Abstract
Single-walled carbon nanotubes synthesized with iron (Fe-SWCNT) or gadolinium (Gd-SWCNT) show promise as high performance multimodal contrast and drug-delivery agents. Our purpose was to evaluate potential vasoactive effects of SWCNT. Stable aqueous solutions of Fe-SWCNTs or Gd-SWCNTs were made using the biocompatible amphiphilic polymer N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoylsn-glycero-3- phosphoethanolamine (PEG-DSPE). Both aggregated and non-aggregated (sonicated) formulations were tested. The initial vasoactivity of the formulations and their potential for inducing pro-inflammatory endothelial dysfunction were investigated in the hamster cheek pouch and murine cremaster muscle intravital microscopy models. These models provide an assay to test several formulations/dosages in a paired fashion. Abluminal exposure to small arterioles exposes both endothelial and vascular smooth muscle cells. Using abluminal exposures of dosages that would approximate the first pass of an i.v. bolus injection, both Fe-SWCNTs and Gd-SWCNTs were immediately vasoactive. Aggregated formulations induced dilation and non-aggregated formulations induced constriction in both hamsters and mice. Endothelial dysfunction was evident after exposure to either aggregated or non-aggregated forms. General loss of dilator capability was seen after exposure to non-aggregated but not aggregated forms. Thus concentrations mimicking bolus dosing of PEG-DSPE coated SWCNT induce both acute and chronic vascular responses.
Keywords: Single walled carbon nanotubes, Vasoactivity, Endothelial dysfunction, arteriole, MRI contrast agent
Introduction
Single-walled carbon nanotubes (SWCNTs) are being developed as multifunctional nanoparticles for drug-delivery, imaging and therapeutic applications (Liu et al., 2009). A large part of development is biocompatibility testing. The emerging consensus is that in vivo toxicity and biocompatibility of SWCNT depends on their size and aspect ratio, whether they are aggregated, the catalysts used in their preparation, the dispersing agents required to make stable aqueous suspensions of the hydrophobic nanotubes, dosage, exposure time, and the in vivo exposure routes (Liu et al., 2009, Lacerda et al., 2006). SWCNTs synthesized by chemical vapor deposition (CVD) using iron (Fe) (Hafner et al., 1998), or gadolinium (Gd) as catalysts (Swierczewska et al., 2009) results in Fe or Gd intercalation within the SWCNT structures (e.g., Fe- or Gd-SWCNTs). SWCNT coated with natural or synthetic amphiphilic polymers form stable aqueous suspensions (Avti et al., 2013a, Choi et al., 2007, Sitharaman et al., 2012, Liu et al., 2009). We have dispersed Fe-SWCNTs or Gd-SWCNTs in physiologic saline with the biocompatible amphiphilic polymer N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoylsn-glycero-3- phosphoethanolamine (PEG-DSPE) (Avti et al., 2013b). The hydrophobic lipid moiety DSPE non-covalently coats the surface of the SWCNT, while the hydrophilic PEG moiety faces the aqueous solution. Initial toxicological studies showed that a single dose (0.5 mg/kg) in rats is not toxic and did not induce persistent inflammatory changes over 30 days; however, we did see a transient elevation in lipid peroxidation (Avti et al., 2013b).
In the present study, we use topical (abluminal) exposure as an assay to test for potential vasoactive responses that may accompany intravenous administration. Many of the potential end-user applications of SWCNT call for intravenous (i.v.) administration, and hence the SWCNTs would first interact with the vasculature. If the initial exposure is given as an i.v. bolus, then small volumes of high concentration are injected at once. The initial dosage ‘seen’ by the vasculature would be as much as 20- to 60-fold higher than the final steady state circulating dosage (Rivers et al., 2001). It is this initial high dosage of contrast agents that can cause urticaria (due to immediate vasoactive effects) or other adverse effects (Shellock et al., 2006). No studies to date have examined the effects of stable aqueous suspensions of SWCNT on vasoactivity. This is a non-trivial biocompatibility concern because very brief (seconds) exposure to oxidative stress can induce long lasting pro-inflammatory effects. (Frame, 2003, Frame et al., 2002, Frame and Mabanta, 2007)
Using intravital microscopy, several dosages and formulations can be independently tested within one animal as a screening process for vascular effects. By choosing small arterioles, with a discontinuous vascular smooth muscle layer, both the inner endothelial cell layer and the outer vascular smooth muscle layer are exposed using topical application. Hence, we can screen for effects that could occur with i.v. injections, and can employ paired statistical analysis. We tested both the hamster cheek pouch and murine cremaster muscle intravital microscopy models to investigate the universality of the findings across species and tissues. In these models, we tested whether SWCNT caused constriction or dilation (immediate vasoactive effect), and whether exposure could induce pro-inflammatory endothelial dysfunction (long-term effect). As these particles tend to aggregate, we tested both aggregated and non-aggregated suspensions. We found that the initial vasoactive response is dependent on aggregation state and not the presence of Fe vs Gd, per se. Further, endothelial dysfunction is apparent following a single high dose of any formulation, with the non-aggregated SWCNT also attenuating general dilatory capability.
Methods
Formulation Materials
Fe-SWCNT’s were obtained from a commercial vendor (Cheap Tubes Inc., Brattleboro, VT). Gd-SWCNT’s were synthesized, and characterized as previously reported (Swierczewska et al., 2009). PEG-DSPE (molecular weight 2000 Da) was from NOF America Corporation, White Plains, NY.
Preparation of suspensions
PEG-DSPE was used to prepare stable aqueous suspensions of aggregated and non-aggregated Fe- or Gd-SWCNT’s by using a sonication based protocol that we have previously described (Avti et al 2013, reference 1). DSPE (a lipid), forms a coating on the surface of SWCNT’s due to its hydrophobicity and PEG (a hydrophilic polymer) on the outer surface gives aqueous stability to the SWCNT’s (Avti et al., 2013a).
Non-aggregated suspensions
Fe-SWCNT’s or Gd-SWCNT’s (15 mg) were dispersed in 15 ml of 0.1 g% PEG-DSPE solution (in physiologic saline) by probe sonication (Cole Parmer Ultrasonicator LPX 750 operating at 25% of maximum power) for 5 min (1 second on, and 2 second off cycle), and by bath sonication (Fischer Scientific bath ultra-sonicator FS30H operating at 250W) for 15 min. The dispersion was then centrifuged at 1000 rpm to obtain stock suspensions that contained a mixture of individual and bundles of 2–3 nanotubes coated with PEG-DSPE (Figure 1). The stock suspensions (5 ml) were dried in a lyophilizer, and the solid residue was weighed. The solid was re-suspended and serially diluted in physiologic saline to obtain the test concentrations (0–50 μg/ml) of non-aggregated Fe-SWCNTs or Gd-SWCNTs. Re-suspension and serial dilution was performed immediately before testing in vivo.
Figure 1.
A. Raman spectra of Gd-SWCNT and Fe-SWCNT showing characteristic D and G bands at 1369 cm−1 and 1580 cm−1.(B–E) Atomic Force Microscopy images of non-aggregated (B) Gd-SWCNT (C) Fe-SWCNT samples showing individual tubes and aggregated (D) Gd-SWCNT (E) Fe-SWCNT samples showing aggregated bundles of tubes. Individual Gd-SWCNT’s appear ~1–3 nm in height and ~600–800 nm in length. Individual Fe-SWCNT’s appear ~1–3 nm in height and ~1.5–3 μm in length.
Aggregated suspensions
The aggregated suspensions were prepared by dispersing 0.5 mg of the Fe-SWCNTs or Gd-SWCNTs in 5 ml 0.1 g% PEG-DSPE followed by bath sonication for 10 seconds to obtain a homogenous dispersion of tens to hundreds of nanotubes coated with PEG-DSPE as the stock suspension (Figure 1). This stock suspension was immediately serially diluted in physiologic saline to obtain the various concentrations (0–50 μg/ml) of aggregated Fe-SWCNTs or Gd-SWCNTs. These solutions were not sonicated again prior to use (1–2 hours later).
Characterization
The prepared suspensions were characterized using Raman Spectroscopy and Atomic Force Microscopy (AFM). (Figure 1) Raman spectra of Fe-SWCNT and Gd-SWCNT suspensions was obtained using an Enwave Pro Raman-L Spectrophotometer equipped with a charge couple device (CCD) detector, and a 532 nm laser, at 20% of maximum laser strength (500 mW). Aggregated and non-aggregated Fe- and Gd-SWCNT’s were dispersed in 1:1 ethanol:water to achieve a concentration of 1 μg/ml. Fifty microliters of homogenously dispersed nanomaterial solution was drop cast on silicon wafers (Ted Pella, USA), dried overnight and used for AFM Imaging. AFM images were obtained using a NanoSurf EasyScan 2 Flex AFM (NanoScience Instruments Inc., Phoenix), operating in tapping mode, using a V-shaped cantilever (APP Nano ACL – 10, frequency fc= 145–230 kHz, L = 225 μm, W= 40 μm, tip radius < 10 nm, spring constant k = 20–95 N/m). Data was collected under ambient conditions at 50% relative humidity and 25°C. Length and height of the individual SWCNT’s was calculated from length and height profile of 30 non –aggregated SWCNT’s obtained from 6 representative AFM images. Figure 1 shows representative images; it is acknowledged that aggregation within suspensions used in vivo might be different despite that processing was identical.
Animal models
With university approval (Institutional Animal Care and Use Committee, Stony Brook University, IACUC-SBU), male hamsters (64±9 days, 105±12 grams, N=21) were anesthetized with pentobarbital (hamster 70 mg/kg i.p.); the airway was secured by tracheotomy. With IACUC-SBU approval, male mice (101±17 days, 27±3.5 grams, N=11) were anesthetized with isoflurane (4% induction); mice show a steep LD50 (lethal dose at which half of the population will die) curve with pentobarbital, hence the inhalation anesthetic was used in mice. The anesthetic plane was maintained by supplementing the same anesthetic as used for induction, and animals were kept warm by both conductive and convective heat sources. In the hamster, the left cheek pouch tissue was prepared, as previously.(Frame and Mabanta, 2007, Frame et al., 2007, Mabanta et al., 2006, Mustafa et al., 1999) In the mouse, the right cremaster muscle was prepared, as previously.(Georgi et al., 2011b) For each, the tissue was pinned across a quartz pedestal with warmed superfusate (physiological saline) flowing over the flat tissue at a rate of 5 ml/min.
Microscopy system
The observation site for both hamsters and mice was the arcade - terminal arteriolar network junction (Figure 2). The site was visualized with a modified Nikon microscope using a 25x objective (Leica, NA 0.35) with a final optical resolution of 0.7 μm. Experiments were visualized and videorecorded using a Dage-MTI Gen/Sys CCD camera and a SVHS Panasonic AG7350 recorder. Arteriolar diameter measurements were made offline, calibrated with a stage micrometer.
Figure 2.
Micrograph from mouse cremaster (A, shown maximally dilated with acetylcholine) and hamster cheek pouch (B, shown with normal resting tone; C, illustrating controlled drug exposure via micropipette delivery with the arteriole depicted by dotted lines). TA, terminal arteriole, and AA, arcade arteriole. Typical configurations for micropipette administered drug exposure are illustrated. The micropipette was placed so that the contents passed over the arterioles of interest and then washed away in the flowing tissue bath. C illustrates exposure at 15 seconds. scale bar = 10 μm.
Experimental Protocol
Identical protocols were used for experiments in hamsters or mice. In each tissue paired data was obtained of vasoactive responses before SWCNT exposure, the dose response to SWCNT, and then the vasoactive responses after SWCNT exposure. Following a 30 minute resting period, the tissues were tested for baseline vasoactive responses (arteriolar diameter changes) to adenosine, acetylcholine and phenylephrine (10−4M each, micropipette application for 60 s, 5 min washout) to confirm tone. All pipettes contained a fluorescent tracer of FITC-BSA (bovine serum albumin, 4kD, 0.01 mmol/L) to confirm exposure location (Georgi et al., 2011a). Fe-SWCNT or Gd-SWCNT formulations were applied in increasing dosages (0 [0.1% PEG-DSPE alone], 5, 25, 50 μg/ml) via micropipette, using 30 s exposures, and a 5 min washout between dosages. Micropipette contents were ejected from the micropipette tip (8–10 μm tip diameter over a length of ~500 μm) pneumatically, using the lowest pressure that ejected the contents (typically 0.05 psi = 3447 gm*cm−1*s−2). Calculated total resistance [R= (8 x viscosity x length)/(π x radius4), where viscosity is 1 cP = 0.01 gm*cm−1*s−1 at 36°C] was 2×10−10 g*cm−4*s−1. Using the common Ohm’s Law equation for flow (Pressure = flow x resistance), the flow rate out of the pipette was 1.7×10−7 cm3/s. Over 30 s exposure time period, a volume of 5×10−6cm3 will have been expelled from the pipette tip. Dilution of this dosage is expected due to placement of the micropipette tip 25 μm from the vessel wall within a flow stream of superfusate. Only half of the concentration within the micropipette is expected to be ‘seen’ by the vessel wall (Figure 2C). The flowing superfusate (5 ml/min) rapidly flushes this location after exposure. (Georgi et al., 2011a) Based on this micropipette delivery system, the estimated individual dose per arteriole location was calculated to be: 0, 12.7, 63.4 and 126.8 pg; the estimated cumulative dose per arteriole location is calculated to be: 0, 12.7, 76.1, 202.9 pg. Fifteen minutes later adenosine, acetylcholine and phenylephrine were again applied. Two locations separated by a minimum distance of 1000 μm were tested per animal for paired comparisons between the Gd-SWCNT and Fe-SWCNT formulations. Animals were then euthanized by high dose anesthetic.
Statistics
Two observation sites were tested per animal for paired comparisons between exposure to Fe- vs. Gd-SWCNT. Diameters (μm) are reported for the baseline values. Diameter change is reported as per-cent of baseline: [(peak change – baseline)/baseline] x 100. Data of Figure 3 was analyzed by ANOVA for repeated measures; data of Figure 4 was analyzed by paired t-test, variability was analyzed by examining the coefficient of variation (CV), using standard equations as found in Snedecor and Cochran. (Snedecor and Cochran, 1974) The concentration response was analyzed using the sigmoidal dose response fit weighted by the standard deviation in OriginPro (v7.0383, Origin Labs, Inc. Northampton, MA); the subsequent fitted EC50 [effective concentration for a half maximal vasoactive response] and fitted maximal vasoactive response values are given. Note the EC50 value is the concentration in the micropipette. The significance level for hypothesis testing was p=0.05.
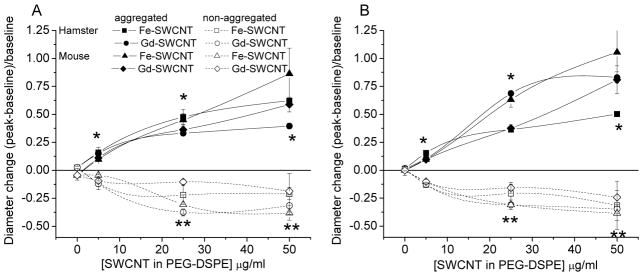
Figure 3.
Shown are sustained diameter changes (mean±SEM) with 30 s continuous exposure via micropipette to Fe-SWCNT or Gd-SWCNT coated with 0.1% PEG-DSPE that were aggregated (hamster N=14; mouse N=7) or non-aggregated (monodispersed; hamster N=4; mouse=4) in hamster cheek pouch or mouse cremaster muscle. The x-axis gives the concentration in the micropipette; kindly refer to the Methods for exposure dose per arteriole. The dose of 0 μg/ml SWCNT contains only PEG-DSPE at 0.1%. Two classifications of arterioles were observed: arcade arterioles (A); terminal arterioles (B). The legend in (A) applies to both (A) and (B). *dilation in each group significant at that dose; **constriction in each group significant at that dose; p<0.05.
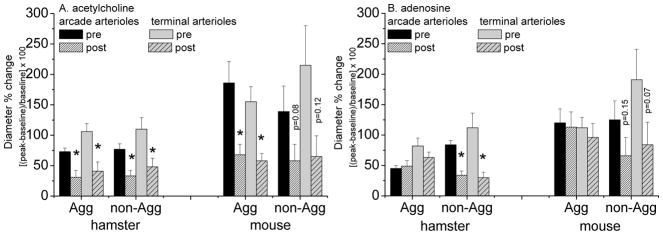
Figure 4.
Diameter changes (mean±SEM) with 30s continuous exposure to micropipette applied acetylcholine (panel A, 10−4 mol/L) or adenosine (panel B, 10−4 mol/L) before (pre) and 15–30 minutes after (post) nanotube exposure to the highest concentration of 50 μg/ml Fe-SWCNT or Gd-SWCNT (the concentration in the micropipette; kindly refer to the Methods for calculation of the exposure dose per arteriole). [Same animals as for Figure 3 so that paired comparisons could be made.] *post differs from pre, p<0.05. Agg = aggregated; non-Agg = non-aggregated. The data for the Gd-SWCNTs and Fe-SWCNTs are pooled together; there was no statistical difference in the data between these formulations (Gd- vs. Fe-SWCNT).
Results
Raman spectra of both Fe-SWCNT and Gd-SWCNT samples showed a G peak at 1580 cm−1 and a smaller D peak at 1369 cm−1 which are characteristic of SWCNTs (Figure 1A). The D peak which is indicative of defects in structure was higher in the Gd-SWCNTs probably because of the bigger size of Gd3+ ions vs Fe3+. Representative AFM images shown in Figure 1(B–E) indicate that samples prepared by sonication to be non-aggregated have mostly individual SWCNTs whereas samples prepared to be aggregated show significant clumping of the SWCNT.
Figure 2 shows representative images from each tissue and illustrates the micropipette exposure technique (see Discussion). In the hamster cheek pouch, the resting baseline diameter for the arcade arterioles was 18.3±6.8 μm (mean±SD), and for the terminal arterioles was 7±1.7 μm. In the mouse cremaster, the average baseline diameter for the arcade arterioles was 17.6±4.5 μm and for the terminal arterioles was 10.5±3.2 μm. Baseline diameters were similar for sites exposed to Fe-SWCNT vs. Gd-SWCNT.
Figure 3 shows that the Fe-SWCNT or Gd-SWCNT suspensions were themselves vasoactive (caused dilation or constriction) during a 30 s exposure. The concentration response to the Fe-SWCNT or Gd-SWCNT formulations shows that aggregated nanotubes exhibit a completely opposite vasoactive response (dilation) as compared to non-aggregated nanotubes (constriction) (Figure 3). Testing 0.1% PEG-DSPE coating alone showed no response, and this appears on Figure 3 as SWCNT dose of 0 μg/ml. Because the presence of Fe- or Gd- did not affect this acute vasoactive response, the concentration response analysis was performed for the group of aggregated formulations (pooling Fe- and Gd-SWCNT and pooling hamsters and mice) vs. the group of non-aggregated formulations. For the aggregated SWCNT causing dilation, the larger arcade arterioles showed an EC50 18.2±5 μg/ml (mean±sem) and maximal dilation +60±12%*, which was not different from that of the terminal arterioles (EC50 20.2±3 μg/ml, maximal dilation +80±10%*). For the non-aggregated SWCNT causing constriction, the larger arcade arterioles showed an EC50 5.2±22 μg/ml, maximal constriction −25±1%*, and the values for the terminal arterioles were again similar (EC50 5.3±19 μg/ml, maximal constriction −26±1.3%*). [*maximal dilation or constriction values differed from baseline; EC50 values do not differ.] There were no differences in responses between the arcade arterioles vs. the terminal arterioles. Further, Figure 2 shows that the results of ANOVA for repeated measures indicating that the dilation responses were individually significant at 5 μg/ml SWCNT in PEG-DSPE, and the constrictor responses became significant at 25 μg/ml. All dilation or constriction responses were recoverable within 60 s after exposure, with return to baseline diameters. Thus, the initial brief exposure caused a uniform transient vasoactive response in both hamsters and mice, dependent on aggregation state of the SWCNT, and unrelated to the presence of Fe or Gd.
Our secondary question was whether these formulations had a lasting impact on vascular function. Specifically, we tested whether endothelial dysfunction (attenuated dilation to acetylcholine) was induced by pre-exposure to the nanotubes. To do this, we first obtained the initial responses to acetylcholine, adenosine and phenylephrine (Figure 4 ‘pre’) and then exposed the arterioles to the doses of SWCNT shown in Figure 3, followed by a second exposure to acetylcholine, adenosine and phenylephrine (Figure 4 ‘post’). In this way, paired measures were obtained within each animal.
Vasoactive responses were altered by exposure to SWCNT in an identical fashion for Fe- or Gd-SWCNT. Therefore the data for Fe-SWCNTs and Gd-SWCNTs were pooled together in Figure 4. Figure 4A shows evidence of endothelial dysfunction 15–30 minutes after exposure to SWCNT. The diluent alone, 0.1% PEG-DSPE, had no effect. In hamsters, dilation to acetylcholine was always compromised following exposure to either aggregated or non-aggregated nanotubes. Therefore, in three additional hamsters, we tested for endothelial dysfunction following exposure to only a single dose of 10 μg/ml nanotubes (aggregated). After exposure to SWCNT, dilation to acetylcholine in those animals was unchanged, +72±12% initially, and +65±8% (N=3). In mice, dilation to acetylcholine was statistically different following exposure to aggregated nanotubes only.
Examining dilation to adenosine tested generalized vascular smooth muscle function. In hamsters, attenuated dilation to adenosine (Figure 4B) after exposure to non-aggregated SWCNT suggested generalized loss of dilatory tone. In mice, dilation to adenosine was not affected by prior exposure to SWCNT (note p-values in Figure 4B). However, following exposure to non-aggregated SWCNT dilation to adenosine was more variable. Together, this suggested that exposure to the non-aggregated SWCNT affected vascular function in general for hamsters, but in a variable manner for mice.
Variability was examined further for responses to acetylcholine or to adenosine. Variability is indicated by the coefficient of variation (CV = SD/mean). In mice, baseline CV for dilation to either acetylcholine or adenosine was 30–60%. CV more than doubled following exposure to non-aggregated nanotubes (120–140%). This means that for mice, after exposure to non-aggregated SWCNT, despite that the diameter had returned to baseline, 50% of the mice no longer responded to adenosine or to acetylcholine (meaning that dilation was less than 5% for each arteriole tested in that mouse). Within the other half of the mice normal robust dilations were seen to adenosine or acetylcholine. In contrast, in hamsters, the baseline CV vs. post-exposure CV for dilation was similar (30–50%). Within hamsters, individual arterioles showed a diminished response, to account for an average uniform decrease in response to adenosine or acetylcholine. Thus, there is a species or tissue difference in loss of dilatory responses that accounts for an increased variability in dilation across mice.
In contrast to the dilation responses, constriction responses to phenylephrine did not differ by species or tissue, and were not affected by nanotube aggregation. [Fe-SWCNT and Gd-SWCNT data are pooled; hamster, arcades, aggregated nanotubes: before −63±4% (mean±sem); after −67±3%; non-aggregated nanotubes: before −65±6%; after −73±5%; terminal arterioles, aggregated nanotubes: before −57±4%; after −63±4%; non-aggregated nanotubes: before −61±8%; after −75±3%. mouse, arcades, aggregated nanotubes: before −57±4%; after −61±4%; non-aggregated nanotubes: before −59±6%; after −55±5%; terminal arterioles, aggregated nanotubes: before −63±4%; after −54±7%; non-aggregated nanotubes: before −60±4%; after −54±5%.]
Discussion
There is a broad range of potential biomedical applications for Fe-SWCNTs or Gd-SWCNTs in which these compounds would come into contact with vascular system (Choi et al., 2007, Sitharaman et al., 2012, Avti et al., 2013a, Lacerda et al., 2006, Liu et al., 2009). The present study examines their vasoactive effect as an essential component of overall toxicity assessment. We found that SWCNT in stable aqueous suspensions with a biocompatible coating can still cause both acute and long-lasting microvascular effects that are species and dosage dependent. Although Fe-SWCNTs and Gd-SWCNTs were chosen as model SWCNTs to determine whether SWCNTs prepared via different catalysts had differential vasoactive effects, we found no differences between Fe- vs Gd-SWCNT. Instead, the vasoactive effects are directly attributable to the relative aggregated vs. monodisperse state, and to a lesser extent on the animal species tested. Our key findings are that aggregated SWCNT cause an acute vasodilation that immediately subsides after exposure, with a long-lasting effect of endothelial dysfunction. Non-aggregated (monodisperse, nano-sized SWCNT) cause an acute vasoconstriction that immediately subsides after exposure, with a long-lasting effect of endothelial dysfunction, and potentially loss of dilatory capability.
Previous studies have established that non-covalent functionalization of Fe-SWCNT or Gd-SWCNT with PEG-DSPE is essential to ensure their dispersibility, and their immediate biocompatibility in blood and biological media.(Liu et al., 2008, Avti et al., 2013a, Avti et al., 2013b) Our goal was to extend these studies, and examine whether these nanoparticles were vasoactive. There are two important discussion points regarding our experimental model: first, the relevance of this dosage range and exposure time; second, use of the intravital microscopy model to screen for potential microvascular effects.
Dosages and exposure times
As is typical for indicator dilution methodology, drugs injected as a venous bolus will travel through all blood vessels first as a high concentration (first pass of the agent). Intravascular mixing occurs within minutes to reach the lower steady state concentration. Within terminal arterioles of this size, we have previously measured the first pass concentration to be ~30% of the bolus concentration; within minutes the agent is uniformly distributed throughout the blood volume to reach a steady state concentration that is 25–30% lower than the first pass concentration. (Rivers et al., 2001) The time factor for the first pass of an i.v. injected drug to reach this size of terminal arterioles occurs on the order of 5 seconds post injection, the high dosage exposure time-window generally occurs between 10–20 seconds, and complete uniform dispersion in the blood occurs on the order of 2 minutes; all time-frames are dependent on the cardiac output.(Rivers et al., 2001) Thus, our nanotube exposure time was restricted to 30 seconds to test for the immediate vasoactive response; most responses were complete within 10–15 seconds. Testing the high concentration (50 μg/ml in the present study, representing the estimated first pass concentration) is very important because we know that brief (seconds) exposure to pro-inflammatory agents can induce long-lasting pro-inflammatory responses that include endothelial dysfunction (Frame et al., 2002, Frame and Mabanta, 2007, Frame and Sarelius, 1995, Frame and Sarelius, 1996a, Frame and Sarelius, 1996b, Mabanta et al., 2006, Mustafa et al., 1999). We have previously determined that i.v. injection of 1 mg/ml Fe- or Gd-SWCNT as a bolus (dosage 0.5 mg/kg in rats) showed lipid peroxidation over 30 days in several organs, but was not toxic (Avti et al., 2013b). Applying the same calculations used in the present study, to our prior study, the initial pass of the SWCNT would have been ~300 μg/ml, and the steady state concentration would have been ~100 μg/ml in that study (Avti et al., 2013b). In comparison to the present study, the highest concentration we tested here was 50 μg/ml. Thus a dosage of half of a previously used non-lethal dosage still potentially compromises the cardiovascular system.
Intravital microscopy to screen for microvascular effects
Representative images of the two intravital microscopy preparations are shown in Figure 2. The images illustrate the minimal connective tissue and parenchymal tissue that would potentially interfere with drugs accessing the arterioles of interest. This level of the microcirculation was deliberately chosen because at this level, the vascular smooth muscle cells form a discontinuous layer on the abluminal side of the arteriole, revealing the outer surface of the inner endothelial cell layer. (Frame et al., 2007; Georgi et al., 2011b) Thus exposure to the abluminal surface applies agents to both vascular smooth muscle cells and endothelial cells. By using localized abluminal exposure several doses can be independently tested within the same animal as a screening assay to determine the effect on the microcirculation as a whole. This is in fact, another reason for testing more than one animal species/tissue. In contrast, systemic exposure as a bolus would lack a controlled ‘exposure time period’ for individual arterioles, required in order to test causal relationships, and, importantly, only one dose could be administered per animal to avoid systemic exposure secondary effects. Thus, while we acknowledge that the intravital microscopy model system used does not fully replicate systemic intravenous injection, it does provide a relevant screening process to test for potential adverse reactions.
The present study shows both an initial vasoactive response, dependent on aggregation state, and a long term effect consistent with endothelial dysfunction. Previously, we have extensively examined how other oxidative/nitrosative compounds will induce endothelial dysfunction that is separate from the initial vasoactive response. (Frame et al., 2002, Frame and Mabanta, 2007, Frame and Sarelius, 1995, Frame and Sarelius, 1996a, Frame and Sarelius, 1996b, Mabanta et al., 2006, Mustafa et al., 1999) The initial vasoactive response is only seen during the initial brief exposure to the toxicant (SWCNT in the present study). The long term effect is only seen 15 minutes or more after exposure to the toxicant, and is assayed for by applying endothelial cell dependent vasodilators. The potential mechanisms for the initial response vs. long term endothelial dysfunction may or may not be linked to an initial oxidative damage, and are in fact, separate responses.
Initial vasoactive response
Aggregation of aqueous suspensions of SWCNTs is a common occurrence. Therefore, aggregated and non-aggregated SWCNT formulations were tested to understand the effects of aggregation of nanotubes on the vasoactive response. The non-aggregated formulations were prepared fresh, and used immediately. The aqueous suspensions of aggregated Fe-SWCNT or Gd-SWCNT formulations induced vasodilation and non-aggregated formulations induced constriction in small arterioles. Each vasoactive effect occurred within seconds, was dose dependent with an EC50 in the therapeutic range, and immediately reversible after the exposure period. The mechanism(s) for the initial dilation or constriction is(are) currently not known. We speculate for the non-aggregated state causing constriction, there is an increased surface area on the nanoparticles with exposed positively charged PEG. The increased local positive charge potentially raises the local Nernst potential, thus directly causing constriction.
In contrast, aggregated SWCNT caused vasodilation. Clinically, this would induce urticaria, seen as an immediate ‘flushing’ effect that is also commonly described as a side-effect in other contrast agents. (Shellock et al., 2006) In vivo, we would expect that the aggregates would re-form, although we do not have the kinetics for this reaction. From our results, it is quite clear that the aggregation state and concentration are quite important. For silver nanoparticles (45 nm), others have reported a concentration dependent (possibly aggregation state dependent) vasoconstriction at low dosages (1–10 μg/ml), and a partially NO-dependent vasodilation at high dosages (50–100 μg/ml) (Rosas Hernandez et al., 2009). It is not clear from that study whether the causal component was silver or the composite charge or the size of the agglomerate itself. Similar studies testing carbon based nanoparticles were not found.
Induced endothelial dysfunction
Our second objective was to establish whether exposure to the SWCNT could induce long-lasting endothelial dysfunction. Clinically, an important indicator of whether microvascular nutrient flow is coordinated with the metabolic needs of the tissue is a response termed endothelial mediated dilation; the response is absent in endothelial dysfunction, a hallmark sign of a pro-inflammatory state (Chen et al., 2011, Savoia et al., 2011, Zhou et al., 2010). One experimental test for endothelial mediated dilation is the ability to dilate to acetylcholine, ACh, which induces dilation via the endothelial muscarinic receptors when the endothelium is healthy, and experimentally induces a constriction effect via the vascular smooth muscle muscarinic receptors in endothelial dysfunction (Shi et al., 2006). While in a healthy individual a single exposure to the SWCNT formulations tested here may not significantly compromise their ability to regulate flow, in otherwise compromised individuals, including those with metabolic syndrome or other clinically recognized pro-inflammatory states, the high dosage bolus exposure may compromise their ability to regulate flow (Mustafa et al., 1999, De Vriese et al., 2000). In many cases, coating potentially inflammatory agents with PEG, dextran, or other compounds, renders them biocompatible (Falabella Ca, 2008, Ghosh et al., 2006, Patel et al., 2012, Kim et al., 2011). In our case, the coating alone (0.1% PEG-DSPE) did not itself cause endothelial dysfunction, but neither did this protective coating protect dilation to acetylcholine after SWCNT exposure.
The mechanism by which SWCNT exposure induces endothelial dysfunction was not determined here. Based on work by others, extensive exposure to SWCNT shows they are endocytosed directly by endothelial cells causing cell damage that initially manifests as endothelial dysfunction. (Yaron et al., 2011; Chen et al., 2013) Our exposure time is only seconds. Importantly, we show that exposure to only seconds of Fe-SWCNT or Gd-SWCNT induces endothelial dysfunction whether the formulation was aggregated or non-aggregated. Thus we speculate that the initial vasoactive response is unrelated to the subsequent endothelial cell compromise.
Lastly, a limitation of the current study is that our results may be restricted to Fe- and Gd-SWCNTs that are non-convalently functionalized with PEG-DSPE. All SWCNT synthesis requires a metal catalyst. Iron, Cobalt and Nickel are also commonly used elements for this purpose. During the synthesis process the catalysts (metal ions) get embedded at one end of nanotubes. Harsh acid washes (e.g. with concentrated nitric acid) can remove the metal catalysts to some extent, but this is not complete. Thus, even washed SWCNT will contain some metal ions (Pumera, 2007). Secondly, by removing the metal ions, the harsh process itself can oxidize the external carbon sheet of the nanotubes and covalently functionalize it with hydroxy and carboxy groups. (Tobias et al., 2009)The presence of the –OH or –COOH groups will render it somewhat water soluble and could reduce the aggregation, but then the biocompatible coating would not adhere to the SWCNT.
Conclusions
Short term exposure to PEG-DSPE coated SWCNTs that were synthesized using iron or gadolinium nanoparticles as catalysts induced (1) immediate significant vasoactive responses, and (2) later endothelial dysfunction at clinically relevant concentrations. Vehicle alone (containing PEG-DSPE) was not vasoactive. Our data clearly show a compromised microvasculature in mice or hamsters due to short term exposure to SWCNT, despite that in our previous study a single i.v. bolus injection of these SWCNT formulations were not toxic to organ or tissues in healthy rats. (Liu et al., 2008, Avti et al., 2013a, Avti et al., 2013b) Thus concentrations that mimic i.v. bolus dosing of PEG-DSPE coated SWCNT can exert adverse vascular effects.
Acknowledgments
Thanks to NIH HL55492, AHA 0655908T (MF); NIH 1DP2OD007394-01, DoD BCRP W81XWH-10-1-0521 (BS). We thank Dr. Pramod Avti for help in preparing the SWCNT suspensions.
Footnotes
Declaration of Interest:
none
References
- Avti PK, Caparelli ED, Sitharaman B. Cytotoxicity, Cytocompatibility, Cell-Labeling Efficiencies, and In Vitro Cellular Magnetic Resonance Imaging of Gadolinium-Catalyzed Single-Walled Carbon Nanotubes. J Biomed Mater Res, Part A. 2013a doi: 10.1002/jbm.a.34643. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avti PK, Talukdar Y, Sirotkin MV, Shroyer KR, Sitharaman B. Toward Single-Walled Carbon Nanotube-Gadolinium Complex as Advanced MRI Contrast Agents: Pharmacodynamics and Genomic Response in Small Animals. J Biomed Mater Res, Part B. 2013b doi: 10.1002/jbm.b.32914. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Hsiao KM, Chou CC. Molecular characterization of toxicity mechanism of single-walled carbon nanotubes. Biomaterials. 2013;34(22):5661–5669. doi: 10.1016/j.biomaterials.2013.03.093. [DOI] [PubMed] [Google Scholar]
- Chen YX, Wang XQ, Fu Y, Yao YJ, Kong MY, Nie RQ, Wang JF. Pivotal role of inflammation in vascular endothelial dysfunction of hyperlipidemic rabbit and effects by atorvastatin. Int J Cardiol. 2011;146:140–4. doi: 10.1016/j.ijcard.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Choi JH, Nguyen FT, Barone PW, Heller DA, Moll AE, Patel D, Boppart SA, Strano MS. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett. 2007;7:861–867. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falabella CA, Jiang H, Frame MD, Chen W. In Vivo Validation of Biological Responses of bFGF Released from Microspheres Formulated by Blending Poly-lactide-co-glycolide and poly(ethylene glycol)-grafted-Chitosan in Hamster Cheek Pouch Microcirculatory Models. J Biomater Sci Polym Ed. 2009;20(7–8):903–22. doi: 10.1163/156856209X444330. [DOI] [PubMed] [Google Scholar]
- Frame MD. Remote activation of arteriolar network preconditioning involves Katp channel activation and NO, ROS. Faseb J. 2003;17:A139–A139. [Google Scholar]
- Frame MD, Fox RJ, Kim D, Mohan A, Berk BC, Yan C. Diminished arteriolar responses in nitrate tolerance involve ROS and angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H2377–H2385. doi: 10.1152/ajpheart.00429.2001. [DOI] [PubMed] [Google Scholar]
- Frame MD, Mabanta L. Remote microvascular preconditioning alters specific vasoactive responses. Microcirculation. 2007;14:739–751. doi: 10.1080/10739680701410074. [DOI] [PubMed] [Google Scholar]
- Frame MD, Rivers RJ, Altland O, Cameron S. Mechanisms initiating integrin-stimulated flow recruitment in arteriolar networks. J Appl Physiol. 2007;102:2279–2287. doi: 10.1152/japplphysiol.00537.2006. [DOI] [PubMed] [Google Scholar]
- Frame MD, Sarelius IH. L-arginine-induced conducted signals alter upstream arteriolar responsivity to L-arginine. Circ Res. 1995;77:695–701. doi: 10.1161/01.res.77.4.695. [DOI] [PubMed] [Google Scholar]
- Frame MD, Sarelius IH. Endothelial cell dilatory pathways link flow and wall shear stress in an intact arteriolar network. J Appl Physiol. 1996a;81:2105–2114. doi: 10.1152/jappl.1996.81.5.2105. [DOI] [PubMed] [Google Scholar]
- Frame MD, Sarelius IH. Vascular communication and endothelial cell function in the control of arteriolar flow distribution. Microcirculation. 1996b;3:233–235. doi: 10.3109/10739689609148294. [DOI] [PubMed] [Google Scholar]
- Georgi MK, Dewar AM, Frame MD. Downstream exposure to growth factors causes elevated velocity and dilation in arteriolar networks. J Vasc Res. 2011a;48:11–22. doi: 10.1159/000317396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi MK, Vigilance J, Dewar AM, Frame MD. Terminal arteriolar network structure/function and plasma cytokine levels in db/db and ob/ob mouse skeletal muscle. Microcirculation. 2011b;18:238–51. doi: 10.1111/j.1549-8719.2011.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- Hafner JH, Bronikowski MJ, Azamian BR, Nikolaev P, Rinzler AG, Colbert DT, Smith KA, Smalley RE. Chem Phys Lett. 1998;296:195–202. [Google Scholar]
- Kim YK, Kim MH, Min DH. Biocompatible reduced graphene oxide prepared by using dextran as a multifunctional reducing agent. Chem Commun (Camb) 2011;47:3195–7. doi: 10.1039/c0cc05005a. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460–70. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tabakman S, Welsher K, Dai H. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabanta L, Valane P, Borne J, Frame MD. Initiation of remote microvascular preconditioning requires K(ATP) channel activity. Am J Physiol Heart Circ Physiol. 2006;290:H264–H271. doi: 10.1152/ajpheart.00455.2005. [DOI] [PubMed] [Google Scholar]
- Mustafa SS, Rivers RJ, Frame MD. Microcirculatory basis for nonuniform flow delivery with intravenous nitroprusside. Anesthesiology. 1999;91:723–731. doi: 10.1097/00000542-199909000-00025. [DOI] [PubMed] [Google Scholar]
- Patel B, Gupta V, Ahsan F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release. 2012;162:310–320. doi: 10.1016/j.jconrel.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Pumera M. Carbon nanotubes contain residual metal catalyst nanoparticles even after washing with nitric zcid at elevated temperature because these metal nanoparticles are sheathed by several graphene sheets. Langmuir. 2007;23(11):6453–6458. doi: 10.1021/la070088v. [DOI] [PubMed] [Google Scholar]
- Rivers RJ, Beckman JB, Frame MD. Technique for using video microscopy and indicator dilution for repeated measurements of cardiac output in small animals. Anesthesiology. 2001;94:489–495. doi: 10.1097/00000542-200103000-00021. [DOI] [PubMed] [Google Scholar]
- Rosas-Hernández H, Jiménez-Badillo S, Martínez-Cuevas P, Gracia Espino E, Terrones H, Terrones M, Hussain SM, Ali SF, González C. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol Lett. 2009;191:305–313. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Savoia C, Sada L, Zezza L, Pucci L, Lauri FM, Befani A, Alonzo A, Volpe M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. doi: 10.4061/2011/281240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellock FG, Parker JR, Venetianer C, Pirovano G, Spinazzi A. Safety of gadobenate dimeglumine (MultiHance): Summary of findings from clinical studies and postmarketing surveillance. Invest Radiol. 2006;41(6):500–509. doi: 10.1097/01.rli.0000209661.99225.c2. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318:276–81. doi: 10.1124/jpet.105.099739. [DOI] [PubMed] [Google Scholar]
- Sitharaman B, Jacobson B, Wadghiri YZ, Bryant H, Frank J. The magnetic, relaxometric and optical properties of gadolinium-catalyzed single walled carbon nanotubes. J Appl Phys. 2013;113:134308. doi: 10.1063/1.4796183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames: The Iowa State University Press; 1974. [Google Scholar]
- Swierczewska M, Rusakova I, Sitharaman B. Gadolinium and europium catalyzed growth of single-walled carbon nanotubes. Carbon. 2009;47:3137–3142. [Google Scholar]
- Tobias G, Shao L, Ballesteros B, Green ML. Enhanced sidewall functionalization of single-wall carbon nanotubes using nitric acid. J Nanosci Nanotechnol. 2009;9(10):6072–6077. doi: 10.1166/jnn.2009.1567. [DOI] [PubMed] [Google Scholar]
- Yaron NY, Holt BD, Shor AP, Lösche M, Islam MF, Dahl KN. Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J Nanobiotechnology. 2011;9:45. doi: 10.1186/1477-3155-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28:527–35. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]