensitive disease, increasingly, therapeutic advances have come as a result of advances in molecular biology, which have led to the development of targeted therapies. For example, trastuzumab and lapatinib have proven activity in combination with chemotherapy for those patients with MBC, which overexpresses the human epidermal growth factor receptor-2 (HER2) [2, 3]. For tumors that lack this receptor, multiple other putative pathways have been elucidated, for which targeted therapies are being investigated.
One potential target in MBC is SRC, a membrane-associated non-receptor tyrosine kinase, which is involved in multiple signaling pathways regulating normal cell growth, angiogenesis, steroid receptor activation, and cell survival [4]. SRC is frequently overexpressed in human breast cancer and has been implicated in the development of metastases [5, 6]. Furthermore, SRC kinase plays an important role in breast cancer cell survival within bone marrow and is important for activation of osteoclasts and late-onset bone metastases [5, 7, 8]. In estrogen receptor (ER)-negative breast cancer, differential expression of other tyrosine kinases, including KIT and ABL, has been suggested. Therefore, inhibition of these tyrosine kinases, including SRC, represents a novel therapeutic approach for MBC. Although prior studies in MBC with the c-KIT and ABL tyrosine kinase inhibitor imatinib failed to demonstrate clinical activity, the combination of a SRC kinase inhibitor with cytotoxic chemotherapy might be more effective [9, 10].
Dasatinib is a potent, broad-spectrum ATP-competitive inhibitor of five critical oncogenic tyrosine kinase families, Bcr–Abl, SRC, c-KIT, platelet-derived growth factor receptor-β and ephrin, each of which has been linked to multiple forms of human malignancies [11]. Dasatinib has proven activity in Bcr–Abl-driven disease such as chronic myeloid leukemia (CML) [12]. Monotherapy with dasatinib up to 100 mg twice is safe, although nausea and vomiting, diarrhea and fluid retention, including pleural and pericardial effusions, emerged at this dose level. In order to maximize efficacy and minimize toxicity, subsequent studies have demonstrated the superiority of a once-daily schedule [13]. In preclinical studies, dasatinib inhibited proliferation of cancer cell lines that express activated SRC or c-KIT [14]. Preclinical evidence suggested that triple-negative breast cancer [which lacks HER2, ER and progesterone receptor (PgR)] is a subtype likely to respond to dasatinib [15]. Alternatively, as SRC activation has been associated with endocrine resistance in ER-positive breast cancer, dasatinib may also have a role in this subgroup [16, 17]. Furthermore, since dasatinib inhibits vascular endothelial growth factor-stimulated proliferation and has potent bone antiresorptive activity, this agent is of particular interest for the treatment of MBC to bone.
Paclitaxel, which is among the most active cytotoxic agents for MBC, is associated with a response rate of up to 42% when administered in a weekly schedule; neurotoxicity is the main dose-limiting toxicity (DLT) [18]. Preclinically, the combination of paclitaxel and dasatinib showed greater antitumor activity than either single agent [19]. To determine the maximum tolerated dose (MTD), we conducted the current phase I study (Bristol-Myers Squibb Study CA180194). Secondary objectives were to obtain preliminary data on the therapeutic activity of this combination.
patients and methods
Patients with MBC; Eastern Cooperative Oncology Group performance status of zero to one; normal hepatic, renal and marrow function were eligible. Prior taxane therapy, stable brain metastases and baseline neuropathy grade 1 or less were allowed. Due to the risk of fluid retention from dasatinib, patients with pleural or pericardial effusions were excluded. Since dasatinib is extensively metabolized in humans, primarily by the cytochrome P-450 3A4 isozyme, where possible, patients discontinued known potent inhibitors of this enzyme.
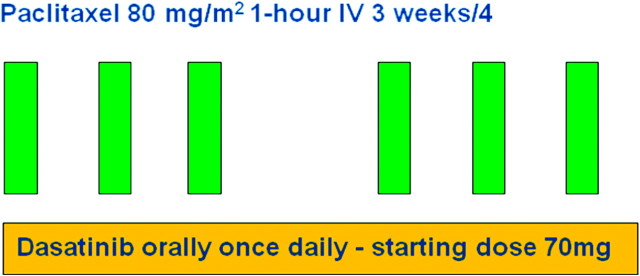
Weekly paclitaxel 80 mg/m2 was given by 1-h i.v. infusion 3 weeks of 4 (one cycle) and dasatinib was given orally in a single-daily dose (Figure 1). Supportive medications for weekly paclitaxel were administered per institutional policy. Dasatinib was supplied by Bristol-Myers Squibb and administered at a starting dose of 70 mg and escalated 100, 120 and 150 mg in cohorts of three patients, using a standard 3 + 3 dose-escalation scheme. There was no intra-patient dose escalation. Given the inhibitory effect of dasatinib on osteoclasts, bisphosphonates were withheld during the first two cycles of treatment, due to the potential risk of hypocalcemia.
Figure 1.
Study schema.
Safety assessments occurred every 2 weeks; additional hematologic toxic effects were assessed weekly. Electrocardiography was carried out on day 1 of each cycle of treatment. Toxic effects were graded per Common Terminology Criteria for Adverse Events version 3.0. DLT was defined as any of the following during cycle 1: grade 3–4 hematologic toxicity lasting >2 weeks, clinically significant grade 3–4 non-hematologic toxicity or grade 2 non-hematologic toxicity that persists despite adequate medical management, requiring withholding dasatinib for >4 days in cycle 1. The MTD for the phase II study was defined accordingly as the dose level immediately below the dose at which two or more patients experienced a DLT. If the MTD of dasatinib was ≤100 mg daily given continuously, the protocol allowed exploration of dasatinib dosing of 5 days of 7.
All patients underwent bone scan and computed tomography (CT) at baseline and after two cycles to assess response. Thereafter, tumor burden was assessed every three cycles. For patients with measurable disease (at least one lesion of minimum diameter of 10 mm on CT scan), response was assessed by standard RECIST.
results
From January to December 2009, 15 patients (14 women and 1 man) were enrolled. The median age was 54 years (range 35–74), and most patients (73%) had received prior adjuvant chemotherapy (Table 1). Before enrollment, patients received a median of 3 lines of therapy (range 0–12) for MBC, but only six (40%) had never received a taxane in either the adjuvant or the metastatic setting.
Table 1.
Baseline characteristics of patients (N = 15)
| n | % | |
| Age, median (range) | 54 (35–74) | |
| Female | 14 | 93 |
| Male | 1 | 7 |
| ECOG PS of zero | 6 | 40 |
| ECOG PS of one | 9 | 60 |
| Histology | ||
| Invasive ductal carcinoma | 14 | 93 |
| Other | 1 | 7 |
| ER status | ||
| Positive | 8 | 53 |
| Negative | 7 | 47 |
| PgR status | ||
| Positive | 6 | 40 |
| Negative | 9 | 60 |
| HER2 status | ||
| Positive | 1 | 7 |
| Negative | 14 | 93 |
| Triple negative | 6 | 40 |
| Visceral disease | 11 | 73 |
| Menopausal status | ||
| Postmenopausal | 11 | 73 |
| Premenopausal | 3 | 20 |
| Not applicable (male) | 1 | 7 |
| Adjuvant therapy | ||
| Chemotherapy | 11 | 73 |
| Anthracyclines | 9 | 60 |
| Taxanes | 7 | 47 |
| Treatment of MBC | ||
| Prior chemotherapy | 11 | 73 |
| Prior taxane | 4 | 27 |
| Prior lines of treatment, median (range) | 3 (0–12) | |
| Prior lines of chemotherapy, median (range) | 2 (0–8) | |
| Taxane naive | 6 | 40 |
ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor-2; MBC, metastatic breast cancer.
DLT and MTD
Three patients were treated in each of the first three cohorts (dasatinib 70–120 mg) and no DLTs occurred. In the fourth cohort (dasatinib 150 mg), one DLT (grade 3 fatigue) occurred as follows. A heavily pretreated patient with multiple liver and bone metastases was admitted with grade 2 nausea and vomiting and grade 3 fatigue (possibly dasatinib related) 2 weeks after starting treatment. In addition, she had grade 3 lymphopenia and grade 3 elevated alkaline phosphatase. She was removed from study for toxicity, but a CT scan after 4 weeks showed multiple stable liver and bone metastases.
This dose level was then expanded with a further three patients (six total) and no further DLTs were observed. However, cumulative toxic effects were seen in several patients during subsequent cycles of treatment. One woman developed erythematous papular pruritic rash (grade 1) during her fourth week of treatment, thought to be a delayed hypersensitivity reaction to dasatinib, which resolved with cessation of therapy. She was removed from study and had stable disease for 6 months on weekly paclitaxel with bevacizumab. One woman developed febrile neutropenia (grade 3) and withdrew. One woman, with a history of rash during her first cycle of treatment, was admitted to hospital with fever and pulmonary infiltrates during her third cycle of treatment. An infective cause was excluded and a provisional diagnosis of dasatinib-related pneumonitis was made. She was removed from study and was rechallenged with paclitaxel. However, she was readmitted with recurrent fever and pneumonitis and a definitive diagnosis of paclitaxel pneumonitis was made.
Overall, at the highest dose level (dasatinib 150 mg), three patients were removed from study treatment for toxicity and one withdrew for personal reasons. Therefore, based on cumulative toxic effects at this dose, the recommended phase II dose was determined to be dasatinib 120 mg.
Patients received a median of 2.8 cycles of treatment (range 1–15). One patient continues on study after 15 cycles and the remaining 14 have stopped treatment for progression/toxicity as set out in the following section.
hematological toxic effects
Overall, hematological toxic effects were common and predominantly of low grade. During cycle 1, 13 (87%) patients had at least one hematological toxicity. The most common dasatinib-related hematological toxic effects were leukopenia, seven (47%) patients (four grade 1); anemia, six (40%) patients (five grade 1); thrombocytopenia, six (40%) patients (all grade 1) and neutropenia, three patients (all grade >1). Hematological toxicity grade >1 during cycle 1 appeared to be more common with higher doses of dasatinib (Table 2). Similar cumulative hematological toxic effects were seen in subsequent cycles in the 14 patients who received more than one cycle of treatment, with increasing hematological toxicity seen with higher doses (Table 3).
Table 2.
Laboratory abnormalities grade >1 during cycle 1 by highest grade (N = 15)
| Cohort (N), dasatinib (mg) | Alkaline phosphatase |
ALT |
Amylase |
AST |
||||||||
| Grade |
Grade |
Grade |
Grade |
|||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | |||||||||||
| Cohort 3 (3), 120 | ||||||||||||
| Cohort 4 (6), 150 | 1 | 1 | 1 | 1 | ||||||||
| Cohort (N), dasatinib (mg) | Glucose, high | Hemoglobin | INR | Leukocytes | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | 1 | |||||||||||
| Cohort 2 (3), 100 | 1 | |||||||||||
| Cohort 3 (3), 120 | 1 | |||||||||||
| Cohort 4 (6), 150 | 2 | 1 | 1 | 2 | ||||||||
| Cohort (N), dasatinib (mg) | Lipase | Lymphocytes | Neutrophils | Phosphate, low | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | |||||||||||
| Cohort 3 (3), 120 | 1 | 1 | ||||||||||
| Cohort 4 (6), 150 | 1 | 1 | 1 | 1 | 2 | 2 | ||||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
Table 3.
Cumulative laboratory abnormalities grade >1 by highest grade at any time on treatment (N = 15)
| Cohort (N), dasatinib (mg) | AST |
Bilirubin |
Creatinine |
Glucose, high |
||||||||
| Grade |
Grade |
Grade |
Grade |
|||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | 1 | 2 | |||||||||
| Cohort 3 (3), 120 | 1 | 2 | ||||||||||
| Cohort 4 (6), 150 | 1 | 3 | ||||||||||
| Cohort (N), dasatinib (mg) | Hemoglobin | Leukocytes | Lymphocytes | Neutrophils | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | 1 | 1 | |||||||||
| Cohort 3 (3), 120 | 1 | 1 | 1 | |||||||||
| Cohort 4 (6), 150 | 3 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | ||||
| Cohort (N), dasatinib (mg) | Phosphate, low | Platelets | Potassium, high | Potassium, low | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | 1 | 1 | 1 | ||||||||
| Cohort 3 (3), 120 | 2 | |||||||||||
| Cohort 4 (6), 150 | 2 | 1 | 1 | |||||||||
AST, aspartate aminotransferase.
non-hematological toxic effects
Non-hematological toxic effects were as expected, were generally low grade and were more common with increasing doses of dasatinib (Tables 4 and 5). Overall, eight (53%) patients complained of diarrhea, two patients grade 1 and six patients grade 2, of whom four had received ≥120 mg dasatinib). In total, nine (60%) patients had skin toxicity, mainly consisting of acneiform rash, which was generally mild: seven patients grade 1 and two patients grade 2. Fatigue was common, reported in 11 (73%) patients, and was the DLT in 1 patient. However, in most cases this was of low grade (two patients grade 2 and three patients grade 3). Prolongation of QTc occurred in eleven (73%) patients (four patients grade 2 and nine patients grade 1), although this did not result in any clinical significant arrhythmias. Neuropathy occurred in 12 (80%) patients: eight grade 1, three grade 2 and one grade 3.
Table 4.
Non-hematologic toxic effects of grade >1 during cycle 1 by highest grade recorded (N = 15)
| Cohort (N), dasatinib (mg) | Diarrhea |
Dyspnea |
Fatigue |
Infection |
||||||||
| Grade |
Grade |
Grade |
Grade |
|||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | ||||||||||||
| Cohort 3 (3), 120 | 2 | |||||||||||
| Cohort 4 (6), 150 | 1 | 1 | 1 | 2 | ||||||||
| Cohort (N), dasatinib (mg) | Nausea | Pain | Prolonged QTc | Vomiting | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | 1 | |||||||||||
| Cohort 2 (3), 100 | 1 | |||||||||||
| Cohort 3 (3), 120 | ||||||||||||
| Cohort 4 (6), 150 | 1 | 2 | 1 | |||||||||
Table 5.
Non-hematologic toxic effects of grade >1 by highest grade at any time on treatment (N = 15)
| Cohort (N), dasatinib (mg) | Diarrhea |
Dyspnea |
Fatigue |
Gastritis |
||||||||
| Grade |
Grade |
Grade |
Grade |
|||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 2 | 1 | ||||||||||
| Cohort 3 (3), 120 | 2 | 2 | ||||||||||
| Cohort 4 (6), 150 | 2 | 2 | 2 | 1 | ||||||||
| Cohort (N), dasatinib (mg) | Infection | Nausea | Neuropathy | Pain | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | ||||||||||||
| Cohort 2 (3), 100 | 1 | 1 | 1 | 1 | ||||||||
| Cohort 3 (3), 120 | 1 | 1 | ||||||||||
| Cohort 4 (6), 150 | 3 | 1 | 1 | 2 | ||||||||
| Cohort (N), dasatinib (mg) | Pleural effusion | Pneumonitis | Prolonged QTc | Vomiting | ||||||||
| Grade | Grade | Grade | Grade | |||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (3), 70 | 1 | |||||||||||
| Cohort 2 (3), 100 | 2 | 1 | ||||||||||
| Cohort 3 (3), 120 | 1 | |||||||||||
| Cohort 4 (6), 150 | 1 | 1 | 1 | |||||||||
effusion and fluid retention
Overall, four (27%) patients had edema: three grade 1 and one grade 2. In total, five (33%) patients developed pleural effusions, two of which occurred in the setting of worsening mediastinal adenopathy. Neither underwent thoracocentesis but both were felt to be disease related. A 74-year-old woman treated with dasatinib 150 mg developed pleural effusions, likely secondary to paclitaxel pneumonitis as set out above. One patient on dasatinib 100 mg developed bilateral effusions with otherwise stable systemic disease after two cycles of treatment and withdrew consent due to excessive cumulative toxicity. One patient on dasatinib 150 mg developed bilateral pleural effusions (grade 2), a pericardial effusion (grade 1) and peripheral edema (grade 1) after five cycles with stable disease in bone and lymph nodes. These improved clinically and radiographically with interruption of therapy for 1 week and dasatinib was successfully reintroduced with a dose reduction. However, she was removed from study during her sixth cycle due to new brain metastases. Therefore, overall, two (13%) patients (one of whom was treated at the highest dose level) had pleural effusions thought possibly or probably related to dasatinib.
response
Overall, two patients received less than two cycles of treatment and per protocol were not assessable for response; both were treated at the highest dose level (dasatinib 150 mg) and withdrew due to toxicity. One had a DLT during cycle 1 and repeat CT scan showed progression of disease. The second patient was removed from study because of rash during cycle 2 and had stable disease on CT scan. Per protocol, 13 patients are assessable for response as shown in Table 6. A partial response was seen in four (31%) patients, including two patients previously treated with taxanes. All responders were treated with ≥120 mg dasatinib. Furthermore, an additional five patients (29%) had stable disease as their best response.
Table 6.
Best response to treatment (N = 13)
| Patient ID | Cohort | Dasatinib dose (mg) | Best response | Duration of response |
| 1a | 1 | 70 | Progression | |
| 2a | 1 | 70 | Progression | |
| 3a | 1 | 70 | Progression | |
| 4a | 2 | 100 | Stable disease | Progressed after four cycles |
| 5 | 2 | 100 | Stable disease | Progressed after seven cycles |
| 6 | 2 | 100 | Stable disease | Off study due to toxicity during cycle 3 |
| 7a | 3 | 120 | PR | Progressed after seven cycles |
| 8 | 3 | 120 | PR | Continued PR after 15 cycles |
| 9a | 3 | 120 | PR | Withdrew for personal reasons after six cycles |
| 11a | 4 | 150 | Progression | |
| 13 | 4 | 150 | Stable disease | Withdrew for personal reasons after two cycles |
| 14 | 4 | 150 | PR | Off study due to toxicity after three cycles |
| 15a | 4 | 150 | Stable disease | Progressed after five cycles |
PR, partial response.
Indicates prior taxane exposure.
discussion
This investigator-initiated phase I study demonstrates that in combination with weekly paclitaxel 80 mg/m2, the recommended phase II dosing of dasatinib is 120 mg. Per protocol, only one DLT occurred at the highest dose level explored (dasatinib 150 mg), but due to cumulative toxic effects (rash, fatigue and diarrhea) we believe that this dose level is not tolerable. Preliminary evidence of activity for this combination has been demonstrated, including in patients previously treated with taxanes. Therefore, our results offer preliminary evidence that in patients with MBC this approach is feasible, active and associated with an acceptable safety profile.
Preclinical evidence suggested that the activity of paclitaxel and dasatinib was superior to either single agent [19]. As the first step in translating this observation, we conducted the current phase I trial. In further support of the choice of the cytotoxic agent, paclitaxel is among the most active chemotherapy agents for MBC, with a well-established and acceptable safety profile (neuropathy is the DLT), which is nonoverlapping with the toxicity profile of dasatinib (hematological, gastrointestinal and fluid retention) [18, 20]. Although paclitaxel 80 mg/m2 has been administered weekly in a continuous schedule, in clinical practice weekly paclitaxel 3 weeks of 4 is often preferred due to improved tolerability. Furthermore, this schedule (albeit at a dose of 90 mg/m2) has been investigated in combination with other targeted therapies such as antiangiogenic agents [21]. The starting dose of dasatinib 70 mg was based on single-agent experience in CML and other tumors. In two phase II studies of single-agent dasatinib in MBC, the initial starting dose of 100 mg twice a day was reduced to 70 mg twice a day for tolerability [22, 23]. In one of these studies, pharmacokinetic monitoring of 70 mg twice daily demonstrated that plasma concentrations were comparable with those seen in patients with leukemia [23]. More recent studies have examined a once-daily schedule of dasatinib. For example, in a randomized phase III study examining four different dosing schedules of dasatinib in CML, dasatinib 100 mg once daily was associated with similar response rates and survival but improved tolerability over the other schedules investigated (50 mg twice daily, 140 mg once daily, or 70 mg twice daily) [24]. However, it should be noted that myelosuppression might be greater in patients with CML due to the targeting of the Bcr–Abl clones. A similar once-daily approach has been adopted in an ongoing phase I study of dasatinib in MBC, examining combination therapy with capecitabine, which initially enrolled patients in escalating doses twice a day but was amended to once-daily dosing [25].
In the current study, only one DLT (fatigue) occurred at the highest dose level investigated (150 mg). However, as can be seen in Tables 2–5, cumulative toxic effects, both hematological (such as anemia and thrombocytopenia) and non-hematological (including diarrhea), were more common at that dose level. Furthermore, three (of six) patients treated at this dose level were removed from study for toxicity and one withdrew for personal reasons. Therefore, the recommended phase II dose is dasatinib 120 mg. However, the optimum dosing strategy for novel targeted therapies is challenging. The traditional approach adopted for cytotoxic agents, whereby increasing doses are investigated until DLT (often hematologic) occurs, may be less relevant for tyrosine kinase inhibitors. An alternative approach may be preferable, whereby the dose of novel agents is determined based on a combination of tolerability and biological surrogates that determine that target inhibition is adequate.
Overall, the toxicity profile of dasatinib was as expected. The most common toxic effects were hematological: 47% leukopenia, 40% anemia, and 40% thrombocytopenia. Increasing doses of dasatinib were associated with greater hematological toxicity. For example, among patients treated with dasatinib 150 mg during cycle 1, two patients had grade 3 neutropenia and one patient had grade 2 neutropenia. In comparison, no patients treated at lower doses of dasatinib had grade >1 neutropenia during cycle 1. One possible side-effect with dasatinib is the development of fluid retention, including edema and pleural effusions. Given these concerns, patients with pleural effusions were excluded from this study. However, we did not observe serious problems with fluid retention, which might have been because of the weekly dose of dexamethasone administered in conjunction with paclitaxel. Three (20%) patients had edema and five (33%) patients developed pleural effusions, mostly due to progressive disease. Therefore, we have amended the ongoing phase II study to allow the participation of patients with pleural effusions at baseline.
In this trial, the combination of dasatinib and paclitaxel demonstrated preliminary antitumor activity at the recommended phase II dose, including in taxane-pretreated patients. The response rate of 31% in a relatively heavily pretreated population is encouraging. Studies of single-agent dasatinib in MBC have reported modest results. In a previous phase II study of 44 patients with triple-negative MBC, the response rate was 4.7%, although 26% of patients had stable disease [22]. Notably, this study used a starting dose of 100 mg twice a day, which had to be reduced to 70 mg twice a day for tolerability [22]. In a second phase II study in patients with HER2-positive or hormone receptor-positive MBC, a similar response rate of 4% (3 of 69 patients) was seen [23]. Of note, all patients who responded and had stable disease had ER- and/or PgR-positive disease. In a phase I study of capecitabine and dasatinib in 27 patients with measurable MBC, 11 (41%) patients had a partial response. These preliminary results suggest that dasatinib has limited single-agent activity in MBC but may be active in combination with cytotoxics, and potentially with endocrine therapy. Several randomized phase II trials are investigating dasatinib in combination with endocrine therapy, such as letrozole, exemestane or fulvestrant, for patients with ER-positive MBC and these results are awaited [26]. Given that recent experience in CML suggests that once daily appears to optimize efficacy, while minimizing toxicity, it may be that the twice-daily schedule initially favored in these studies in MBC was suboptimal [24]. However, it should be noted that the recommended dose for our ongoing phase II study (120 mg once a day) is higher than the recently proposed optimal dose for single-agent dasatinib in CML (100 mg once a day) [24].
The results of the current study indicate that dasatinib 120 mg with weekly paclitaxel (80 mg/m2) is feasible. The results of our ongoing phase II study, and others, will give a more accurate assessment of the feasibility and activity of dasatinib in MBC. In an attempt to improve patient selection for combination therapy with dasatinib and paclitaxel, we are also assessing putative biomarkers of response, incorporating phospho-SRC, circulating tumor cells and tumor gene profiling, including expression of EphA2, a dasatinib target [27].
In summary, this investigator-initiated phase I study has demonstrated that in combination with weekly paclitaxel 80 mg/m2, the MTD of dasatinib is 120 mg. The side-effect profile of the combination is consistent with prior experience with the individual agents. Preliminary evidence of antitumor activity for this combination has been seen in patients with MBC, including patients with prior taxane exposure.
funding
Bristol-Myers Squibb.
disclosure
MNF has received research funding from Bristol-Myers Squibb. PGM has received research funding and honoraria from Bristol-Myers Squibb. All remaining authors have declared no conflicts of interest.
Acknowledgments
Presented in part at the 32nd Annual San Antonio Breast Cancer Symposium, December 2009, San Antonio, TX; American Society of Clinical Oncology 46th Annual Meeting, June 2010, Chicago, IL; and European Society for Medical Oncology 35th Congress, Milan, October 2010.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16:3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 5.Verbeek BS, Vroom TM, Adriaansen-Slot SS, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol. 1996;180:383–388. doi: 10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Myoui A, Nishimura R, Williams PJ, et al. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63:5028–5033. [PubMed] [Google Scholar]
- 7.Miyazaki T, Sanjay A, Neff L, et al. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modi S, Seidman AD, Dickler M, et al. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- 10.Cristofanilli M, Morandi P, Krishnamurthy S, et al. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-beta: clinical activity and biological correlations. Ann Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 12.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 14.Nautiyal J, Majumder P, Patel BB, et al. Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett. 2009;283:143–151. doi: 10.1016/j.canlet.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Dering J, Ginther C, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/‘triple-negative’ breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 16.Hiscox S, Morgan L, Green TP, et al. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–274. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 17.Riggins RB, Thomas KS, Ta HQ, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–7015. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- 18.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 19.Company BMS. Preclinical pharmacology of dasatinib, a SRC protein kinase inhibitor. 2003 Control No. 930003300. [Google Scholar]
- 20.Morris PG, McArthur HL, Hudis CA. Therapeutic options for metastatic breast cancer. Expert Opin Pharmacother. 2009;10:967–981. doi: 10.1517/14656560902834961. [DOI] [PubMed] [Google Scholar]
- 21.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Bengala C, Ibrahim N, et al. San Antonio, TX: San Antonio Breast Cancer Symposium (Abstr 3118) 2008. Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059. [Google Scholar]
- 23.Mayer E, Baurain J, Sparano J, et al. Dasatinib in advanced HER2/neu amplified and ER/PR-positive breast cancer: phase II study CA180088. Proc Am Soc Clin Oncol. 2009 (Abstr 1011) [Google Scholar]
- 24.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somlo G, Atzori F, Strauss L, et al. Dasatinib plus capecitabine (Cap) for progressive advanced breast cancer (ABC): phase I study CA180004. Proc Am Soc Clin Oncol. 2009 (Abstr 1012) [Google Scholar]
- 26.Strauss LC, O'Shaughnessy J, Jackson J, et al. Three parallel randomized phase II trials of dasatinib plus hormone therapy (HT) in advanced ER+ breast cancer (ER+ ABC) Proc Am Soc Clin Oncol. 2010 (Abstr TPS133) [Google Scholar]
- 27.Morris PG, Chang J, Abbruzzi A, et al. Correlative biomarkers in a phase II study of dasatinib (D) and weekly (w) paclitaxel (P) for patients (Pts) with metastatic breast carcinoma (MBC) Proc Am Soc Clin Oncol. 2010 (Abstr TPS124) [Google Scholar]