Summary
Axoplasmic organelles move on actin as well as microtubules in vitro and axons contain a large amount of actin, but little is known about the organization and distribution of actin filaments within the axon. Here we undertake to define the relationship of the microtubule bundles typically found in axons to actin filaments by applying three microscopic techniques: laser-scanning confocal microscopy of immuno-labeled squid axoplasm; electronmicroscopy of conventionally prepared thin sections; and electronmicroscopy of touch preparations-a thin layer of axoplasm transferred to a specimen grid and negatively stained. Light microscopy shows that longitudinal actin filaments are abundant and usually coincide with longitudinal microtubule bundles. Electron microscopy shows that microfilaments are interwoven with the longitudinal bundles of microtubules. These bundles maintain their integrity when neurofilaments are extracted. Some, though not all, microfilaments decorate with the S1 fragment of myosin, and some also act as nucleation sites for polymerization of exogenous actin, and hence are definitively identified as actin filaments. These actin filaments range in minimum length from 0.5 to 1.5 μm with some at least as long as 3.5 μm. We conclude that the microtubule-based tracks for fast organelle transport also include actin filaments. These actin filaments are sufficiently long and abundant to be ancillary or supportive of fast transport along microtubules within bundles, or to extend transport outside of the bundle. These actin filaments could also be essential for maintaining the structural integrity of the microtubule bundles.
Introduction
Axoplasmic organelles move on actin filaments in vitro (Bearer et al., 1993, 1996a; Langford et al., 1994). Indeed, organelles moving on microtubules can transfer to and move along invisible tracks presumed to be actin filaments (Kuznetsov et al., 1992). Furthermore, when gelsolin, a protein that serves actin filaments, is injected into some living axons, organelle movements cease, a result that suggests that intact actin filaments are required for organelle transport (Brady et al., 1984). Finally, several myosins, which could use actin filaments as substrates for movement, have been identified in axoplasm (Bearer et al., 1993, 1996a; Molyneaux & Langford, 1997). At least two of these co-purify with motile axoplasmic organelles (Bearer et al., 1993, 1996a,b). As a result of these studies it has become clear that axoplasm contains myosins, that some of these myosins associate with organelles, and that organelles separated from axoplasm can move on actin filaments. Now that it has become apparent that some organelles in axoplasm move on actin filaments, it becomes of interest to re-examine the distribution of actin filaments in axoplasm.
Actin is present in most axons (Kuczmarski & Rosenbaum, 1979; reviewed in Gavin, 1997). In the giant axon of the squid, actin filaments have been reported to be in the form of short segments, less than 1 μm long, although actin protein concentration is at least equal to that of tubulin or neurofilament protein (Fath & Lasek, 1988). Thus, actin filaments are present but their reported distribution tells us little about what role they might have in fast transport of organelles. At the same time actin filaments might also act as structural elements, stabilizing the microtubule bundles or acting as a cage to separate neurofilament-rich axoplasm from microtubule bundles, thus maintaining the integrity of the microtubule bundles.
To understand the role of actin filaments in axoplasmic transport, we undertook to investigate the axoplasmic cytoskeleton by confocal and electron microscopy, focusing on the relative distributions of microtubules and actin filaments. Extrusion of the axoplasm and subsequent imaging, either by confocal microscopy or by thin section or with a touch preparation for electron microscopy (Bearer et al., 1996c), allowed us to overcome the difficulties caused by the large diameter of the squid giant axon and the impermeability of its glial sheath that had previously limited visualization of its cytoskeletal organization.
Thus, understanding the distribution of actin filaments in axoplasm becomes fundamental to understanding the function of axoplasmic myosins, whether for delivery of transported materials or as a structural component of the transport system. In this paper, we address the following questions: Where is the actin in the axon? Is there enough filamentous actin in the right location to serve as a substrate for transport? Does actin, like microtubules, form bundles that could serve as substrate to move organelles over great distances or is transport on actin filaments necessarily local? What are the polarities of the actin filaments?
Materials and methods
Squid (Loligo pealeii) were obtained from the Marine Biological Laboratory at Woods Hole, MA. The giant axon was dissected as described (Bearer et al., 1996b) from squid within two days of capture. Phalloidin and rhodamine-labeled phalloidin were purchased from Molecular Probes (Eugene, OR) and mouse monoclonal anti-tubulin antibodies from Amersham (Arlington Ht., IL). Rabbit polyclonal anti-squid neurofilament antibody was a gift from Harrish Pant (Gallant et al., 1986).
CONFOCAL IMMUNOFLUORESCENCE OF AXOPLASM
Axoplasm extrudes onto coverslips in a toothpaste-like ribbon of intact viscous material. After air drying for 1 min it usually adheres firmly to the coverslip, which can then be passed gently through various solutions. When it is fixed in 4% formaldehyde in 50 mM Pipes, 50 mM Hepes, 4 mM MgSO4 and 1 mM EGTA, pH 7.0 (PHEMS) containing 1/100 dilution of either 3 μM rhodamine phalloidin or 3 μM unlabeled phalloidin, neurofilaments are retained. To extract neurofilaments, axoplasm was extruded and incubated in PHEMS containing 1/100 phalloidin dilution and 2 nM taxol and a 1/100 dilution of a protease cocktail (Bearer, 1992) for 10–15 min before fixation, as it has been previously shown that squid neurofilaments are solubilized and extracted in low salt (Zackroff & Goldman, 1980; Gallant et al., 1986). After 1 hr in fixative, coverslips containing axoplasm were washed in 60 mM KCl and 4 mM MgCl2 (KM), blocked in phosphate buffered saline, pH 7.4 (PBS) with 1% bovine serum albumin and 0.1% Triton X100 (PBT) for one hour. Primary antibodies (anti-tubulin at 1/500 dilution or anti-neurofilament at 1/1000 dilution) in PBT were applied for 1 hr, the coverslip washed three times for 10 min each in PBT, and incubated in anti-mouse or anti-rabbit secondary antibodies conjugated with fluorescein (Boehringer-Mannheim, Indianapolis, IN) at 1/1000 dilution for 1 hour. After washing three times in PBS, the coverslip is mounted in anti-quench (Bearer, 1992) and viewed in a BioRad laser-scanning confocal microscope. To control for bleed-through from one fluorescent channel to another, images were obtained with the same enhancement parameters in each channel, and various specimens were either labeled for one or the other cytoskeletal protein, or double-labeled for both.
THIN SECTION ELECTRON MICROSCOPY
Axoplasm extruded onto glass coverslips was extracted to remove neurofilaments and fixed in 3.7% formaldehyde, 1% glutaraldehyde in PHEMS. After three washes axoplasm was post-fixed treated in cold 1% OsO44 in sodium barbital buffer, 1% tannic acid, and 1% aqueous uranyl acetate (Bearer, 1990) to enhance staining of cytoskeletal elements. Axoplasm was cut into 1–2 mm segments and embedded in Araldite (Metuzals et al., 1997). Silver gray sections were post-stained with 2% aqueous uranyl acetate for 10 min followed by lead citrate for 5 min. Specimens were viewed and photographed in a JEOL 200CX electron microscope.
TOUCH PREPARATIONS OF AXOPLASM
Axoplasm extruded onto a coverslip is scored with an eyebrow hair into 3–5 mm segments perpendicular to the long axis of the axon. Then a Formvar-carbon-coated grid previously glow discharged for 5 min is touched to each segment. This lifts a thin layer of axoplasm from the extruded axoplasm adhering to the surface of the grid (Bearer et al., 1996c). The grid is immediately placed for 10 min on 10 μl droplets of one or more of the following solutions: (A) first, PHEMS containing phalloidin and taxol to stabilize actin and microtubules and extract neurofilaments; (B) actin-polymerization buffer (APB: 25 mM Imidazole pH 7.1, 25 mM KCl, 4 mM MgCl2, 1 mM EGTA) plus 1 μM G-actin in G-buffer to add actin to endogenous filament ends; and/or, (C) myosin S1 fragment in the same buffer to decorate filaments. To label only endogenous filament, step (B) was omitted. After the last incubation, grids were passed through three 10 μl droplets of 2% aqueous uranyl acetate, dried, and viewed in JEOL CX100 electron microscope.
Examination by computer enhanced differential interference contrast microscopy of a touch preparation on a glass coverslip instead of an EM specimen grid reveals that it contains long cytoskeletal elements and organelles (Fig. 1) (Schnapp et al., 1985). No movement of organelles is observed, probably because they also adhere to the glass.
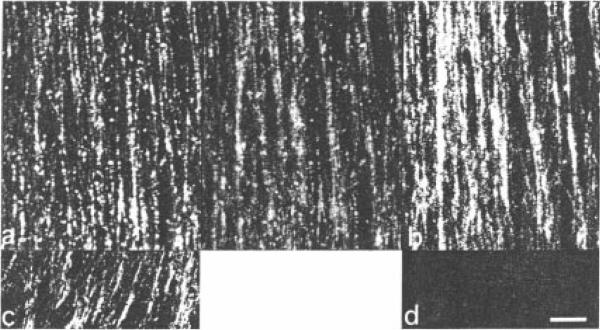
Fig. 1.
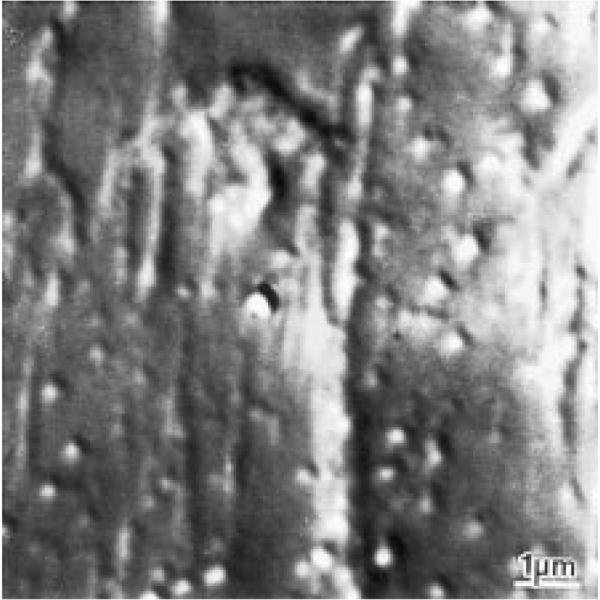
Differential interference contrast microscopy of a thin spread made from extruded axoplasm by touching it with a coverslip-a “touch preparation”. Parallel elements presumably correspond to the longitudinal actin/microtubule and neurofilament bundles. These preparations appear to be very thin because all tissue elements are included in the focal plane of a 1.4 NA lens. Bar = 1 μm.
MEASUREMENTS OF ACTIN FILAMENT LENGTHS
S1 labeled actin filaments present in 45 electronmicrographs printed at a final magnification of 75,000 were measured using a string laid along the length of each filament. The string is marked at the beginning and end of the filament and the lengths between the markings measured with a ruler. Only filaments that could be clearly identified along their full length were included. Histograms were generated using Kaleidograph software on a Macintosh computer.
Results
ACTIN FILAMENTS ARE CO-EXTENSIVE WITH MICROTUBULES IN EXTRUDED AXOPLASM
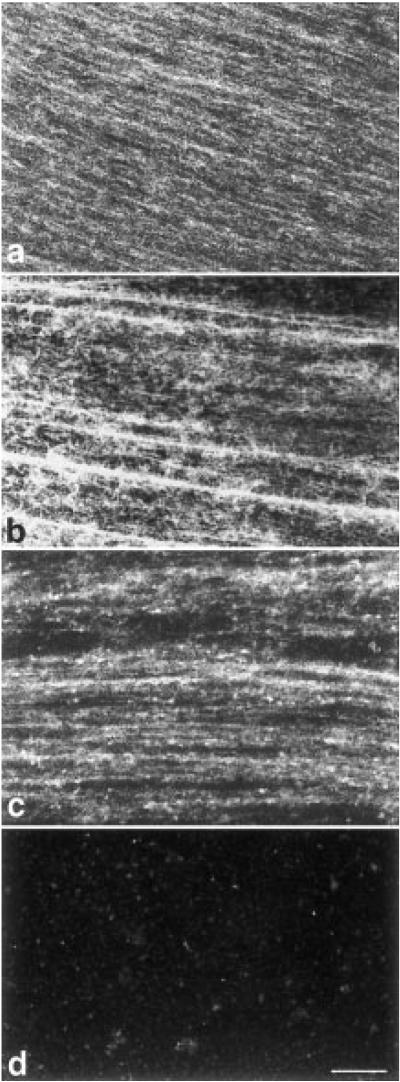
Abundant actin filaments run parallel to long, longitudinally oriented bundles of microtubules in axoplasm extruded from the giant axon of the squid (Fig. 2). Since we examined extruded axoplasm, our observations apply to the actin deep in the axon, not the cortical actin which is left behind with the sheath. Based on previous descriptions of axoplasmic actin (Fath & Lasek, 1988), we did not expect the abundance of actin filaments and their co-localization with the longitudinal bundles of microtubules. By confocal microscopy of extruded axoplasm stained with rhodamine phalloidin, actin filaments appear at least as abundant as microtubules. In preparations stained for both actin (rhodamine-phalloidin) and tubulin (FITC-indirect immunofluorescence), the two systems are frequently coincident with similar but not identical distributions.
Fig. 2.
Confocal microscopy of squid axoplasm extruded onto a glass coverslip and double stained for tubulin (a channel) and actin (b channel). Longitudinal elements presumably correspond to microtubules and filamentous actin respectively, though the definition of fluorescence microscopy leaves it uncertain whether stained elements correspond to individual or bundled filamentous elements. Fields (a) and (b) correspond, and the distributions of microtubules and actin filaments are largely overlapped as shown in color (middle panel) where actin is represented in red and microtubules in green. In some places microtubule staining does not correspond to actin staining (predominately green areas) whereas in others actin staining (red areas) does not correspond to microtubules. That little or none of the staining in the actin (rhodamine) channel could be attributed to bleed-through from the bright tubulin (FITC) channel is shown in (c & d) where a field stained only for tubulin was examined in the rhodamine channel. Bar = 10 μm.
Small areas are apparent in which one or the other type of filament is present while the other is absent (Fig. 2a and b), demonstrating that the FITC image of tubulin is not being detected in the rhodamine channel. This is most clear in the color superimposition of double-labeled specimens (middle panel, Fig. 2). Indeed, in preparations in which only microtubules were stained by FITC, no signal could be detected in the rhodamine channel (Fig. 2c and d), demonstrating that the abundance of actin filaments shown by rhodamine phalloidin is not caused by bleed-through from the microtubule labeling in the FITC channel.
The length and abundance of these filaments is striking, as is their co-localization with microtubules. With fluorescence microscopy at this resolution, it is not possible to determine how close the two filament systems are to each other, nor to distinguish the number of filaments in each bundle-such observations require the resolution of electron microscopy.
EXTRACTION OF NEUROFILAMENTS BY LOW SALT LEAVES ACTIN INTACT
While longitudinal bundles containing actin are distributed throughout the axon (Fig. 3a), staining for neurofilament shows a coarser, less uniform pattern, with bright staining of longitudinal aggregates interspersed with areas of little or no fluorescent signal (Fig. 3b). These observations suggest that the neurofilaments are organized into coarse longitudinal fascicles separate from the actin/microtubule bundles. The aggregates of neurofilament have roughened surfaces with short filamentous elements extending tangential from them. Squid neurofilaments are now known to be more closely related to lamins than to mammalian neurofilaments in their biochemistry and organization (Martin, 1996), and are soluble in low salt (Gallant et al., 1986). After extraction of extruded axoplasm in low salt with rhodamine phalloidin (added to stabilize as well as stain actin filaments), actin filaments are preserved but have a slightly less homogeneous distribution, as if they had coalesced (Fig. 3c). In contrast, the neurofilaments are no longer detectable, with neurofilament staining reduced to a weakly fluorescent, diffuse, particulate material (Fig. 3d).
Fig. 3.
Confocal microscopy of squid axoplasm extruded onto glass showing distribution of actin and neurofilament. Actin staining in (a) is shown for comparison with staining for neurofilament in (b). The neurofilament distribution also is in longitudinal linear elements suggestive that this staining represents the distribution of bundles of neurofilaments. However, these are unlikely to be co-extensive with the microtubule/actin bundles because they are coarser and more widely dispersed. After extraction of neurofilaments (c) and (d) show that fewer actin filaments appear as individual filaments while bundles appear brighter (c), as if the actin filiments aggregated after extraction of the neurofilaments (d, same field). Bar = 10 μm
The similarity in diameter between neurofilaments and actin filaments (Leapman et al., 1997) makes distinguishing them in samples examined by electron microscopy quite problematic. Hence, low salt extraction of neurofilaments provides a way to examine the actin filament network at the higher resolution provided by the electron microscope without the complication of confusing them with neurofilaments.
THIN SECTION ELECTRON MICROSCOPY OF EXTRACTED AXOPLASM
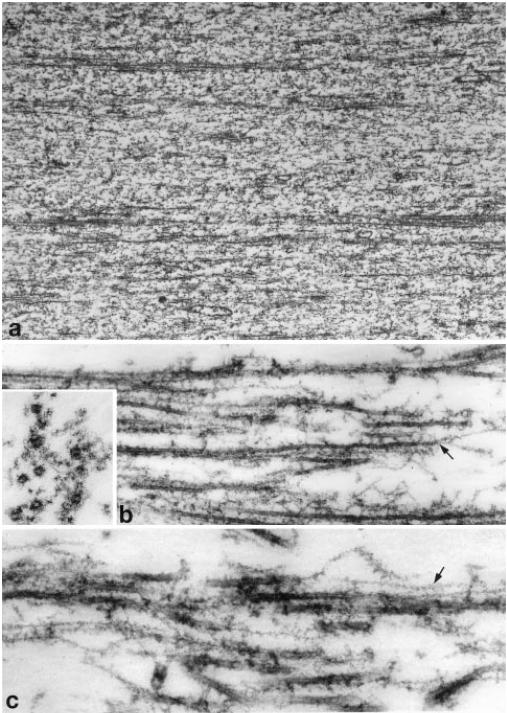
At low magnification, extruded and briefly extracted axoplasm displays scattered bundles of microtubules interspersed between a flocculent cytosol devoid of other filamentous components (Fig. 4a). At higher resolution, these microtubule bundles are seen to include finer microfilaments, interwoven among the microtubules (Fig. 4b). In cross section (Fig. 4b, inset), the microfilaments have a diameter of 6–8 nm; in some areas they contact the microtubule, while in others they are separated by 20–50 nm. Microtubules within a given bundle are 50–100 μm apart, and microfilaments occur both between them and on the periphery of the bundle.
Fig. 4.
Thin section electron microscopy of extruded axoplasm from which neurofilament has been extracted. In (a) longitudinal bundles of microtubules are interspersed with non-filamentous material representing fragmented neurofilaments (extraction in this axon was brief, leaving behind neurofilament fragments). The higher magnification in (b) is required to see that microfilaments are associated with the microtubule bundles as well as extending beyond them (arrow). A cross section view (inset in b) shows that the microfilaments have a diameter in the range of 7 nm, and lie at various distances from the associated microtubules. When the preparation is stained with S1 (c), it is apparent that many of the microfilaments (arrow) have a thickened, irregular outline suggesting that they are actin filaments. Magnifications in (a), 10,000; (b), 40,000; inset, 100,000; (c), 75,000
The microfilaments interwoven among the microtubules in the bundles are most likely to be actin, since neurofilaments, so far as can be seen by confocal microscopy after immunostaining, have been solubilized by the extraction procedure. To confirm the identity of the microfilaments, we decorated them with the S1 subfragment of myosin II. Many microfilaments appeared thicker and fuzzier, although not all were affected (Fig. 4c). Unfortunately, it proved difficult in fixed, plastic embedded material to achieve a level of S1 decoration sufficient to determine the polarity of the actin filaments associated with microtubules.
TOUCH PREPARATIONS OF AXOPLASM
In order to see the different axonal filaments in enough detail to achieve clear S1 labeling, we developed a technique for making thin spreads of axoplasm that could be examined by negative stain electron microscopy (Bearer et al., 1996c). When the surface of extruded axoplasm is lightly touched to the surface of glow-discharged, coated electron microscope grids, a thin layer of cytoskeletal elements and cytoplasm adheres to the grid. This preparation can be immediately negatively stained for electron microscopy, or stained after various chemical treatments or fixation that would be difficult in whole axons or whole extruded axoplasm because of permeability constraints. This preparation offered considerable improvement over negatively stained, whole axoplasm where the staining was always so dense that only the very periphery of the axoplasm could be seen and here the filaments were jumbled, leaving little information about their orientation within the axon.
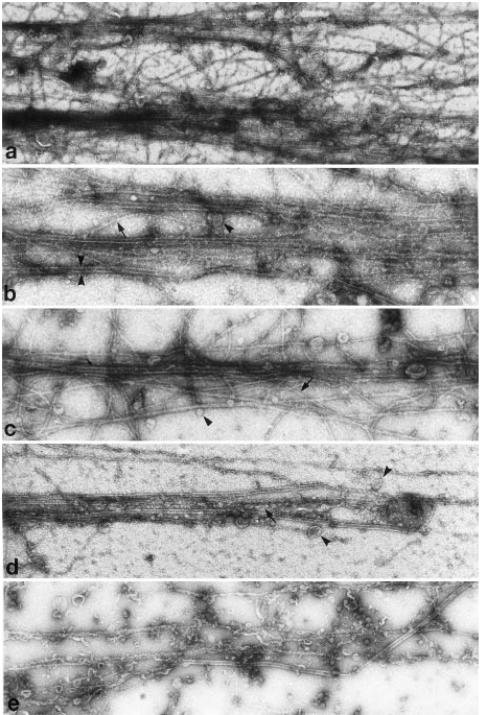
In the central region of a touch preparation that had been extracted in low salt with taxol and phalloidin, separate bundles of cytoskeletal elements are clearly visible, although their individual components are sometimes difficult to distinguish because of the thickness of the preparation (Fig. 5a). The bundles appear to correspond to the longitudinal elements seen by DIC microscopy of touch preparations described in the Methods (Fig. 1). Some membranous organelles, whose number and density varies from bundle to bundle, are also present. Microfilaments, appearing flexible and serpentine, interweave among microtubules as was observed in thin sections, and a few also traverse from one bundle to another. In less dense areas individual cytoskeletal elements within the bundle are clear, including the sinuous microfilaments crossing back and forth among long, straight microtubules. In addition, membrane-bound organelles of various sizes adhere to either microtubules or microfilaments (Fig. 5b and c). Microfilaments often extend beyond the ends of microtubules, and can run perpendicular as well as parallel to microtubules.
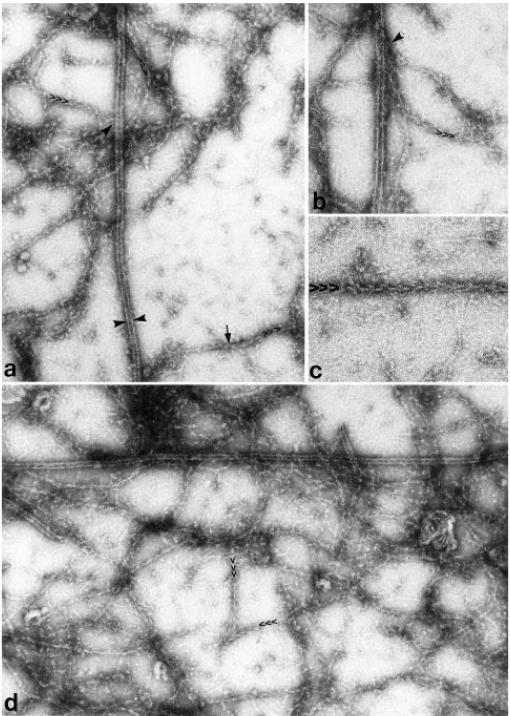
Fig. 5.
Fields selected from thinner regions of negative stained touch preparations. Field in (a) shows two bundles of microtubules lying side by side with numerous microfilaments crossing between them. In (b) microfilaments (arrow) are entwined within individual bundles of microtubules. A single microtubule is indicated between pairs of arrowheads. In (c) organelles (arrowhead) are also found within bundles of microtubules and microfilaments (arrow). In (d) endoplasmic reticulum (arrow) as well as vesicular organelles (arrowheads) are associated with the microtubule/microfilament bundles. Long cisterns of endoplasmic reticulum parallel the bundles, and contact microfilaments as well as microtubules, particularly evident when touch preparations are quickly fixed with glutaraldehyde (e). All fields are representative of the thinner regions of the preparation; thicker regions were obscured by excess stain. Magnifications in (a) 35,000; (b) 90,000; (c) 90,000; (d) 60,000; (e) 60,000.
In some regions of the preparation, tubulo-vesicular membrane, probably representing endoplasmic reticulum (ER), runs along with the microtubule/actin bundles, typically closely apposed to the microfilaments (Fig. 5d). When preparations are fixed after extraction but before staining, more ER is preserved, and in some areas it either replaces or obscures the microfilaments (Fig. 5e). Endoplasmic reticulum is not found in all the bundles even after fixation. Granular material, possibly representing vesiculated ER, can coat filaments within the bundles, obscuring them and making it difficult to determine which type of filament is coated.
S1 DECORATION OF AXOPLASMIC MICROFILAMENTS
The S1 fragment of myosin decorates the axonal actin filaments in the classical feather-like configuration in negative stained preparations (Tilney & Tilney, 1994). The direction of the feathering reveals which of the two ends of the actin filament is the “barbed” or fast growing end (Tilney et al., 1981). Thus, S1 labeling not only identifies a given filament as actin but also provides information on its polarity. Since all known myosins move only in the barbed end direction, the orientation of polarity of the actin filaments can determine the direction of actin-based transport.
We hoped that by preparing a thin touch preparation we might overcome two problems with S1 decoration in whole axoplasm: (1) poor penetration of the S1 into the deeper axoplasm resulting in patchy labeling; and (2) inability to image consistently the direction of the feathering in thin sections or in whole axoplasm. Thus, we hoped that labeling microfilaments in touch preparations with S1 would permit identification of all the actin filaments, including those that were not decorated in whole axoplasm, and possibly give insight as to their orientation.
S1 decorated a sub-population of the microfilaments in the touch preparations (Fig. 6a). While the classic feathered appearance occurred on some microfilaments, many remained resistant to labeling. Some of these undecorated filaments were also 6–8 nm sinuous microfilaments similar to those that decorated. Of these filaments some were labeled only part of the way along their length (arrow in Fig. 6a). Other filaments were thickened and rendered fuzzy after labeling, but the feathering of the S1 label could not be resolved well enough to assign polarity to these filaments. The best labeling, showing the most labeled filaments and the clearest feathering, was obtained in the thinnest part of the preparation, but in these regions the cytoskeletal elements were no longer in strictly parallel arrays, suggesting that the integrity of the longitudinal bundles had been perturbed by the preparation process. Even in the best areas, both decorated and undecorated actin-like filaments ran both parallel and perpendicular to microtubules (Fig. 6a, b and d). Despite these caveats it was clear when the direction of feathering could be determined, that the microtubule bundles included actin filaments with both polarities.
Fig. 6.
Touch preparation labeled with S1 to determine which microfilaments correspond to f-actin. Many of the microfilaments (a,b,d) appear to have S1 attached, and in some instances the polarity of the filament is evident (>>>). Other microfilaments appear unlabeled, and in some instances there are transitions between labeled and unlabeled segments of the same filament (arrow in a, below). Thus, the frequency of actin filaments may be even higher than indicated by the label. Either the pointed end or the barbed end of actin filaments can contact microtubules, suggesting that both orientations of actin filaments are present in the touch preparation. However, the variability in the overall distribution of actin filaments (d) suggests that their orientations may have become disrupted during extrusion or while making the touch preparation. Finer filaments are the nanofilaments shown in more detail in Figure 8. A single microtubule is indicated between a pair of arrowheads in (a). Magnifications in (a) 75,000; (b) 100,000; (c) 135,000; (d) 85,000.
The majority of S1-labeled filaments ranged from 0.5 to 1.5 μm in length, with a few filaments as long as 3.5 μm, which was the maximum length we could measure on an electronmicrograph (Fig. 7). This figure must represent an underestimate of the true length of these filaments, because their ends were typically lost when they joined bundles or mingled with other cytoskeletal elements. The boundaries of the micrograph when photographed at a magnification that permitted identification of the decoration on the filament also limited the length of filament that could be measured.
Fig. 7.
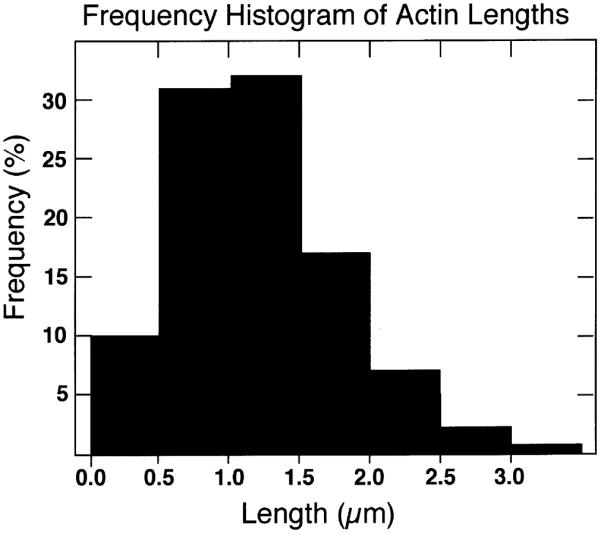
Lengths of actin filament in touch preparations. These numbers represent a minimum because in numerous instances ends of actin filaments left the field of view or were otherwise obscured. All labeled filaments with distinct ends in 45 micrographs were measured.
Another population of short filaments never labeled. These were slender filaments typically 3–5 nm in diameter and 150–200 nm in length (Fig. 8) which we call nanofilaments to distinguish them from microfilaments. While the nanofilaments appeared to originate from axoplasm because of their consistent vicinity to it, they lay directly on the glass and were never associated with membranous or other particulate structures (Fig. 6c and arrows, Fig. 8).
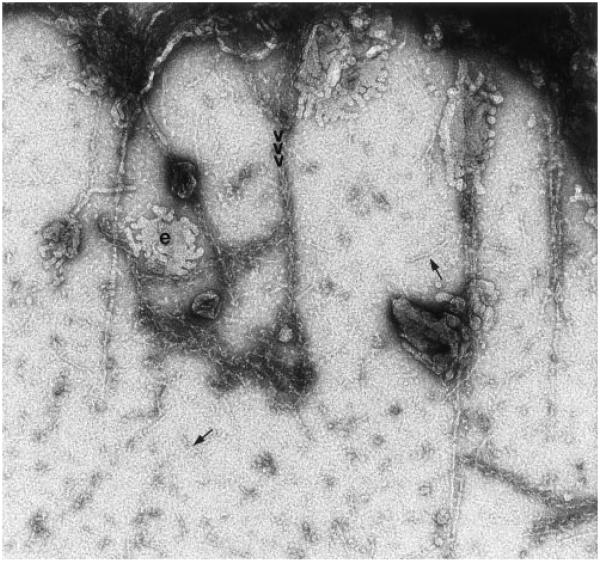
Fig. 8.
Touch preparation to which monomeric actin was added under conditions to promote polymerization of actin prior to S1 labeling. Some filaments extending from the edges of bundles (above) showed S1 label, but nanofilaments (arrows) never showed extended labeled segments, indicating that they are distinct from the actin filaments. Structure indicated by “e” appears to represent collapsed endoplasmic reticulum (Dabora and Sheetz, 1988). Magnification: 90,000.
SOME FILAMENTS DO NOT DECORATE OR NUCLEATE
Actin-binding proteins, such as scruin, block S1 from binding to actin filaments (Tilney et al., 1981). To determine if the undecorated filaments might be actin that could not bind S1, we also added exogenous actin to touch preparations under polymerizing conditions, and then decorated with S1 (Fig. 8). Exogenous actin would be expected to polymerize onto the ends of actin filaments, thereby identifying them. The exogenous actin concentration that we used was high enough to promote both barbed and pointed end elongation. Even so, some of the actin-like filaments did not nucleate or decorate, although more decorated filaments were observed overall. The numerous nanofilaments never decorated with S1, nor did they ever act as nucleation sites for polymerization of exogenous actin. Organelles and membranous structures likely to be ER were seen to be associated with decorated filaments (Fig. 8).
Discussion
Here we show that a large number of long actin filaments join, leave and run co-axially with microtubule bundles down the giant axon in the squid. Rhodamine phalloidin staining imaged by confocal laser scanning microscopy revealed abundant filaments and filament bundles in patterns that were similar but not identical to the pattern of tubulin antibody staining for microtubules. By thin section electron microscopy, numerous long microfilaments 6–8 nm in diameter were entwined within groups of microtubules. A central issue was whether the microfilaments seen by electron microscopy correspond to the microtubule-associated actin seen by confocal microscopy. By electron microscopy we could also determine the exact spatial relationships between actin filaments and microtubules and, with S1 labeling, determine the polarity of the actin.
IDENTIFICATION OF AXONAL ACTIN FILAMENTS
Because neurofilaments in the squid axon are, like actin filaments, 6–8 nm in diameter, additional criteria were needed to distinguish them from neurofilaments in electron micrographs (Leapman et al., 1997). The neurofilaments can be selectively depolymerized by extracting the extruded axon in low salt buffer while the actin and microtubules remain stabilized with phalloidin and taxol. By confocal microscopy using anti-neurofilament antibodies, this procedure removed all detectable neurofilament staining without extracting the actin filaments. In thin section electron microscopy, the spaces separating microtubule bundles were devoid of filaments and contained instead small aggregates of electron-dense material. In contrast, numerous sinuous 6–8 nm filaments, which are likely to be actin filaments remain within and around the microtubule bundles.
In order to determine whether the microfilaments stable in low salt were actually actin, the extruded axoplasm was labeled with the S1 fragment of myosin. This approach provides two pieces of information: (1) any labeled filaments must be actin because only actin binds S1; and (2) the orientation of the S1 arrowhead distinguishes the barbed or fast growing end of the actin filament. Since all myosins studied to date only move towards the barbed end of an actin filament, the structural polarity of axoplasmic filaments is crucial to understanding their participation in organelle motility. Although S1 decorated some filaments in whole axoplasm prepared for thin section electron microscopy, not all microfilaments were decorated and the decoration was too fuzzy to determine polarity. This difficulty was attributed to poor penetration of the large S1 molecule into the axoplasm, and to the presence of other proteins on the actin filaments inhibiting its binding and obscuring detection of the orientation of the arrowheads (Tilney & Tilney, 1994).
In order to circumvent these problems, we developed a touch preparation in which a thin sheet of extruded axoplasm is attached to a specimen grid, and then extracted, labeled and negatively stained for electron microscopy (Bearer et al., 1996c). This procedure allowed us to identify the microfilaments seen in electron micrographs as actin in two ways: first, by directly labeling some filaments with S1, and second by extending some endogenous filaments by adding actin monomer under polymerizing conditions (Tilney et al. 1981). However, even after extraction and polymerization, some filaments remain unlabeled, and thus not definitively identified. These could be actin filaments that are both capped and coated by other molecules that block both S1 decoration and monomer addition to filaments ends but it could also mean that some filaments are not actin. Some could even be neurofilaments, which in the squid have diameters indistinguishable from those of actin filaments, if some escaped solubilization.
AXONAL ACTIN FILAMENTS MAY BE OF MIXED POLARITY
Because the only consistent S1 decoration that we achieved was in touch preparations, where the actin might be jumbled during extrusion of the axoplasm, we cannot definitively say what the overall polarity of the filaments is. However, in every instance that it could be determined in these touch preparations, filaments of opposite polarities were found in the same field, some even contacting microtubules. If this organization is representative of axons, then organelle movements on the actin filaments associated with the microtubules bundles would be possible in both the anterograde and retrograde directions. It would appear that these actin filaments do support organelle movements because organelles are associated with them as well as with microtubules in the microtubule-actin bundles. Some of the membranous organelles of various sizes as well as tubulo-vesicular structures may represent endoplasmic reticulum (Tabb et al., 1996).
NANOFILAMENTS, A FOURTH FILAMENT SYSTEM IN THE AXON?
A population of filaments that did not nucleate actin or decorate with S1 is 3–5 nm in diameter and 150–200 nm in length. These “nanofilaments” could represent plectin (Svitkina et al., 1996) or the 45 kDa filamentous protein identified in goldfish neurons (Wang et al., 1997). Since plectin has not been found in squid, the nanofilaments cannot be assigned that identity. Plectin, which links microtubules to intermediate filaments, is 5–6 nm in diameter and 150 nm in length (Svitkina et al., 1996). The nanofilaments are associated with neither microtubules nor actin filaments, but neurofilaments have been extracted in our preparations, so the nanofilaments might have been associated with them before the extraction.
The microfilaments that we definitively determined were actin on the basis of S1 labeling, averaged 0.5–1 μm in length and some longer than 3.5 μm were seen. This differs from a previous report (Fath & Lasek, 1988) where microfilaments in the squid giant axon averaged 0.1 μm in length. In this report S1 labeling was not performed on the axonal filaments, and phalloidin was not used to stabilize them. Our results may be different because we stabilized the actin, but it could also be that the nanofilaments described here were previously confused with actin, since they are only slightly less in diameter. Indeed, without S1 labeling, the similarity of the diameter of neurofilaments in squid to that of actin filaments could confound the two filament systems.
STABILITY OF THE MICROTUBULE/ACTIN BUNDLES
Since the microtubule/actin bundles remain intact despite the solubilization of all detectable neurofilaments, it appears that this transport complex has a structural integrity that is not dependent on neurofilaments. Further supporting this idea, the microtubule/actin bundles remained intact when separated from axoplasm in the touch preparations. This integrity of the microtubule/actin bundles suggests a physical association rather than an aggregation of these filaments by exclusion from the neurofilament domains. Interdependence between microtubule and actin distribution has been demonstrated in Bryopsis (Menzel & Schliwa, 1986b), mouse melanoma cells (Cunningham et al,. 1997), and many other species (reviewed in Gavin, 1997).
Both microtubules and actin filaments can act independently as tracks to support organelle movements in vivo (Menzel & Schliwa, 1986a, b; Rodionov et al., 1998; Rogers & Gelfand, 1998; Yamashita & May, 1998). In the axon, injection of gelsolin, which severs actin filaments, arrests organelle movements (Brady et al., 1984). This result might suggest that microtubules by themselves are not sufficient to support organelle motility in the axon. Mitochondria move on either actin or microtubules in hippocampal neurons (Morris & Hollenbeck, 1995; Olink-Coux & Hollenbeck, 1996), although the rate of movement is different. In Bryopsis, drugs that destabilize microtubules also cause actin depolymerization and the cessation of organelle movements, while drugs that destabilize actin have no effect on microtubules but also effect transport (Menzel & Schliwa, 1986b). Taken together, these results support the notion that the actin and microtubule systems are synergistic and both are necessary for normal organelle transport.
Three possible contributions that actin might make to transport are shown in Figure 9. It is possible that actin is only a structural element of the microtubule bundles (Fig. 9, upper panel). However, the stunning discovery of myosin motors in axoplasm (vide supra) suggests that there could be two, synergistic transport systems, an idea which is supported by the present finding that membranous organelles make contact with actin filaments as well as microtubules. The actin filaments could thus act as ancillary substrates to mediate transport of organelles that also travel on microtubules. Microtubules do not run the length of the axon, so the actin tracks could provide bridges from one microtubule to the next (Fig. 9, middle panel).
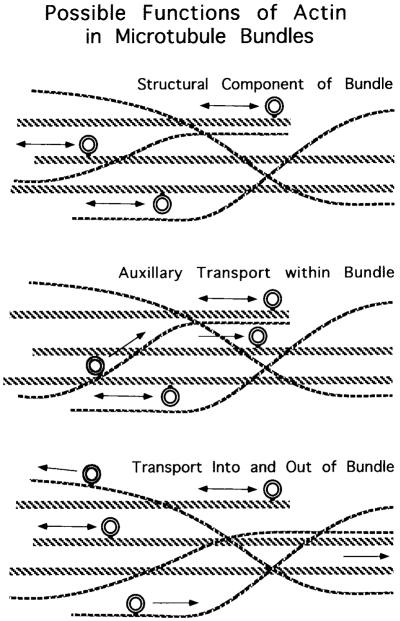
Fig. 9.
Three different possible, but not mutually exclusive, functions of actin filaments associated with microtubule bundles in axoplasm. Top: actin filaments could serve as a structural component knitting the microtubule bundle together, holding microtubules close enough to each other such that organelles can move readily from one to the next, and possibly even restricting random organelle movements. Middle: actin filaments could act as auxiliary transport tracks within microtubule bundles, mediating transfer of organelles from one microtubule to the next. In this model, organelles move for short distances on actin filaments within the bundle to cross from one microtubule to another. Bottom: actin filaments might be side streets that traffic organelles into and out of bundles to other destinations not served by microtubule pathways. In this model, organelles move on microtubules within the bundle, but enter and exit bundles on actin tracks.
Since the actin filaments run between microtubules within a bundle as well as from one bundle to another, actin could also serve as side streets to traffic organelles to different parts of the cytoplasm or even out to the axolemma, as has been proposed for the actin-based transport of organelles in the apex of gut epithelium (Fig. 9, lower panel; Fath et al., 1994). Alternatively, the actin side branches might contribute to the mixing of organelles between different bundles. In the squid, in particular, such mixing may be necessary because the giant axon arises from multiple cell bodies, each presumably contributing organelles to the axon. In any instance, the actin filaments could provide a structural integrity to the microtubule bundles, maintaining the various components of the transport system as a unit. The most attractive possibility that fits all the data at this time is that actin filaments do all of these things.
Acknowledgments
We thank Jeannette Liu, Axel Hsu, John Chludzinski and Kasia Hammer for technical assistance, Harish Pant for the generous use of his anti-neurofilament antibody. We also acknowledge Elizabeth McCloy for measurements of actin filament lengths and Carolyn Smith for advice on the use of the confocal microscope. This work was supported in part by NIH GM47368 (ELB) and CTR 3192 (ELB).
References
- BEARER EL. Platelet membrane skeleton revealed by quick-freeze deep-etch. Anatomical Record. 1990;227:1–11. doi: 10.1002/ar.1092270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARER EL. An actin-associated protein present in the microtubule organizing center and the growth cone of PC-12 cells. Journal of Neuroscience. 1992;12:750–61. doi: 10.1523/JNEUROSCI.12-03-00750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARER EL, DEGIORGIS JA, BODNER RA, KAO AW, REESE TS. Evidence for myosin motors on organelles in squid axoplasm. Proceedings of the National Academy of Sciences USA. 1993;90:11252–256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARER EL, DEGIORGIS JA, JAFFE H, MEDEIROS NA, REESE TS. An axoplasmic myosin with a calmodulin-like light chain. Proceedings of the National Academy of Sciences USA. 1996;93:6064–68. doi: 10.1073/pnas.93.12.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARER EL, DEGIORGIS JA, MEDEIROS NA, REESE TS. Actin-based motility of isolated axoplasmic organelles. Cell Motility and the Cytoskeleton. 1996b;33:106–14. doi: 10.1002/cm.970330202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARER EL, LIU J, HSU A, REESE TS. Method for visualizing filaments in axoplasm by electron microscopy. Biological Bulletin. 1996c;191:272–73. doi: 10.1086/BBLv191n2p272. [DOI] [PubMed] [Google Scholar]
- BRADY ST, LASEK RJ, ALLEN RD, YIN HL, STOSSEL TP. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature. 1984;310:56–8. doi: 10.1038/310056a0. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM CC, LECLERC N, FLANAGAN LA, LU M, JANMEY PA, KOSIK KS. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding-protein-280-deficient melanoma cell line. Journal of Cell Biology. 1997;136:845–57. doi: 10.1083/jcb.136.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DABORA SL, SHEETZ MP. The microtubuledependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988;154:27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- FATH KR, TRIMBUR GM, BURGESS DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. Journal of Cell Biology. 1994;126:661–75. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATH KR, LASEK RJ. Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. Journal of Cell Biology. 1988;107:613–21. doi: 10.1083/jcb.107.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT PE, PANT HC, PRUSS RM, GAINER H. Calcium-activated proteolysis of neurofilament proteins in the squid giant neuron. Journal of Neurochemistry. 1986;46:1573–81. doi: 10.1111/j.1471-4159.1986.tb01779.x. [DOI] [PubMed] [Google Scholar]
- GAVIN RH. Microtubule-microfilament synergy in the cytoskeleton. International Review of Cytology. 1997;173:207–42. doi: 10.1016/s0074-7696(08)62478-x. [DOI] [PubMed] [Google Scholar]
- KUCZMARSKI ER, ROSENBAUM JL. Studies on the organization and localization of actin and myosin in neurons. Journal of Cell Biology. 1979;80:356–71. doi: 10.1083/jcb.80.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUZNETSOV SA, LANGFORD GM, WEISS DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–25. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- LANGFORD GM, KUZNETSOV SA, JOHNSON D, COHEN DL, WEISS DG. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: Evidence for a barbed-end-directed organelle motor. Journal of Cell Science. 1994;107:2291–98. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- LEAPMAN RD, GALLANT PE, REESE TS, ANDREWS SB. Phosphorylation and subunit organization of axonal neurofilaments determined by scanning transmission electron microscopy. Proceedings of the National Academy of Sciences USA. 1997;94:7820–24. doi: 10.1073/pnas.94.15.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENZEL D, SCHLIWA M. Motility in the siphonous green alga Bryopsis. I. Spatial organization of the cytoskeleton and organelle movements. European Journal of Cell Biology. 1986a;40:275–85. [PubMed] [Google Scholar]
- MENZEL D, SCHLIWA M. Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. European Journal of Cell Biology. 1986b;40:286–95. [PubMed] [Google Scholar]
- METUZALS J, CHANG D, HAMMAR K, REESE TS. Organization of the cortical endoplasmic reticulum in the squid giant axon. Journal of Neurocytology. 1997;26:529–39. doi: 10.1023/a:1015482407202. [DOI] [PubMed] [Google Scholar]
- MOLYNEAUX BJ, LANGFORD GM. Characterization of antibodies to the head and tail domains of squid brain myosin V. Biology Bulletin. 1997;193:222–23. doi: 10.1086/BBLv193n2p222. [DOI] [PubMed] [Google Scholar]
- MORRIS RL, HOLLENBECK PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. Journal of Cell Biology. 1995;131:315–26. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLINK-COUX M, HOLLENBECK PJ. Localization and active transport of mRNA in axons of sympathetic neurons in culture. Journal of Neurosciences. 1996;16:1346–58. doi: 10.1523/JNEUROSCI.16-04-01346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. The structure of the neurofilament cytoskeleton in the squid giant axon and synapse. Journal of Neurocytology. 1996;25:547–54. doi: 10.1007/BF02284822. [DOI] [PubMed] [Google Scholar]
- RODIONOV VI, HOPE AJ, SVITKINA TM, BORISY GG. Functional coordination of microtubule-based and actin-based motility in melanophores. Current Biology. 1998;8:165–68. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- ROGERS SL, GELFAND VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Current Biology. 1998;8:161–64. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- SCHNAPP BJ, VALE RD, SHEETZ MP, REESE TS. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985;40:455–62. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- SVITKINA TM, VERKHOVSKY AB, BORISY GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. Journal of Cell Biology. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABB JS, HARMON KO, DEPINA AS, LANGFORD GM. Localization of myosin on tubulovesicular organelles in the squid giant axon by immuno-EM. Biology Bulletin. 1996;191:274–75. doi: 10.1086/BBLv191n2p274. [DOI] [PubMed] [Google Scholar]
- TILNEY LG, BONDER EM, DEROSIER DJ. Actin filaments elongate fromtheir membrane-associated ends. Journal of Cell Biology. 1981;90:485–94. doi: 10.1083/jcb.90.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILNEY LG, TILNEY MS. Methods to visualize actin polymerization associated with bacterial invasion. Methods in Enzymology. 1994;236:476–81. doi: 10.1016/0076-6879(94)36036-7. [DOI] [PubMed] [Google Scholar]
- WANG S-M, CHEN J-S, FONG T-H, HSU S-Y, LIM S-S. Characterization of a novel filament system in goldfish xanthophores. Cell Motility and the Cytoskeleton. 1997;36:216–27. doi: 10.1002/(SICI)1097-0169(1997)36:3<216::AID-CM2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- YAMASHITA RA, MAY GS. Motoring along the hyphae: molecular motors and the fungal cytoskeleton. Current Opinion in Cell Biology. 1998;10:74–9. doi: 10.1016/s0955-0674(98)80088-4. [DOI] [PubMed] [Google Scholar]
- ZACKROFF RV, GOLDMAN RD. In vitro reassembly of squid brain intermediate filaments (neurofilaments): Purification by assembly-disassembly. Science. 1980;208:1152–54. doi: 10.1126/science.7189605. [DOI] [PubMed] [Google Scholar]