In a large multicentre study, Toledo et al. examine core Alzheimer’s disease CSF biomarkers in 1233 cognitively normal subjects aged 40–85 years. Alzheimer disease-like changes in Aβ1-42 are seen as early as middle age, while APOE genotype strongly modifies age-related effects on both Aβ1–42 and phosphorylated/total tau.
Keywords: Alzheimer’s disease, dementia, biomarkers, cognitive ageing, imaging
In a large multicentre study, Toledo et al. examine core Alzheimer’s disease CSF biomarkers in 1233 cognitively normal subjects aged 40–85 years. Alzheimer disease-like changes in Aβ1-42 are seen as early as middle age, while APOE genotype strongly modifies age-related effects on both Aβ1–42 and phosphorylated/total tau.
Abstract
In a large multicentre sample of cognitively normal subjects, as a function of age, gender and APOE genotype, we studied the frequency of abnormal cerebrospinal fluid levels of Alzheimer’s disease biomarkers including: total tau, phosphorylated tau and amyloid-β1-42. Fifteen cohorts from 12 different centres with either enzyme-linked immunosorbent assays or Luminex® measurements were selected for this study. Each centre sent nine new cerebrospinal fluid aliquots that were used to measure total tau, phosphorylated tau and amyloid-β1-42 in the Gothenburg laboratory. Seven centres showed a high correlation with the new Gothenburg measurements; therefore, 10 cohorts from these centres are included in the analyses here (1233 healthy control subjects, 40–84 years old). Amyloid-β amyloid status (negative or positive) and neurodegeneration status (negative or positive) was established based on the pathological cerebrospinal fluid Alzheimer’s disease cut-off values for cerebrospinal fluid amyloid-β1-42 and total tau, respectively. While gender did not affect these biomarker values, APOE genotype modified the age-associated changes in cerebrospinal fluid biomarkers such that APOE ε4 carriers showed stronger age-related changes in cerebrospinal fluid phosphorylated tau, total tau and amyloid-β1-42 values and APOE ε2 carriers showed the opposite effect. At 40 years of age, 76% of the subjects were classified as amyloid negative, neurodegeneration negative and their frequency decreased to 32% at 85 years. The amyloid-positive neurodegeneration-negative group remained stable. The amyloid-negative neurodegeneration-positive group frequency increased slowly from 1% at 44 years to 16% at 85 years, but its frequency was not affected by APOE genotype. The amyloid-positive neurodegeneration-positive frequency increased from 1% at 53 years to 28% at 85 years. Abnormally low cerebrospinal fluid amyloid-β1-42 levels were already frequent in midlife and APOE genotype strongly affects the levels of cerebrospinal fluid amyloid-β1-42, phosphorylated tau and total tau across the lifespan without influencing the frequency of subjects with suspected non-amyloid pathology.
Introduction
Alzheimer’s disease is characterized by the deposition of intracellular tau proteins into neurofibrillary tangles and amyloid-β peptides into extracellular amyloid plaques. However, these pathologies also are present in cognitively normal subjects with advancing age (Hyman et al., 2012) and neurofibrillary tangles can appear even before the fourth decade of life (Braak and Del Tredici, 2011), although these early changes may be below the biomarker diagnostic threshold (Jack et al., 2013a). Tau and amyloid-β can be measured in the CSF. CSF tau levels correlate with the number of neurofibrillary tangles in the brain, whereas amyloid-β1-42 levels show an inverse correlation with brain amyloid plaques (Strozyk et al., 2003; Tapiola et al., 2009; Toledo et al., 2012), which makes them informative as Alzheimer’s disease biomarkers. Changes in CSF tau and amyloid-β biomarker levels appear between one and two decades before the expected time of onset of dementia in subjects who develop Alzheimer’s disease due to autosomal dominant mutations (Bateman et al., 2012; Reiman et al., 2012; Fagan et al., 2014). Similarly population-based studies have shown that low CSF amyloid-β1-42 levels in cognitively normal elderly subjects predict future Alzheimer’s disease dementia up to 8 years in advance (Skoog et al., 2003; Gustafson et al., 2007), while approximately one-third of elderly cognitively normal subjects have an Alzheimer’s disease-like profile of tau and amyloid-β CSF biomarker levels (Shaw et al., 2009; De Meyer et al., 2010) and similarly pathological amyloid burden as measured by PET has been found in cognitively normal subjects (Aizenstein et al., 2008). Taken together with data on Alzheimer’s disease imaging biomarkers, these findings have led to a model that predicts successive appearance of abnormal biomarker values before the onset of cognitive changes, which leads at a later stage to dementia and impairments in activities of daily living (Jack et al., 2013a). Recently, a study that used Pittsburgh compound B (PIB) PET as biomarker for amyloid-β load as well as fluorodeoxyglucose (FDG) PET and hippocampal MRI volume as biomarkers for neurodegeneration described how changes started at the end of the sixth decade and differed based on gender and APOE genotype in a population-based sample of ageing (Jack et al., 2014). In the current study, amyloid-β status [negative (A−) or positive (A+)] and neurodegeneration status [negative (N−) or positive (N+)] were established based on pathological CSF Alzheimer’s disease cut-off values for CSF amyloid-β1-42 and total tau, respectively, and the goal of this study was to describe the association of these CSF biomarkers with ageing, gender and APOE genotype in a large multicentre cohort of healthy controls.
Materials and methods
Cohorts
All of the subjects included in the current study were healthy controls although some of the subjects presented with a diagnosis of subjective cognitive decline. The subjective cognitive decline group included subjects who indicated that they presented cognitive decline, but did not show any impairment the applied neuropsychological battery, i.e. did not test below a score of 1.5 standard deviations or more below the mean of healthy controls. Subjects belonged to the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (Weiner et al., 2013), the Parkinson Progression Marker Initiative (PPMI) (Kang et al., 2013a), the University of Pennsylvania Penn Memory Center/Alzheimer disease Center Core (Toledo et al., 2014a), Amsterdam Dementia Cohort (van Harten et al., 2013; van der Flier et al., 2014), NYU Center for Brain Health, CITA Alzheimer, IRCCS Centro San Giovanni di Dio, Brescia, Italy (Paternico et al., 2012), Lund University (Stomrud et al., 2007), University Hospital of Alicante (Berenguer et al., 2014), IDIBAPS-Hospital Clinic de Barcelona, DZNE Rostock (Teipel et al., 2014), Emory University and BIOCARD (Moghekar et al., 2013). ADNI and PPMI measurements were performed at the University of Pennsylvania and the NYU Center for Brain Health samples were measured in the Clinical Neurochemistry Laboratory at Gothenburg University (Supplementary material).
CSF measurements were performed in the different cohorts either by a single analyte enzyme-linked immunosorbent assay (ELISA; INNOTEST® for Research Use Only reagents; Fujirebio Europe) or the multiplex Luminex® assay format (INNO-BIA AlzBio3 for Research Use Only reagents; Fujirebio Europe). The monoclonal antibodies that were used in the assays for capture and reporting for detection of amyloid-β1-42, total tau and phosphorylated tau are described in Supplementary Table 1 and have been previously described in more detail (Vanderstichele et al., 2008; Kang et al., 2013b). Supplementary Table 2 summarizes the CSF collection and storage procedures in the different centres. Each centre sent nine aliquots to the Gothenburg University laboratory; three aliquots were selected to represent the CSF amyloid-β1-42 range of values, three aliquots were selected to represent the CSF total tau range of values and the last three aliquots were selected to represent the CSF phosphorylated tau range of values. Each of the aliquots represented the first, second and third tertile of the biomarker values. The ELISA method to measure CSF tau and amyloid-β1-42 levels in all the nine aliquots sent by each centre for this study was performed as described previously (Palmqvist et al., 2014). In addition, the Luminex® method was also used to measure the CSF samples if enough CSF volume was left after the ELISA measurements.
Statistics
Comparisons of quantitative and qualitative variables between the different cohorts were performed using an ANOVA and Fisher’s exact test, respectively. Correlations between the original CSF tau and amyloid-β1-42 values that were obtained in each of the centres and the reference values generated by the Gothenburg laboratory were tested using Spearman rank correlation. Centres whose data showed a correlation coefficient >0.7 when compared to the ELISA values obtained by the Gothenburg University laboratory were included in the analyses. To transform values from each centre into a common scale a robust linear regression was applied, using the values of each of the shipping centres as a predictor and the values obtained by the Gothenburg laboratory as an outcome. Supplementary Tables 3 and 4 summarize Spearman rank correlation rho values and the results of the robust regression including the intercept and slope that were used to transform the data from each centre.
In all of these analyses, APOE genotypes were grouped into three categories: (i) ε2 carriers (ε2/ε2 and ε2/ε3); (ii) ε3/ε3 genotype; and (iii) ε4 carriers (ε3/ε4 and ε4/ε4). ε2/ε4 subjects were not included due to small sample size. To test which variables were associated with the CSF biomarkers studied here, we tested linear models that included APOE genotype, gender and age and squared age as predictors. Power transformations were applied as necessary to achieve a normal distribution of the data. A backward stepwise procedure was applied to select the predictors. In all models, squared age and gender were excluded as predictors. We then modelled the biomarker changes across the different ages of the subjects included here by applying multivariate adaptive regression splines (MARS) to the data, analysing each of the APOE genotype groups separately to better capture biomarker dynamics as a function of age across the lifespan. A multinomial regression model that included age, gender and APOE groups (see above), was used to estimate the frequencies associated with each of the groups of CSF tau and amyloid-β results for the range of ages of these subjects from 45 to 85 years old, including three cubic restricted splines at 55, 65 and 75 years to allow age-dependent trends. Mean values and 95% confidence intervals (CI) were estimated applying a parametric bootstrap using 1000 multivariable normal deviates as previously described (Jack et al., 2014). This method was also applied to estimate frequency differences between groups and the corresponding 95% CIs. Differences were deemed significant if 0 was not included in the CI. Analyses were performed using R version 3.0.3 (R Foundation for Statistical Computing).
Results
Cohorts
The study includes data from 15 different cohorts whose samples were measured in 10 different centres, each one composed of nine to 270 subjects (Table 1). Cohorts differed in gender (P < 0.0001) and age (P < 0.0001) of the subjects, but not with respect to the presence of their APOE ε4 alleles (P = 0.15).
Table 1.
Gender, age and APOE ε4 status of the cognitively normal subjects in the different cohorts included in this study
| Cohort A | Cohort B | Cohort C | Cohort D | Cohort E | Cohort F | Cohort G | Cohort H | Cohort I | Cohort J | Cohort K | Cohort L | Cohort M | Cohort N | Cohort O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender (% male) | 55.6 | - | 46.8 | 32.8 | 50.0 | 73.6 | 41.2 | 44.0 | 28.6 | 44.4 | 47.5 | 57.0 | 34.9 | 34.0 | 65.5 |
| Age | 57 (56–71) | 69 (57–71.2) | 74 (68.5–79) | 70 (65–77) | 73.8 (70.8–78.2) | 53 (41.8–62) | 68 (63–72) | 57.3 (53–63.2) | 69 (68–69.5) | 74 (60–75) | 54 (43–76) | 60.4 (53.5–66.7) | 62 (56.1–69.2) | 73 (68–79) | 61 (55–68.3) |
| SMD (%) | 0 | 0 | 0 | 0 | 0 | 0 | 58.7 | 33.6 | 0 | 0 | 32.5 | 94.5 | 0 | 0 | 0 |
| APOE (%) | |||||||||||||||
| ε3/ε4 ε4/ε4 | 33.3 | 55.6 | 33.3 | 28.6 | 25.5 | 16.9 | 23.0 | 22.1 | 0 | 33.3 | 30.7 | 29.3 | 30.1 | 32.1 | 24.1 |
| ε3/ε3 | 55.6 | 33.3 | 61.6 | 60.7 | 59.9 | 74.6 | 67.2 | 68.6 | 71.4 | 55.6 | 59.0 | 55.8 | 55.4 | 66.0 | 64.2 |
| ε2/ε3 ε2/ε2 | 11.1 | 11.1 | 5.1 | 10.7 | 14.6 | 8.5 | 9.8 | 9.3 | 28.6 | 11.1 | 10.3 | 14.9 | 14.5 | 18.9 | 11.7 |
SMD = Subjective memory decliners. Age is represented by the median (25th–75th percentile).
Comparison of CSF tau and amyloid-β values to data generated by the Gothenburg laboratory
CSF total tau, phosphorylated tau and amyloid-β1-42 measurements for the different cohorts were performed in 12 centres, one of them being the University of Gothenburg laboratory that also generated reference values to perform the transformations in this study. Ten of the centres that had performed the measurements sent nine CSF aliquots of participants included in this study to the University of Gothenburg to be able to transform values across the different cohorts. Two laboratories did not include aliquots for this analysis: the first laboratory had performed a previous adjustment run in a larger sample and the second one was the Gothenburg laboratory that measured total tau, phosphorylated tau and amyloid-β1-42 in all these CSF aliquots. In most cases, there was enough CSF available to perform ELISA and Luminex® measurements for each of the aliquots. Supplementary Fig. 1 presents the values for each of the three analytes measured in the reference laboratory using both platforms on the same samples. Amyloid-β1-42 and total tau values were highly correlated across platforms (r = 0.91 and r = 0.98, respectively), whereas phosphorylated tau values showed a lower correlation (r = 0.66).
Notably, when the values obtained at the Gothenburg laboratory were compared with the original values obtained in the different centres that shipped the samples, we observed that correlations varied across centres (Supplementary Tables 3, 4 and Supplementary Figs 2 and 3). For the following analyses, we selected centres that showed a spearman rank correlation ≥ 0.70, which correspond to Cohorts C–H and L–O, which included 1233 subjects and transformed CSF amyloid-β1-42, total tau and phosphorylated tau values according to the results of the robust regression (Supplementary Tables 3 and 4). Subjects aged 40 to 84 were included in the following analyses to avoid extreme age ranges with small number of subjects.
Association of amyloid-β1-42 and tau with age and APOE groups
Age and APOE genotype, but not gender, were associated with CSF biomarker values (Table 2). When we compared CSF values in young (age 50–64 years) and old participants (age 65–80 years) in an analysis adjusted for APOE, total tau (P < 0.0001) and phosphorylated tau (P < 0.0001) were increased in the group composed of older subject, whereas there were no differences in amyloid-β1-42 values (P = 0.07) between both age-defined groups.
Table 2.
Association between CSF biomarkers and APOE genotypes
| Age |
APOE ε2/ε3 & ε2/ε2 |
APOE ε3/ε4 & ε4/ε4 |
Gender (male) |
|||||
|---|---|---|---|---|---|---|---|---|
| Coef. | P-value | Coef. | P -value | Coef. | P -value | Coef. | P -value | |
| Amyloid-β1-42 | −0.12 | <0.0001 | 0.23 | 0.009 | −0.40 | <0.0001 | 0.018 | 0.75 |
| Total tau | 0.45 | <0.0001 | 0.012 | 0.88 | 0.21 | 0.0007 | −0.03 | 0.59 |
| Phosphorylated tau | 0.40 | <0.0001 | 0.080 | 0.37 | 0.25 | 0.0001 | −0.03 | 0.62 |
Only the results for the best model are shown here.
Coef. = standardized coefficient of the linear regression. Models are adjusted for age and gender.
We then analysed the changes in the CSF biomarker values across different ages stratified by APOE genotype (Fig. 1). We included gender, in addition to age, in all the MARS models, but gender was not selected as a predictor in any of the models.
Figure 1.

CSF amyloid-β1-42, total tau and phosphorylated tau181 levels in association with ageing in healthy controls stratified by APOE genotype. Dashed lines represent the cut-off points for the biomarkers.
Subjects with APOE ε4 carriers showed higher CSF tau and lower amyloid-β values than APOE ε3/ε3 subjects. The largest effect was observed for amyloid-β1-42 values; whereas amyloid-β1-42 values remained stable up to the beginning of the seventh decade in the healthy controls without any ε4 alleles, amyloid-β1-42 levels of healthy controls with one or two ε4 alleles showed a decrease starting during the fifth decade of life until a plateau was reached at the middle of the eighth decade. APOE ε2 carriers showed a similar pattern of amyloid-β1-42 changes levels as APOE ε3/ε3 subjects, although APOE ε2 carriers presented overall higher values. On the other hand, total tau and phosphorylated tau levels remained stable until the beginning of the seventh decade in subjects with APOE ε3/ε3 and ε4 carriers and it was in this age range that these groups differed in the rate of increase in their values. Total tau and phosphorylated tau value changes were similar in APOE ε2 carriers as subjects with APOE ε3/ε3 genotype.
To study possible differences between the cognitively normal and subjective memory decline subjects, there were three cohorts that included both groups of participants (Cohorts G, H and L); however, Cohort L was excluded because it mainly consisted of subjective memory decline subjects. Analysis was limited to the APOE ε3/ε3 genotype due to sample size (84 cognitively normal and 52 subjective memory decline participants). There were no differences between the two groups (Supplementary Table 5).
When we transformed the Luminex® CSF amyloid-β1-42 cut-off defined by Shaw et al. (2009) into ELISA reference values using the transformation formula obtained from the robust regression applied to the University of Pennsylvania values, we obtained a value of 543.5 pg/ml, which is close to the one applied in the Gothenburg laboratory (550 pg/ml) determined following International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) guidelines (IFCC, 1987). Conversely, the transformed total tau cut-off value was higher than the one described by the Gothenburg laboratory, namely 616 pg/ml compared with 400 pg/ml. In our study we selected the mean value of the cut-offs from the two aforementioned cohorts to define pathological amyloid-β1-42 (546.7 pg/ml) and total tau (508 pg/ml) levels.
Amyloid and neurodegeneration positive groups based on CSF amyloid-β1-42 and total tau values
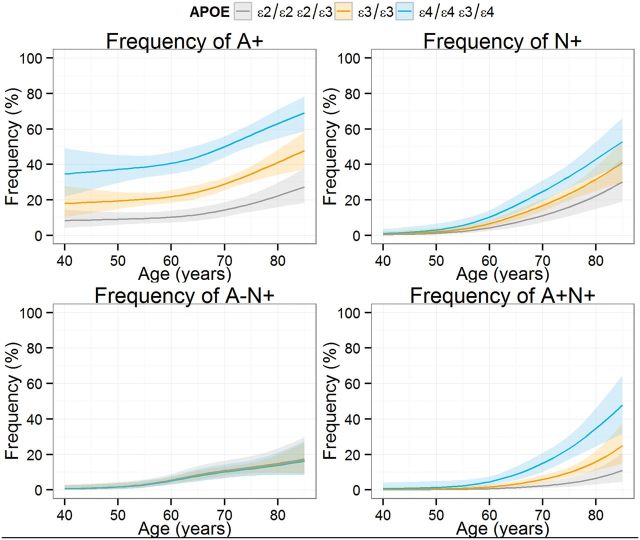
For these analyses, amyloid status [negative (A−) or positive (A+)] and neurodegeneration status [negative (N−) or positive (N+)] was established based on CSF Alzheimer’s disease cut-off values for CSF amyloid-β1-42 and total tau, respectively. In all groups, the frequency of subjects without abnormal biomarkers was lower in older subjects, whereas the frequency in the A+N− group showed only slightly higher frequency. Both the frequency of A−N+ and A+N+ subjects was higher in older subjects, but the former reached a plateau whereas the latter showed a stable increase (Fig. 2). At 45 years of age, 76% were classified as A−N− whereas their frequency was only 32% at 85 years; and A+N− frequency showed small differences during the same period (22% versus 24%). The A−N+ and A+N+ groups showed larger age-related differences: 1% at 45 years versus 16% at 85 years and 1% at 54 years versus 28% at 85 years, respectively. Male and female subjects showed similar frequencies for the different groups. On the other hand APOE genotype strongly influenced the frequency of the different groups. In the youngest participants included, ε4 carriers presented a higher frequency in the A+ group than the ε3/ε3 carriers (absolute 17% difference) and the ε2 carrier (absolute 26% difference) groups that were larger in the eldest subjects (absolute 21.2% for the ε3/ε3 participants and 41.6% for the ε2 carriers). On the other hand, there were no differences in the frequency of N+ subjects in the different groups defined by APOE genotype; and even when the frequency difference became larger in the older participants, there was a significant overlap, which was a result of the complete overlap in the A−N+ group and the larger differences observed in the eldest participants in the A+N+ group (Fig. 3). A more detailed analysis of the effect of APOE genotypes on the frequency of each of the four groups is presented in Fig. 4, where the frequency of each group is compared based on the APOE genotype, and the APOE ε3/ε3 genotype is selected as the reference and compared to the ε2 and ε4 carriers. Therefore values above zero represent a higher frequency in the carrier groups (either APOE ε2 or ε4) compared to the APOE ε3/ε3 group and values below zero represent the opposite finding. In the A−N− groups, the frequency difference between APOE ε3/ε3 subjects and APOE ε4 carriers remained largely similar indicating that differences between groups appeared mainly at earlier ages. On the other hand, older APOE ε2 carriers showed a larger difference compared to the older APOE ε3/ε3 subjects, indicating that the protective effect of these alleles acted throughout the age span studied here. Older APOE ε2 carriers showed a larger difference in the A+N− group frequency compared to APOE ε3/ε3 subjects, whereas APOE ε4 carriers showed similar differences independently of age. However, APOE ε4 carriers showed a smaller A+N− frequency difference compared to APOE ε3/ε3 subjects with increasing age. This decrease in the A+N− frequency difference was accompanied by a larger A+N+ frequency difference in APOE ε4 carriers. Conversely, APOE ε2 carriers showed a lower frequency of A+N+ that showed a larger difference in older ages when compared to APOE ε3/ε3 subjects. Finally, the different APOE genotype groups showed no difference and overlapped with each other for the A−N+ category, indicating that only age was associated with changes in this group. Although female subjects showed increased frequency of A+N− subjects and decreased frequency of A−N− across the studied ages, differences were small and included the zero value, therefore lacking statistical significance.
Figure 2.
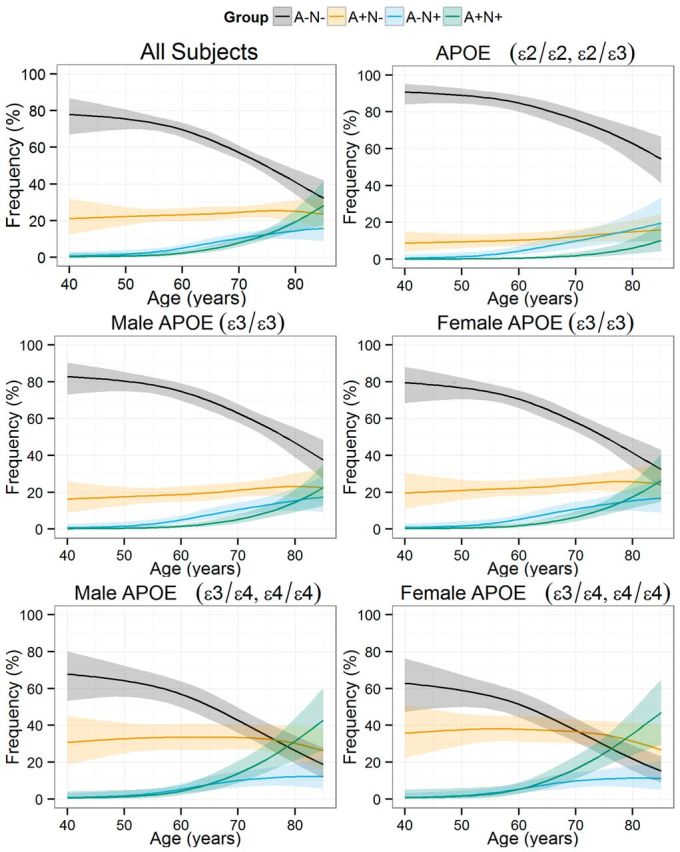
Estimated frequency of pathological amyloid-β (A) and neurodegeneration (N) categories according to age of the subjects. Plots represent all subjects and subjects stratified by gender and APOE genotype. Due to smaller sample size subjects with ε2 alleles were not stratified by gender. Shaded areas represent 95% CI.
Figure 3.
Frequency of A+, N+, A+N− and A+N+ stratified by APOE-defined groups.
Figure 4.
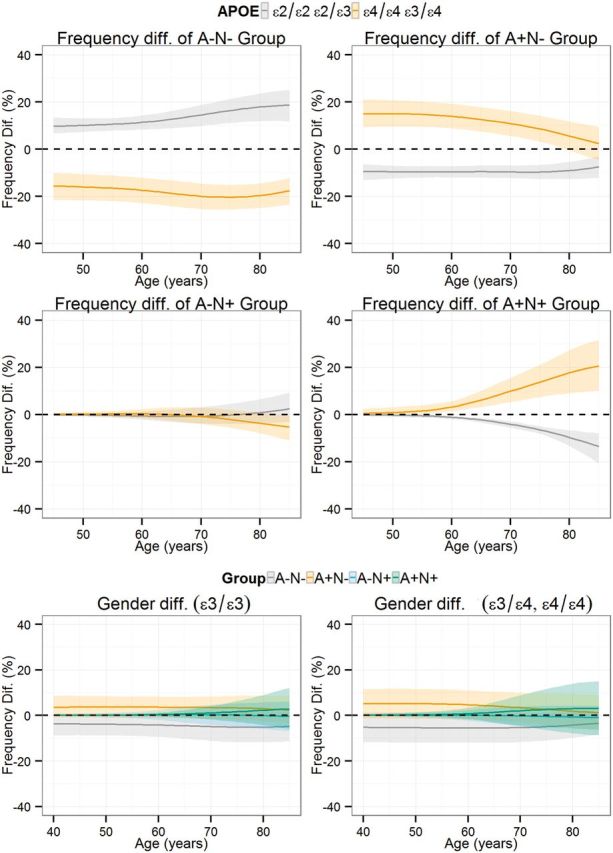
Differences in the frequency of the four biomarker groups in subjects with APOE ε3/ ε3 genotype compared subjects who are ε2 or ε4 allele carriers. The lines above the black dashed line indicate that the plotted group has a higher frequency of the studied biomarker category. For the gender plots values above the 0 represent a higher frequency for females, whereas values below 0 represent a higher frequency in males. Shaded areas represent 95% CI.
Discussion
In this large cohort of healthy control subjects covering a wide age range over the life span we found that already starting in the fifth decade of life there is a significant number of healthy control subjects who show evidence of abnormal CSF amyloid-β1-42 values, and that APOE genotypes significantly modified CSF amyloid-β1-42 values with the ε4 allele strongly associated with the lower of amyloid-β1-42 values at younger ages and the ε2 allele associated with overall lower values at older ages. The APOE ε4 allele also associated with the age at which CSF amyloid-β1-42 began declining (A+N− group) and additionally, in subjects with abnormal CSF amyloid-β1-42, associated with the age at which total tau started changing (A+N+). Conversely, we did not observe any APOE genotype effects on total tau levels in subjects without pathological amyloid-β1-42 values (A−N+ group).
The availability of longitudinal studies and their combination with Alzheimer’s disease biomarkers findings has led to a deeper understanding of the long preclinical stages of Alzheimer’s disease (Jack et al., 2013a) and this is corroborated by the finding of Alzheimer’s disease pathology in autopsies of elderly cognitively normal subjects (Montine et al., 2012). Recently, results from studies that included cognitively normal subjects with Alzheimer’s disease, autosomal dominant mutations and a well characterized expected age of onset of dementia have shown that several Alzheimer’s disease biomarkers show changes already one to two decades before the onset of cognitive decline (Bateman et al., 2012; Reiman et al., 2012; Fagan et al., 2014). Models based on longitudinal CSF and PET amyloid measures have shown that changes in these Alzheimer’s disease biomarkers take place more than one decade before clinical disease onset (Skoog et al., 2003; Gustafson et al., 2007; Jack et al., 2013b; Toledo et al., 2013c; Villemagne et al., 2013). However, the modelling of these changes also has included stable cognitively normal subjects therefore altering the timeframes of these changes as well as probably underestimating the real rate of biomarker changes (Toledo et al., 2013c).
In our study we found that already by the fifth decade of life >20% of subjects show abnormal CSF amyloid-β1-42 values and that the frequency of A+N− subjects remained relatively stable across the different ages, whereas the A−N+ and A+N+ categories increased their frequencies starting early in the sixth decade. However, these two categories differed at the end of the eighth decade, with A−N+ group reaching a plateau and the A+N+ group still showing an exponential increase. We also observed that while the difference in tau biomarker values in middle aged and elderly healthy controls was significant, this was not the case for amyloid-β1-42.
The stable frequency of the A+N− can be explained by the fact that this is a transitory category of subjects who were A−N− and later progress to A+N+ and later on to mild cognitive impairment and Alzheimer’s disease. This would indicate that there is equilibrium in the rate of subjects entering and leaving this category. Another factor is the increasing frequency of this category in the ε3/ε3 subjects that is accompanied by a decrease in the subjects with ε4 alleles. Nevertheless the overall frequency of A+ participants (independently of neurodegeneration status) was higher with increasing age. The increase in A−N+ frequency antecedes overall the A+N+ frequency increase, but reaches an early plateau. The underlying pathologies and longitudinal prognosis of the A−N+ is still largely unknown, but vascular pathology, frontotemporal lobar degeneration or primary age-related tauopathy (Crary et al., 2014; Jellinger et al., 2015). It has been proposed that it can represent non-Alzheimer’s disease pathologies and also precede the A+N+ category (Jack et al., 2014). The fact that besides Alzheimer’s disease, pathologies associated with increased CSF total tau values are mainly the less frequent acute head trauma and stroke, and prion diseases, would indicate that the latter hypothesis is more plausible. Both of these hypotheses explain a plateau of the frequency with ageing either due to a transition to A+N+ with an exhausted pool of A−N− subjects in aged individuals or due to an earlier age of onset and later decrease of incidence in non-Alzheimer’s disease pathologies. A third explanation is the high prevalence of coincident neurodegenerative and non-neurodegenerative diseases that cause dementia in elderly individuals (Kovacs et al., 2013; Toledo et al., 2013a; Rahimi and Kovacs, 2014; Jellinger and Attems, 2015) that cannot be accurately predicted by the current biomarkers (Toledo et al., 2012, 2013b) and therefore it can be expected that these subjects are classified in the A+N+ group. It is interesting that the exponential increase in the frequency of healthy controls in the A+N+ category mirrors the exponential prevalence observed for Alzheimer’s disease, only differing by an earlier onset in the middle of the sixth decade instead of in the middle of the seventh decade.
APOE genotype showed an important but differential effect on the frequency of the different groups across ages. APOE ε4 carriers showed relatively stable difference in A−N− frequency across ages when compared to APOE ε3/ε3 subjects, ∼18% lower, but APOE ε2 carriers showed an increasingly larger percentage of subjects in the A−N− category compared to APOE ε3/ε3 subjects with ageing (the frequency went from 10% higher to 19% higher than ε3/ε3 subjects; Fig. 4). Nevertheless, for the oldest subjects, the difference in A+N− frequency between APOE ε3/ε3 subjects and APOE ε4 carriers was smaller due to a slightly higher percentage of A+N− in APOE ε3/ε3 subjects and a smaller percentage of A+N− in APOE ε4 carriers (Fig. 2). This most likely is linked to the fact that APOE ε4 carriers start to progress to A+N− and A+N+ at a younger age followed by progression to mild cognitive impairment and Alzheimer’s disease which leads to a depletion of the A−N− category and acts as a survival bias.
Interestingly, the strongest effect of the APOE genotype was observed for the A+N+ group. Whereas in the A−N− and A+N− only one of the APOE-defined groups showed changes in differences compared to the ε3/ε3 group (and the other showed stable differences parallel to the x-axis) and no differences were found in the A−N+ group, in the A+N+ group APOE ε2 and ε4 carriers showed opposite changes when compared to subjects with ε3/ε3 genotype. With ageing there was a higher frequency of the A+N+ group in APOE ε4 carriers compared to APOE ε3/ε3 subjects whereas there was a decreasing frequency of A+N+ subjects in APOE ε2 carriers. This indicates that APOE genotype is a strong modifier for the transition from A+N− to A+N+ and of total tau changes in subjects with pathological amyloid-β1-42 levels.
It is also noteworthy that APOE genotype status did not affect the frequency of the A−N+ group, which emphasizes that these subjects, who would fit the suspected non-amyloid pathology category (SNAP) (Jack et al., 2012), represent mostly subjects who do not have underlying Alzheimer’s disease pathology. This result is important for modelling total tau changes because APOE genotype might differentially affect CSF total tau values depending upon the presence or absence of pathological Alzheimer’s disease-like CSF amyloid-β1-42 levels. Nevertheless, it has been described that this category might later transition to A+N+ (Jack et al., 2013c) as discussed above. Our results would indicate that the presence of significant amyloid pathology, estimated in our study by CSF amyloid-β1-42 values below the cut-off point, should be present to present a significant APOE genotype-related increase of tau pathology as measured by CSF tau levels. This finding agrees with a previous neuropathological study that estimated that the increase in tau pathology associated to the presence of APOE ε4 alleles was mainly indirectly mediated through an increase in amyloid pathology (Mungas et al., 2014), although a lesser direct effect was also present. Nonetheless, in this study we are classifying subjects as having normal and abnormal values and a detailed analysis with CSF or tau PET measurements would be needed to evaluate the presence of a direct effect on tau pathology as described in previous cell and animal models (Huang et al., 2001; Harris et al., 2003). However, it must be taken into account that significant increases of CSF total tau and phosphorylated tau values are only seen in two neurodegenerative disease, namely Alzheimer’s disease and prion diseases, and therefore CSF tau values are not representative of tau burden present in frontotemporal lobar degeneration due to tau pathology, which we cannot estimate with the current biomarkers (Toledo et al., 2012).
One previous study performed a similar analysis to the one we present here, but this study was carried out in a population-based cohort (Jack et al., 2014) and presented additional differences. First, in the Jack et al. (2014) study, younger subjects were almost entirely classified as A−N− and there was an increase in the frequency of A+N− subjects that reached a plateau followed by a decrease in aged subjects. This difference between our study and the Jack et al. (2014) study could be due to differences in CSF and PET amyloid measures. Recently it was shown that CSF and amyloid PET measures are associated for a limited mid-range values that includes the cut-offs that are used for diagnostic purposes and that the association between both measures is modified by the APOE genotype (Toledo et al., 2015). Therefore the cut-offs for abnormal amyloid-β values offer consistent results across platforms (CSF immunoassays and PET scans) and methodologies (different PET scan processing pipelines) to establish the cut-offs (Toledo et al., 2015). The difference between these two measures of amyloid-β pathology might explain why, despite significant agreement between both measures (Landau et al., 2013; Toledo et al., 2015), there is a significant number of subjects who are classified discordantly for each biomarker measure with most discordant subjects being classified as having abnormal CSF amyloid-β1-42 levels while having normal amyloid-β amyloid PET scans. The disagreement decreases as subjects become more cognitively impaired (Mattsson et al., 2015) and this could indicate that CSF biomarker changes precede amyloid PET changes at least in a subset of subjects. One potential limitation of the study is the lack of amyloid-β1-40 measurements to calculate the CSF amyloid-β1-42/amyloid-β1-40 ratio, which could classify some participants as A− even if their CSF amyloid-β1-42 values are below the cut-off, due to the constitutively low values for the amyloid-β peptides. However, it has been described that the value of the amyloid-β1-42/amyloid-β1-40 ratio might be related to the immunoassay method (Hertze et al., 2010) and the assay we used in this study did not seem to be affected. In addition, the diagnostic performance of the amyloid-β42/tau ratio was not improved when the amyloid-β1-42/amyloid-β1-40 ratio was used instead of amyloid-β1-42 values (Spies et al., 2010). Therefore we favour the hypothesis that CSF amyloid biomarker changes precede PET amyloid biomarker changes. Longitudinal follow-up of these subjects will be needed to ascertain the implication of low CSF amyloid-β1-42 values in middle-aged healthy controls. On the other hand there is little agreement between the different neurodegeneration biomarkers (as opposed to amyloid biomarkers) (Toledo et al., 2014b). However, the overall frequency observed in the eldest subjects was similar in the Mayo clinic and our sample offering converging results on the prevalence of biomarker-based preclinical Alzheimer’s disease stages.
We found a non-significant higher percentage of A+N− participants and lower percentage of A−N− participants in females compared to males. This is consistent with previous results that also reported higher but not significant amyloid PET values in females (Jack et al., 2015) and the previously discussed study from the same group that reported higher frequency of A+N− participants in females compared to males, although the latter study did not indicate if differences were significant and did not perform a formal comparison (Jack et al., 2014).
Previously, the association between age, gender and CSF Alzheimer’s disease biomarkers has been studied in smaller studies using different analytical approaches. For example, Sjögren et al. (2001) described a positive correlation between age and CSF total tau levels without any association with CSF amyloid-β1-42 levels in a sample of 231 subjects, and suggested age-adjusted cut-offs for total tau levels. This most likely represents an increased frequency of preclinical Alzheimer’s disease associated with ageing and therefore we consider that cut-offs should not be adjusted based on age. In another study with 81 subjects, Paternico et al. (2012) described the association with age and CSF total tau, but they found no interaction with APOE and no association with age for CSF amyloid-β1-42. On the other hand, Peskind et al. (2006) found an association between CSF amyloid-β1-42 levels and age and that this association was modified by APOE genotype, with APOE ε4 cognitively normal carriers showing an earlier change and lower amyloid-β1-42 levels in elder subjects, but the latter study did not include CSF tau measurements. In an ageing study by Glodzik-Sobanska et al. (2009) an association between APOE genotype and CSF total tau and phosphorylated tau values but not with amyloid-β1-42/ amyloid-β1-40 was described (Glodzik-Sobanska et al., 2009). The association between the APOE ε4 allele and low CSF amyloid-β1-42 levels has recently been shown to depend on APOE ε4 carriers having also increased cortical amyloid deposition as evaluated by PET scanning, indicating a higher number of preclinical Alzheimer’s disease cases in APOE ε4 carriers (Lautner et al., 2014). In our study, we found an association between all three studied CSF biomarkers and age and APOE genotype as described above. The association of APOE genotype with all three CSF biomarkers can be explained by the large number of samples we studied across a large age span which allowed us to have a representative number of subjects in each of the APOE groups. In addition, most of the studies apply linear analyses, which do not follow the biomarker dynamics that have been described in elderly individuals with longitudinal biomarker studies (Jack et al., 2013b; Toledo et al., 2013c; Villemagne et al., 2013) and we confirmed in the large analyses performed herein in a cross-sectional population encompassing a wider age range. It will be important to study longitudinal clinical changes in middle-aged individuals to confirm previous findings between baseline CSF amyloid-β1-42 values and memory decline (Li et al., 2014).
Our study has four main limitations: samples were not drawn from population based samples, measurements were performed in different laboratories using two different assays, CSF amyloid-β1-40 levels were not available and clinical and biomarker longitudinal data were not available. Thus, recruitment of cognitively normal subjects in specialized centres might lead to biased recruitment and not represent the general population. Notably, however, this bias can go in either direction as these subjects might have personal and familial reasons to be included in Alzheimer’s disease biomarker studies, but also the inclusion criteria might be stricter and therefore include healthier subjects like the ones included in clinical trials. In addition, these healthy controls tend to have a higher education level than the general population. Although two different platforms were used for the measurements of CSF amyloid-β1-42 and total tau, the values obtained were highly correlated between both assays, as previously described (Fagan et al., 2011; Irwin et al., 2012; Wang et al., 2012; Le Bastard et al., 2013). Another important observation was the fact that there were inter-laboratory differences. To control for this we measured nine aliquots from each centre in the Gothenburg laboratory and selected those subjects whose CSF tau and amyloid-β values were highly correlated for further study here and could therefore be transformed. This emphasizes the well-established fact that each laboratory must validate its own CSF tau and amyloid-β cut-offs and cannot adopt the ones described in other laboratories even using the same assay. A better solution is the availability of a common standard with associated cut-off values in all biomarker laboratories. Finally, the CSF amyloid-β1-42/amyloid-β1-40 ratio has been suggested as a method to account for subjects who constitutively have low values for the amyloid-β peptides in the CSF and therefore some of our cases might be false positives.
Our results indicate that Alzheimer’s disease-like CSF amyloid-β1-42 positivity appears already in the fifth decade of life in healthy controls, which has important implications for clinical trials targeting prevention or elimination of amyloid-β deposits, but also indicates that there is a significant interval between the time A−N− subjects progress to the A+N+ category, which represents an important therapeutic window for disease modifying therapies. This is because only the A+N+ category mimics the Alzheimer’s disease CSF biomarker profile and total tau reflects brain neurofibrillary tangle burden which is closely associated with neurodegeneration, and shows a stronger correlation with cognitive symptoms than amyloid-β amyloid deposition (Toledo et al., 2013a) thereby suggesting that there is time window that might span almost 10 years for intervening with Alzheimer’s disease prevention strategies. Finally APOE genotype strongly modifies the observed CSF biomarker profile and classification into preclinical stages with ε2 alleles showing a lifetime protective effect.
Supplementary Material
Acknowledgements
We want to thank Dr Steven Weigand for his helpful input on the analytical analysis.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Glossary
Abbreviations
- ADNI
Alzheimer’s disease Neuroimaging Initiative
- ELISA
enzyme-linked immunosorbent assay
Funding
J.B.T. is supported by P01 AG032953, PO1 AG017586, P30 AG010124 and P50 NS053488. J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology. H.Z.: The Swedish Research Council, the Knut and Alice Wallenberg Foundation and Swedish State Support for Clinical Research. The NYU CSF studies were supported by the following NIH grants to MdeL (PI): AG022374, AG13616, and AG1210. The Antwerp centre was supported by the University of Antwerp Research Fund; the Alzheimer Research Foundation (SAO-FRA); the central Biobank facility of the Institute Born-Bunge / University Antwerp; the Research Foundation Flanders (FWO); the Agency for Innovation by Science and Technology (IWT); the Belgian Science Policy Office Interuniversity Attraction Poles (IAP) program; the Flemish Government initiated Methusalem excellence grant; the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant n° 115372) and this work is part of the BIOMARKAPD project within the EU Joint Programme for Neurodegenerative Disease Research (JPND). W.T.H. is supported by K23 AG042856, R21 AG043885, and P50 AG016976. O.H. is supported by the European Research Council, the Swedish Research Council, the Swedish Brain Foundation, and the Swedish federal government under the ALF agreement. This publication is part of the Consolider-Ingenio 2010 (CSD 2010-00045 PI: José L Molinuevo), FIS-Fondo europeo de desarrollo regional, una manera de hacer Europa (PI11/01071 PI: Lorena Rami) and Miguel Servet program (CP 08/00147; PI: Lorena Rami). H.H. is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie and the ‘Fondation pour la Recherche sur Alzheimer’, Paris, France. The research leading to these results has received funding from the program ‘Investissements d’avenir’ ANR-10-IAIHU-06. Data collection and sharing for this project was funded ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Supported by the Michael J. Fox Foundation for Parkinson’s Research and funding partners: abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Piramal, La Roche Ltd, Pfizer Inc and UCB Pharma SA.
Supplementary material
Supplementary material is available at Brain online.
Conflicts of interest
Dr Shaw serves as consultant for Janssen AI R & D Janssen AI R & D and Lilly, outside the submitted work. Dr Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-inventor and he received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania. C. Prof. Dr. Scheltens serves/has served on the advisory boards of: Novartis, Pfizer, Roche, Danone, Jansen AI, Baxter and Lundbeck. He has been a speaker at symposia organised by Lundbeck, Lilly, Merz, Pfizer, Jansen AI, Danone, and Roche. He is co-editor-in-chief of Alzheimer’s Research & Therapy. He is a member of the scientific advisory board of the EU Joint Programme on Neurodegenerative Disease Research (JPND) and the French National Plan Alzheimer. He acts as vice-chair of the Dutch Deltaplan Dementia. Dr de Leon has served on the advisory board of Roche and is a member of the scientific advisory board of the French National Plan Alzheimer and has patents with NYU in the area of brain imaging that have been licensed to Abient technologies. Prof. dr. Engelborghs serves / served on advisory boards of or received research funding from Innogenetics / Fujirebio Europe, Janssen, Novartis, Pfizer, Lundbeck, UCB, Roche, Danone, Nutricia. Prof. Dr. P.P. De Deyn serves on advisory boards or received research funding from Janssen Pharmaceutica, Orion and Abbvie. M.V. is an employee of Fujirebio-Europe nv. Dr Scheltens receives no personal compensation for the activities mentioned above. Dr Vanderstichele is a co-founder of ADx NeuroSciences and a founder of Biomarkable bvba. José L Molinuevo serves/has served on the advisory boards of: Novartis, Pfizer, Roche, Lilly, Piramal, IBL, GE Healthcare and Lundbeck. He has been a speaker at symposia organized by Novartis, Lundbeck, Lilly, Merz, Pfizer, Piramal and GE Healthcare. Dr. Blennow has served on advisory boards for IBL International, Lilly, Pfizer, Roche, and Kyowa Kirin Pharma. Dr Hu may accrue revenue in the future on patents submitted by Emory University wherein he is inventor. Prof Dr van der Flier receives research money from Boehringer Ingelheim and Piramal Neuroimaging, all funding is paid to her institution. H.H. declares no competing financial interests related to the present article. During the last 36 months H.H. has received lecture honoraria and/or research grants and/or travel funding and/or participated in scientific advisory boards and/or as a consultant to diagnostic, biotechnology and pharmaceutical companies involved in the manufacture and marketing of biomarkers and/or diagnostics and/or drugs or medicinal products for cognitive impairment and Alzheimer’s disease including Boehringer-Ingelheim, Bristol-Myers Squibb, Elan Corporation, Novartis, Eisai Inc., Pfizer, Sanofi-Aventis, Roche Pharmaceuticals and Diagnostics, GE Healthcare, Avid, Eli Lilly and Company, GlaxoSmithKline-Biologicals, Jung-Diagnostics, Cytox, Takeda, Isis Pharmaceutical Inc. H.H. is co-inventor in pending patent submissions relating to biological markers and/or diagnostics and has not received any royalties. J.B. Toledo, H. Zetterberg, O. Hansson, A.C. van Harten, L. Bocchio-Chiavetto and W.M. van der Flier report no disclosures.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65: 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012; 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer RG, Monge Argiles JA, Ruiz CM, Paya JS, Blanco Canto MA, Santana CL. Alzheimer disease cerebrospinal fluid biomarkers predict cognitive decline in healthy elderly over 2 years. Alzheimer Dis Assoc Disord 2014; 28: 234–8. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol 2011; 121: 171–81. [DOI] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 2010; 67: 949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, et al. Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol 2011; 68: 1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Sci Trans Med 2014; 6: 226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Pirraglia E, Brys M, de Santi S, Mosconi L, Rich KE, et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging 2009; 30: 672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry 2007; 78: 461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer's disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA 2003; 100: 10966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer's disease: a clinical follow-up study of 4.7 years. J Alzheimer's Disease 2010; 21: 1119–28. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA 2001; 98: 8838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's Dement 2012; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IFCC. Approved recommendation on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin Chim Acta 1987; 170: 13–32. [Google Scholar]
- Irwin DJ, McMillan CT, Toledo JB, Arnold SE, Shaw LM, Wang LS, et al. Comparison of cerebrospinal fluid levels of tau and Abeta 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol 2012; 69: 1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013a; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012; 71: 765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, et al. Brain beta-amyloid load approaches a plateau. Neurology 2013b; 80: 890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 2013c; 81: 1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE epsilon4 effects on memory, brain structure, and beta-amyloid across the adult life span. JAMA Neurol 2015; 72: 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol 2014; 13: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 2015; 129: 757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm 2015; 122: 505–21. [DOI] [PubMed] [Google Scholar]
- Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013a; 70: 1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-beta1-42 and tau proteins as Alzheimer disease biomarkers. Clin Chem 2013b; 59: 903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 2013; 126: 365–84. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013; 74: 826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautner R, Palmqvist S, Mattsson N, Andreasson U, Wallin A, Palsson E, et al. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry 2014; 71: 1183–91. [DOI] [PubMed] [Google Scholar]
- Le Bastard N, Coart E, Vanderstichele H, Vanmechelen E, Martin JJ, Engelborghs S. Comparison of two analytical platforms for the clinical qualification of Alzheimer's disease biomarkers in pathologically-confirmed dementia. J Alzheimer's Dis 2013; 33: 117–31. [DOI] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, et al. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol 2014; 71: 742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer's disease. Brain 2015; 138(Pt 3): 772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A, Li S, Lu Y, Li M, Wang MC, Albert M, et al. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology 2013; 81: 1753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA. A 2-process model for neuropathology of Alzheimer's disease. Neurobiol Aging 2014; 35: 301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 2014; 71: 1282–9. [DOI] [PubMed] [Google Scholar]
- Paternico D, Galluzzi S, Drago V, Bocchio-Chiavetto L, Zanardini R, Pedrini L, et al. Cerebrospinal fluid markers for Alzheimer's disease in a cognitively healthy cohort of young and old adults. Alzheimer's Dement 2012; 8: 520–7. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol 2006; 63: 936–9. [DOI] [PubMed] [Google Scholar]
- Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimer's Res Ther 2014; 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol 2012; 11: 1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009; 65: 403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelso C, et al. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem 2001; 47: 1776–81. [PubMed] [Google Scholar]
- Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement Geriatr Cogn Disord 2003; 15: 169–76. [DOI] [PubMed] [Google Scholar]
- Spies PE, Slats D, Sjogren JM, Kremer BP, Verhey FR, Rikkert MG, et al. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer's disease from non-Alzheimer's dementia. Curr Alzheimer Res 2010; 7: 470–6. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord 2007; 24: 118–24. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003; 60: 652–6. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66: 382–9. [DOI] [PubMed] [Google Scholar]
- Teipel S, Heinsen H, Amaro E, Jr, Grinberg LT, Krause B, Grothe M. Cholinergic basal forebrain atrophy predicts amyloid burden in Alzheimer's disease. Neurobiol Aging 2014; 35: 482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013a; 136(Pt 9): 2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Bjerke M, Da X, Landau SM, Foster NL, Jagust W, et al. Nonlinear association between cerebrospinal fluid and florbetapir f-18 beta-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol 2015; 72: 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol 2012; 124: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 2013b; 1: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimer's Dement 2014a; 10: 477–84 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun 2014b; 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol 2013c; 126: 659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. Journal of Alzheimer's disease 2014; 41: 313–27. [DOI] [PubMed] [Google Scholar]
- van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Abeta42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimer's Dement 2013; 9: 481–7. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, DM G, Shapiro F, Engelborghs S, De Deyn P, Shaw L, et al. Alzheimer’s disease biomarkers: from concept to clinical utility (Chapter 5). In: Galimberti D, Scarpini E, editors. BioMarkers for early diagnosis of Alzheimer's disease. Hauppauge: Nova Science Publishers Inc.; 2008. p. 81–122. [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–67. [DOI] [PubMed] [Google Scholar]
- Wang LS, Leung YY, Chang SK, Leight S, Knapik-Czajka M, Baek Y, et al. Comparison of xMAP and ELISA assays for detecting cerebrospinal fluid biomarkers of Alzheimer's disease. J Alzheimer's Dis 2012; 31: 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimer's Dement 2013; 9: e111–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.