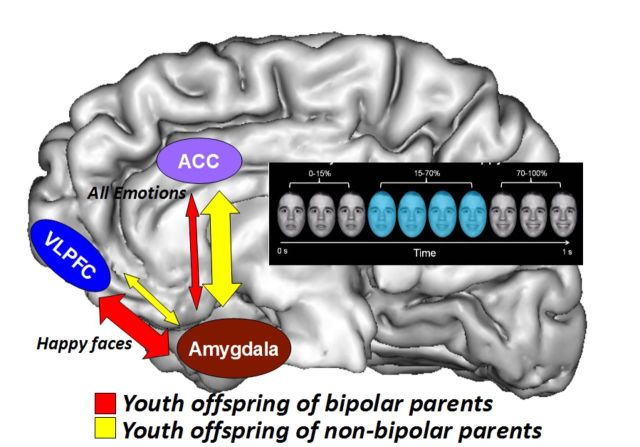
Manelis et al. use fMRI to compare implicit processing of emotional faces in the offspring of individuals with bipolar disorder, non-bipolar psychopathology and no psychopathology. Reduced amygdala-ACC functional connectivity and increased amygdala-VLPFC functional connectivity distinguishes the offspring of bipolar parents from other participants, and may be a bipolar disorder biomarker.
Keywords: youth offspring of parents with bipolar disorder, risk of bipolar disorder, functional MRI, emotion processing, emotion regulation, biological markers
Manelis et al. use fMRI to compare implicit processing of emotional faces in the offspring of individuals with bipolar disorder, non-bipolar psychopathology and no psychopathology. Reduced amygdala-ACC functional connectivity and increased amygdala-VLPFC functional connectivity distinguishes the offspring of bipolar parents from other participants, and may be a bipolar disorder biomarker.
Abstract
This study aimed to identify neuroimaging measures associated with risk for, or protection against, bipolar disorder by comparing youth offspring of parents with bipolar disorder versus youth offspring of non-bipolar parents versus offspring of healthy parents in (i) the magnitude of activation within emotional face processing circuitry; and (ii) functional connectivity between this circuitry and frontal emotion regulation regions. The study was conducted at the University of Pittsburgh Medical Centre. Participants included 29 offspring of parents with bipolar disorder (mean age = 13.8 years; 14 females), 29 offspring of non-bipolar parents (mean age = 13.8 years; 12 females) and 23 healthy controls (mean age = 13.7 years; 11 females). Participants were scanned during implicit processing of emerging happy, sad, fearful and angry faces and shapes. The activation analyses revealed greater right amygdala activation to emotional faces versus shapes in offspring of parents with bipolar disorder and offspring of non-bipolar parents than healthy controls. Given that abnormally increased amygdala activation during emotion processing characterized offspring of both patient groups, and that abnormally increased amygdala activation has often been reported in individuals with already developed bipolar disorder and those with major depressive disorder, these neuroimaging findings may represent markers of increased risk for affective disorders in general. The analysis of psychophysiological interaction revealed that offspring of parents with bipolar disorder showed significantly more negative right amygdala-anterior cingulate cortex functional connectivity to emotional faces versus shapes, but significantly more positive right amygdala-left ventrolateral prefrontal cortex functional connectivity to happy faces (all P-values corrected for multiple tests) than offspring of non-bipolar parents and healthy controls. Taken together with findings of increased amygdala-ventrolateral prefrontal cortex functional connectivity, and decreased amygdala-anterior cingulate cortex functional connectivity previously shown in individuals with bipolar disorder, these connectivity patterns in offspring of parents with bipolar disorder may be risk markers for, rather than markers conferring protection against, bipolar disorder in youth. The patterns of activation and functional connectivity remained unchanged after removing medicated participants and those with current psychopathology from analyses. This is the first study to demonstrate that abnormal functional connectivity patterns within face emotion processing circuitry distinguish offspring of parents with bipolar disorder from those of non-bipolar parents and healthy controls.
Introduction
Identifying early clinical signs of bipolar disorder is critically important to improve treatment outcome (Birmaher et al., 2009). One factor that increases probability of developing affective disorders is having a parent with psychopathology (Birmaher et al., 2009; Goldstein et al., 2010). Offspring of parents with bipolar disorder (BO) are at higher risk of bipolar disorder than offspring of parents with non-bipolar disorder psychopathology (NBO), for example. Yet, little is known about the neurophysiological processes that predispose to or protect against risk for bipolar disorder. Neuroimaging studies can help elucidate such processes by identifying abnormal functioning in neural circuitries supporting information processing domains known to be aberrant in individuals with established bipolar disorder, such as face emotion processing. For example, youth and adults with bipolar disorder are impaired in both memory for emotional faces (Dickstein et al., 2007; Adleman et al., 2013) and identification of facial emotional expressions (Rich et al., 2006; Guyer et al., 2007). Comparing BO and NBO in neuroimaging studies of face emotion processing circuitry may thus help identify biomarkers reflecting risk for, or protection against, bipolar disorder specifically.
When comparing BO and NBO groups in neuroimaging studies there are additional facts that should be considered. First, both BO and NBO are at increased future risk of a range of non-bipolar disorder psychiatric disorders (Birmaher et al., 2009). Second, both the parents of BO and the parents of NBO often have non-bipolar disorder comorbid disorders. Thus, including NBO will control for the potential impact upon neuroimaging measures in BO of: (i) being at risk for non-bipolar disorder psychopathology; and (ii) living with parents with non-bipolar disorder psychopathology. Furthermore, as parents of BO often have multiple non-bipolar disorder disorders in addition to bipolar disorder (e.g. generalized anxiety disorder, phobias, alcohol/drug dependence), in studies comparing BO and NBO, parents of NBO need to also have several of these disorders rather than only one non-bipolar disorder condition, to control for the presence of all of these different disorders in the parents of BO.
Face processing depends on the coordinated function of a distributed network of temporo-occipital, frontal and limbic regions (Haxby et al., 2002; Ishai, 2008; Fusar-Poli et al., 2009) and relies on interactions among amygdala, prefrontal and visual cortices (Banks et al., 2007; Fairhall and Ishai, 2007; Dima et al., 2011). The amygdala supports processing of emotional faces (Morris et al., 1996; Phillips et al., 1997; Vuilleumier et al., 2001). Prefrontal cortices (PFCs) (including ventrolateral PFC and orbitofrontal cortex) support semantic and reward-related aspects associated with faces (Leveroni et al., 2000; Ishai et al., 2002), recognition of facial expressions (Tsuchida and Fellows, 2012; Willis et al., 2014), and voluntary emotional regulation (Phillips et al., 2008; Strakowski et al., 2012). The anterior cingulate cortex (ACC) and medial prefrontal cortices are involved in emotion appraisal and regulation (Etkin et al., 2011).
Previous neuroimaging studies of youth and adults with bipolar disorder showed abnormally elevated amygdala activation (Blumberg et al., 2005; Rich et al., 2006). In adults with bipolar disorder, the abnormalities in functional connectivity between amygdala and prefrontal cortical regions may be emotion-specific, with patterns of abnormally reduced amygdala-PFC functional connectivity being shown to happy faces in particular (Almeida et al., 2009; Versace et al., 2010; Wang et al., 2012). In youths with bipolar disorder, however, findings suggest abnormally decreased amygdala-prefrontal cortical functional connectivity to fear faces (Ladouceur et al., 2011).
A small number of neuroimaging studies suggest that BO and youths with bipolar disorder may show similar patterns of face emotion processing impairment and functional abnormalities in neural circuitry supporting emotional face processing (Brotman et al., 2008a, b). These findings indicate that both youths with bipolar disorder and BO made more face emotion labelling errors than healthy offspring of psychiatrically healthy parents (healthy controls) (Brotman et al., 2008a) and needed more intense emotional expressions to correctly identify emotions (Brotman et al., 2008b). Both youth with bipolar disorder and BO, relative to healthy control subjects, had increased amygdala activation when viewing happy and fearful faces (Olsavsky et al., 2012), but reduced dorsolateral PFC activation for successful versus unsuccessful encoding of emotional faces (Tseng et al., 2014). Moreover, the modulation of amygdala and ventrolateral PFC activation by the changes in the intensity of emotions was reduced in youth with bipolar disorder and BO, compared with healthy control subjects (Brotman et al., 2014a). Given that offspring of parents with major depressive disorder also showed increased amygdala activation during passive viewing of fear faces when compared with healthy control subjects (Monk et al., 2008), increased amygdala activation during processing of emotional faces may be a risk factor for affective disorders in general, rather than that for bipolar disorder specifically. On the other hand, findings that depressed individuals with bipolar disorder were distinguished from those with major depressive disorder by patterns of prefrontal cortical-amygdala functional connectivity during face emotion processing (Almeida et al., 2009), and by increased subcortical and ventrolateral PFC activation during processing of positive and negative facial expressions (Lawrence et al., 2004), suggest that offspring of parents with bipolar disorder may also show functional abnormalities in the wider prefrontal cortical-amygdala neural circuitry supporting emotional face processing compared with offspring of parents with non-bipolar disorder psychopathology.
The extent to which these functional abnormalities in BO reflect specific risk markers for future bipolar disorder versus risk for psychiatric disorders in general remains unclear, however, given that there are no studies directly comparing functioning in face emotion processing neural circuitry in BO and in youth who are at higher than normal risk for psychiatric disorders in general, but lower risk of future bipolar disorder than BO (i.e. NBO). Therefore, the aim of the present study was to identify the effect of familial genetic risk for bipolar disorder (BO > NBO > healthy controls) on activation and functional connectivity in face emotion processing neural circuitry. BO and NBO included youths with and without current non-bipolar disorder psychopathology, some of whom were treated with psychotropic medications. This allowed us to determine, in secondary analyses, the effect of genetic risk for bipolar disorder on emotional face processing in participants with and without current psychopathology and psychotropic medication. We used an implicit face emotion processing task that has previously been shown to elicit patterns of abnormally decreased ventrolateral PFC activation in youths with bipolar disorder (Hafeman et al., 2014).
Based on neuroimaging findings described above, we hypothesized that during emotional face processing:
BO, compared with NBO and healthy control subjects, would show significantly greater amygdala and reduced prefrontal cortical activation to all emotional faces (versus shapes). An alternative hypothesis was that both BO and NBO would show significantly greater amygdala activation to all emotional faces (versus shapes), which may be a risk marker for psychiatric disorders in general, rather than for bipolar disorder specifically.
BO, compared with NBO and healthy control subjects, would show significantly reduced amygdala-prefrontal cortical positive functional connectivity to all emotional faces.
The differential patterns of brain activation and functional connectivity in the above neural regions in BO versus NBO versus healthy control subjects youth would be present in the subset of participants without current psychiatric diagnoses and psychotropic medications.
Given previous findings suggesting differential patterns of abnormal neural response to happy versus negative facial emotions in adults and youth with bipolar disorder (Almeida et al., 2009; Versace et al., 2010; Ladouceur et al., 2011), we also examined the extent to which there were differential patterns of neural response to happy and all negative facial emotions in BO, NBO and healthy control subject youths in the above analyses.
Materials and methods
Participants
Three groups of participants aged 7–17 years who were not affected with bipolar disorder took part in this study: offspring of parent(s) with bipolar disorder (BO; n = 36), offspring of parent(s) with non-bipolar disorder psychopathology (NBO; n = 38) and healthy offspring of healthy parents (healthy control subjects; n = 25) without family history of any lifetime psychiatric disorders including bipolar disorder. The majority of BO (n = 34) and NBO (n = 33) were recruited from the Bipolar Offspring Study (BIOS). BIOS is an ongoing longitudinal study examining psychiatric symptomatology in youth offspring of parents with bipolar disorder (Birmaher et al., 2009) and functioning in neural circuitries underlying information processing domains implicated in the pathogenesis of bipolar disorder. See Supplementary Fig. 1 for detailed information about selection of BIOS participants for the functional MRI study.
Two BO and five NBO subjects were recruited from the Longitudinal Assessment of Manic Symptoms (LAMS) study (Findling et al., 2010; Horwitz et al., 2010), a parallel study examining neural circuitry functioning in youth with behavioural and emotional dysregulation. We ensured that recruitment source did not impact main neuroimaging findings by conducting additional analyses of neural functioning using only the BIOS sample (see Supplementary material for details). Twenty-four healthy control subjects were recruited from the healthy comparison youth group of the LAMS study. Exclusion criteria for healthy control subjects were: history of meeting criteria for any psychiatric, alcohol, or substance use disorder, and family history (first-degree relatives) of any psychiatric disorder. All participants recruited from the LAMS study were scanned on the same scanner concurrently with BIOS participants. One healthy control subject was recruited from BIOS. Exclusion criteria for all participants were: systemic medical illness, neurological disorders, head trauma, alcohol or illicit substance use, standard exclusion criteria for MRI research (metal anywhere in the head or body, claustrophobia), IQ < 70 (using the Weschler Abbreviated Scale of Intelligence; Wechsler, 1999), unable to read and write in standard English, and corrected far visual acuity worse than 20/40 on the Snellen visual acuity test. Seven BO (from BIOS), nine NBO (from BIOS), and two healthy control subjects (from LAMS) were excluded from data analysis due to inability to complete the scanning session or due to excessive motion in the scanner (translation > 4 mm). The total numbers of participants with usable functional MRI data were: 29 BO, 29 NBO, and 23 healthy control subjects. Eleven BO and 14 NBO had current non-bipolar disorder psychopathology, five BO and four NBO were taking one class of psychotropic medications (Table 1). Given ethical concerns with stopping medication for research participation, participants were permitted to use prescribed medication(s) before and on the day of scanning.
Table 1.
Demographic and clinical variables in youth offspring of parents with bipolar disorder (BO), youth offspring of parents with non-bipolar psychopathology (NBO), and healthy offspring of psychiatrically healthy parents (HC)
| BO n = 29 | NBO n = 29 | HC n = 23 | Statistics | P-value | |
|---|---|---|---|---|---|
| Number of youths without psychiatric diagnoses | 18 (62%) | 15 (52%) | 23 (100%) | BO versus NBO | ns |
| χ2(2) < 1 | |||||
| Number of youths untreated with psychotropic medications | 24 (83%) | 25 (86%) | 23 (100%) | BO versus NBO | ns |
| χ2(2) < 1 | |||||
| Age at scan | 13.81 (2.45) | 13.83 (2.36) | 13.74 (1.80) | F(2,78) < 1 | ns |
| Gender (female) | 14 | 12 | 11 | χ2(2) < 1 | ns |
| IQ (WASI) | 103.21 (14.51) | 101.79 (14.06) | 105.78 (13.79) | F(2,78) < 1 | ns |
| Handedness (right hand) | 26 | 27 | 21 | Yates' χ2(2) < 1 | ns |
| SES based on parental education | 5.48 (0.95) | 5.54 (0.96) | 5.30 (1.02) | F(2,77) < 1 | ns |
| Symptom Assessment Scale Scores administered on the day of scan | |||||
| SCARED Parent Total | 9.45 (6.86) | 9.79 (11.33) | 4.17 (4.32) | F(2,77) = 3.6 | 0.03 |
| SCARED Child Total | 11.66 (8.61) | 10.14 (13.52) | 9.33 (11.42) | F(2,78) < 1 | ns |
| CALS Parent Total | 8.25 (10.33) | 3.86 (4.53) | 1.78 (2.59) | F(2,76) = 6.0 | 0.004 |
| CALS Child Total | 10.52 (12.22) | 7.62 (10.84) | 5.96 (13.39) | F(2,78) < 1 | ns |
| MFQ Parent | 6.11 (9.06) | 3.85 (3.52) | 1.57 (2.09) | F(2,75) = 3.7 | 0.03 |
| MFQ Child | 8.86 (10.73) | 9.38 (10.85) | 5.09 (10.57) | F(2,78) = 1.2 | ns |
| Medications | |||||
| Antidepressants | na | Sertraline HCI: n = 1 | na | ||
| Antipsychotics | Risperidone: n = 1 | na | na | ||
| Quetiapine: n = 1 | |||||
| Mood stabilizers | na | na | na | ||
| Stimulants | Dextroamphetamine mixed salts: n = 1 | Methylphenidate: n = 1; dextroamphetamine mixed salts: n = 2 | na | ||
| Non-stimulants | Atomoxetine: n = 2 | na | na | ||
| Benzodiazepines | na | na | na | ||
| Youth offspring current psychiatric diagnoses | |||||
| Number of youth with more than 1 diagnosis | 5 | 6 | na | ||
| MDD/DDNOS | 3 | 2 | na | ||
| Attention deficit hyperactivity disorder | 6 | 7 | na | ||
| Anxiety disorders | 2 | 3 | na | ||
| Oppositional defiant disorder | 1 | 2 | na | ||
| Phobias | 2 | 2 | na | ||
| Tourette's disorder | 1 | 0 | na | ||
| Obsessive compulsive disorder | 0 | 2 | na | ||
| Eating disorder | 1 | 0 | na | ||
Standard deviations (SD) are reported in parentheses. MDD = major depressive disorder; SCARED = Self-Report for Childhood Anxiety Related Emotional Disorders; MFQ = Mood and Feelings Questionnaire; CALS = The Children’s Affective Lability scale; na = not applicable; ns = not significant; SES = Socioeconomic Status; DDNOS = Depressive Disorder Not Otherwise Specified.
Assessment procedures
A trained clinician interviewed parents about their children and a separate clinician interviewed children themselves using the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (KSADS-PL; Kaufman et al., 1997). Inter-rater reliability for all psychiatric diagnoses ascertained through the KSADS was κ > 0.8. This clinician was blind to parental psychopathology that was evaluated by another clinician using the Structural Clinical Interview for DSM-IV (SCID-I; First et al., 2002) for BIOS youth, and using the Family History Screen (Weissman et al., 2000) for LAMS youths. All cases were presented to a child psychiatrist who was ultimately responsible for determining all diagnoses.
On the day of scanning, all participants completed clinical and demographic questionnaires in the morning and were scanned and administered the tasks outside the scanner in the afternoon. Staff administering these questionnaires and the scanning procedures were not blinded to the participant’s group, but they were not involved with the psychiatric management of the study participants. All participants completed medication forms that documented psychotropic medications used during the past 24 h, and those used on a regular basis; drug/alcohol/pregnancy screens; the Edinburgh Handedness Inventory (Oldfield, 1971); and the Snellen visual acuity test. IQ was measured using the Weschler Abbreviated Scale of Intelligence (Wechsler, 1999). Additionally, parents/guardians of youth participants completed the PGBI-10 M [Parent Version, General Behavior Inventory (Youngstrom et al., 2008), to assess the severity of behavioral and emotional dysregulation in their offspring during the last 6 months; only parents of BO and NBO completed this questionnaire]; the SCARED-P (Self-Report for Childhood Anxiety Related Emotional Disorders, Parent Version, to assess offspring anxiety over last 2 weeks; Birmaher et al., 1997); the CALS-P (The Children’s Affective Lability Scale, Parent Version; Gerson et al., 1996); the MFQ-P (Mood and Feelings Questionnaire, Parent Version, to assess the severity of depression during the last 2 weeks; Angold et al., 1995); and a questionnaire to assess sociodemographic status represented by parental education (Hollingshead, 1975). Youth participants completed child report versions of affective symptomatology scales: the CALS-C, SCARED-C, MFQ-C. Pubertal status of youth was assessed using a self-report questionnaire (Petersen et al., 1988). Table 1 summarizes demographic and clinical variables in children. Table 2 summarizes lifetime psychiatric diagnoses in parents. Many (but not all) parents of NBO had major depressive disorder, but they also had other comorbid psychiatric disorders (Table 2). Supplementary Table 1 reports demographic and clinical variables for youth without psychopathology and youth untreated with psychotropic medications.
Table 2.
Lifetime psychiatric diagnoses in parents of BO and NBO
| Parents with bipolar psychopathology | Parents with non-bipolar psychopathology | Statistics | P-value | |
|---|---|---|---|---|
| BD-I | 23 | 0 | Yates' χ2(1) = 34.8 | < 0.001 |
| BD-II | 6 | 0 | Yates' | 0.03 |
| χ2(1) = 4.6 | ||||
| BD-NOS | 0 | 0 | ||
| Major depressive disorder/depressive disorder NOS | 1 | 21 | Yates' χ2(1) = 26.6 | < 0.001 |
| Generalized anxiety disorder/anxiety disorders NOS | 16 | 9 | χ2(1) = 3.4 | ns |
| Phobias | 21 | 14 | χ2(1) < 3.0 | ns |
| Alcohol/drug abuse/dependence | 23 | 13 | χ2(1) = 6.6 | 0.01 |
| Post-traumatic stress disorder | 12 | 4 | χ2(1) = 5.2 | 0.02 |
| Panic disorder | 16 | 6 | χ2(1) = 6.8 | < 0.01 |
| Eating disorder | 4 | 2 | χ2(1) = 1.8 | ns |
| Obsessive-compulsive disorder | 10 | 0 | χ2(1) = 11.7 | < 0.001 |
| Attention deficit hyperactivity disorder | 4 | 2 | χ2(1) < 1 | ns |
Standard deviations (SD) are reported in parentheses. BD = bipolar disorder.
Dynamic faces task
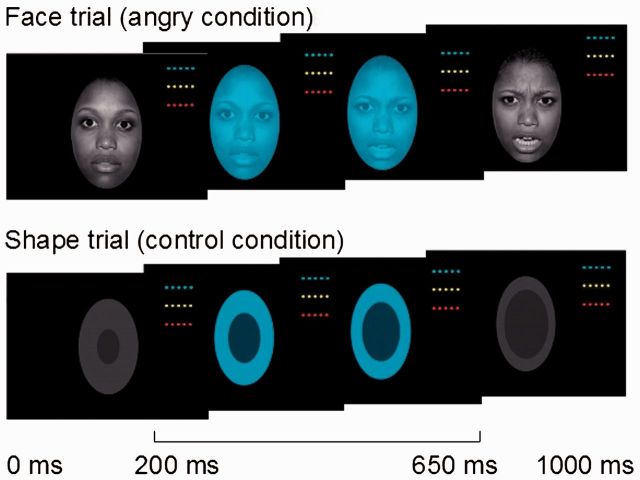
The dynamic faces task (Fig. 1) was the first task administered following 15 min of structural image acquisition. During this task (Perlman et al., 2013; Hafeman et al., 2014) participants were presented with greyscale emotional faces (happy, angry, fearful, and sad) taken from the NimStim face database (Tottenham et al., 2009) and control stimuli (greyscale ovals matched in luminance with the face stimuli). The 13-min task included three, 12-trial blocks for each of four emotional face types, and 12, six-trial blocks of shapes. Trials were separated by 2–3 s jittered intertrial intervals. During face trials, a face changed in emotional expression from neutral to emotional over 1 s in 5% increments. During shape trials, a dark oval was superimposed on a light grey oval and changed in size to parallel the changes in the face trials. In the middle of each trial (between 200 and 650 ms), a coloured semi-transparent oval (blue, orange or yellow) overlaid the image. Participants had to identify the colour of the oval and respond using a corresponding button on the response stick. This task is a measure of implicit emotion processing and regulation, given that participants do not overtly label facial emotional expressions.
Figure 1.
The emotional dynamic faces task. Graphic representation of an emotional face (angry condition) and a shape (control condition) trials of the emotional dynamic faces task. During this task, participants viewed a face changing from a neutral to a happy, sad, angry (depicted here), or fearful emotional expression during a 1000-ms trial. In a control condition, participants viewed a dark grey oval increasing in size over the same period of time. Participants had to identify the colour of an oval superimposed over the face/shape and presented between 200 and 650 ms of a trial.
Post-scanning emotion labelling task
An explicit emotion labelling task (Ladouceur et al., 2013) was conducted after the scanning session outside the scanner to ensure that youth were not impaired on emotional expression labelling in general. Participants labelled facial emotional expressions as angry, fearful, disgusted, sad, happy or neutral, and determined these expressions intensity on a scale ranging from 1 to 10 (10 being the most intense).
Behavioural data analysis
Participants' accuracy and response times during colour identification during the neuroimaging task were analysed to parallel the structure of the functional MRI data analyses. (i) We computed the Happy-Shape and AllNegative-Shape differences; (ii) accuracies and response time were compared using a 2 (Emotion: Happy-Shapes versus AllNegative-Shapes) × 3 (Group: BO/NBO/healthy controls) ANOVA. Only response times for correctly identified colours were included in the analysis of response time. Due to a computer failure, the data for one healthy control youth were missing.
The post-scan emotion labelling task data were analysed in the same way. Disgusted faces were excluded from the analysis to match the analyses of the implicit dynamic faces task. In addition to analysing participants' accuracy and response time, we also analysed ratings of emotion intensity.
Functional MRI data acquisition and analysis
Functional MRI data were acquired using a Siemens MAGNETOM TrioTim 3 T MR system. A high-resolution structural image (1 × 1 × 1 mm) was acquired using MPRAGE (repetition time = 2300 ms, echo time = 3.93 ms, field of view = 256, flip angle = 9°, 192 slices). Functional data were collected using a gradient-echo, echo-planar sequence [voxel size: 3.2 × 3.2 × 3.1 mm, repetition time = 2000 ms, echo time = 28 ms, field of view = 205, flip angle = 90°, 38 slices; 386 volumes (repetition times)]. Field maps were collected at the 4 × 4 × 4 mm resolution using a gradient echo sequence (repetition time = 488 ms, echo time1 = 4.92 ms, echo time2 = 7.38 ms, field of view = 256, flip angle = 60°, 32 slices).
The images were preprocessed and analysed using FSL 5.0.2 (www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction with MCFLIRT (Jenkinson et al., 2002), non-brain removal using BET (Smith, 2002), fieldmap-based EPI unwarping using PRELUDE + FUGUE (Jenkinson, 2003), spatial smoothing with a Gaussian kernel of full-width at half-maximum 6 mm; grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor; high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 100.0 s). A field map image used in the functional MRI data analysis was prepared using the fsl_prepare_fieldmap script. No slice-timing correction was applied. The high-resolution structural images were segmented using the fsl_anat script to separate white matter, grey matter and CSF, and to also segment subcortical structures. The white matter and CSF masks were then coregistered with functional images, and their time courses were extracted from the preprocessed functional data for further analyses. Motion outliers (time points where the functional MRI signal was corrupted due to subject motion) were identified using the fsl_motion_outliers script (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers). A confound matrix from this analysis was then combined with the white matter and CSF time courses and used as a confound variable of no interest in the first-level analyses.
Co-registration was carried out using FLIRT (FMRIB's Linear Image Registration Tool; Jenkinson and Smith, 2001; Jenkinson et al., 2002) and FNIRT (FMRIB's Non-linear Image Registration Tool; Andersson et al., 2007). BOLD images were registered to the high-resolution structural (MPRAGE) images using FLIRT, the high-resolution images were registered to the MNI152_T1_2mm template using FNIRT, and the two resulting transformations were concatenated and applied to the original BOLD image (http://www.fmrib.ox.ac.uk/fsl/flirt/gui.html) to transform it to MNI space. The registration quality was checked for each subject. In rare cases FNIRT was substituted with FLIRT to obtain a better quality registration.
An anatomical mask used as a target region in the PPI analysis consisted of prefrontal regions (ACC, orbitofrontal cortex and ventrolateral PFC) previously implicated in emotion regulation (Phillips et al., 2008; Strakowski et al., 2012) and used in a previous study (Hafeman et al., 2014) that examined emotionally dysregulated youth using the same dynamic faces task. This mask was created by combining the thresholded at 30% population probability anatomical masks of ACC, orbitofrontal cortex and inferior frontal gyrus (pars triangularis and pars opercularis) from the Harvard-Oxford Cortical structural atlas shipped with FSL.
Preprocessed data were submitted to a first-level General Linear Model analysis implemented using FEAT (FMRI Expert Analysis Tool, v6.0). The model included five regressors (happy, angry, fear and sad faces, and shapes). The magnitude of activation was examined for each facial expression versus shape, and for all faces versus shape. All group-level analyses were conducted using FLAME1 (FMRIB's Local Analysis of Mixed Effects). Whenever possible, gender, age, IQ, and presence/absence of psychopathology were used as covariates in the group-level analyses in order to factor out the effects of these variables. To establish that participants activated face emotion processing neural circuitry during task performance, the first group-level analysis identified regions that activated for all emotional faces, compared with shapes, in all participants (n = 81). The Z-statistical images were thresholded using GRF (Gaussian-Random-Field) theory-based maximum height threshold at voxel-wise-corrected P < 0.05.
Statistics
Hypothesis 1 testing
The second group-level analysis examined brain activation using a 3 (Group: BO/NBO/healthy controls) × 2 (Emotion: Happy versus AllNegative faces) ANOVA limited to the face emotion processing neural circuitry identified in the first group-level analysis. Significant clusters of activation were determined using a cluster method in FSL by thresholding Z-statistic images in the face emotion processing circuitry mask using voxel-wise-uncorrected P < 0.005 (z > 2.57) and a corrected cluster significance threshold of P < 0.05 (Worsley, 2001).
Hypothesis 2 testing
Functional connectivity was examined using psychophysiological interaction (PPI) analysis (Friston et al., 1997). The regions within the emotional face processing circuitry identified in the second group-level analysis served as the seed regions. The target region was an anatomical mask consisting of prefrontal regions (ACC, orbitofrontal cortex, and ventrolateral PFC). The PPI first-level analysis model included five psychological regressors (happy, angry, fear and sad faces, and shapes), one physiological regressor—a mean time course extracted from the seed region, and five interaction terms between the physiological and psychological regressors. The group-level connectivity analyses (a 3 × 2 ANOVA) paralleled the activation analysis.
Post hoc tests
Tukey's HSD post hoc tests of activation and connectivity values (parameter estimates extracted from the significant activation and connectivity clusters) were performed in SPSS to determine the direction of the between-group effects.
Hypothesis 3 testing
Here, we examined the effect of diagnosis and medications on activation and connectivity in the brain regions identified in the previous analyses. For this purpose, we first extracted activation and connectivity values from the significant clusters. Then, we conducted two 3 × 2 ANOVAs, using SPSS, on (i) participants without diagnoses; and (ii) unmedicated participants.
Exploratory analyses
These analyses examined the relationship between neural measures of emotional face processing and demographic, clinical and behavioural variables separately in each group of participants. We also examined the effect of puberty on activation and connectivity measures.
Results
Pubertal status
The Petersen's self-report pubertal status data were available for 27 BO, 27 NBO, and 18 healthy control subjects. The biological ages of youth were distributed across all five pubertal categories: prepubertal, early pubertal, mid-pubertal, late pubertal and post-pubertal, with only very small number of youth in prepubertal and early pubertal stages. Given this, we combined data across prepubertal, early pubertal and mid-pubertal categories into one category (‘earlier’ pubertal), and combined late pubertal and post-pubertal categories into another category (‘later’ pubertal). Twelve BO, 10 NBO and nine healthy control subjects were in the ‘earlier’ pubertal category, whereas 15 BO, 17 NBO and nine healthy control subjects were in the ‘later’ pubertal category. A chi-square test for the three groups and two pubertal categories indicated no significant effect of group on pubertal status [χ2(2) = 0.78, P = 0.68].
Behavioural analyses
Dynamic faces task (scanned)
A 2 (Emotion: Happy-Shapes versus AllNegative-Shapes) × 3 (Group:BO/NBO/healthy controls) ANOVA revealed that there was no significant effect of Group, and no significant Group × Emotion interaction, on accuracy and response time (P > 0.05). There was, however, an effect of Emotion on accuracy [F(1,77) = 20.4, P < 0.001] and response time [F(1,77) = 7.3, P = 0.009]. All participants were significantly faster and more accurate when processing AllNegative relative to Happy faces.
Post-scanning emotion labelling task (outside the scanner)
To make these analyses similar to the functional MRI analyses, we computed the average judgement accuracy, average response time for accurate responses and emotion intensity for angry, fearful and sad faces (AllNegative) and conducted a 3 (Group:BO/NBO/healthy control subjects) × 2 (Emotion: Happy versus AllNegative) ANOVA. There was no main effect of Group or Group × Emotion interaction effect on accuracy and intensity judgement. All participants judged Happy, compared to AllNegative, faces more accurately [F(1,78) = 62.4, P < 0.001] and gave them higher intensity ratings [F(1,78) = 242.1, P < 0.001]. The analysis of response time for correctly judged emotions revealed a main effect of Group [F(2,78) = 3.7, P = 0.03], a main effect of Emotion [F(1,78) = 71.8, P < 0.001] and a Group × Emotion interaction effect [F(2,78) = 3.9, P = 0.03]. Based on the Tukey’s HSD post hoc test, BO were overall faster than healthy control subjects (P = 0.006), and NBO did not differ from BO and healthy control subjects. The interaction effect was driven by greater changes in response time for Happy versus AllNegative faces in healthy control subjects than in BO.
Functional MRI analyses
All emotional faces versus shape across all participants
Consistent with previous studies (Fusar-Poli et al., 2009; Sabatinelli et al., 2011), bilateral amygdala, temporal and occipital fusiform cortices, frontal polar, frontal medial and orbito-frontal cortices, right ventrolateral PFC and right temporal polar cortex showed increased activation for emotional faces versus shapes, across all participants (Table 3 and Supplementary Fig. 2). These regions were used as a region of interest mask for testing Hypotheses 1 and 2.
Table 3.
The effect of presentation of happy, angry, fear and sad faces versus presentation of shapes on whole brain activation in all participants
| Region | nvox | z-score | x | y | z | |
|---|---|---|---|---|---|---|
| Activation All Emotional Faces > Shapes | ||||||
| R | LOC, inf | 5612 | 11 | 40 | −82 | −10 |
| L | LOC, inf | 3857 | 9.73 | −38 | −86 | −12 |
| R | Amygdala | 2130 | 10.3 | 24 | 0 | −20 |
| L | Amygdala | 1019 | 9.15 | −26 | −2 | −18 |
| R | vlPFC | 397 | 6.29 | 52 | 24 | 20 |
| R | Frontal medial cortex | 72 | 5.72 | 6 | 54 | −16 |
| R | Frontal pole/frontal orbital cortex | 64 | 6.47 | 34 | 34 | −14 |
| R | Subcallosal cortex | 13 | 5.14 | 2 | 8 | −14 |
| L | Frontal pole/frontal orbital cortex | 13 | 5.12 | −38 | 32 | −16 |
The statistical maps were thresholded at voxel-wise corrected P < 0.05. The resulting thresholded image is referred to as the face processing region of interest mask. nvox = number of voxels in the cluster; R = right hemisphere; L = left hemisphere; LOC, inf = lateral occipital cortex, inferior division; vlPFC = ventrolateral prefrontal cortex.
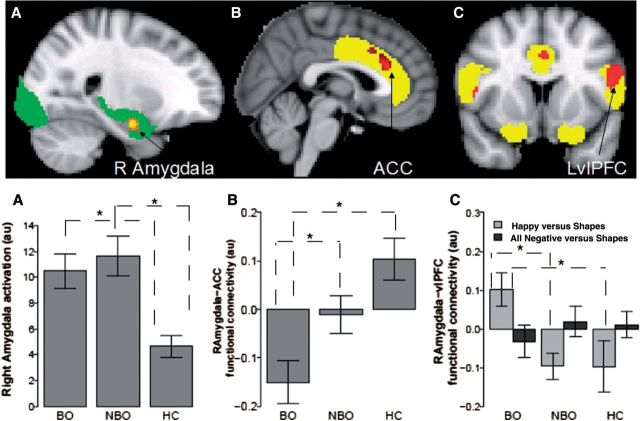
Hypothesis 1: Activation in the emotional face processing circuitry region of interest
A significant main effect of group in the emotional face processing circuitry was found in the right amygdala (Table 4 and Fig. 2A). The Tukey's HSD post hoc test indicated that BO and NBO had significantly greater right amygdala activation for Faces versus Shapes than healthy control subjects (BO > healthy controls: P = 0.009; NBO > healthy controls: P = 0.001), but did not differ from each other. There was also a main effect of Emotion (Supplementary Table 2), but no Group × Condition interaction.
Table 4.
Main effect of Group (BO/NBO/HC) on right amygdala activation
| Region | nvox | z-score | x | y | z | |
|---|---|---|---|---|---|---|
| Activation analysis: main effect of Group (all faces versus shape) in the face processing region of interest mask | ||||||
| R | Amygdala | 171 | 3.79 | 24 | −6 | -20 |
| PPI analyses: RAmygdala – ACC,OFC, vlPFC region of interest mask main effect of Group | ||||||
| B | ACC | 146 | 3.45 | 2 | 28 | 22 |
| PPI analyses: RAmygdala – ACC,OFC, vlPFC region of interest mask Group x Condition interaction | ||||||
| L | vlPFC | 111 | 3.83 | −64 | 12 | 24 |
Main effect of Group on right amygdala bilateral anterior cingulate cortex functional connectivity, and a Group x Condition (Happy-Shape versus All Negative versus Shape) interaction effect on right amygdala-left ventrolateral prefrontal cortex functional connectivity. The statistical maps were thresholded at voxel-wise-uncorrected P < 0.005 (z > 2.57) and a corrected cluster significance threshold of P < 0.05 (Worsley, 2001). R = right hemisphere; L = left hemisphere; B = bilateral; PPI = psychophysiological interactions; ACC = anterior cingulate cortex; vlPFC = ventrolateral prefrontal cortex.
Figure 2.
The differences in brain activation and functional connectivity in BO, NBO and healthy controls. (A) Right amygdala activation, (B) right amygdala-ACC and (C) right amygdala-left ventrolateral PFC (LvlPFC) functional connectivity. The emotional face processing region of interest mask is shown in green. The emotion regulation regions (ACC, OFC, and vlPFC) mask is shown in yellow. au = arbitrary units; asterisk indicates significant post hoc Tukey’s HSD results. BO, n = 29; NBO, n = 29 and healthy control subjects (HC), n = 23.
Hypothesis 2: Functional connectivity
The PPI analysis with right amygdala (the region of interest identified in the activation analysis) as the seed region and the ACC, orbitofrontal cortex, ventrolateral PFC region of interest mask as the target region revealed a main effect of Group on right amygdala–ACC (Fig. 2B and Table 4) functional connectivity and a Group × Emotion interaction effect on right amygdala-left ventrolateral PFC functional connectivity (Fig. 2C and Table 4). The Tukey's HSD post hoc test indicated that BO, relative to NBO and healthy control subjects, showed significantly decreased positive right amygdala-ACC functional connectivity (BO < NBO: P = 0.049; BO < healthy controls: P < 0.001; NBO = healthy controls).
To determine which emotional condition drove the Group × Emotion interaction on right amygdala-left ventrolateral PFC functional connectivity, we conducted two planned comparison tests using one-way ANOVA on Happy-Shape and AllNegative-Shape contrasts with Group as a between-subject factor and Bonferroni corrected P-values for two comparisons (0.05/2 = 0.025). These tests revealed that the interaction was driven by group differences for the Happy-Shape condition [F(2,78) = 6.1, P = 0.004]. Tukey's HSD post hoc test indicated that BO showed significantly more positive right amygdala-left ventrolateral PFC functional connectivity for happy faces (versus Shapes) than both NBO (P = 0.008) and healthy control subjects (P = 0.014).
Hypothesis 3: Activation and functional connectivity in unmedicated youths and youths without psychopathology
There was a significant effect of Group on right amygdala activation [participants without psychopathology: F(2,53) = 8.2, P < 0.001; unmedicated participants: F(2,69) = 8.5, P < 0.001; Supplementary Figs 3 and 4], and on right amygdala-ACC functional connectivity [participants without psychopathology: F(2,53) = 7.8, P = 0.001; unmedicated participants: F(2,69) = 8.6, P < 0.001]. There was also a significant Group × Emotion interaction effect on right amygdala-left ventrolateral PFC functional connectivity [participants without psychopathology: F(2,53) = 9.0, P < 0.001; unmedicated participants: F(2,69) = 9.8, P < 0.001].
Participants without psychopathology: post hoc comparisons
The results of post hoc comparisons using the Tukey's HSD test indicated that both BO without psychopathology and NBO without psychopathology, compared with healthy control subjects, had higher right amygdala activation (BO > healthy controls: P = 0.001, NBO > healthy controls: P = 0.02), but were not different from each other. BO had significantly lower right amygdala-ACC functional connectivity than healthy control subjects (BO < healthy controls: P = 0.001), but significantly higher right amygdala-left ventrolateral PFC functional connectivity for happy faces versus shapes than healthy control subjects (BO > healthy controls: P = 0.025). Measures of functional connectivity did not significantly differ in BO versus NBO and in NBO subjects versus healthy control subjects.
Unmedicated participants: post hoc comparisons
The results of post hoc comparisons using the Tukey's HSD test indicated that both unmedicated BO and unmedicated NBO subjects, compared with healthy control subjects, had higher right amygdala activation (BO > healthy controls: P = 0.001, NBO > healthy controls: P = 0.002), but were not different from each other. Right amygdala-ACC functional connectivity was significantly less positive in BO versus NBO (P = 0.04) and in BO versus healthy control subjects (P < 0.001), but was not different for NBO versus healthy control subjects. Right amygdala-left ventrolateral PFC functional connectivity for Happy faces versus Shapes was significantly more positive in BO versus NBO subjects (P = 0.023) and in BO versus healthy control subjects (P = 0.016), but was not significantly different for NBO versus healthy control subjects.
Exploratory analyses
Relation between neural measures and demographic, clinical, and behavioural variables
Right amygdala-ACC functional connectivity was significantly positively correlated with CALS-C, MFQ-P, SCARED-P and SCARED-C measures in NBO (CALS-C: r = 0.4, P = 0.03; MFQ-P: r = 0.46, P = 0.02; SCARED-P: r = 0.45, P = 0.02; SCARED-C: r = 0.41, P = 0.03), but not in BO or healthy control subjects. Those correlations with SCARED were significantly greater in NBO versus BO (SCARED-P: Fisher's z = 2.7, P = 0.007; SCARED-C: Fisher's z = 2.5, P = 0.01). CALS-P and MFQ-C measures did not correlate significantly with neural measures in either group. Right amygdala-ACC connectivity was significantly negatively correlated with IQ in BO (r =−0.43, P = 0.03) and NBO (r = −0.58, P = 0.001), but not in healthy control subjects (r = 0.06). This correlation was significantly more negative in NBO versus healthy control subjects (Fisher's z = −2.4, P = 0.02) and marginally significant in BO versus healthy control subjects (Fisher's z = −1.7, P = 0.08).
Right amygdala-left ventrolateral PFC functional connectivity to Happy faces-Shapes and participants' age were significantly negatively correlated in BO (r = −0.4, P = 0.03), positively correlated in healthy control subjects (r = 0.45, P = 0.03; BO < healthy controls: Fisher's z = −3.1, P = 0.002), and not significantly correlated in NBO (r = 0.16). Right amygdala-left ventrolateral PFC connectivity to Happy faces-Shapes and response time to correctly identify a happy expression were significantly positively correlated in BO (r = 0.48, P = 0.009), negatively correlated in NBO (r = −0.44, P = 0.02) and marginally negatively correlated in healthy control subjects (r = −0.41, P = 0.052; BO > NBO: Fisher's z = 3.6, P = 0.0003; BO > healthy controls: Fisher's z = 3.2, P = 0.001).
The effect of pubertal status on main neuroimaging measures
A 3 (Groups) × 2 (pubertal status: earlier/later) ANOVA conducted on the three main neuroimaging measures identified in the above analyses testing main hypotheses revealed neither a significant main effect of pubertal status, nor an interaction between group and pubertal status, on these activation and connectivity measures.
Discussion
Our goal was to determine the extent to which alterations in neural functioning supporting processing of emotional faces characterized BO relative to NBO and healthy control subjects. Consistent with Hypotheses 1 and 2, we found abnormally elevated right amygdala activation and abnormally reduced positive right amygdala-ACC functional connectivity in BO relative to healthy control subjects, and abnormally reduced positive right amygdala-ACC functional connectivity in BO relative to NBO, during implicit processing of emerging emotional faces. Contrary to Hypothesis 2, we found abnormally increased right amygdala-left ventrolateral PFC functional connectivity for emerging happy, but not negative, emotional faces in BO compared with NBO and healthy control subjects. These patterns of activation and functional connectivity remained in participants free of current psychiatric diagnoses and untreated with medications, supporting Hypothesis 3.
Hypothesis 1 specifically predicted that BO, compared with NBO and healthy control subjects, would show significantly greater amygdala and reduced prefrontal cortical activation to all emotional faces (versus shapes). An alternative hypothesis was that both BO and NBO would show significantly greater amygdala activation than healthy control subjects. The amygdala is a key region for processing of emotional faces and emotions in general (Haxby et al., 2002; Gläscher et al., 2004; Sabatinelli et al., 2011). Our finding of abnormally elevated right amygdala activation to all emotional faces in BO and NBO taken together with previous findings of abnormally increased amygdala activation in individuals with established bipolar disorder (Lawrence et al., 2004; Blumberg et al., 2005; Pavuluri et al., 2007, 2009; Ladouceur et al., 2011; Hulvershorn et al., 2012; Brotman et al., 2014b), in individuals at-risk for bipolar disorder (Olsavsky et al., 2012), and also in those at-risk for major depressive disorder (Monk et al., 2008), suggest that elevated right amygdala activation may be a risk marker for psychiatric disorders in general, rather than for BO specifically. Interestingly, we did not show a pattern of abnormally reduced prefrontal cortical activation to emotional faces in BO relative to the other groups. This may reflect the fact that the three participant groups recruited prefrontal cortical regions to the same extent, but differed in the magnitude of functional connectivity between prefrontal cortical and amygdala regions, during task performance, as is highlighted below.
Hypothesis 2 specifically predicted that BO, compared with NBO and healthy control subjects, would show significantly reduced amygdala-prefrontal cortical positive functional connectivity to all emotional faces. The finding of abnormally reduced positive right amygdala-ACC functional connectivity in BO, relative to NBO and healthy control subjects, supported this hypothesis. Given the role of the ACC in implicit emotion regulation (Phillips et al., 2008; Etkin et al., 2011), our finding of abnormally reduced right amygdala-ACC functional connectivity to all emotional faces in BO supports theories proposing deficient regulation of the amygdala by prefrontal cortical regions during emotion regulation in individuals with, and at risk for, bipolar disorder (Phillips et al., 2008; Strakowski et al., 2012; Phillips and Swartz, 2014) and accords with previous findings in adults and youth with bipolar disorder (Almeida et al., 2009; Wang et al., 2009, 2012; Ladouceur et al., 2011).
While the finding of abnormally elevated right amygdala-left ventrolateral PFC functional connectivity in BO (BO > NBO, healthy controls) to happy faces did not support Hypothesis 2, and may contradict one previous finding of reduced right amygdala-right ventrolateral PFC functional connectivity in BO versus healthy control subjects in an emotional regulation paradigm (Ladouceur et al., 2013), other studies report similar findings to those of the present study. For example, individuals with bipolar disorder type 1 versus healthy control subjects showed reduced inverse left amygdala-bilateral ventrolateral PFC functional connectivity during emotional downregulation (Townsend et al., 2013), individuals with bipolar disorder type 2 versus healthy control subjects had higher positive right amygdala-left ventrolateral PFC functional connectivity during emotional face processing (Vizueta et al., 2012), and right amygdala-left ventrolateral PFC connectivity increased during mania in individuals with bipolar disorder type 1 (Cerullo et al., 2012). In addition, increased right amygdala-left ventrolateral PFC functional connectivity was reported in anxious versus healthy adolescents during social evaluation anticipation (Guyer et al., 2008).
In parallel, abnormally increased left ventrolateral PFC activation during outcome and reward expectancy has been highlighted in adults with bipolar disorder (Nusslock et al., 2012; Caseras et al., 2013; Chase et al., 2013; Phillips and Swartz, 2014). Given the role of the ventrolateral PFC in encoding the value of different decision-making options (Walton et al., 2011), linking specific stimulus representations to specific reward outcome representations (Noonan et al., 2012), and the potential role of the anterior left ventrolateral PFC in controlling access to stored conceptual representations (Badre and Wagner, 2007), these findings from our previous studies were interpreted as reflecting increased engagement with reward and positive emotions in particular in individuals with bipolar disorder (Nusslock et al., 2012; Caseras et al., 2013; Chase et al., 2013). Together, these previous and present findings thereby suggest that the pattern of abnormally increased functional connectivity between left ventrolateral PFC and amygdala in BO relative to NBO and healthy control subjects may reflect increased engagement with positive emotions in BO, and may be a risk marker of bipolar disorder.
Hypothesis 3 predicted that altered neural functioning within emotional face processing circuitry in BO versus NBO versus healthy control youths would be present in the subset of participants without current psychiatric diagnoses and psychotropic medications. Our data supported this hypothesis by showing the same activation and connectivity patterns in the subset of participants that were free of current psychopathology and did not take psychotropic medications.
The fact that BO significantly differed from NBO and from healthy control subjects in right amygdala-ACC and right amygdala-left ventrolateral PFC functional connectivity suggests that these connectivity measures may both represent a unique neural mechanism characterizing BO, compared with NBO and healthy controls. Taken together with previous findings that connectivity between these regions is abnormal in individuals with already established bipolar disorder (Cerullo et al., 2012; Vizueta et al., 2012; Townsend et al., 2013), these findings suggest that altered right amygdala-ACC and right amygdala-left ventrolateral PFC connectivity in BO may be a risk marker for future bipolar disorder, rather than a protective marker against the development of bipolar disorder, in these youth. Future longitudinal follow-up studies are needed to determine the extent to which this is the case, however.
There were interesting exploratory findings. Right amygdala-left ventrolateral PFC functional connectivity positively correlated with explicit Happy face labelling response time and negatively correlated with age in BO, but not in NBO and healthy control subjects, suggesting that abnormally increased right amygdala-left ventrolateral PFC functional connectivity may be related to greater engagement with, and greater difficulty in labelling, happy faces (slower response time) in BO. This difficulty may be greater for younger BO, for whom happy faces may be more ambiguous than for older BO. IQ negatively correlated with right amygdala-ACC functional connectivity in BO and NBO. This suggests that decreased positive right amygdala-ACC functional connectivity may be a compensatory mechanism in BO and NBO with higher IQ, to modulate abnormally increased right amygdala activation by increasing ACC activation.
In NBO, right amygdala-ACC functional connectivity also positively correlated with measures of anxiety and depression severity, suggesting that greater right amygdala-ACC functional connectivity in NBO may be associated with the emergence of affective pathology and anxiety, and that the changes in right amygdala-ACC functional connectivity may represent a state marker of anxiety and depression severity in NBO. In contrast, right amygdala-ACC connectivity in BO was not significantly correlated with current levels of anxiety and depression severity, suggesting that this connectivity pattern may be a trait marker of BO that exists independently of a current level of anxiety and depression severity. Clearly, these exploratory findings need to be replicated in future studies.
One limitation of this study was that we did not focus on examining between-group differences in neural activation and functional connectivity to the different negative emotional faces, given our main focus on group differences to positive versus all negative emotions. Future studies can examine this with larger sample sizes. Another possible limitation was using offspring of parents with psychopathology from two recruitment sources (BIOS and LAMS). Our exploratory analyses showed, however, that recruitment source did not impact main findings because excluding BO and NBO from LAMS did not alter the results. Future studies should also include youths with established bipolar disorder to directly compare neural measures of emotion processing in these youth with BO and NBO.
In summary, our findings in BO indicate three main themes of abnormal functioning in face emotion processing circuitry: abnormally increased right amygdala activation, abnormally reduced right amygdala-ACC functional connectivity during emotion processing in general, and abnormally increased right amygdala-left ventrolateral PFC positive functional connectivity during processing of positive emotional faces, that parallel the themes previously identified in individuals with bipolar disorder (Phillips and Swartz, 2014). Importantly, these patterns of activation and functional connectivity remained unchanged even after removing medicated participants and those with current psychopathology from the analyses. Given that both BO and NBO, with or without psychopathology, showed abnormally increased right amygdala activation, this pattern of abnormal activation may confer risk for affective disorders in general, rather than bipolar disorder specifically. By contrast, the fact that right amygdala-ACC and right amygdala-left ventrolateral PFC functional connectivity differentiated BO from healthy control subjects and NBO, and that both patterns of abnormal functional connectivity parallel findings in individuals with established bipolar disorder, as discussed above, suggests that these measures may be risk markers for the future development of bipolar disorder in youth. BIOS is an ongoing study, with all participants being invited for follow-up clinical assessments. Our future research will thus be able to focus on using the measures of abnormal right amygdala activation and right amygdala-ACC and right amygdala-left ventrolateral PFC functional connectivity to determine if these neuroimaging markers can help predict future mood and anxiety symptom severity, as well as changes in diagnosis (e.g. development of bipolar disorder) over time, in BO and NBO.
Supplementary Material
Acknowledgements
The authors thank the families for participating in this research study.
Glossary
Abbreviations
- ACC
anterior cingulate cortex
- BIOS
Bipolar Offspring Study
- BO
offspring of parents with bipolar disorder
- LAMS
Longitudinal Assessment of Manic Symptoms
- NBO
offspring of parents with non-bipolar disorder psychopathology
- PFC
prefrontal cortex
Funding
This work was supported by grants from the National Institute of Health2 R01 MH060952-12S1 to Birmaher, Axelson, Phillips (MPIs) and R01 MH073953 to Birmaher and Phillips (MPIs), and Pittsburgh Foundation to Phillips.
Supplementary material
Supplementary material is available at Brain online.
References
- Adleman NE, Kayser RR, Olsavsky AK, Bones BL, Muhrer EJ, Fromm SJ, et al. Abnormal fusiform activation during emotional-face encoding assessed with functional magnetic resonance imaging. Psychiatry Res 2013; 212: 161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 2009; 66: 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration aka spatial normalisation. Oxford: FMRIB Centre; Technical Report FMRIB Technical Report TR07JA2; 2007. [Google Scholar]
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA). Psychol Med 1995; 25: 739–53. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 2007; 45: 2883–901. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2007; 2: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry 2009; 66: 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 1997; 36: 545–53. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005; 183: 308–13. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Deveney CM, Thomas LA, Hinton KE, Yi JY, Pine DS, et al. Parametric modulation of neural activity during face emotion processing in unaffected youth at familial risk for bipolar disorder. Bipolar Disord 2014a; 16: 756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry 2008a; 165: 385–9. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry 2008b; 47: 1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Tseng WL, Olsavsky AK, Fromm SJ, Muhrer EJ, Rutenberg JG, et al. Fronto-limbic-striatal dysfunction in pediatric and adult patients with bipolar disorder: impact of face emotion and attentional demands. Psychol Med 2014b; 44: 1639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry 2013; 170: 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, et al. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord 2012; 14: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JR, Forbes EE, Labarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord 2013; 15: 839–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord 2007; 9: 679–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Stephan KE, Roiser JP, Friston KJ, Frangou S. Effective connectivity during processing of facial affect: evidence for multiple parallel pathways. J Neurosci 2011; 31: 14378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 2007; 17: 2400–6. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, et al. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry 2010; 71: 1664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I disorders, research version, non-patient edition. (SCID-I/NP) New York, NY: Biometrics research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6: 218–29. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34: 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res 1996; 65: 189–98. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tüscher O, Weiller C, Büchel C. Elevated responses to constant facial emotions in different faces in the human amygdala: an fMRI study of facial identity and expression. BMC Neurosci 2004; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Axelson DA, Kalas C, Monk K, Brent DA, et al. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2010; 49: 388–96. [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 2008; 65: 1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry 2007; 48: 863–71. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Bebko G, Bertocci MA, Fournier JC, Bonar L, Perlman SB, et al. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: Attenuated by medication. J Psychiatr Res 2014; 58C: 129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry 2002; 51: 59–67. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, et al. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. J Clin Psychiatry 2010; 71: 1511–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Karne H, Gunn AD, Hartwick SL, Wang Y, Hummer TA, et al. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry 2012; 71: 603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. Let's face it: it's a cortical network. Neuroimage 2008; 40: 415–19. [DOI] [PubMed] [Google Scholar]
- Ishai A, Haxby JV, Ungerleider LG. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage 2002; 17: 1729–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med 2003; 49: 193–7. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–56. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–8. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci 2013; 5: 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry 2011; 50: 1275–89.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55: 578–87. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci 2000; 20: 878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 2008; 165: 90–8. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383: 812–15. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MF. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur J Neurosci 2012; 35: 997–1010. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, Labarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord 2012; 14: 249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry 2012; 51: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 2007; 62: 158–67. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2009; 48: 308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Fournier JC, Bebko G, Bertocci MA, Hinze AK, Bonar L, et al. Emotional face processing in pediatric bipolar disorder: evidence for functional impairments in the fusiform gyrus. J Am Acad Child Adolesc Psychiatry 2013; 52: 1314–25.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 1988; 17: 117–33. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13: 833–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171: 829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature 1997; 389: 495–8. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 2006; 103: 8900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage 2011; 54: 2524–33. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 2012; 14: 313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168: 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 2013; 73: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WL, Bones BL, Kayser RR, Olsavsky AK, Fromm SJ, Pine DS, et al. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur Psychiatry 2014; 30: 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex 2012; 22: 2904–12. [DOI] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JRC, Hassel S, Klein CR, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 2010; 67: 422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, et al. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am J Psychiatry 2012; 169: 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 2001; 30: 829–41. [DOI] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Noonan MP, Rushworth MF. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci 2011; 1239: 14–24. [DOI] [PubMed] [Google Scholar]
- Wang F, Bobrow L, Liu J, Spencer L, Blumberg HP. Corticolimbic functional connectivity in adolescents with bipolar disorder. PLoS One 2012; 7: e50177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009; 66: 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry 2000; 57: 675e82. [DOI] [PubMed] [Google Scholar]
- Willis ML, Palermo R, McGrillen K, Miller L. The nature of facial expression recognition deficits following orbitofrontal cortex damage. Neuropsychology 2014; 28: 613–23. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, eds. Functional MRI: an introduction to methods, Chapter 14. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J Clin Psychiatry 2008; 69: 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.