Abstract
Background: Bariatric procedures are effective options for weight loss (WL) in the morbidly obese. However, some patients fail to lose any weight after bariatric surgery, and mid-term weight maintenance is variable. The aim of this study was to investigate whether initial WL could predict mid-term weight maintenance.
Methods: Eighty patients were enrolled, of whom 44 were treated with the BioEnterics Intragastric Balloon (BIB), 21 with laparoscopic adjustable gastric lap-banding (LAGB), and 15 with laparoscopic sleeve gastrectomy (LSG). Percentage of body WL and percentage of excess weight loss (EWL) were calculated at baseline and after 1, 3, 6, and 12 months. Successful WL was defined as EWL >20% for patients treated with BIB and >50% for patients treated with LAGB and SG.
Results: Success in the 6th and 12th month was achieved in 80% and 58% of patients in the BIB group, 33% and 40% in the LAGB group, and 60% and 73% in the LSG group. In the BIB group, WL in the 1st month correlated positively with WL at the 6th and 12th month, and an initial WL >6.5% best predicted success (sensitivity 50%, specificity 80%). A similar association was observed in the LAGB group at the 6th and 12th month and an initial WL >9.4% best predicted success (sensitivity 90.0%, specificity 81.2%). In patients treated with LSG, WL in the 3rd month correlated positively with EWL at the 6th and 12th month, with a cutoff value of 17% (sensitivity 66.7%, specificity 100%).
Conclusions: WL in the 1st month in patients treated with BIB and LAGB and WL in the 3rd month in patients treated with LSG could be used as a prognostic factor to predict mid-term weight maintenance.
Introduction
With the obesity epidemic on the rise, bariatric procedures are increasingly being performed worldwide. Bariatric surgery is an overall effective method in obesity treatment, with a mean percentage of excess weight loss (EWL) after 2 years of 47.5% for laparoscopic gastric banding (LAGB) and 68.2% for laparoscopic sleeve gastrectomy (LSG).1 The BioEnterics Intragastric Balloon (BIB) is less effective, with EWL ranging from 27.0% to 48.3% after 6 months of treatment.2,3 In addition, only a quarter of patients treated with BIB achieve long-term weight maintenance.4 Despite the overall success of bariatric surgery, approximately 15–20% of patients fail to lose any weight after the procedure.5 To the authors' knowledge, no study to this date has managed to explain the exact cause for these differences in outcome. Few studies have aimed to detect prognostic factors that could influence the outcome of bariatric surgery. Established predictors of weight loss (WL) after bariatric surgery include age, preoperative WL, initial body mass index (BMI), depression score, eating behavior and level of physical activity after surgery.6–14 Only one study analyzed the influence of initial WL on long-term weight maintenance in patients treated with BIB. The authors concluded that a 5% WL after the first month predicted long-term outcome.15 These findings are of great interest because 50–75% of patients fail to maintain WL after BIB removal,16 and it is crucial to detect those patients early in order to initiate other treatment modalities. To the authors' knowledge, no studies have analyzed the effect of initial WL after bariatric surgery on later WL maintenance. Therefore, the aim of this study was to investigate whether initial WL in the first 3 months following treatment with BIB, LAGB, and LSG could predict WL after 1 year of treatment.
Patients and Methods
A prospective nonrandomized study was conducted in a tertiary care referral hospital in Croatia. Eighty patients were enrolled; 44 patients (37 female, age range 20–60 years) were treated with BIB, 21 (16 female, age range 36–61 years) with LAGB, and 15 patients (11 female, age range 25–60 years) with LSG. Decision on the treatment modality was made during multidisciplinary rounds after detailed endocrinological, gastroenterological, and psychological evaluation. Each patient's preference was also taken into consideration. The protocol was approved by the university hospital center's Institutional Review Board, and all patients gave informed consent.
The BIB by INAMED Corporation (Santa Barbara, CA) was used in this study. It was positioned under endoscopic control and filled with 600 mL saline and methylene blue. It was removed after 6 months.
A single experienced surgeon performed the surgeries (M.B.-B.). LAGB was performed using a Swedish Adjustable Gastric Band (Ethicom EndoSurgery, Cincinnati, CH). The surgeon made the choice regarding the size of the gastric band system used. The standardized pars flaccida technique was used.17 The band reservoir was gradually filled with 6.5 mL of saline. The band reservoir was additionally filled with 2 mL of saline in the 4th and 8th week after the procedure. If the patient did not lose >0.5 kg/week, the band reservoir was filled with an additional 2–2.5 mL in the 12th week.
For the LSG procedure, the greater curvature of the stomach was dissected from the antrum to the level of the left crus of the diaphragm. A 36 Fr calibration probe was inserted into the stomach and placed next to the lesser curvature. Longitudinal gastric resection was then peformed parallel to this probe using a 60 mm cartridge stapler (green or black). The resection line extended from the antrum (5 cm proximal to the pylorus) to the angle of Hiss. Caution was undertaken in order to avoid placing the resection line too close to the esophagus.
Patients were followed over a 1 year period. They were encouraged to change their eating habits, participate in regular physical activity, and attend their follow-up visits. Follow-up visits were scheduled at the end of the 1st, 3rd, 6th, and 12th month. Pre- and postoperative workup included physical examination, anthropometric measurements, detailed laboratory and endocrine evaluation, transabdominal ultrasonography, and psychological evaluation. The main outcome measures were percentage WL and EWL. EWL was calculated as WL divided by excess weight at baseline, with the quotient multiplied by 100: EWL=(body mass at baseline – body mass at follow-up)/(body mass at baseline–ideal body mass)×100, where the ideal body mass was calculated using a BMI of 25 kg/m2, where BMI was calculated as weight in kilograms divided by the height in meters squared.
Mid-term WL was calculated after the 6th and 12th month. Successful mid-term WL was defined as EWL >20% for patients treated with BIB, and EWL >50% for patients treated with LAGB and SG.
Statistical analyses
Patient characteristics were assessed using descriptive statistics presented as a median with interquartile range values. Independent variables were compared using the Mann–Whitney U-test, and dependent variables (treatment outcomes) were compared using Wilcoxon's test. Linear regression was used to evaluate the association between initial WL and EWL after 6 and 12 months (data were expressed with R2 and p-values). Receiver operating characteristic (ROC) analysis was performed for significant associations in order to establish cutoff values of initial WL. Only anthropometric characteristics were compared between patients treated with different procedures. No other statistical analysis was performed between different treatment groups. p-Values <0.05 were considered significant. Statistical analysis was performed using MedCalc v12.7.5 (MedCalc Software, Ostend, Belgium).
Results
The baseline BMI was 42.1 kg/m2 (32.6–60.8 kg/m2) in patients treated with BIB, 41.8 kg/m2 (36.2–50.0 kg/m2) in patients treated with LAGB, and 46.8 kg/m2 (40.8–58.8 kg/m2) in patients treated with LSG (p<0.001). Seven patients in the BIB group, six in the LAGB group, and 1 in the LSG group were lost to follow-up at 12 months. Data were gathered from all patients at the 6th month follow-up visit. BMI and EWL decreased significantly in all groups after the 6th and 12th month. As expected, a more pronounced decrease was observed in the LAGB and the LSG group (Table 1).
Table 1.
Anthropometric Parameters and Weight Loss in Patients Treated with BioEnterics Intragastric Balloon (BIB), Laparoscopic Adjustable Gastric Banding (LAGB), and Laparoscopic Sleeve Gastrectomy (LSG)
| BIB (n=44) | LAGB (n=21) | LSG (n=15) | p-Value | |
|---|---|---|---|---|
| Age, years | 35 (20–59) | 36 (21–61) | 45 (25–60) | 0.054 |
| Weight, kg | 114 (90–197) | 120 (109–165) | 135 (112–180) | 0.003 |
| Body mass index, kg/m2 | 40.3 (32.6–60.8) | 41.8 (36.2–50.0) | 46.8 (40.8–58.8) | <0.001 |
| Waist circumference, cm | 117 (96–180) | 120 (103–147) | 132 (114–165) | 0.007 |
| Hip circumference, cm | 129.5 (107–166) | 129 (120–153) | 142 (114–165) | 0.046 |
| Excess weight, kg | 42.9 (21.6–116) | 50.2 (35.3–77.6) | 67.6 (47.2–103.4) | <0.001 |
| Gender | ||||
| Male n (%) | 8 (18.2) | 5 (23.8) | 4 (26.7) | 0.744 |
| Female n (%) | 36 (81.8) | 16 (76.2) | 11 (73.3) | |
| Weight loss 1st month (%) | 5.71 (4.39–7.23) | 9.17 (7.64–12.20) | 10.70 (8.83–12.6) | <0.001 |
| Weight loss 3rd month (%) | 9.63 (8.16–11.5) | 14.3 (10.9–18.6) | 16.9 (15.4–17.9) | <0.001 |
| Weight loss 6th month (%) | 11.7 (8.26–15.0) | 18.3 (14.2–22.2) | 23.3 (19.8–26.0) | <0.001 |
| p-Value (initial vs. 6th) | <0.001 | <0.001 | <0.001 | |
| BMI decrease 1st (kg/m2) | 2.51 (1.86–3.03) | 4.10 (3.04–5.02) | 4.84 (4.21–5.75) | <0.001 |
| BMI decrease 3rd (kg/m2) | 3.90 (3.21–5.00) | 6.55 (4.41–7.42) | 7.97 (7.17–8.59) | <0.001 |
| BMI decrease 6th (kg/m2) | 4.76 (3.03–5.82) | 7.9 (5.65–9.08) | 10.9 (9.25–13.18) | <0.001 |
| p-Value (initial vs. 6th) | <0.001 | <0.001 | <0.001 | |
| EWL 6th month (%) | 32.0 (21.7–41.3) | 43.1 (33.9–51.5) | 50.7 (43.8–55.1) | <0.001 |
| Weight loss 12th month (%) | 9.26 (4.3–17.4) | 20.0 (14.3–25.6) | 26.3 (22.6–34.7) | 0.002 |
| p-Value (initial vs. 12th) | <0.001 | <0.001 | <0.001 | |
| BMI decrease 12th (kg/m2) | 3.54 (1.63–7.32) | 9.30 (6.08–10.80) | 11.80 (9.98–17.55) | <0.001 |
| p-Value (initial vs. 12th) | <0.001 | <0.001 | <0.001 | |
| EWL 12th month (%) | 27.3 (10.9–43.5) | 44.6 (38.9–61.1) | 58.6 (50.7–68.6) | 0.002 |
BMI, body mass index; EWL, excess weight loss.
In the BIB group, success was achieved in 80% and 58% of patients at the 6th and 12th month, respectively. However, 6/44 patients did not respond to treatment and had a WL of <6% at the 6th month. After BIB removal, 56% (21/37) of patients gained weight. In the LAGB group, 33% and 40% of patients achieved success at the 6th and 12th month, respectively. In the LSG group, 60% and 73% of patients achieved success at the 6th and 12th month, respectively. In the LAGB group, moderate WL was observed in 5/15 (<15%) patients. Moderate WL was observed in one patient treated with LSG (<35%). No patients in the LSG and LAGB group gained weight during the 6–12 month period.
Linear regression analysis revealed that WL after the first month of treatment with BIB correlated positively with EWL at the 6th (R2=0.184, p=0.004) and 12th month (R2=0.2024, p=0.009). The same association was observed in the LAGB group at the 6th (R2=0.4470, p=0.001) and 12th month (R2=0.3006, p=0.0343). In patients treated with LSG, there was no association between WL in the 1st month and EWL at the 6th and 12th month. However, WL in 3rd month correlated positively with EWL at the 6th (R2=0.357, p=0.019) and 12th month (R2=0.294, p=0.037).
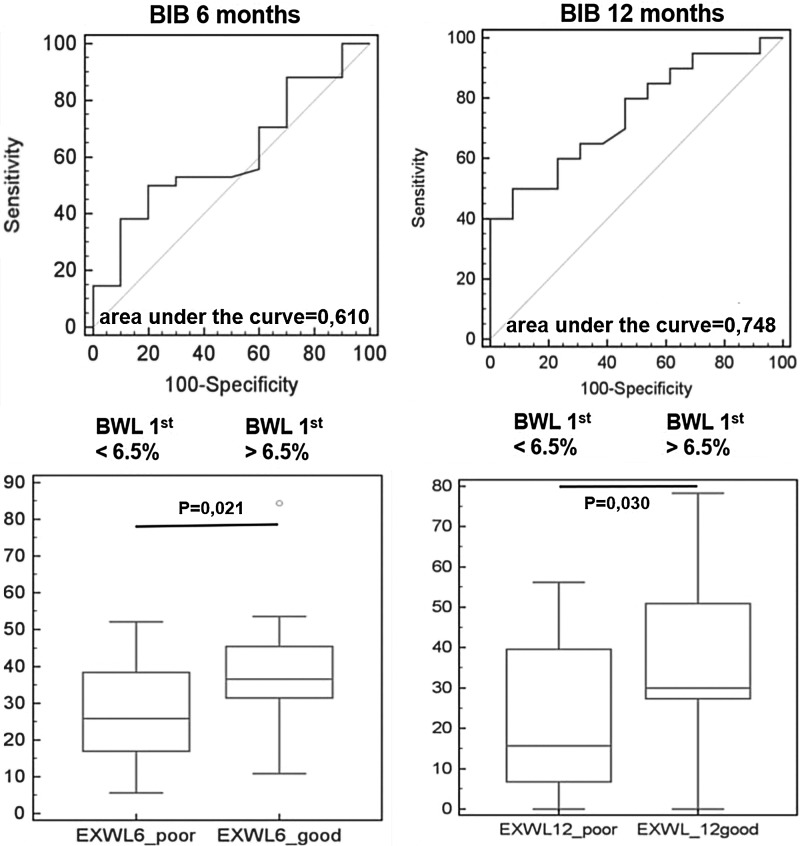
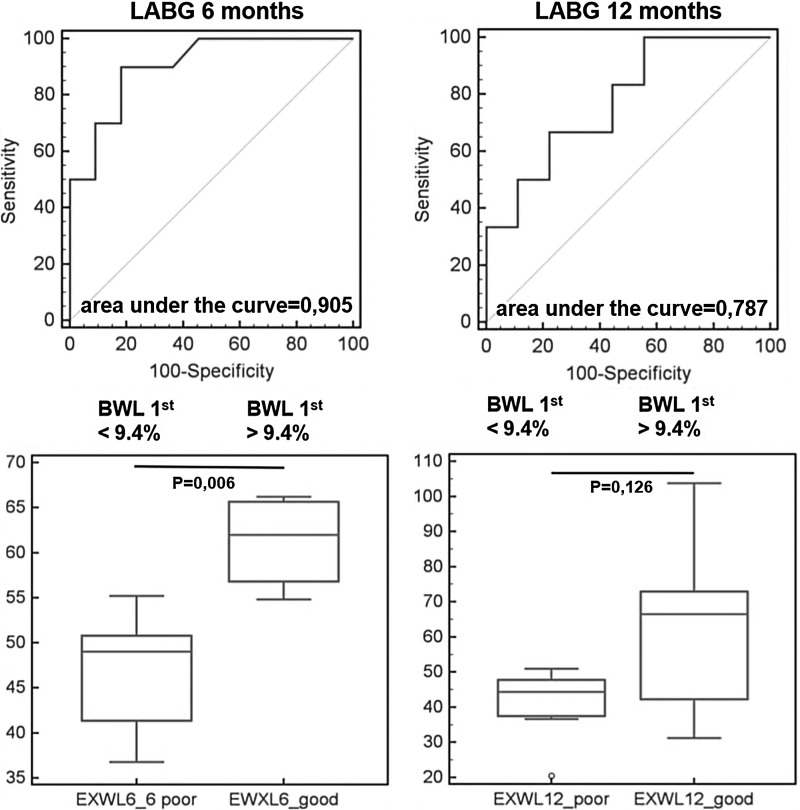
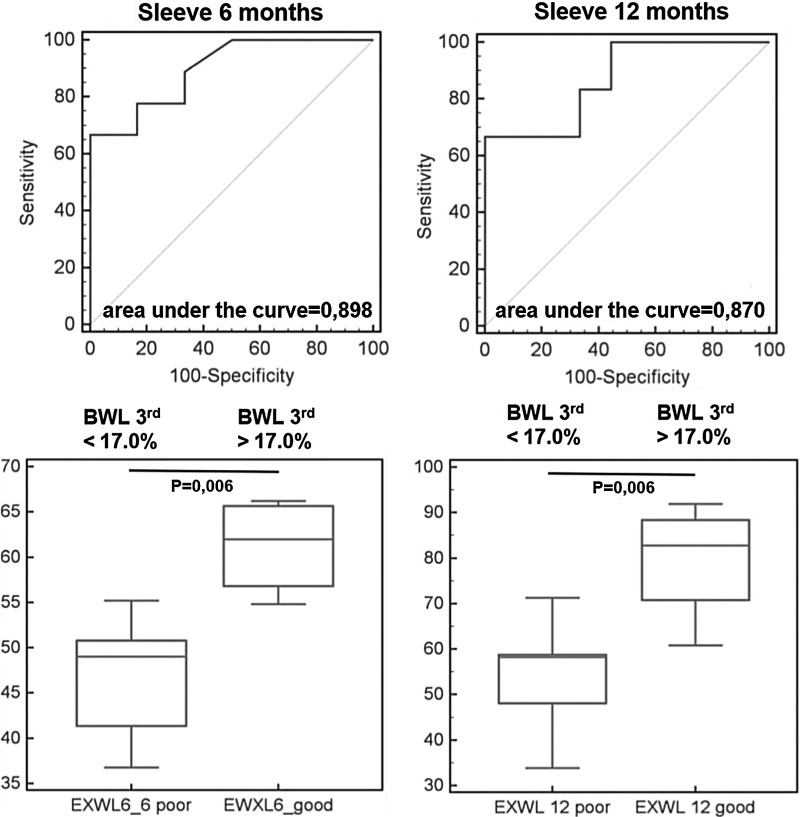
In order to establish cutoff values for initial WL, ROC analysis was performed. It was found that an initial WL ≥6.5% best predicted success in the BIB group (sensitivity 50%, specificity 80%; Fig. 1). Successful WL at the 6th month was achieved in all patients with an initial WL >6.8% (specificity 100% [95% CI 63.1–100.0]). Successful WL at the 12th month was achieved in all patients with an initial WL >7.3% (specificity 100% [95% CI 75.3–100.0]). On the other hand, no patients with an initial WL <3.1% (sensitivity 100% [95% CI 89.4–100.0]) met the success criteria at the 6th month. Similarly, no patients with an initial WL <3.4% (sensitivity 100% [95% CI 83.2–100.0]) met the success criteria at the 12th month. Initial WL >9.4% in the LAGB group best predicted successful WL (sensitivity 90.0%, specificity 81.2%; Fig. 2). All patients with an initial WL>13.1% (specificity 100% [95% CI 76.8–100.0]) and >12.5% (specificity 100% [95% CI 66.4–100.0]) reached the success criteria at the 6th and 12th month, respectively. No patients reached the success criteria with an initial WL<7.8% (sensitivity 100% [95% CI 59.0–100.0]) and <7.5% (sensitivity 100% [95% CI 54.1–100.0]) at the 6th and 12th month, respectively. In the LSG group, the 3rd month WL cutoff value of 17.0% best predicted WL (sensitivity 66.7%, specificity 100%; Fig. 3). All patients with initial WL>18.2% (specificity 100% [95% CI 59.9–100.0]) reached the success criteria at the 6th and 12th month. No patients reached the success criteria with an initial WL<15.4% (sensitivity 100% [95% CI 63.1–100.0]) and <15.6% (sensitivity 100% [95% CI 54.1–100.0]) at the 6th and 12th month, respectively.
FIG. 1.
Receiver operating characteristic (ROC) curve and excess weight loss in patients with 1st month weight loss (WL1) <6.5%, and >6.5% in 6th and 12th month of treatment with BioEnterics Intragastric Balloon.
FIG. 2.
ROC curve and excess weight loss in patients with 1st month weight loss (WL1) <9.4%. and >9.4% in 6th and 12th month after laparoscopic adjustable gastric banding.
FIG. 3.
ROC curve and excess weight loss in patients with 3rd month weight loss (WL3) <17%, and >17% in 6th and 12th month after laparoscopic sleeve gastrectomy.
Discussion
This study demonstrated that initial WL after restrictive bariatric procedures could predict WL maintenance up to 1 year after the procedure. In patients treated with BIB and LAGB, WL in the 1st month proved to be a good predictor of mid-term weight maintenance. On the other hand, WL in the 3rd month best correlated with mid-term weight maintenance in patients treated with LSG. In comparison with the meta-analysis by Buchwald et al., this study was inferior in terms of EWL.1 This could be explained from a socioeconomic standpoint. The present study was publicly funded, and it is known that publicly funded treatment is adversely associated with long-term weight maintenance.18 The difference cannot be explained by the operator's skill because, as proposed by Prevot et al., an experienced surgeon who performs more than 50 LSG and LAGB procedures per year performed the operations.19 Despite the overall success of bariatric surgery, approximately 15–20% of patients fail to lose any weight after LSG and LAGB.5 Furthermore, 50–75% of patients treated with BIB gain weight once the BIB is removed.16 The exact cause of these trends is still a matter of debate. Some authors emphasize the importance of eating habits and behavior. Psychological aspects also play a role, and patients who fail to lose weight after bariatric surgery are also more likely to crave sweets and have disordered eating behaviors such as bulimia.20,21 Additionally, preoperative binge eating is also associated with poor results after gastric bypass.8 However, since the exact mechanism of failure is still unknown, several studies have aimed to detect additional factors that could predict long-term outcome. Important behavioral and prognostic factors include a will to change eating habits and increase physical activity, depression score, and cognitive function.6,9,13 Age and BMI correlate negatively with WL6,7 and preoperative WL achieved with other treatment modalities correlates positively.11,12 The results of this study indicate that WL in the 1st month after BIB placement and LAGB can be used as predictors for mid-term and possibly long-term WL. Unexpectedly, only WL in the 3rd month has emerged as a prognostic factor in patients treated with LSG. This could possibly be explained by the fact that LSG is a more radical procedure than LAGB and BIB. Therefore, all patients lose weight rapidly in the 1st month, and the WL pattern emerges after the third month. These findings can help improve the care of patients undergoing bariatric procedures. It can help identify which patients are at high or low risk for treatment failure. Additional medication or psychotherapy could be initiated in “high risk” patients immediately after the procedure in order to avoid bariatric procedures in the future.22
This study has some limitations. Despite the prospective design, a relatively small number of patients was analyzed with a moderate dropout rate, particularly in the LAGB group. Moreover, only patients treated with restrictive bariatric procedures were analyzed.
In conclusion, a WL>6.5% in the 1st month in patients treated with BIB and a WL>9.4% in the 1st month in patients treated with LAGB could be used as a good prognostic factor for mid-term and possibly long-term WL. In patients treated with LSG, a WL of >17% in the 3rd month could also be used as a good prognostic factor for mid-term and possibly long-term WL. Further studies with a longer follow-up period should be carried out. Additionally, the role of initial WL in predicting long-term weight maintenance should be analyzed in patients treated with malabsorptive procedures as well.
Acknowledgments
The results of this study were presented at Asian Pacific Digestive Week 2013, Shangai, and were published in an abstract form in the Journal of Gastroenterology and Hepatology 2013; 28, suppl. 3.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. . Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 2.Sallet JA, Marchesini JB, Paiva DS, et al. . Brazilian multicenter study of intragastric balloon. Obes Surg 2004;14:991–998 [DOI] [PubMed] [Google Scholar]
- 3.Coskun H, Bostanci O, Dilege E, Bozbora A. BioEnterics Intragastric Balloon: clinical outcomes of the first 100 patients—a Turkish experience. Obes Surg 2008;18:1154–1156 [DOI] [PubMed] [Google Scholar]
- 4.Dastis NS, François E, Deviere J, et al. . Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy 2009;41:575–580 [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Li P, Sun XF, Ye NY, Xu ZK, Wang D. Comparison between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding for morbid obesity: a meta-analysis. Obes Surg 2013;23:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier JM, Paita M, Rodde-Dunet MH, et al. . Predictive factors of outcome after gastric banding: a nationwide survey on the role of center activity and patients' behavior. Ann Surg 2007;246:1034–1039 [DOI] [PubMed] [Google Scholar]
- 7.Scozzari G, Passera R, Benvenga R, Toppino M, Morino M. Age as a long-term prognostic factor in bariatric surgery. Ann Surg 2012;256:724–729 [DOI] [PubMed] [Google Scholar]
- 8.Sallet PC, Sallet JA, Dixon JB, et al. . Eating behavior as a prognostic factor for weight loss after gastric bypass. Obes Surg 2007;17:445–451 [DOI] [PubMed] [Google Scholar]
- 9.Averbukh Y, Heshka S, El-Shoreya H, et al. . Depression score predicts weight loss following Roux-en-Y gastric bypass. Obes Surg 2003;13:833–836 [DOI] [PubMed] [Google Scholar]
- 10.Guajardo-Salinas GE, Hilmy A, Martinez-Ugarte M. Predictors of weight loss and effectiveness of Roux-en-Y gastric bypass in the morbidly obese Hispano-American population. Obes Surg 2008;18:1369–1375 [DOI] [PubMed] [Google Scholar]
- 11.Alami RS, Morton JM, Schuster R, et al. . Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis 2007;3:141–146 [DOI] [PubMed] [Google Scholar]
- 12.Alger-Mayer S, Polimeni JM, Malone M. Preoperative weight loss as a predictor of long-term success following Roux-en-Y gastric bypass. Obes Surg 2008;18:772–775 [DOI] [PubMed] [Google Scholar]
- 13.Spitznagel MB, Alosco M, Strain G, et al. . Cognitive function predicts 24-month weight loss success after bariatric surgery. Surg Obes Relat Dis 2013;9:765–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel A, Sebbag G, Fraser D, Levy I. Nutritional behavior as a predictor of early success after vertical gastroplasty. Obes Surg 2005;15:88–94 [DOI] [PubMed] [Google Scholar]
- 15.Dogan UB, Gumurdulu Y, Akin MS, Yalaki S. Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance. Obes Surg 2013;23:892–896 [DOI] [PubMed] [Google Scholar]
- 16.Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg 2012;22:896–903 [DOI] [PubMed] [Google Scholar]
- 17.Cobourn C, Mumford D, Chapman MA, et al. . Laparoscopic gastric banding is safe in outpatient surgical centers. Obes Surg 2010;20:415–422 [DOI] [PubMed] [Google Scholar]
- 18.Afoke J, Agrawal S, Edmond J, Mahon D, Welbourn R. Effect of source of funding on weight loss up to 3 years after gastric banding. Surg Endosc 2013;27:1219–1224 [DOI] [PubMed] [Google Scholar]
- 19.Prevot F, Verhaeghe P, Pequignot A, et al. . Two lessons from a 5-year follow-up study of laparoscopic sleeve gastrectomy: persistent, relevant weight loss and a short surgical learning curve. Surgery 2014;155:292–299 [DOI] [PubMed] [Google Scholar]
- 20.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA 2002;288:2793–2796 [DOI] [PubMed] [Google Scholar]
- 21.Sarwer DB, Dilks RJ, West-Smith L. Dietary intake and eating behavior after bariatric surgery: threats to weight loss maintenance and strategies for success. Surg Obes Relat Dis 2011;7:644–651 [DOI] [PubMed] [Google Scholar]
- 22.Elnahas A, Graybiel K, Farrokhyar F, Gmora S, Anvari M, Hong D. Revisional surgery after failed laparoscopic adjustable gastric banding: a systematic review. Surg Endosc 2013;27:740–745 [DOI] [PubMed] [Google Scholar]