Abstract
Nitrate radical (NO3•) surface chemistry of indoor environments has not been well studied due to the difficulty in generating and maintaining NO3• at low concentrations for long term exposures. This article presents the Surface Chemistry Reactant Air Delivery and Experiment System (SCRADES), a novel feedback controlled system developed to deliver nitrate radicals at specified concentrations (50–500 ppt, ±30 ppt) and flow rates (500–2000 ml min−1) to a variety of indoor surfaces to initiate reaction chemistry for periods of up to 72 h. The system uses a cavity ring-down spectrometer (CRDS), with a detection limit of 1.7 ppt, to measure the concentration of NO3• supplied to a 24 l experiment chamber. Nitrate radicals are introduced via thermal decomposition of N2O5 and diluted with clean dry air until the desired concentration is achieved. Additionally, this article addresses details concerning NO3• loss through the system, consistency of the NO3• concentration delivered, and stability of the CRDS cavity over long exposure durations (72 h).
I. Introduction
A recent review by Weschler highlighted research progress in the last 20 years to characterize the dynamic nature of the indoor environment.1 This review focused on indoor chemistry and revealed that there are still several fundamental questions regarding indoor gas-phase and surface-phase chemistry. The article discusses the multitude of experimental evidence demonstrating that several initiator species such as ozone (O3), hydroxyl radicals (OH•), and nitrate radicals (NO3•) are present indoors and can react with volatile/semi-volatile organic compounds (VOCs/SVOCs) to produce oxygenated organic compounds such as: aldehydes, ketones, carboxylic acids, dicarbonyls, and organic nitrates.2–9 Although the gas-phase chemistry of these indoor initiators with VOCs/SVOCs has been studied extensively, the equivalent reactions on a variety of surfaces are still poorly understood. Furthermore, nitrate radical reactions are the least studied of these reactants due to the difficulties in generating NO3• for flow studies, measuring NO3• concentrations, and delivering known concentrations of NO3• for long term experiments.
The nitrate radical (NO3•) has been identified as the main reactive species in the nighttime outdoor environment10, 11 and has been recently measured in the indoor environment.12 A variety of sensitive analytical techniques have previously been employed to measure NO3• such as laser-induced fluorescence,13–17 direct optical absorption spectroscopy,18–24 Fourier-transform infrared spectroscopy,25 and cavity ring-down spectroscopy (CRDS).26–31 These measurements have been made due to an interest in measuring the transient nighttime concentrations of NO3• in the atmosphere, which can be in the sub-parts-per-trillion (ppt) to hundreds of ppt range. Over the last two decades, CRDS has gained popularity due to its high sensitivity and ability to monitor localized NO3• concentration fluctuations compared to long-path spectroscopy techniques. Recently, NO3• measurements were made indoors using a surrogate mass spectrometric technique based on the reaction of cyclohexene + NO3• and measuring the yield of cyclohexane nitrate.12 Using this technique, Nøjgaard was able to measure NO3• concentrations indoors from 1 to 58 ppt.
There have been only a few studies of the reactions of VOCs/SVOCs + indoor reactant (OH•, O3, or NO3•) on surfaces found in indoor environments.32–42 These studies have focused on surface reactions of VOCs derived from material emissions and reactions of indoor surfaces that have been coated with consumer cleaning products. Recently, our group developed a novel computer controlled feedback system, Field and Laboratory Emission Cell (FLEC) Automation and Control System (FACS) that could be used to deliver air mixed with a specified concentration of O3 at flow rates up to 2 l min−1 and at a relative humidity between 5% and 90%.43 This system has been used to investigate the surface reactions of O3 with two terpene alcohols (α-terpineol, dihydromyrcenol) and one consumer based pine-oil cleaner on glass and vinyl flooring tiles.35–37 Using this system, several new oxygenated products were observed compared to gas-phase product experiments indicating that surfaces can stabilize reaction intermediates and/or catalyze reactions to generate new oxidation products.
In this article, the development of a new feedback system, which delivers nitrate radicals (NO3•) to a surface at specified concentrations, relative humidity, and flow rate, will be discussed. The new system, although based on the previous FACS, incorporates: (1) N2O5/NO3• introduction, (2) cavity ring-down spectroscopy to measure NO3• concentrations, (3) new feedback controls, and (4) a newly developed Teflon experiment chamber for surface chemistry experiments. This article will discuss steps to generate NO3•, calibration and stability measurement results, and how this system will be used to initiate NO3• + VOC/SVOC reactions on a variety of indoor surfaces.
II. Instrument
A. Overview
The new Surface Chemistry Reactant Air Delivery and Experiment System (SCRADES) (Figure 1) is based on the previous FACS43 and is composed of three stages: the air purification stage, the air humidification stage, and the reactant injection and delivery stage. The air purification and air humidification stages have been previously described35–37,43 and remain unchanged from the original system except for modifications allowing for higher flow rates. The redesigned reactant injection and delivery stage begins by introducing NO3• into the air stream. After two dilution steps, the CRDS measures the NO3• concentration, after which the air continues to the experiment chamber.
Fig. 1.
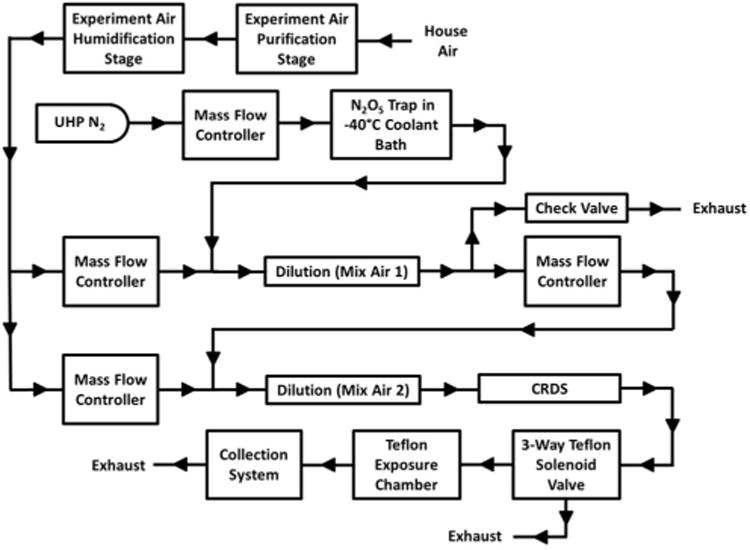
Flow diagram of the surface chemistry reactant air delivery and experiment system (SCRADES).
The entire system is controlled by a standard desktop computer which houses an analog output card, a multifunction data acquisition card, a multi-port serial card, and a highspeed analog data acquisition card. An additional analog output device and a digital input/output device are connected to the computer via Universal Serial Bus (USB) ports. The control program is a modified version of the original C/C++ Windows application that was written in-house and utilizes libraries provided by both National Instruments Corp. (NI) (Austin, TX) and Gage-Applied (Lockport, IL).
B. Design and assembly
1. N2O5 synthesis and transfer
Nitrate radicals are generated by thermal decomposition of N2O5 using a similar method as described by Atkinson et al.3 N2O5 (white, crystalline solid) is synthesized via mixing 0.5% NO2 (from a commercial gas cylinder) at 1.01 min−1 with O3 at 1.01 min−1 generated at 1.25 g h−1 (OSR-4, Ozone Solutions, Sioux Center, IA) using zero grade air into a 1.2 1 mixing tube (Ace glass, Vineland, NJ) as seen in reactions 1 and 2. The NO2/O3 mixture was allowed to flow from the mixing tube into a glass vacuum trap (8750-20, Ace glass, Vineland, NJ) which was partially submerged in an ethanol bath held at −75 °C. N2O5 was stored in the −75 °C bath until use in the feedback system. To minimize its surface loss by conversion to nitric acid, N2O5 was used within six days after synthesis.44
| (Reaction 1) |
| (Reaction 2) |
For CRDS experiments, the N2O5 trap was moved to another ethanol bath held at −40°C (±0.5 °C). This was done to increase the vapor pressure of N2O5 above the solid to 0.55 Torr.45 Ultrahigh purity (UHP) N2, beginning at 10.0 ml min−1, is then used to sweep N2O5/NO3• into the CRDS cavity. However, at this pressure, the concentration of N2O5 that is generated is ∼945 ppm (63 ppm NO3•), which is considerably greater than indoor measurements made by Nøjgaard (<60 ppt).12 As a consequence, serial dilution was incorporated using two mixing cavities. N2O5/NO3• from the trap is mixed with clean, dry air at a ratio of 1:100 into a 1.18 l borosilicate glass column (Mix Air 1). The reduced N2O5/NO3• concentration was further diluted using clean, dry air at a ratio 1:49 in a 79 ml borosilicate glass column (Mix Air 2) before delivery to the CRDS optical cavity. All mixing cavities and the optical cavity are at room temperature, typically 295–298 K. At these temperatures, the ratio of N2O5/NO3• is ∼15:1.16
Based on the NO3• concentration derived from the CRDS, the airflows are adjusted to meet the target concentration (100 ppt, ±30 ppt). Large, coarse concentration adjustments are made by altering the flows through the second stage dilution chamber (Mix Air 2) while fine adjustments are made by altering the flow of UHP N2 through the trap. Additionally, in order to reduce the loss of NO3• due to photolytic degradation, the dilution system and the CRDS are in a light tight enclosure. All of the tubing outside of the enclosure has been incased in black heat-shrink tubing.
Initially, an attempt was made to generate NO3• in a constant flow system by introducing NO2 (150 ppb) and O3 (75 ppb) into a 1 1 mixing tube at 300 ml min−1. However, given the formation rate of NO3• at 7.87 × 10−7 ppb−1 s−1 and the residence time of the mixing cavity, only 2.1% of the O3 reacted. Given these parameters, the amount of NO3• that can be generated at room temperature is ∼13 ppt. Although this NO3• concentration lies in the regime of both the predicted and measured range, the ability to generate NO3• consistently without fluctuations in concentration was difficult. Also, due to an absorption band in the 662 nm region, large concentrations of NO2 could interfere with measurements of NO3• as described below. For this reason, solid N2O5 was synthesized and transferred to the optical cavity as described above.
2. CRDS
a. CRDS theory
CRDS has been reviewed extensively,28,46–52 so only a brief description will be given here. This absorption technique is based on measuring the loss of light over time in an optical cavity constructed of two highly reflective mirrors (typically, R > 99.990) and is given by the equation I(t) = Io exp (−t/τ), where Io = initial intensity of light, t = time, and τ is the ring-down time.
A CRDS measurement consists of a short duration of light (100 μs for this system) injected into an optical cavity and then observing the intensity decay over time. This measurement of the characteristic decay time constants in the presence and absence of an absorber (i.e., NO3•) yields its concentration for a known absorption cross section (σ), and is calculated using a derivation of Beer's Law, Eq. (1), where α is the absorption coefficient (cm−1), σ is the absorption cross section (cm2 molecule−1), RL is the ratio of the cavity length (L) to the length over which the absorber is present (LA), c is the speed of light, and τNO3 and τempty are the ring-down times with and without the presence of absorber
| (1) |
b. CRDS system design
The CRDS system consists of a diode laser, optical isolator, Teflon optical cavity, two highly reflective mirrors, and a photomultiplier tube (PMT). A 662± 0.4 nm continuous wave diode laser (IQμ1H, Power Technology, Inc., Little Rock, AR), modulated at a frequency of 1500 Hz with a 15% duty cycle, passes through an optical isolator (IO-3-662-LP, OFR/Thorlabs, Verona, NJ) and into a 1.0 in. i.d. × 24.0 in. Teflon tube which forms the optical cavity. The ends of the optical cavity consist of two 1.0 in. highly reflective mirrors (R > 99.995, radius of curvature = 1 m, Advance Thin Films, Boulder, CO) which are held by custom designed Delrin mounts connected to the cavity via steel bellows (150-96-3-l, Standard Bellows Co., Windsor Locks, CT). The mirror alignment is adjusted using Starrett (Athol, MA) 263L micrometer heads. A set of tension springs maintains the integrity and stability of the mirror mounts and the alignment heads. The light exiting the optical cavity is detected using an 8 MHz PMT (H10492-013, Hamamatsu Corp., Bridgewater, NJ). The PMT signal is captured by a Gage-Applied 14-bit data acquisition card (Octopus CompuScope 8327, Lockport, IL) which is triggered by the laser modulation signal. There are two main sources of signal noise. The first derives from the 8 MHz bandwidth of the PMT. The second comes from the building electrical system and appears as low amplitude 60 Hz ripple. Therefore, the 1500 data records from a one second event are averaged in order to reduce this signal noise, then processed to determine the ring-down time. When the optical cavity was evacuated to 50 Torr, the empty cavity ring-down time was measured to be 173 μs.
There are several interferences that are inherent with measuring NO3• concentrations by CRDS at 662 nm. The absorption cross section (σNO3• at 662 nm) is 2.1 × 10−17 cm2 molecule−1 at 298 K; however, there is also a small absorption by NO2 (a byproduct of N2O5 decomposition), σNO2 = 5.6 × 10−21 cm2 molecule−1 at 298 K.53 The four orders of magnitude difference in absorption cross sections reduce the impact of NO2 on the measured ring-down time. As seen in reaction (2), thermal decomposition of N2O5 generates NO3• and NO2 in a 1:1 ratio. Given this, the concentration of NO2 contributes approximately 0.1%–0.2% to the measured ring-down time. Residual N2O5 from incomplete decomposition has a negligible absorption cross section of σN2O5 = 1.0 × 10−24 cm2 molecule−1 at 298 K.53 Water may also interfere with the ring-down time due to absorption lines (σN2O = 1.06 × 10−26 cm2 molecule−1) at 662 nm. Since τempty is determined using the same relative humidity as the experiment, this interference becomes incorporated into the background for subsequent concentration measurements. Furthermore, hydrolysis reactions of N2O5 may be catalyzed on surfaces at higher humidity. Therefore, all experiments thus far have been done at 5% RH.10
3. Teflon experiment chamber
Due to the reactivity of NO3• with water absorbed to stainless steel and the possible production of nitric acid, a new inert experiment chamber was designed. The chamber consists of a black anodized aluminum box, measuring 15 in. × 15 in. × 6.5 in. (38.1 cm × 38.1 cm × 16.5 cm) inside, with all of the interior surfaces lined with 2-mil Teflon sheeting (American Durafilm, Hollistan, MA). The internal volume of the chamber is ∼23.97 1 and has an air-exchange rate of 1.25 h−1 at 500 ml min−1. The N2O5/NO3• air mixture enters the top of the chamber at the center via a Teflon nozzle designed to evenly distribute the air/NO3• mixture to the surface. The test surface rests on a Teflon support plate with raised sides, in order to minimize edge exposure to the reactant. The test bed sits in the center of the chamber and rests on a Teflon pedestal that doubles as the exhaust port. The gas mixture exits the bottom of chamber from the center and proceeds to the collection system, as previously described.35–37,43
C. Control
As stated above, the system is controlled by a desktop computer with four additional Peripheral Component Interconnect (PCI)-bus cards installed. The additional cards consist of one NI PCI-6040E Multifunction I/O Data Acquisition board, one NI PCI-6713 Analog Output board (National Instruments Corp., Austin, TX), one Sealevel Systems (Liberty, SC) Comm+8.LPCI eight-port serial board, and the aforementioned Gage-Applied card.
The computer runs an application that was written using Microsoft (Redmond, WA) Visual C/C++ 6.0. This application allows the researcher to define the parameters of the experiment such as flow rate through the experiment chamber, relative humidity of the experiment air, target NO3• concentration, duration of the experiment, and the recording interval for data collection. The system continually monitors all parameters and makes adjustments accordingly. A proportional-integral-differential (PID) algorithm controls the adjustment of the NO3• concentration. Due to the low flow rates, mixing chamber sizes, and CRDS cavity size, there is a lag time of nearly 3 min between adjustments and the detection of concentration changes. The air temperature and humidity are measured using the in-house developed in-line sensor units described previously.43
III. Experiments
A. NO3• loss through system
Previous reports have shown that NO3• can react with water absorbed to metal surfaces and/or be removed by decomposition/reaction on Teflon walls.29,30,54 Dube et al. observed a first order loss rate of NO3• on the Teflon inlet walls of their CRDS instrument of approximately 0.2 s−1.29 To determine the potential loss of NO3• throughout the system described here, three-way Teflon solenoid valves were temporarily installed to allow alternate air flow paths. First, air flowed through the system as originally described with the amount of N2 going through the N2O5 trap set to a constant flow rate. Once the system achieved stability, the concentration and ring-down time were recorded. The flow was then altered so that air flowed from the mixing chambers, through the experiment chamber, and then to the CRDS. When the new flow path regained stability, the new concentration and ring-down times were recorded.
This series of experiments were performed using UHP N2 flow rates of 5, 10, and 15 ml min−1 through the N2O5 trap while flows through both dilution chambers were held constant. Using this data, a loss curve was generated allowing the determination for the required concentration of NO3• through the CRDS cavity in order to have the specified concentration of reactant delivered to a surface inside the experiment chamber. The percent loss of NO3• at each flow rate was determined five times for statistical analysis. This relationship was used to calculate the required concentration of NO3• within the CRDS in order to achieve the target concentration inside the chamber.
B. Stability profiles of CRDS: τempty and τNO3•
The NO3• concentration is calculated every 5 s using a stored τempty value that is measured before an experiment has begun. However, if τempty drifts as a function of time, then the calculated NO3• concentrations will have an associated error due to this drift. In typical CRDS measurements of NO3•, NO is added as a titrant to remove NO3• from the cavity so that τempty can be periodically determined throughout a measurement. This reaction occurs quickly with a rate constant of 2.6 × 10−11 cm3 molecule−1 s−1 and results in the formation of 2NO2 molecules. Unfortunately, this additional NO2 to the system may interfere with surface chemistry experiments as well as introduce an error to the CRDS ring-down time. Furthermore, the system is designed to provide continuous specified concentrations of NO3• for up to 72 h without interruptions in flow which would occur with periodic injections of NO. To determine the amount of drift in the system, a series of τempty measurements were collected and analyzed to determine its stability profile. τempty was determined five times at a flow rate of 500 ml min−1 and 5% relative humidity over 72 h.
IV. Results and Discussion
A. Detection of NO3•
Using the CRDS system described above, ring-down times were collected with NO3• (τNO3) and without NO3• (τempty) present. The natural logs of the ring-down signals were plotted and are shown in Figure 2. The linearity of the ring-down signal indicates no additional interferences were contained within the signal. Additionally, Figure 2 shows that when NO3• is present, the expected subsequent decrease in the ring-down time (110.4 μs) was observed; in this case at a concentration of 124 ppt. For this system, the detection limit was determined to be 1.7 ppt. Due to environmental issues, such as barometric pressure, and the aging of the N2O5,44,55 the N2O5/NO3• concentration ratio leaving the trap can vary over time. Therefore, the feedback system is designed to maintain the concentration within the experiment chamber at ±30 ppt of that specified by the user.
Fig. 2.
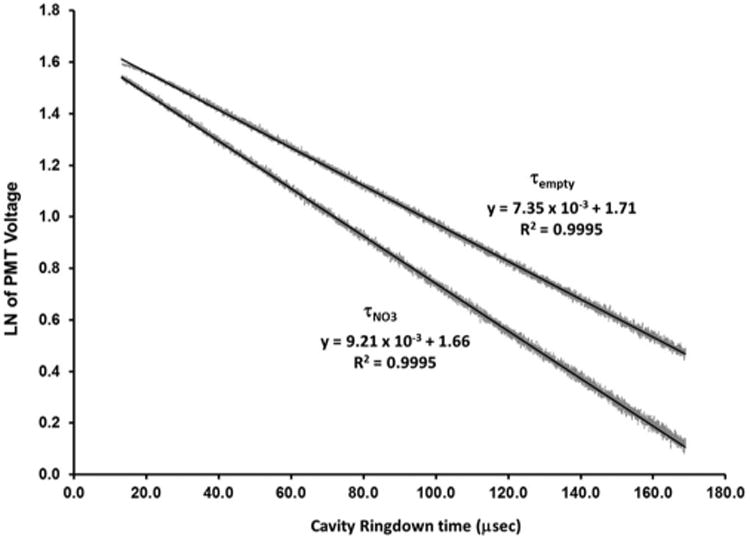
Log plots of ring-down traces for τempty (no NO3•) and τNO3 (124 ppt NO3•) with linear fit.
The system determines τempty by generating a 5% RH atmosphere and calculating a 1 min rolling average for 30 min. This ring-down value is then stored in a data file and used for all subsequent experiments until a new value is measured. In practice, the user determines a new τempty prior to each experiment.
B. Drift of τempty over time
Huang and Lehmann observed that changes in barometric pressure can affect the ring-down times of a CRDS cavity.55 For this system, several experiments were ran using clean air (no NO3•) at 5% RH for a period of 48 h or longer. Figure 3 shows the results of one such experiment. In this plot, the mean value for τempty is 140.3 μs with a standard deviation of 1.6 μs. As can be seen in Table I, the standard deviations range from 1.0 to 2.9. In the worst case, with a standard deviation of 2.9 μs, the calculated error is ±11 ppt (or 11%) for a target concentration of 100 ppt. Due to the system's ±30 ppt control band around the target concentration, the potential drift error becomes irrelevant.
Fig. 3.
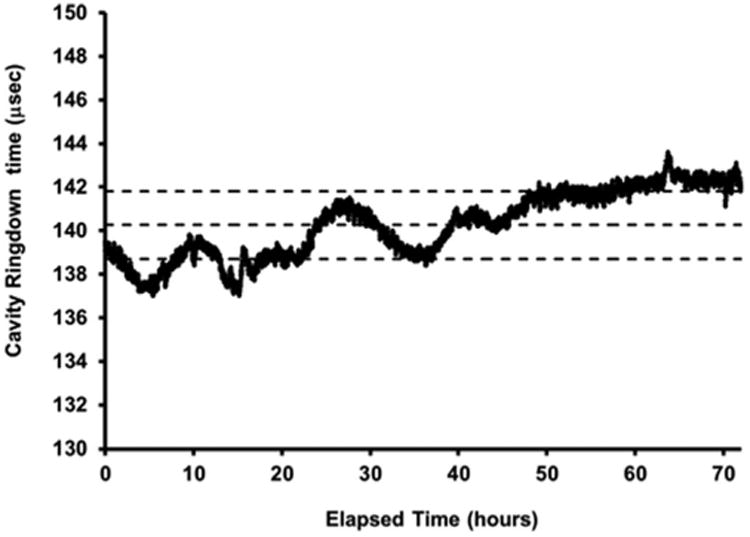
Drift of τempty over a 72 h period with mean ring-down time (140.3 μs) bracketed by standard deviation (±1.6).
Table I.
Results of CRDS τempty (no NO3•) drift experiments.
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Experiment 5 | |
|---|---|---|---|---|---|
| Elapsed time (hours) | 48 | 67 | 96 | 72 | 72 |
| τempty | 147.5 | 137.5 | 141.2 | 149.6 | 140.3 |
| Average (s.d.) (μs) | (±2.9) | (±2.5) | (±2.9) | (±1.0) | (±1.6) |
C. NO3• loss and target NO3• determination
The results of the NO3• loss experiments can be seen in Figure 4. N2 flow rates through the trap gave overall percent NO3• losses through the system for 5 ml min−1, 10 m l min−1, and 15 ml min−1 as 57.0% (±9.9), 50.2% (±12.9), and 41.5% (±10.6), respectively. The flow rates through the dilution chambers, CRDS cavity, and experiment chamber were held constant. Subsequent plots of the data indicated a linear relationship that can be described using Eq. (2). The decrease in percentage loss of NO3• at higher flow rates can be attributed to the limited uptake capacity of the Teflon tubing walls, as previously described by Dube et al.29
Fig. 4.
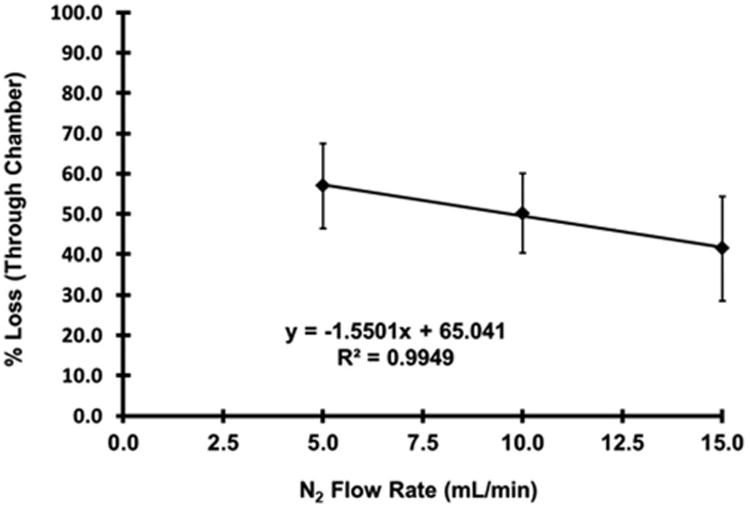
Percent loss of NO3• between the CRDS cavity and the experiment chamber based on N2 flow through the N2O5 trap. Error bars represent the standard deviations from five separate experiments at each flow rate.
| (2) |
| (3) |
Using Eq. (2), the system determines the loss based upon the N2 flow rate through the trap. Using Eq. (3), the system then determines the ideal measurement from the CRDS to produce the desired NO3• concentration (i.e., 100 ppt) within the experiment chamber. The difference between the actual measured concentration and the ideal measured concentration is the feedback provided to the PID control algorithm.
Figure 5 shows the data from one 72 h experiment. The green data points indicate the ideal concentration to be measured by the CRDS based upon Eq. (3), which changes as the flow of N2 through the N2O5 trap changes. The red data points show the actual concentration measured by the CRDS. As can be seen, the concentration fluctuates but the system adjusts flows in order to maintain it. These flow adjustments result in the changes seen in the green data. The blue data indicate the calculated NO3• concentration within the experiment chamber, which is specified at 100 ppt, ±30 ppt. The fluctuations in the calculated NO3• concentration within the experiment chamber (blue) are significantly less than those shown by the measured concentration (red). This is due to the large amount of NO3• lost (40%–60%), as calculated from Eqs. (2) and (3).
Fig. 5.
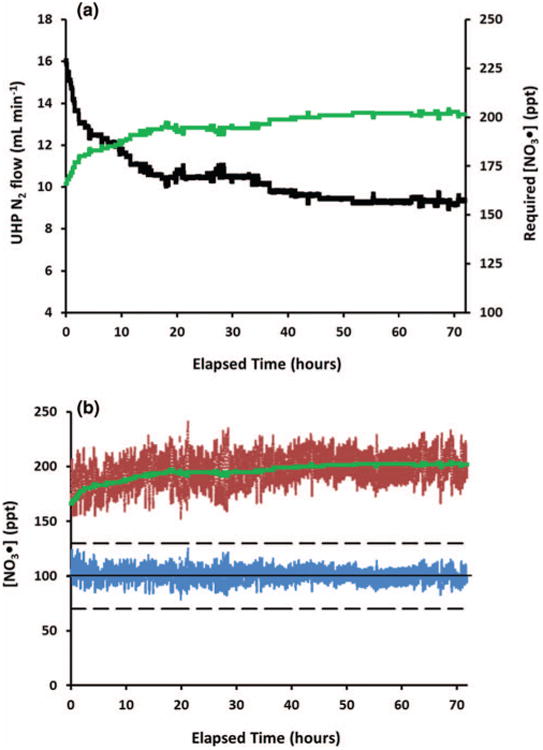
Calculated ideal NO3• concentration to be measured by CRDS based on N2 flow through N2O5 trap (green). Actual NO3• concentration measured by CRDS (red). Calculated NO3• concentration in experiment chamber (blue).
D. Indoor nitrate radical surface chemistry
Indoor environment models by Sarwar et al.56 and recent measurements by Nøjgaard12 indicate that NO3• exists indoors in the 1–60 ppt range. At these concentrations and given a typical air exchange 0.6 h−1,57 gas-phase reactions of NO3• with VOCs/SVOCs are important. For example, NO3• reactions with terpenes such as limonene, terpinolene, and α-terpinene have pseudo-first order rate constants of 1.1, 8.6, and 16 h−1, respectively.58 This data suggests that these reactions will likely occur before removal by air exchange leading to the formation of oxidation products indoors. However, surface-phase reactions may be more influential indoors than gas-phase reactions as air exchange becomes less important. Furthermore, research has shown that several surface-bound species have higher reaction probabilities than their respective gas-phase reactions.59
V. Conclusions
The novel SCRADES is able to generate NO3• at specified concentrations (50–500 ppt) and at specified flow rates (500– 2000 ml min−1) to the experiment chamber for over 72 h. This system will be used to deliver NO3• at concentrations that exist indoors in order to initiate reactions on a variety of indoor surfaces. Initial experiments have focused on low humidity (i.e., 5%) in order to reduce loss of NO3• due to reactions with water. Future tests of the system will include increasing the RH to 50% in order to mimic typical indoor air conditions. Data from these experiments will be useful in developing more accurate exposure assessments, better analytical tools for health hazard evaluations and industrial hygiene sampling, and improved engineering control methods to reduce chemical contaminants.
Acknowledgments
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Weschler CJ. Indoor Air. 2011;21:205. doi: 10.1111/j.1600-0668.2011.00713.x. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson R, Arey J. Chem Rev. 2003;103:4605. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson R, Plum CN, Carter WPL, Winer AM, Pitts JN. J Phys Chem. 1984;88:1210. [Google Scholar]
- 4.Atkinson R, Tuazon EC, Aschmann SM. Environ Sci Technol. 1995;29:1860. doi: 10.1021/es00007a025. [DOI] [PubMed] [Google Scholar]
- 5.Forester CD, Ham JE, Wells JR. Atmos Environ. 2007;41:1188. [Google Scholar]
- 6.Jones BT, Ham JE. Atmos Environ. 2008;42:6689. [Google Scholar]
- 7.Wells JR. Environ Sci Technol. 2005;39:6937. doi: 10.1021/es0481676. [DOI] [PubMed] [Google Scholar]
- 8.Weschler CJ. Sci World J. 2001;1:443. [Google Scholar]
- 9.Weschler CJ. Indoor Air. 2004;14:184. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson-Pitts BJ, Pitts JJN. Chemistry of the Upper and Lower Atmosphere. Academic; New York: 2000. p. 8. [Google Scholar]
- 11.Seinfeld JH, Spandis SN. Atmospheric Chemistry and Physics. Wiley; NJ: 2006. p. 250. [Google Scholar]
- 12.Nojgaard JK. Chemosphere. 2010;79:898. doi: 10.1016/j.chemosphere.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Brown SS, Dube WP, Osthoff HD, Stutz J, Ryerson TB, Wollny AG, Brock CA, Warneke C, De Gouw JA, Atlas E, Neuman JA, Holloway JS, Lerner BM, Williams EJ, Kuster WC, Goldan PD, Angevine WM, Trainer M, Fehsenfeld FC, Ravishankara AR. J Geophys Res, [Atmos] 2007;112:17. doi: 10.1029/2007JD008883. [DOI] [Google Scholar]
- 14.Ishiwata T, Fujiwara I, Naruge Y, Obi K, Tanaka I. J Phys Chem. 1983;87:1349. [Google Scholar]
- 15.Ishiwata T, Tanaka I, Kawaguchi K, Hirota E. Bull Soc Chim Belg. 1983;92:502. [Google Scholar]
- 16.Matsumoto J, Kosugi N, Imai H, Kajii Y. Rev Sci Instrum. 2005;76:064101. [Google Scholar]
- 17.Wood EC, Wooldridge PJ, Freese JH, Albrecht T, Cohen RC. Environ Sci Technol. 2003;37:5732. doi: 10.1021/es034507w. [DOI] [PubMed] [Google Scholar]
- 18.Allan BJ, Plane JMC, Coe H, Shillito J. J Geophys Res, [Atmos] 2002;107:4588. doi: 10.1029/2002jd002112. [DOI] [Google Scholar]
- 19.Biermann HW, Winer AM, Pitts JN. Abstr Pap - Am Chem Soc. 1986;192:144. [Google Scholar]
- 20.Geyer A, Alicke B, Ackermann R, Martinez M, Harder H, Brune W, di Carlo P, Williams E, Jobson T, Hall S, Shetter R, Stutz J. J Geophys Res, [Atmos] 2003;108:4368. doi: 10.1029/2002jd002967. [DOI] [Google Scholar]
- 21.Li S, Liu W, Xie P, Li A, Qin M, Dou K. Adv Atmos Sci. 2007;24:875. [Google Scholar]
- 22.Stutz J, Alicke B, Ackermann R, Geyer A, White A, Williams E. J Geophys Res, [Atmos] 2004;109:D12306. doi: 10.1029/2003jd004209. [DOI] [Google Scholar]
- 23.Stutz J, Wong KW, Lawrence L, Ziemba L, Flynn JH, Rappenglueck B, Lefer B. Atmos Environ. 2010;44:4099. [Google Scholar]
- 24.von Friedeburg C, Wagner T, Geyer A, Kaiser N, Vogel B, Vogel H, Platt U. J Geophys Res, [Atmos] 2002;107:4168. doi: 10.1029/2001jd000481. [DOI] [Google Scholar]
- 25.Mentel TF, Bleilebens D, Wahner A. Atmos Chem Phys. 1996;30:4007. [Google Scholar]
- 26.Ayers JD, Apodaca RL, Simpson WR, Baer DS. Appl Opt. 2005;44:7239. doi: 10.1364/ao.44.007239. [DOI] [PubMed] [Google Scholar]
- 27.Brown SS, Stark H, Ciciora SJ, McLaughlin RJ, Ravishankara AR. Rev Sci Instrum. 2002;73:3291. [Google Scholar]
- 28.Brown SS, Stark H, Ravishankara AR. Appl Phys B. 2002;75:173. [Google Scholar]
- 29.Dube WP, Brown SS, Osthoff HD, Nunley MR, Ciciora SJ, Paris MW, McLaughlin RJ, Ravishankara AR. Rev Sci Instrum. 2006;77:034101. [Google Scholar]
- 30.Odame-Ankrah CA, Osthoff HD. Appl Spectrosc. 2011;65:1260. doi: 10.1366/11-06384. [DOI] [PubMed] [Google Scholar]
- 31.Wagner NL, Dube WP, Washenfelder RA, Young CJ, Pollack IB, Ryerson TB, Brown SS. Atmos Meas Tech. 2011;4:1227. [Google Scholar]
- 32.Disselkamp RS, Carpenter MA, Cowin JP, Berkowitz CM, Chapman EG, Zaveri RA, Laulainen NS. J Geophys Res, [Atmos] 2000;105:9767. doi: 10.1029/1999JD901189. [DOI] [Google Scholar]
- 33.Gratpanche F, Sawerysyn JP. J Chim Phys Phys Chim Biol. 1999;96:213. [Google Scholar]
- 34.Grossi CM, Brimblecombe P. J Phys Iv. 2002;12:197. [Google Scholar]
- 35.Ham JE, Wells JR. Indoor Air. 2008;18:394. doi: 10.1111/j.1600-0668.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- 36.Ham JE, Wells JR. Atmos Environ. 2009;43:4023. [Google Scholar]
- 37.Ham JE, Wells JR. Chemosphere. 2011;83:327. doi: 10.1016/j.chemosphere.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Reiss R, Ryan PB, Koutrakis P, Tibbetts SJ. Environ Sci Technol. 1995;29:1906. doi: 10.1021/es00008a007. [DOI] [PubMed] [Google Scholar]
- 39.Shu S, Morrison GC. Environ Sci Technol. 2011;45:4285. doi: 10.1021/es200194e. [DOI] [PubMed] [Google Scholar]
- 40.Shu S, Morrison GC. Atmos Environ. 2012;47:421. [Google Scholar]
- 41.Springs M, Wells JR, Morrison GC. Indoor Air. 2011;21:319. doi: 10.1111/j.1600-0668.2010.00707.x. [DOI] [PubMed] [Google Scholar]
- 42.Toftum J, Feund S, Salthammer T, Weschler CJ. Atmos Environ. 2008;42:7632. [Google Scholar]
- 43.Flemmer MM, Ham JE, Wells JR. Rev Sci Instrum. 2007;78:014101. doi: 10.1063/1.2432243. [DOI] [PubMed] [Google Scholar]
- 44.Perraud V, Bruns EA, Ezell MJ, Johnson SN, Greaves J, Finlayson-Pitts BJ. Environ Sci Technol. 2010;44:5887. doi: 10.1021/es1005658. [DOI] [PubMed] [Google Scholar]
- 45.McDaniel AH, Davidson JA, Cantrell CA, Shetter RE, Calvert JG. J Phys Chem. 1988;92:4172. [Google Scholar]
- 46.Brown SS. Chem Rev. 2003;103:5219. doi: 10.1021/cr020645c. [DOI] [PubMed] [Google Scholar]
- 47.Clemitshaw KC. Crit Rev Environ Sci Technol. 2004;34:1. [Google Scholar]
- 48.Fiddler MN, Begashaw I, Mickens MA, Collingwood MS, Assefa Z, Bililign S. Sensors. 2009;9:10447. doi: 10.3390/s91210447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedrichs G, Phys Z. Chem-Int J Res Phys Chem Chem Phys. 2008;222:31. [Google Scholar]
- 50.Paldus BA, Kachanov AA. Can J Phys. 2005;83:975. [Google Scholar]
- 51.Scherer JJ, Paul JB, Collier CP, Okeefe A, Rakestraw DJ, Saykally RJ. Spectroscopy. 1996;11:46. [Google Scholar]
- 52.Scherer JJ, Paul JB, Okeefe A, Saykally RJ. Chem Rev. 1997;97:25. doi: 10.1021/cr930048d. [DOI] [PubMed] [Google Scholar]
- 53.Keller-Rudek H, Moortgat GK. Mainz; Germany: 2012. MPI-Mainz-UV-Vis Spectral Atlas of Gaseous Molecules. see http://www.atmosphere.mpg.de/enid/2295. [Google Scholar]
- 54.Fuchs H, Dube WP, Cicioira SJ, Brown SS. Anal Chem. 2008;80:6010. doi: 10.1021/ac8007253. [DOI] [PubMed] [Google Scholar]
- 55.Huang HF, Lehmann KK. Appl Opt. 2010;49:1378. doi: 10.1364/AO.49.001378. [DOI] [PubMed] [Google Scholar]
- 56.Sarwar G, Corsi R, Allen D, Weschler CJ. Indoor Air 2002. Vol. 2002. Monterey, CA: 2002. p. 80. [Google Scholar]
- 57.Wilson AL, Colome SD, Tian Y, Becker EW, Baker PE, Behrens DW, Billick IH, Garrison CA. J Expo Anal Environ Epidemiol. 1996;6:311. [PubMed] [Google Scholar]
- 58.Nazaroff WW, Weschler CJ. Atmos Environ. 2004;38:2841. [Google Scholar]
- 59.Voges AB, Stokes GY, Gibbs-Davis JM, Lettan RB, Bertin PA, Pike RC, Nguyen ST, Scheidt KA, Geiger FM. J Phys Chem C. 2007;111:1567. [Google Scholar]


