Abstract
Background
Although morbidity and mortality rates from asthma are highest in patients > 65 years of age, the effect of older age on airway inflammation in asthma is not well established.
Objective
To investigate age-related differences in the promotion of allergic inflammation after influenza A viral respiratory infection on antigen specific IgE production, antigen-induced airway inflammation and airway hyper-responsiveness in mice.
Methods
To accomplish this objective, the following model system was used. Young (six-week) and aged (18-month) BALB/c mice were first infected with a non-lethal dose of influenza virus A (H/HK×31). Mice were then ovalbumin (OVA) sensitized during the acute-infection (3-days post inoculation) and then chronically underwent challenge to the airways with OVA. Forty-eight hours after the final OVA-challenge, airway hyperresponsiveness (AHR), bronchoalveolar fluid (BALF) cellular and cytokine profile, antigen-specific IgE and IgG1, and lung tissue inflammation were measured.
Results
Age-specific differences were noted on the effect of a viral infection, allergic sensitization, airway inflammation and airway hyperresponsiveness. Serum OVA-specific IgE was significantly increased in only the aged mice infected with influenza virus. Despite greater morbidity (e.g. weight loss and sickness scores) during the acute infection in the 18-month old mice that were OVA-sensitized there was little effect on the AHR and BALF cellular differential. In contrast, BALF neutrophils and AHR increased, but eosinophils decreased in 6-week mice that were OVA-sensitized during an acute influenza infection.
Conclusion
With increased age in a mouse model, viral infection prior to antigen sensitization affects the airway and systemic allergic response differently. These differences may reflect distinct phenotypic features of allergic inflammation in older patients with asthma
Introduction
The effect of age on antigen sensitization, antigen-induced airway inflammation, and subsequent development of asthma are not well established. Asthma in older adults is an important and unmet area of research as disease morbidity and mortality rates are the highest in patients over 65 years of age [1]. Although there has been considerable study on the influence of early developmental processes of allergic airway inflammation, including respiratory infections, on the onset of childhood asthma, little is known about the other extreme age of life, older patients. Older patients are at increased risk for respiratory infections, which may serve to induce late onset asthma in patients over the age of 60 years [2].
Respiratory viral infections in infancy have been associated with an increased risk for the development of asthma, particularly in the presence of allergen sensitization [3-7] [8-10]. Observational studies have also reported that nearly 50% of subjects with asthma onset after the age of 60 years have had a prior respiratory infection [2]. Despite our understanding of the role of viral infections on childhood onset asthma, the effect of viral infection on allergen sensitization and eventual development of features of asthma have not been well characterized.
The following study was designed to address whether age affects the response to an acute respiratory infection with influenza A virus with subsequent antigen sensitization, allergic airway inflammation and airway responsiveness in a mouse model. The underlying goals of these animal models are to gain further insight into characteristics and mechanisms of asthma in older patients.
Materials and Methods
Animals
Young (6-weeks) female BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Aged (18-months) female BALB/c mice were obtained from the National Institutes of Aging (NIA, Bethesda, MD, USA). The ages of mice represent approximately 15-18 and 60 human years, respectively, based upon the 24 month life span of BALB/c mice [11] and the life expectancy of 80.4 years of human females (source National Center for Health Statistics, www.cdc.gov/nchs). The ages were chosen to represent early and late adulthood. Mice were maintained in the animal facility at Mount Sinai School of Medicine following standard guidelines for laboratory animal care and with institutional permission for animal handling. Mice were housed in the same facility to normalize gut flora. (Preliminary data on lung histology, lung cytokine expression and airway function revealed no statistical differences between antigen sensitized and challenged mice purchased from Jackson Laboratories who were allowed to age in our facilities, and similarly antigen-treated and aged mice obtained from the N.I.A.) Peripheral blood for complete blood cell count and differential was collected at the time of sacrifice by cardiac puncture and transfer of blood to EDTA-coated tubes.
Infection of mice with influenza A virus
The HK×31 (H3N2) flu virus was grown in 10 day embryonated chicken eggs (SPAFAS; Charles River Laboratories, Wilmington, MA, USA). Egg's allantoic fluid was snap frozen in ethanol-dry bath and stored at -80°C. Mice were infected using an Inhalation Exposure System A42X (Glass-Col, Terre Haute, IN, USA). Influenza stock virus titer was prepared at 106.8 pfu/25μl; 240μl of this stock virus was added to 12ml of PBS to prepare the viral solution. The virus solution was placed inside of a sterile glass nebulizer connected to the infection chamber. Total exposure time with aerosolized virus was 30 minutes. Under these conditions, 100% of animals were infected and showed reproducible lung titers in several experiments at every time point analyzed as described previously[12, 13]. Animals were weighed and monitored for signs of illness (using a published sickness score [14]) until sacrifice. Young and aged mice were infected with the ×31 Influenza virus on day -3 of the protocol (Figure 1).
Figure 1.

Experimental protocol. Mice (6-weeks, 18-months) were infected with influenza A 3 days prior to antigen sensitization. Mice were sensitized intraperitoneally (i.p) with 100μg OVA absorbed with 2mg alum in 0.4ml PBS on days 0 and 7. Ten days after the last sensitization dosing, mice were anesthetized i.p. with ketamine and xylazine and challenged intratracheally (i.t.) for a total of four times with 100μg OVA on days 17, 24, 31 and 32. Control mice consisted of: age-matched antigen sensitized and challenged alone, virally infected alone (day -3), and naïve mice. Forty-eight hours after the final OVA challenge the mice were sacrificed.
To verify that mice were infected with influenza, influenza A-specific IgG1 was measured in serum that was collected at sacrifice. A 96-well plate was coated overnight at 4°C with 100μl of Influenza A virus H3N2 (Abcam, Cambridge, MA, USA) cell lysate in PBS. Coating concentration was 0.05μg/ml. Coated wells were blocked with PBS+3%BSA for 2 hrs at RT. Diluted serum was added to the wells and incubated again for 2 hrs at RT. Virus was detected using HRP conjugated goat anti-mouse IgG (incubated 1 hr at RT, 1:4000 dilution), followed by TMB substrate and read at wavelength 450 nm.
Antigen sensitization and bronchial challenge protocols
Three days following the influenza A infection, mice were antigen sensitized intraperitoneally (i.p.) with 100μg OVA (Grade VI, Sigma, St. Louis, MO, USA) absorbed with 2mg alum (Pierce Biotechnology, Inc.) in 0.4ml PBS on days 0 and 7 (Figure 1). Mice were sensitized intrapertioneally, to determine differences in sensitization independent of an altered airway response which may occur after infection. Ten days after the last sensitization, mice were anesthetized i.p. with ketamine and xylazine and challenged intratracheally (i.t.) for a total of four times on days 17, 24, 31, 32 with 100μg OVA in 0.05ml PBS. Intratracheal (i.t.) antigen challenge was performed as previously described [15]. Mice sensitized and challenged with OVA during acute infected are labeled as “Acute-infection/OVA-mice” in the text (n=5-7/age group). Control mice were: age-matched, non-infected, OVA sensitized and challenged mice (“OVA-mice,” n=5-7/age group), naïve mice and infected-alone mice (“Infected alone-mice”, n=5-7/age group).
Determination of serum ovalbumin-specific immunoglobulins
Sera were obtained from each group of mice 48 hours after final OVA challenge and stored at -80°C. OVA-specific IgE and OVA-specific IgG1 were assayed using ELISA kits according to manufacturer's instructions (Chondrex, Inc., Redmond, WA, USA). To measure IgE, plates were coated with rat anti-mouse IgE antibody and incubated overnight at 4°C. After washing and blocking, 100μl of sample or standard was added and once again incubated overnight at 4°C. On day 3, plates were washed and biotinylated ovalbumin was added for 90 minutes, RT. OVA-specific IgE was detected following incubation with strepavidin peroxidase and TMB substrate. To measure IgG1, plates were coated with 10μg/ml OVA and incubated overnight at 4°C. After washing and blocking, 100μl of sample or standard was added and again incubated overnight at 4°C. On day 3, plates were washed and peroxidase-conjugated goat anti-mouse IgG1 antibody was added for 2 hours, RT. OVA-specific IgG1 was detected following incubation with TMB substrate. Plates were read at wavelength of 450 nm.
Measurement of allergic airway inflammation
BALF preparation and cell differential counts
To determine differences on the effect of age on allergic airway inflammation, BALF was collected 48 hours after the final antigen challenge. To collect BALF, the chest was opened and the lungs lavaged with 1.0ml cold PBS, which was then placed into chilled tubes. The cell pellet was re-suspended in PBS and total cell numbers were determined using a hemocytometer. Cytospin preparations were made with a cytocentrifuge and then stained with Diff-Quick (Dade Diagnostics of PR, Aguada, PR). Cell differential counts were obtained by counting at least 500 cells per slide by light microscopy. The supernatant was transferred and stored at -80°C for cytokine measurement.
BALF cytokines and chemokines
BALF supernatant was assayed for a panel of cytokines and chemokines (selected to access Th1/Th2 expression and chemokines associated with recruitment of neutrophils and eosinophils) using a multiplex-assay (Milliplex Mouse Cytokine/Chemokine Magnetic Bead, Billerica, MA, USA) according to manufacturer's instructions and performed in the Mount Sinai CTAD facility. Briefly, samples, standards, and controls were added to the appropriate wells. The premixed magnetic beads were then added to each well and incubated on a plate shaker for 16 to 18 hours at 4°C. After washing, Detection Antibody was added to each well and incubated on a plate shaker for 1 hour at RT followed by Strepavidin-Phycoerythrin for 30 minutes. The plate was run on the Luminex 200 system (Luminex Corp., Austin, TX, USA) and data was analyzed using MILLIPLEX Analyst Software (EMD Millipore Corp., Billerica, MA). Additional cytokines and chemokines (IL-5, eotaxin-1 and 2) were assayed by ELISA.
Lung Histology
Lung tissue was dissected from the trachea 48 hours after final OVA-challenge. The left lobe was fixed in neutral buffered formaldehyde, and 5-micrometer paraffin sections were stained with hematoxylin and eosin (H&E). Perivascular (PV) and peribronchial (PB) inflammation was graded using a modification of a published scoring method by a blinded pathologist: [0) no inflammatory cells noted around vessel or bronchi; 1) occasional inflammatory cells around the vessel or bronchi; 2) a thin layer (1-5 inflammatory cells) surrounding the vessel or bronchi; 3) a thick layer (> 5 inflammatory cells surrounding the vessel or bronchi), 4) thick layer and confluent areas of inflammation] [16]. Sections were stained with periodic acid-Schiff (PAS) for evaluation of goblet cells. PAS staining was graded using an arbitrary scale of 0 to 4 (modified from a published protocol) [16] according to percentage of cells stained in the majority of airways present: [0) no staining; 1) 25-50% stained; 2) >50% stained; 3) >50% stained; 4) 100% stained]. Sections were stained for picrosirius red and examined with the circular polarized microscopy for collagen content. Immunohistochemistry for alpha-smooth muscle actin (Ab5694, Abcam, Cambridge, MA, USA) was performed at 1:2000 dilution and counterstained with hematoxylin.
Measurement of airway responsiveness (AHR)
Forty-eight hours following the final OVA challenge, airway responsiveness to acetylcholine (ACh) was measured [17]. Mice were anesthetized i.p. with pentobarbital (80mg/kg) and ventilated with a tracheal cannula (18 gauge) at the rate of 120 breaths per minute and a constant tidal volume of air (0.2mL) with an RSP1002 pressure-controlled respirator system (Kent Scientific, Torrington, CT, USA). Muscle paralysis was induced by intravenous injection of decamethonium bromide (25mg/kg). Airway pressure was measured with a pressure transducer through a port in the trachea. A stable baseline was recorded for at least 2 minutes, and ACh (50μg/kg) was injected into the IVC and the recording continued for 4 minutes. The airway pressure changes were viewed and recorded for 4 minutes with VENTP software respiratory data acquisition system (Kent Scientific). (The change in the airway pressure from the baseline measure in an individual mouse allowed for comparison of mice with potential differences in lung size.) The time-integrated changes in peak airway pressure referred to as the airway pressure–time index (APTI) (cmH2O/second) were calculated and served as the measurement of airway responsiveness.
Statistical Analysis
Data are expressed as means ± SEMs. Statistical analyses were performed using 2 way ANOVA followed by a Bonferroni post test or Student's unpaired two-tailed t-test for comparison between two groups. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism® Software (La Jolla, CA, USA).
Results
The effect of age on the response to influenza A virus infection
To evaluate the effects of age on the physiologic responses to influenza A infection, the mice were monitored for weight loss and sickness scores. Following infection, both the young and aged mice had weight loss starting 3 days after the viral infection which peaked by day 4 for young and day 8 for aged mice (Figure 2A, B). By day 8, the young infected mice had regained the weight loss and returned to a pre-infection level. In contrast, the aged infected mice had a significant weight loss from baseline, which did not return to baseline before sacrifice on day 34 (data not shown). Additionally, the aged mice either infected with influenza-only or infected and OVA-sensitized/challenged, had greater sickness scores compared to the young mice (Figure 2C, D). Both young and aged OVA-mice, or naïve-mice, did not display signs of illness. To verify exposure to the influenza A virus, sera was collected at the time of sacrifice to determine specific IgG1 to the influenza A strain (H3N2). All mice that were infected with the influenza A virus demonstrated a detectable IgG1 to a viral lysate.
Figure 2.
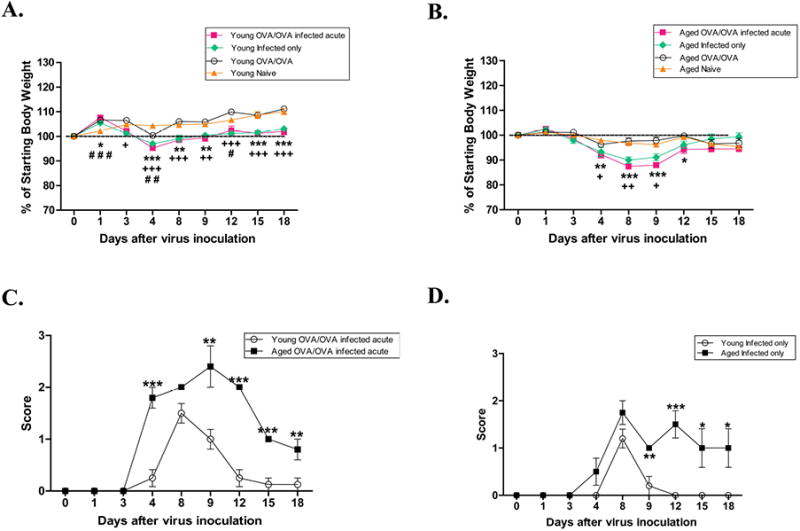
Mouse morbidity. Following infection with a sub-lethal dose of Influenza A, mice were evaluated for weight loss [(A) young and (B) aged] and sickness score (C). Data expressed as mean ±SEM. */+/# p<0.05, **/++/## p<0.01, ***/+++/### p<0.001. *OVA/OVA-infected acute compared to naïve, +infected-only compared to naïve, #OVA/OVA-compared to naïve.
The effect of age on antigen sensitization during acute influenza A
All OVA sensitized and challenged mice (OVA-mice) developed a significant increase in (p<0.001) serum IgE to OVA (Figure 3A). Consistent with our previous data, aged mice undergoing sensitization exhibited a significantly higher serum OVA IgE level compared to young OVA-mice (p=0.0015). To investigate whether the influenza infection had an effect on sensitization, OVA specific IgE was measured in acute-infection/OVA-mice and compared between young and aged OVA-mice. OVA-specific IgE was significantly increased (p=0.0034) only in aged mice that were sensitized during an acute infection (Figure 3A). As an additional measure of sensitization, OVA specific serum IgG1 was determined. All antigen sensitized and challenged mice (OVA-mice) developed elevated serum IgG1 to OVA compared with age-matched naïve mice (Figure 3B). However, OVA-specific IgG1 was significantly higher in the young OVA-mice compared with the aged OVA-mice (p<0.001). Unlike IgE, there was no significant difference in IgG1 to OVA if either young or aged mice were sensitized during the acute infection (Figure 3B).
Figure 3.
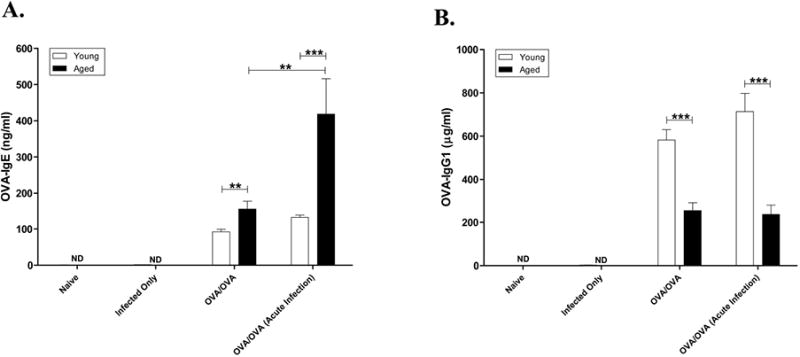
Effect of viral infection on specific antigen-induced immunoglobulin production. Sera were collected forty-eight hours after the final OVA-challenge and antigen-specific (A) IgE (B) IgG1 was determined by ELISA (Serum dilutions: IgG1-1:250,000, IgE-1:10). Data expressed in mean ±SEM from combined data from 2 independent experiments. * p<0.05, **p<0.01, ***p<0.001.
The effect of age on the peripheral leukocyte responses after influenza infection and antigen sensitization
To determine if there were differences in the systemic response to antigen sensitization (in addition to immunoglobulin response) and if this were affected by the viral infection, the peripheral levels of neutrophils, eosinophils, and lymphocytes were measured. There was no significant difference between the percentages of peripheral eosinophils among all groups of the young and aged mice (i.e. OVA-, acute infection/OVA-, infected alone-, or naïve-), although there was a trend towards increased eosinophils in young OVA-mice compared to young naïve mice. In contrast, there was a significant increase in the percentage of peripheral neutrophils in aged OVA-mice (51.7±5.9%, p=0.006) and infected alone aged-mice (51±5.7%, p=0.01) compared to aged naïve mice (29.8±3.3%). In addition, there was significantly elevated peripheral neutrophil percentage in aged OVA-mice compared with young OVA-mice (27.4±3.1%, p=0.008).
The effect of age on the allergic airway inflammation after an acute infection with influenza A
BALF cellular count and differential
To explore age-related differences in the response to antigen challenge, BALF was collected at the time of AHR measurement (48 hours after the final antigen challenge). Both young and aged mice who were antigen sensitized and challenged (OVA-mice) developed significantly elevated BALF total cell counts when compared to age-matched naïve controls (Figure 4A). Aged OVA-mice had a significantly greater total BALF cellular response compared to young OVA-mice (p=0.0098). There was no significant difference in total BALF cell counts in mice that were naïve versus infected-alone mice, in either age group. Aged OVA-mice had a significantly greater number of BALF PMNs (p=0.0015) and eosinophils (p=0.0047) than younger OVA-mice (Figures 4B, C). Sensitization with OVA during an acute infection in 6-week old mice significantly increased the numbers of BALF neutrophils (p=0.033) and significantly decreased the eosinophils (Figure 4C, p=0.0021). Although the total numbers of cells in the BALF tended to be greater in the young naïve (847 × 103) and the young acute infection/OVA-mice (1088 × 103), the difference was not significant. Overall, these results suggest that at a time point past the acute viral infection [18, 19] differences in airway inflammation persist. In contrast, the absolute numbers of neutrophils and eosinophils were not significantly changed in the lungs of the aged mice whether or not they were infected with a virus prior to antigen sensitization. Although the absolute numbers of eosinophils in the aged old acute-infection/OVA-mice were less than the aged OVA-mice, the differences were not statistically significant (Figure 4C). There were no significant changes in the numbers of macrophages or lymphocytes between the young acute infection/OVA-mice and the young OVA-mice and between the aged acute-infected OVA-mice and the aged OVA-mice. (Figures 4D, E).
Figure 4.
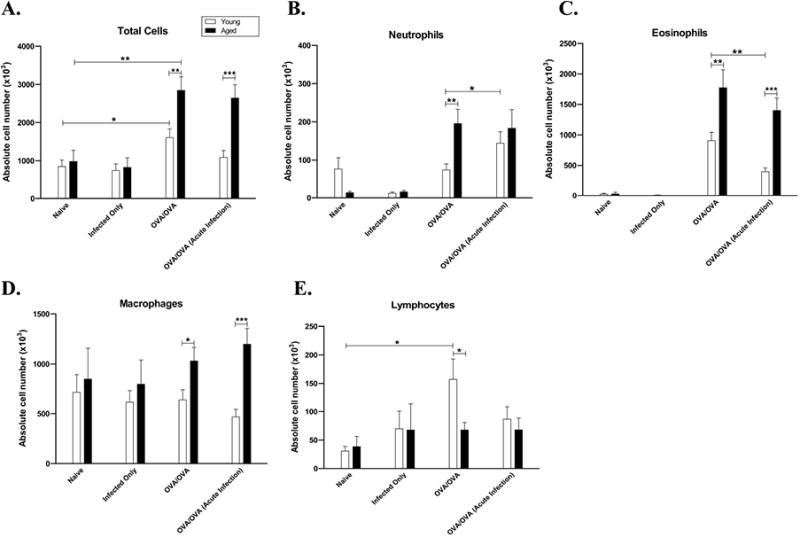
Effect of antigen sensitization during an acute Influenza A infection on antigen-induced BALF inflammation. Forty-eight hours after the final OVA-challenge, BALF was collected for total cell count and differential. Data expressed as mean ±SEM from combined data from 2 independent experiments for total (A) cell count, (B) neutrophil, (C) eosinophil, (D) macrophage, and (E) lymphocyte counts. * p<0.05, **p<0.01, ***p<0.001.
Antigen induced cytokine and chemokine production
To evaluate potential mechanisms why young mice had an altered airway response if sensitized during an acute infection, cytokine and chemokine expression in the BALF was measured (Figure 5). In young OVA-mice, IL-5 was not significantly different between infected and non-infected, however, eotaxin-1 was significantly decreased, suggesting a possible explanation for the decrease in airway eosinophils. Although KC (IL-8) was increased in young acute infected OVA-mice compared to OVA-mice, which could explain, in part, the increase in BALF neutrophils, it did not reach significance. IL-13 increased in young mice sensitized during an acute infection (0.15), but this did not reach significance. In aged compared to younger OVA-mice, there was a trend towards a greater cytokine expression in the former, in particular IL-5 (0.16), IL-6 (0.11), KC (0.14), RANTES (0.16), TNF–α (0.08) and eotaxin-2 (p=.0256). The only significant change in the expression of BALF cytokines between aged acute infected/OVA-mice and non-infected OVA-mice was a decrease in eotaxin-2 in the latter, however, there was a trend towards increased IL-4 and TNF–α.
Figure 5.
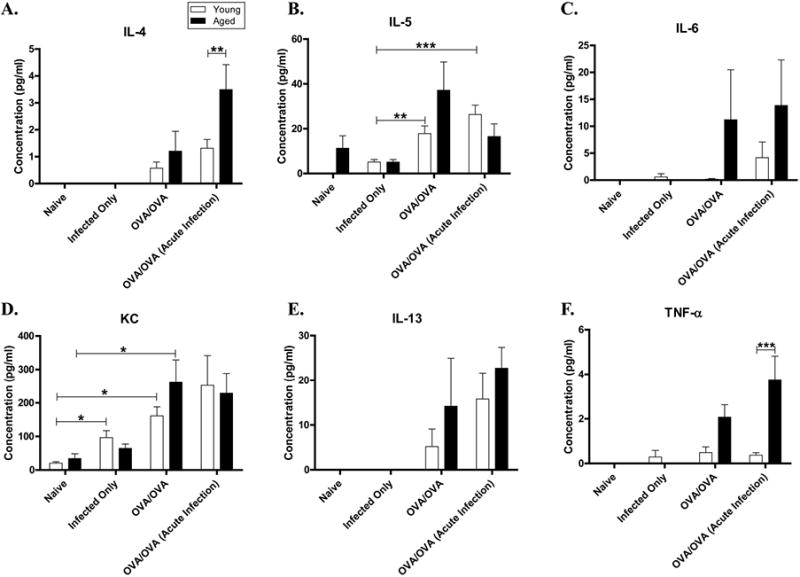
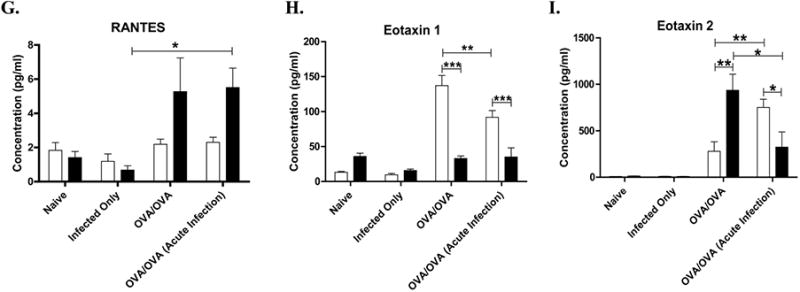
Effect of influenza A infection on antigen induced cytokine production. BALF supernatant was assayed for a panel of cytokines and chemokines [(A) IL-4 (B) IL-5 (C) IL-6 (D) KC (E) IL-13 (F) TNFα] and chemokines [(G) RANTES (H) Eotaxin 1 (I) Eotaxin 2] using a multiplex-assay. Data expressed in mean ±SEM from combined data from 2 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Histology
Lung sections were collected 48 hours after the final OVA-challenge and then fixed and stained with H&E. (Representative sections are shown in Figure 6A). There were significant increases in the inflammatory perivascular (PV) and peribronchial (PB) cells in the young and aged OVA-mice compared to their age-matched naïve controls (p<0.001, Figure 6B, C). PB inflammation was increased in the 6-week acute-infection/OVA-mice compared to young OVA-mice, but did not reach statistical significance (p=0.15). Additionally, no differences in PB inflammation and PV inflammation were noted in the young and aged acute-infection/OVA-mice compared to age-matched OVA-mice. Similar to the BALF, there was no difference in PV or PB inflammation between young and aged naïve and infected-alone mice. Picrosirius red stain and immunohistochemistry for alpha-smooth muscle actin was performed for identification of collagen and smooth muscle respectively, in peribronchiolar tissue as part of the assessment for airway remodeling. Both collagen staining and smooth muscle labeling were visibly increased in OVA-mice of either age compared to age matched naïve mice (Figure 6A). There were no visible differences in collagen deposition and smooth muscle between the aged and younger OVA-mice. There were no visible differences in collagen deposition and smooth muscle hypertrophy in either age group of acute-infection/OVA-mice compared to age-matched OVA-mice. Lung tissue was also stained with PAS to measure goblet cell hyperplasia (Figure 6A), which may have contributed to thickening of the bronchiolar epithelium. There was significantly increased PAS staining in bronchioles in both young and aged OVA-mice compared with aged-matched naïve mice (p<0.001 for both ages, Figure 6D), in which there was none to minimal PAS epithelial cell staining. PAS staining of lung tissue from young and aged mice was not significantly different in acute-infection/OVA-mice compared to age-matched OVA-mice in either age of mice (Figure 6D). In addition, there was no evidence of epithelial degeneration of necrosis in the bronchiolar epithelium of the sections examined in any of the infected or non-infected mice.
Figure 6.

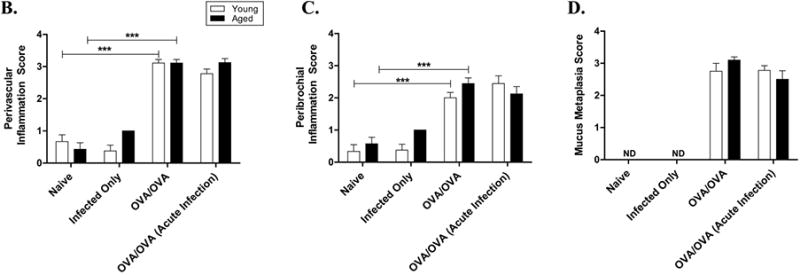
Impact of aging, antigen sensitization and challenge and viral infection on lung histopathology (A) Lung histopathology was evaluated by H&E (to access the degree of total lung inflammation), periodic-acid-Schiff (PAS) (to measure mucus metaplasia; magenta), Picrosirius red visualized under polarizing light (to measure collagen deposition) and immunohistochemistry for α-smooth muscle actin (α-SMA) (for contractile elements in the airway wall; brown). All images were taken at ×200 magnification. (B) Perivascular inflammation (PV), (C) Peribronchial inflammation (PB), and (D) mucus metaplasia (MM) of lung tissues were graded as described in the “Methods” section.
The effect of age on antigen-induced airway hyperresponsiveness in mice sensitized during an acute influenza A infection
To measure AHR we determined APTI following ACh injection 48 hours after the final OVA-challenge. Both young and aged OVA-mice developed increased APTI after antigen sensitization and challenge when compared to age-matched naïve control mice (p=0.03 and p=0.05, respectively) (Figure 7). APTI significantly increased in the young mice infected with influenza A virus 3 days prior to antigen sensitization (acute-infection/OVA-mice compared to young OVA-mice, p<0.001). Although APTI increased in the aged mice infected with influenza A virus prior to sensitization (acute-infection/OVA-mice) compared to aged antigen sensitized and challenged alone (OVA-mice), this value did not reach statistical significance (p=0.27). There were no significant differences between APTI values of young and aged naïve mice and infected-alone mice.
Figure 7.
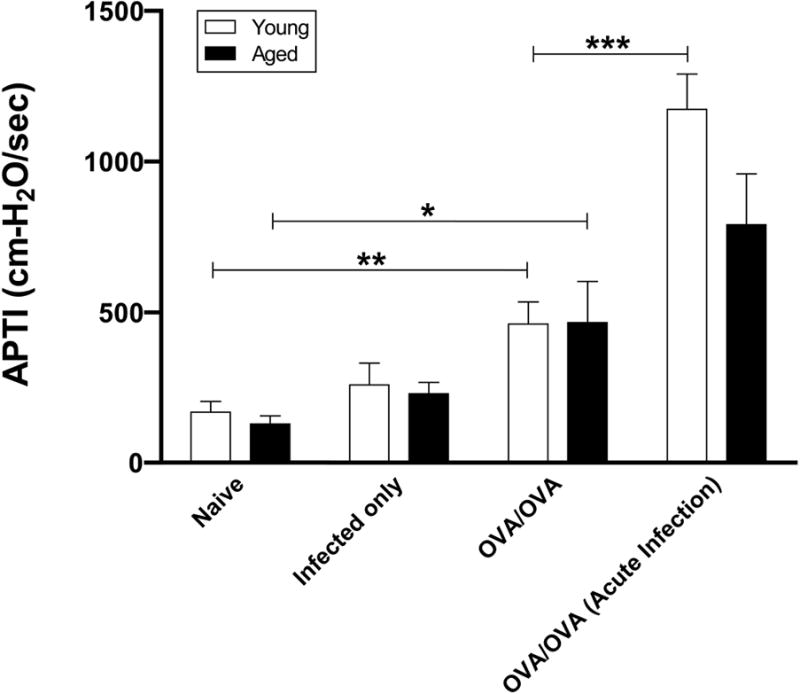
Effect of antigen sensitization during an acute influenza A infection on antigen-induced airway hyperresponsiveness. Forty-eight hours after the final OVA-challenge, AHR was determined by APTI after acetylcholine injection. Data were means ±SEM from combined data from 3-4 independent experiments (n=5-7/group). * p<0.05, **p<0.01, ***p<0.001.
Discussion
To address age-related differences of a respiratory infection on antigen sensitization and its effect on subsequent allergen-induced AHR and inflammation, we have developed a mouse model to gain insight into asthma in older patients. We found significant age-associated differences in the response to infection, IgE production and antigen-induced airway inflammation and AHR. Although antigen-specific IgE production was not altered by a prior viral infection in younger mice sensitized to antigen, BALF neutrophils and AHR increased, and BALF eosinophils decreased when compared to age-matched non-infected, sensitized and challenged mice. In contrast, IgE antigen production was significantly increased in aged mice sensitized during a viral infection (compared to non-infected mice) which was accompanied by a greater morbidity. Despite these differences, there was little effect on the AHR and BALF cellular differential. The results of these experiments suggest that viral infection prior to antigen sensitization affects the allergic airway and the systemic response differently with age in a murine model. These age-dependent differences are summarized in Table 1.
Table 1. Age-related response to Influenza A, Ag-sensitization, Ag-induced AHR and inflammation.
| GROUP | MORBIDITY | OVA-IgE | BALF EOSINOPHILS | BALF NEUTROPHILS | AHR |
|---|---|---|---|---|---|
| Young OVA | NC | + | + + | + | + |
| Young AI/OVA | + | + | + | + + | + + + |
| Aged OVA | + + | + + | + + + | + + | + |
| Aged AI/OVA | + + + | + + + | + + + | + + | + |
Determined by sickness score and weight loss
Ag, antigen; AHR, airway hyperresponsiveness; OVA, ovalbumin; BALF, bronchoalveolar fluid; AI, acute infection; NC, no change.
Morbidity was enhanced with increased age after the acute influenza infection. Although both young and aged mice had weight loss after viral infection which began at 4 days post-infection, the number of days of weight loss was prolonged in the aged mice, and they did not recover to their baseline weight. Reasons for these trends may include decreased cytotoxic T lymphocyte activation, decreased CD8+ T-cell activity and an increase in Treg cells in aged mice interfering with the primary immune response to influenza [20, 21]. Weight loss was exacerbated in both the young and aged mice when they were also OVA sensitized. In addition, aged mice had an increased sickness score at day 4 post-viral infection which remained elevated during the protocol time period. Similar trends of increased weight loss from influenza A infection in aged mice have been published [14]. However, enhanced morbidity and mortality from antigen sensitization specifically addressing age-associated differences, has not been previously reported. As an additional measure of systemic response to viral infection, peripheral blood samples were collected at the time of sacrifice and indicated age-specific differences. Viral infection of aged mice significantly increased peripheral neutrophils compared to young mice in which there was little change.
The production of serum specific IgE to OVA after sensitization was increased in aged mice sensitized during an acute infection. In contrast, there was no difference in OVA-IgE between the infected and non-infected sensitized young mice. These alterations might be in part due to increased IL-4 expression detected in aged, but not young mice sensitized during an acute infection. Although these results require translation to humans, in a murine model they suggest that with increased age IgE production to antigen occurs, and is altered (i.e. enhanced) by viral infection. This model may represent a phenotype characteristic of late onset asthma.
There were specific age-related cellular differences in the airways of young and aged mice. Similar to our prior results, aged OVA-mice were found to have a greater total numbers of cells, eosinophils and neutrophils in the BALF than young OVA-mice [22-24]. Additionally, sensitization of young mice during an acute viral infection resulted in a significant shift of the cellular composition of the BALF (i.e. increased eosinophil and decreased neutrophil number) compared with young non-infected, sensitized mice without a significant difference in the total numbers of cells. This distinction was noted in the post-infectious period (∼ day 10) [18, 19]. There was little effect on the total numbers of cells, neutrophils and eosinophils in the BALF of aged mice antigen sensitized during an acute infection or sensitized without an infection. The results obtained in the young mice are consistent with a report by Wohlleben, in which C57BL/6 mice infected with influenza A prior to intraperioneal OVA sensitization had decreased recruitment of eosinophils to the BALF [25]. However, our results differed from Al-Garawi in which Influenza A exposure 7 days prior to intranasal sensitization with house-dust mite in 6-8 week old BALB/c mice nearly doubled the BALF eosinophil number [26]. These differences may be possibly explained by alternative routes of antigen sensitization (i.e. i.n. versus i.p.), which other groups have demonstrated to alter the airway inflammatory response to antigen challenge [27, 28], or by variation in the timing between viral infection and antigen sensitization between experiments. Future work is necessary to address the kinetics of airway inflammatory cell changes at time points after infection and prior to challenges.
Increased age altered the pattern of the antigen sensitization and allergic inflammatory response in the post-infection period. In young mice sensitized during acute infection, there was a trend towards increased IL-6 and KC (IL-8) compared to non-infected mice which may have contributed to the increased neutrophil count in the former group. Influenza A infection in mice has been reported to active C5a, which increases neutrophil recruitment to the BALF [29]. Although we detected an increase in IL-13 in young mice sensitized during a viral infection, it was not statistically significant from young mice sensitized without infection. Chang demonstrated that influenza A infection alone (without antigen sensitization) in young mice increased AHR and IL-13 secretion from natural helper cells 5 days post infection [30], however, our study differed in that we measured IL-13 at a later time point and found no difference. Consistent with elevated eosinophils in the BALF of aged OVA-mice, IL-5 and eotaxin were increased in the aged versus young OVA-mice. Additionally, KC (IL-8), IL-6 and GCSF, which induce neutrophil mobilization, were also increased in the aged OVA-mice which could explain, in part, the increased airway neutrophilia in the aged group. Unlike young mice, there was no significant changes noted in BALF cytokine or chemokine expression between aged mice either sensitized during an infection or without prior infection.
APTI was measured after administration of acetylcholine to determine if AHR was altered by age and antigen sensitization during an acute viral infection. In both the young and aged mice, OVA-sensitization and challenge significantly increased AHR when compared with naïve aged matched controls, although to a greater degree in the 6-week old mice. We have previously noted discordance between increased airway inflammation and AHR in aged mice. We did not detect differences in airway collagen deposition (by Picrosirius red) or smooth muscle hypertrophy (by α-SMA immunohistochemistry) between the young and aged OVA-mice and have previously not seen differences in the contraction of smooth airway muscle between ages of mice [23]. However, there may be other age-related differences in inflammation, including alteration of airway Treg cells number and function with aging[23] and decreased peripheral eosinophil degranulation in older patients with asthma [31] which could modulate the relationship between inflammation and AHR and aging. APTI significantly increased in the young acute infection/OVA-mice compared to young OVA-mice. Increased airway neutrophils (in particular secondary to a viral infection), as we detected in younger acute-infected/OVA-mice, may increase AHR for several reasons. Respiratory viruses activate release of superoxide production and secretion of enzymes by neutrophils, inducing oxidative stress and epithelial damage producing AHR [32-34]. Additionally, respiratory viral infections can directly affect the airway, disrupting the alveolar fluid clearance [35], releasing ATP [36] and increasing the responsiveness to methacholine [37] as well as altering the inhibitory M2 muscarinic receptors on airway parasympathetic nerves [38]. Aging may alter some of these relationships as illustrated by decreased neutrophil superoxide production and signal transduction [39]. In addition, there are age-related changes with the cholinergic response of muscarinic receptor subtypes [40], which could produce decreased sensitivity to cholinergic stimulation by a viral trigger.
The results of our study suggest that viral infection prior to antigen sensitization affects the allergic airway and systemic response differently with age in a murine model. While both young and aged mice have an altered response to sensitization during an acute viral infection, these responses differ. In the young mice, the response is an increase in AHR associated with airway neutrophilia and a decrease in eosinphils. In contrast, antigen sensitization during a viral infection in aged mice had a minimal effect on AHR and airway inflammation, but a greater systemic response with increased morbidity, increased production of OVA specific IgE and peripheral neutrophilia. These differences could be driven by the modulations in immune response with age and should be addressed in future work. This study represents an initial step to investigate age-associated differences in response to antigen and its modulation and effect on airway inflammation in a murine model.
Acknowledgments
The authors would like to thank Dr. Thomas Kraus for assistance with Multiplex and Drs. Thomas Moran and Wenjing Li for assistance with viral infection.
Funding: 5K08AI063932 (PJB). Partial support by NIH/NCCAM center grant # 1P01 AT002644725-01 Center for Chinese Herbal Therapy (CHT) for Asthma (XML).
Abbreviations
- ACh
Acetylcholine
- α-SMA
α-smooth muscle actin
- Ag
Antigen
- AHR
Airway hyperresponsiveness
- APTI
Airway pressure time index-indices
- BALF
Bronchoalveolar Lavage fluid
- i.p.
Intraperitoneal
- i.t.
Intratracheal
- IVC
Inferior vena cava
- OVA
Ovalbumin
- PAS
Periodic acid-Schiff
- PMNs
Polymorphonuclear leukocytes
- RT
Room temperature
References
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.Bauer BA, Reed CE, Yunginger JW, Wollan PC, Silverstein MD. Incidence and outcomes of asthma in the elderly. A population-based study in Rochester, Minnesota. Chest. 1997;111:303–10. doi: 10.1378/chest.111.2.303. [DOI] [PubMed] [Google Scholar]
- 3.Nafstad P, Brunekreef B, Skrondal A, Nystad W. Early respiratory infections, asthma, and allergy: 10-year follow-up of the Oslo Birth Cohort. Pediatrics. 2005;116:e255–62. doi: 10.1542/peds.2004-2785. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 5.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 6.Hollams EM, Deverell M, Serralha M, Suriyaarachchi D, Parsons F, Zhang G, de Klerk N, Holt BJ, Ladyman C, Sadowska A, Rowe J, Loh R, Sly PD, Holt PG. Elucidation of asthma phenotypes in atopic teenagers through parallel immunophenotypic and clinical profiling. J Allergy Clin Immunol. 2009;124:463–70. 70 e1–16. doi: 10.1016/j.jaci.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–6. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 9.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt PG, Strickland DH, Sly PD. Virus infection and allergy in the development of asthma: what is the connection? Curr Opin Allergy Clin Immunol. 2012;12:151–7. doi: 10.1097/ACI.0b013e3283520166. [DOI] [PubMed] [Google Scholar]
- 11.Flurkey KCJ, Harrison DE. The Mouse in Aging Research. In: Fox J, editor. The Mouse in Biomedical Research: American College Laboratory Animal Medicine (Elsevier) Burlington, MA: 2007. pp. 637–72. [Google Scholar]
- 12.Moltedo B, Lopez CB, Pazos M, Becker MI, Hermesh T, Moran TM. Cutting edge: stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol. 2009;183:3569–73. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- 13.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–9. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noel A, Cataldo DD. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res. 2009;58:845–54. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- 17.NAEPP Working Group Report. Considerations for Diagnosis and Managing Asthma in the Elderly. 1996 [Google Scholar]
- 18.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70:5230–5. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 20.Williams-Bey Y, Jiang J, Murasko DM. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev. 2011;132:163–70. doi: 10.1016/j.mad.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murasko DM, Jiang J. Response of aged mice to primary virus infections. Immunol Rev. 2005;205:285–96. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 22.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birmingham JM, Patil S, Li XM, Busse PJ. The effect of oral tolerance on the allergic airway response in younger and aged mice. J Asthma. 2013;50:122–32. doi: 10.3109/02770903.2012.753455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busse PJ, Lurslurchachai L, Sampson HA, Halm EA, Wisnivesky J. Perennial Allergen-Specific Immunoglobulin E Levels Among Inner-City Elderly Asthmatics. J Asthma. 2010 doi: 10.3109/02770903.2010.489140. [DOI] [PubMed] [Google Scholar]
- 25.Wohlleben G, Muller J, Tatsch U, Hambrecht C, Herz U, Renz H, Schmitt E, Moll H, Erb KJ. Influenza A virus infection inhibits the efficient recruitment of Th2 cells into the airways and the development of airway eosinophilia. J Immunol. 2003;170:4601–11. doi: 10.4049/jimmunol.170.9.4601. [DOI] [PubMed] [Google Scholar]
- 26.Al-Garawi AA, Fattouh R, Walker TD, Jamula EB, Botelho F, Goncharova S, Reed J, Stampfli MR, O'Byrne PM, Coyle AJ, Jordana M. Acute, but not resolved, influenza A infection enhances susceptibility to house dust mite-induced allergic disease. J Immunol. 2009;182:3095–104. doi: 10.4049/jimmunol.0802837. [DOI] [PubMed] [Google Scholar]
- 27.Hansen JS, Alberg T, Rasmussen H, Lovik M, Nygaard UC. Determinants of experimental allergic responses: interactions between allergen dose, sex and age. Scand J Immunol. 2011;73:554–67. doi: 10.1111/j.1365-3083.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–9. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- 29.Garcia CC, Weston-Davies W, Russo RC, Tavares LP, Rachid MA, Alves-Filho JC, Machado AV, Ryffel B, Nunn MA, Teixeira MM. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One. 2013;8:e64443. doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathur SK, Schwantes EA, Jarjour NN. Busse WW, Age-related changes in eosinophil function in human subjects. Chest. 2008;133:412–9. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkerts G, Busse WW, Nijkamp FP, Sorkness R, Gern JE. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998;157:1708–20. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto K, Aizawa H, Inoue H, Koto H, Nakano H, Hara N. Role of neutrophil elastase in ozone-induced airway responses in guinea-pigs. Eur Respir J. 1999;14:1088–94. doi: 10.1183/09031936.99.14510889. [DOI] [PubMed] [Google Scholar]
- 34.Busse WW, Vrtis RF, Steiner R, Dick EC. In vitro incubation with influenza virus primes human polymorphonuclear leukocyte generation of superoxide. Am J Respir Cell Mol Biol. 1991;4:347–54. doi: 10.1165/ajrcmb/4.4.347. [DOI] [PubMed] [Google Scholar]
- 35.Aeffner F, Traylor ZP, Yu EN, Davis IC. Double-stranded RNA induces similar pulmonary dysfunction to respiratory syncytial virus in BALB/c mice. Am J Physiol Lung Cell Mol Physiol. 2011;301:L99–L109. doi: 10.1152/ajplung.00398.2010. [DOI] [PubMed] [Google Scholar]
- 36.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med. 2008;178:969–76. doi: 10.1164/rccm.200803-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozanich EM, Gualano RC, Zosky GR, Larcombe AN, Turner DJ, Hantos Z, Sly PD. Acute Influenza A infection induces bronchial hyper-responsiveness in mice. Respir Physiol Neurobiol. 2008;162:190–6. doi: 10.1016/j.resp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Nie Z, Scott GD, Weis PD, Itakura A, Fryer AD, Jacoby DB. Role of TNF-alpha in virus-induced airway hyperresponsiveness and neuronal M(2) muscarinic receptor dysfunction. Br J Pharmacol. 2011;164:444–52. doi: 10.1111/j.1476-5381.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–41. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Lee HK, Lim MY, Bok SM, Cho ES, Lee EM, Kim SW, Kim YH, Kim HW. Age differences in cholinergic airway responsiveness in relation with muscarinic receptor subtypes. Life Sci. 2007;81:204–9. doi: 10.1016/j.lfs.2007.05.002. [DOI] [PubMed] [Google Scholar]


