Abstract
Recent progress in identification and characterization of novel types of non-coding RNAs has proven that RNAs carry out a variety of cellular functions ranging from scaffolding to gene expression control. In both prokaryotic and eukaryotic cells, several classes of non-coding RNAs control expression of dozens of genes in response to specific cues. One of the most interesting and outstanding questions in the RNA field is whether regulatory RNAs are capable of employing basic biological concepts, such as allostery and cooperativity, previously attributed to the function of proteins. Aside from regulatory RNAs that form complementary base pairing with their nucleic acid targets, several RNA classes modulate gene expression via molecular mechanisms which can be paralleled to protein-mediated regulation. Among these RNAs are riboswitches, metabolite-sensing non-coding regulatory elements that adopt intrinsic three-dimensional structures and specifically bind various small molecule ligands. These characteristics of riboswitches make them well-suited for complex regulatory responses observed in allosteric and cooperative protein systems. Here we present an overview of the biochemical, genetic, and structural studies of riboswitches with a major focus on complex regulatory mechanisms and biological principles utilized by riboswitches for such genetic modulation.
Keywords: RNA structure, X-ray crystallography, metabolite, gene expression, transcription, non-coding RNA
Graphical Abstract
1. INTRODUCTION
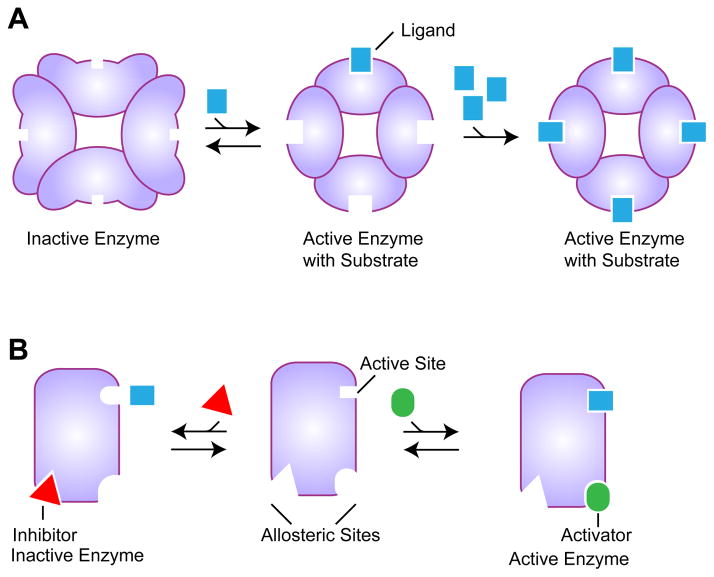
For many years now it has been recognized that some proteins do not simply bind to small molecules to perform a chemical reaction or carry on with a different function. These proteins interact with one or more ligands, providing a finely tuned response to environmental conditions by enhancing or diminishing protein activity. A biological concept describing such complex behavior of protein systems had begun emerging from the beginning of the last century. This concept, termed cooperativity, was later recognized as an important principle describing functional properties of many biological systems. Cooperativity is manifested when the binding of a ligand to a protein alters the affinity for subsequent binding of the same ligand (Fig. 1A). Structural and biochemical studies of oxygen-carrying tetrameric hemoglobins have had a pivotal role in explaining the phenomenon of cooperativity (reviewed in [1]). We now understand, in detail, how binding of the first oxygen molecule facilitates binding of subsequent oxygen molecules. Hemoglobin has also played a major role in the progress towards our understanding of a related concept, allostery. Allostery is typically observed when binding of a ligand changes the protein conformation so that a distant site becomes available for binding or loses the ability to bind to a different ligand (Fig. 1B). Many protein enzymes utilize allosteric regulation to modulate a chemical reaction by binding to a regulatory molecule, an activator or inhibitor, that opens or closes binding sites for reaction substrates.
Fig. 1.
Cooperative and allosteric ligand binding. A. Schematic of cooperative binding. Initial ligand binding to one subunit changes conformation of other subunits to facilitate their binding of other ligand molecules. B. Schematic of allosteric binding. The apo enzyme (middle) can bind either an inhibitor, which would change the conformation of the active site to prevent binding of the substrate to the distal active site, or an activator, which would facilitate substrate binding to the active site.
The concepts of cooperativity and allostery have gradually expanded to explain complex behavior of various biological systems and processes including but not limited to regulation [2]. The term cooperativity is used to describe folding of macromolecules and the formation of molecular structures and macromolecular ensembles while allostery is often referenced to illustrate ligand-induced conformational transitions that impact the function of a biological molecule. Cooperativity and allostery are not exclusive characteristics to protein molecules and are commonly used to describe processes involving nucleic acids such as formation of DNA duplexes, folding of RNA structures, and assembly of nucleic acid-protein complexes [2–5].
The discovery of catalytic RNAs made researchers think of allosteric and cooperative systems that closely resemble ligand-binding regulatory proteins. Such systems could control protein expression on the level of mRNA in response to small molecule ligands or serve as specific and sensitive sensors of chemical compounds. To illustrate this concept, several groups designed allosteric ribozymes that respond to effector molecules such as oligonucleotides or small organic molecules [6–10]. These RNAs employ the architecture and regulatory principles analogous to those established for allosterically regulated proteins. They form effector-binding structures that specifically interact with selected ligands, which bring about a conformational change in the ribozyme, affecting its catalytic rate. The performance capability of an allosteric ribozyme can be designed to resemble that of the natural cooperative proteins by combining two different ligand sensors both of which are required to induce maximum catalytic function [11].
Modulation of gene expression on the mRNA level does not necessarily need a ribozyme’s activity and can be elicited through the interplay of alternative conformations in the region that bears regulatory elements, such as a ribosome-binding site (RBS) or a transcriptional terminator. This mechanism of regulation is employed by a number of proteins that bind 5′ untranslated regions (UTRs) of their mRNAs and prevents translation initiation in an autoinhibitory feedback response [12, 13]. The collection of natural mRNAs exhibiting similar regulatory properties has greatly increased with the discovery of riboswitches, typically cis regulatory mRNA regions found in many bacterial operons and some eukaryotic genes (reviewed in [14, 15]). Riboswitches modulate the expression of genes in response to cellular metabolites over the concentration threshold. Metabolite binding to the evolutionarily conserved metabolite-sensing or aptamer domain of a riboswitch stabilizes the ligand-bound tertiary structure and facilitates folding of a regulatory element (e.g. the transcription terminator) in the nonconserved downstream “expression platform”. In the absence of the cognate ligand, the riboswitch adopts an alternative conformation and directs gene expression in the opposite way. Given the large variety of riboswitch ligands and molecular mechanisms employed by riboswitches for gene control, it is particularly interesting whether riboswitches, which are often considered as a paradigm of RNA-mediated gene regulation, can utilize cooperativity and other basic biological concepts as their protein counterparts do. In this review, we will give an overview of riboswitches which are engaged in complex regulatory responses involving more than one ligand. We will discuss the up-to-date studies focused on deciphering the regulatory mechanisms employed by such riboswitches and will attempt to answer the question of whether these RNAs can be compared with allosteric and cooperative protein systems.
2. Allostery and cooperativity in riboswitch folding
In general terms, all riboswitches can be viewed as both allosteric and cooperative systems. On the one hand, all riboswitches are allosteric molecules because they modulate expression of genes through conformational changes in response to binding with the cognate ligand [16]. The ligand-induced conformational rearrangement affects binding of the effector molecule, ribosome, or RNA polymerase, to the riboswitch thus impacting the regulatory outcome. On the other hand, all riboswitches are cooperative molecules because they adopt intricate three-dimensional structures through cooperative folding that involves formation of double-stranded regions and tertiary elements [17, 18]. In this review however we are most interested in the “classical” aspects of cooperativity and allostery, by comparing riboswitches with protein enzymes whose activity is modulated by interactions with more than one specific molecule.
3. Multiple metal cation binding: a case of cooperativity?
Metal cations play diverse roles in cellular activities and certain cations are indispensable for living organisms. Some metal cations assist folding of macromolecules and serve as structural cofactors. Other cations participate in enzymatic reactions as components of coenzymes and catalytic centers. In order to have adequate metal concentrations, bacteria developed cellular systems capable of monitoring intracellular metal concentrations and balancing acquisition of metals from the environment [19]. Although the majority of such systems involved proteins, several systems rely on metal sensing by riboswitches.
Metal cations are important for folding of cellular RNAs and are especially critical for the majority of riboswitches, which have to fold quickly during transcription to be competent for ligand binding. Such folding is assisted by metal cations which neutralize the negative charges of the sugar-phosphate backbone of RNA. Although a diffuse atmosphere of loosely associated monovalent cations could provide overall charge neutralization for structural compaction, riboswitch folding is greatly accelerated by Mg2+ ions abundant in cells [20]. Structural and biochemical studies of riboswitches revealed multiple specific binding sites for Mg2+ cations which most likely interact with RNA in a cooperative manner and mediate close packing of structural elements in the three-dimensional RNA structures (reviewed in [21]). Although certain riboswitches, such as purine and lysine binding RNAs, can bind cognate ligands and adopt structures in the presence of monovalent cations and the absence of divalent cations [22–24], several studies emphasized the importance of Mg2+ for riboswitch structure formation and provided evidence for cooperative folding of riboswitches in the presence of Mg2+ cations [20, 25].
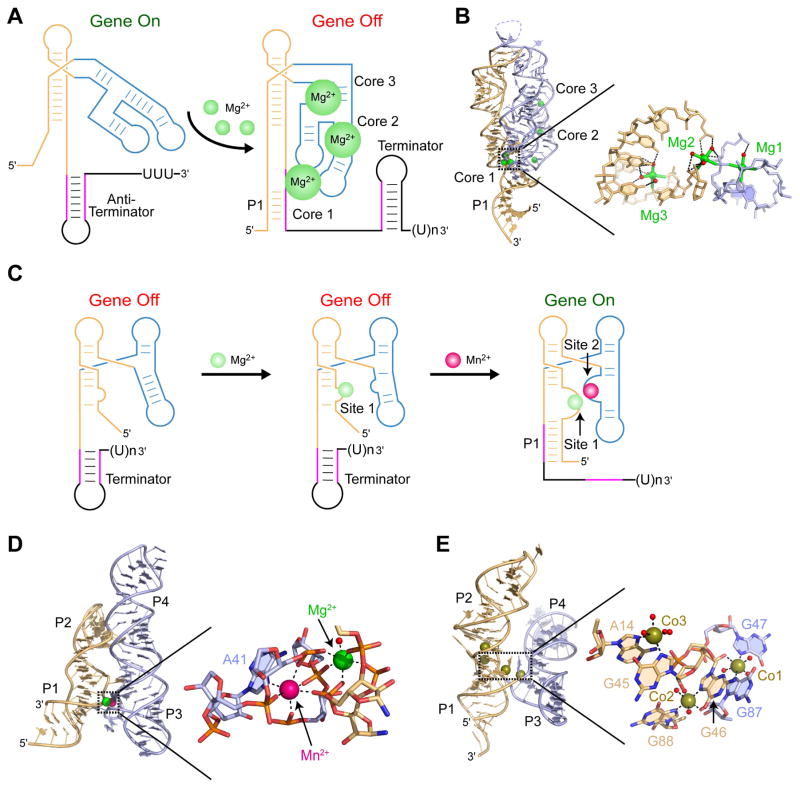
Some riboswitches, like the M-box riboswitch found in Bacillus subtilis mgtE gene, take advantage of specific Mg2+-RNA interactions and employ Mg2+-driven riboswitch folding for functional output [26]. In the M-box, Mg2+ cations assist overall riboswitch folding and serve as specific riboswitch ligands at high intracellular Mg2+ concentrations (Fig. 2A). Among at least eight well-ordered cation binding pockets, several sites are essential for maintaining tertiary long-distance interactions and interplay between alternative riboswitch conformations [26–28]. Distribution of the Mg2+ binding sites in three “cores” that are critical for the structure formation immediately suggests synergism and cooperativity between distinct sites. Structural and biochemical studies suggest that sites in cores 2 and 3 should facilitate formation of the helical junctions and turns, which bring core 1 and the regulatory regions in close proximity for Mg2+-mediated tertiary interactions (Fig. 2A,B). Although the cooperative binding of Mg2+ cations remains to be formally demonstrated and characterized, direct chelation of several cations to M-box RNA suggests the possibility that intracellular responsiveness to magnesium by M-box RNAs may be exquisitely controlled via a coordinated progression of Mg2+-binding sites and metal-induced structural features.
Fig. 2.
Binding of metal cations facilitates riboswitch folding and affects gene expression. Independently folded RNA regions involved in ligand-driven conformational re-arrangements are in light orange and blue colors. A. Several Mg2+ cations bind at different RNA regions and facilitate the folding of the M-box riboswitch leading to the formation of the transcription terminator and premature termination of transcription. Mg2+ cations and water molecules are shown as green and red spheres, respectively. B. A ribbon representation of the X-ray structure of the M-box riboswitch (PDB ID 3PDR) [28] with a zoomed-in view of three Mg2+ cations stabilizing core 1. Mg2+ coordination bonds are depicted by sticks. C. Genetic regulation by the Mn2+ riboswitch. In the unbound form, an expression platform forms a transcription terminator that blocks transcription elongation. Mg2+ binding to site 1 potentiates RNA folding, helps to bind a Mn2+ cation to site 2, and assists ‘docking” of the two helical stacks. Formation of the docked structure stabilizes helix P1 and prevents formation of the transcription terminator. D. A ribbon representation of the X-ray structure of the Mn2+ riboswitch (PDB ID 4Y1I) [32] with a zoomed in view of sites stabilized by the Mg2+ and Mn2+ cations. Metal coordination bonds are depicted by dashed lines. E. A ribbon representation of the X-ray structure of the NiCo riboswitch (PDB ID 4RUM) [36] with a zoomed in view of the ligand binding pocket containing three Co2+ cations.
Despite the ability of RNA to bind metal cations, there were doubts that RNA molecules can rival highly specific recognition of metals by proteins inside the cells. Recent examples illustrate that natural RNAs can achieve high selectivity in metal recognition and use this specific binding as the basis for controlling gene expression. Two recent studies showed that an evolutionarily conserved RNA element [29], typically located upstream of Mn2+ efflux pump genes [30, 31], is a riboswitch responding to high concentrations of Mn2+ cations [32, 33]. In the absence of Mn2+, the riboswitch adopts a fold composed of two helical stacks P1-P2 and P3-P4 in the sensing domain and a transcription terminating hairpin in the expression platform (Fig. 2C, D). The transcription terminator prevents the RNA polymerase from completing transcription, leaving the gene in the off state (Fig. 2C). At physiologically relevant Mg2+ concentrations, a Mg2+ cation is thought to bind to the internal loop (site 1) in the vicinity of the region involved in the formation of the regulatory helix P1. This Mg2+ binding likely prefolds RNA so that once the Mn2+ concentration increases, the Mn2+ cation binds in the internal loop of P3-P4 stack (site 2) and interacts with the region near site 1, thereby docking two helical stacks. Mn2+ binding stabilizes the “docked” structure, facilitates the formation of the P1 helix, and induces transcription read through (Fig. 2C). The riboswitch structure has revealed that the Mg2+ cation directly coordinates a water molecule and five phosphoryl oxygen atoms, three from the P1-P2 stack and two from the P3-P4 stack (Fig. 2D,). The Mn2+ cation is also coordinated by five phosphoryl oxygen atoms, four from the P3-P4 stack and one from the P1-P2 stack, as well as by the N7 of a conserved adenosine A41 from the P1-P2 stack (Fig. 2D). Although both cations are characterized by octahedral coordination geometry, specificity of metal recognition by each site is likely explained by different coordination partners. Mg2+ prefers to coordinate with oxygen atoms, present in site 1, whereas Mn2+ can also bind a nitrogen atom [34, 35], present in site 2. Close location of sites 1 and 2 and binding of both cations to the same nucleotides strongly suggests cooperativity of Mg2+ and Mn2+ cations in RNA binding and genetic response.
Another class of riboswitches has been shown to specifically recognize similar Ni2+ and Co2+ cations and control genes involved in transport of heavy metals [36]. Structural studies have revealed that the NiCo riboswitch binds four Co2+ cations, three of which stabilize the junction between two sets of coaxially stacked helices (Fig. 2E). Each junctional Co2+ cations coordinates to a nucleotide bound by another Co2+ cation, suggesting that binding at one site stabilizes the divalent metal ion binding at the adjoining site (Fig. 2E). Indeed, replacing the N7 atom of G87, which coordinates to cobalt 1, with a carbon, affects binding of G46 to cobalt 2. This change, however, does not affect binding of cobalt 3 unless the N7 atom of G88, which coordinates to cobalt 2, is also replaced with a carbon. These effects demonstrate that intermolecular interactions link the metal binding sites in a cooperative manner. Binding data further support this conclusion and show that metal ions do not bind the NiCo riboswitch independently but rather cooperatively, with Hill coefficients of 2.0 and 1.6 for Co2+ and Ni2+cations, respectively. These data indicate a strong degree of cooperativity since a Hill coefficient of 1.0 correspond to a non-cooperative binding while coefficients of 2.0 and 3.0 correspond to fully cooperative systems with 2 and 3 ligands, respectively. Cooperative binding of Ni2+ and Co2+ cations to the riboswitch ensures folding of the three-way junction and stabilization of regulatory P1 helix that protrudes from the junction.
The NiCo riboswitch so far represents the only RNA metallosensor proven to use cooperative metal binding to elicite genetic control. However, cooperative binding of metal cations by regulatory RNAs could be a common trait. Three-dimensional structures of three different metallosensors revealed that genetic control depends on RNA binding of two or more metal cations and close positioning of the metal binding sites so that interactions of the cations with RNA depend on each other. Although the reason for multiple metal binding to riboswitches is not clear, the difficulty RNA has in discriminating between different metal cations may be why additional metal binding events are required to ensure a genetic response. Alternatively, single metal coordination with RNA may provide insufficient binding affinity to efficiently trigger RNA folding and drive the genetic system to the “ligand-bound” genetic pathway.
4. Synergetic binding of riboswitch ligands and metal cations: potentially cooperative RNAs
Similar to multiligand binding proteins, three-dimensional structures of several riboswitches revealed intermolecular interactions between bound riboswitch ligands and cations. These interactions involve direct coordination bonds between negatively charged moieties of ligands and positively charged metal cations. In several structures—for example in thiamine pyrophosphate (TPP) [37–39], flavin mononucleotide (FMN) [40], and likely in cyclic di-GMP class I (c-di-GMP-I) [41, 42] riboswitches—one or two Mg2+ cations directly bind to oxygen atoms of phosphate moieties of bound metabolites and mediate interactions between ligands and RNA (Fig. 3A). In glycine [43, 44] and lysine [24] riboswitches, cations are coordinated with carboxylates of amino acid ligands. Remarkably, although glycine binding to the glycine riboswitch involves a Mg2+ cation (Fig. 3B), as observed for phosphate-containing metabolites, lysine binding to lysine riboswitches is uniquely mediated by a K+ cation [24]. Another exceptional cation coordination with the ligand was observed in the fluoride riboswitch, where the negatively charged fluoride anion directly coordinates three Mg2+ cations in a near planar geometry [45].
Fig. 3.
A. Zoomed-in view of the FMN binding pocket in the three-dimensional FMN riboswitch structure (PDB ID 3F2Q) [40]. Hydrogen bonds between RNA bases and oxygen atoms of the phosphate moieties help in binding the FMN ligand (red color) to the riboswitch. B. Zoomed-in view of the glycine binding pocket in the glycine riboswitch structure with (PDB ID 3OWW) [44] and without (PDB ID 3OX0) [44] bound glycine, respectively. The left panel shows the Mg2+ cations stabilizing the ligand binding site through hydrogen bonding. The right panel shows the loss of the glycine-bound Mg2+ in the glycine-free structure.
What is the function of metal cations in binding the riboswitch ligands? Given the overall negative charge of RNA, binding of cations with negatively charged ligands could neutralize the charge and decrease repulsion between RNA and ligands, thus making intermolecular interactions possible on a physiologically relevant time scale. Although definitive proof is missing, it is possible that some riboswitch ligands, especially those carrying a high density negative charge in the phosphate moieties, may interact with cations prior to RNA binding and be recognized by RNA as cation-metabolite complexes. This suggestion is indirectly supported by the absence of ligand-interacting cations in the apo structures of glycine [44] (Fig. 3B), lysine [24] and FMN [25] riboswitches and in the structure of the FMN riboswitch bound to riboflavin, the ligand lacking the terminal phosphate of FMN [40]. Structural results are corroborated by solution SHAPE experiments on the FMN riboswitch conducted in the absence of FMN. The analyses showed a significant increase in the Mg2+ concentration needed to modulate conformation of a nucleotide that contacts the ligand-bound cation [25]. These data, however, do not exclude the possibility that ligand binding precedes cation binding and cations serve to adjust the conformation of the ligand-binding pocket and trap the ligand inside the pocket, as illustrated by local conformational differences between apo and ligand-bound structures of the glycine [44] and FMN [25] riboswitches. In addition, recent structural studies of the TPP riboswitch bound to fragments mimicking an aminopyrimidine moiety of TPP revealed single Mg2+ cations in the unoccupied site for the pyrophosphate moiety of TPP [46]. These studies argue against binding of the complex between TPP and two Mg2+ cations to the riboswitch and demonstrate that one Mg2+ cation could be involved to some extent in pre-organization of the ligand-binding site while the second Mg2+ cation could bind the riboswitch in complex with TPP.
In some riboswitches, interactions of cations with ligands clearly occur after ligand binding with RNA. For instance, in the adenine and guanine riboswitches [22, 47], a Mg2+ cation, bound to the Hoogsteen edge of purine ligands via a water molecule, forms the inner-sphere coordination with an RNA phosphate and blocks the exit from the ligand-binding pocket. Observations of Mg2+-bound and -unbound ligand-binding pockets in the apo riboswitch structures resemble that of proteins, for example, from the Nudix hydrolase superfamily. Nudix hydrolases cleave substrates bearing di-phosphate moieties using various metal cations bound to glutamate residues of the Nudix motif. Some of these proteins are capable of coordinating metal cations prior to substrate binding while other proteins do not form stable cation-bound structures without reaction substrates.
Structural studies revealed that metal cations mediate interactions between a ligand and RNA in the majority of riboswitches in which ligand-cation interactions are observed. Could metal cations be critical for specific recognition of ligands by riboswitches? Despite the fact that several riboswitches form direct coordination bonds with metal cations, in most cases the nature of the divalent cation appears to not be essential. Ligand-bound Mg2+ cations can be replaced by Mn2+ and other divalent cations in TPP, glycine, fluoride and FMN riboswitches [37, 39, 40, 44, 45]. The FMN riboswitch can even accommodate a large [Co(NH3)6]3+ cation, which is isosteric with a fully-hydrated Mg2+ cation, but is unable to make inner-sphere coordination as Mg2+ does [40]. Some riboswitches do not necessarily need inner-sphere coordination of cations for binding to phosphorylated ligands. For instance, position, number of Mg2+ cations, and their coordination to the phosphorylated sugar glucosoamine-6-phospate vary in the structures of the glmS riboswitches [48, 49]. These data indicate structural plasticity of the ligand-binding pockets of riboswitches and a lack of exquisite specificity and strict bonding patterns with metal cations in many riboswitch systems.
The lysine riboswitch appears to be an exception from the rule. Although the K+ cation can be replaced in the structures by Cs+ and Tl+ cations, when replaced by Na+ cations, abundant in cells, lysine binding affinity decreases by over 30 folds [24]. Such specificity is likely explained by the larger size of the K+ cation and its ability to deviate from the octahedral coordination geometry characteristic of Na+ and Mg2+ cations (Fig. 3A). In the lysine riboswitch structure, the K+ cation forms eight coordination bonds which directly connect four nucleotides and the ligand in the geometry incompatible with hexacoordinated binding of Na+. The fluoride riboswitch represents another cation-specific system tailored for Mg2+ cations. The fluoride riboswitch structure revealed coordination of three Mg2+ cations with the ligand and RNA, and encapsulation of the cation-bound ligand within the RNA fold [45]. The interactions of various cations with RNA promote the formation and stabilization of the ligand-bound conformation of the riboswitch to exert a genetic response.
The aforementioned examples demonstrate synergism in binding and interdependence of ligand and cation interactions with riboswitches. Metal cations can dramatically impact ligand binding by pre-forming ligand-binding pockets, mediating networks of specific interactions, neutralizing negative charges of ligands, and trapping the ligands in the binding pockets. Binding of cations and ligands with RNA could be considered as events that include close-range allosteric changes, since in many instances interactions with either molecule cause local conformational adjustments in RNA and affect binding affinity for another small molecule. Cation and ligand binding with riboswitches may also involve cooperativity; however, given the contribution of cations to global RNA folding, it is difficult to discriminate between effects of cations on ligand binding and cooperative RNA folding. We should point out that riboswitches highlighted in this section cannot be directly compared with cooperative protein systems in which ligand binding to one site potentiates ligand binding to a distinct remote site, because cations and metabolites are bound together within the same ligand-binding pockets in these riboswitches.
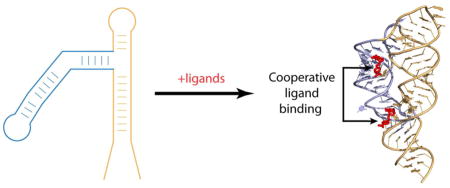
5. Tandem glycine riboswitches: cooperative or non-cooperative RNA?
Although the majority of riboswitches consists of a single metabolite-sensing module followed by an expression platform, several riboswitches are arranged in tandem [50–52]. The tandem arrangement usually involves two full-length riboswitches of either the same specificity, to ensure tighter control, or different specificities, to modulate gene expression in response to different metabolites. One of these riboswitches, a glycine riboswitch, typically consists of two adjacent glycine-sensing modules connected by a short linker and followed by a single expression platform [50] (Fig. 4A). Such tandem arrangement of similar metabolite-sensing domains presents a thrilling possibility of a cooperative RNA that uses a mechanism of ligand binding comparable with cooperative multisubunit proteins. In the original report describing the identification of glycine riboswitches, this possibility was noted and explored biochemically [50]. In-line probing assays revealed steep glycine binding curves indicative of cooperative binding of two glycine molecules to a tandem riboswitch. Hill coefficients, determined from these curves, were ~1.6 and 1.4 for Vibrio cholerae and Bacillus subtilis gsvT riboswitches which activate genes for the glycine cleavage system. In addition, the mutation of one domain decreased glycine binding to the second aptamer. Cooperative glycine binding may result in a more pronounced, more “programmed”, response to the rising and falling concentration of glycine, resulting in rapid activation and repression of genes encoding the glycine cleavage system. Thus, cooperative glycine binding to riboswitches would be an important selective advantage to bacteria that harbor tandem riboswitches [50].
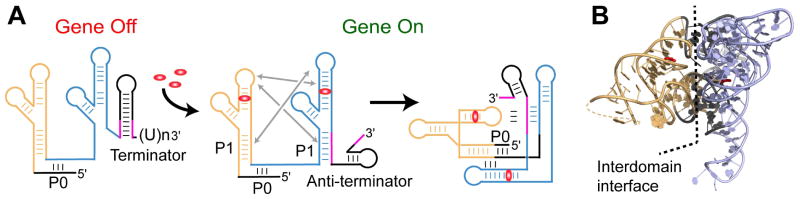
Fig. 4.
Two glycine molecules bind each domain of a tandem glycine riboswitch to elicit a regulatory response. Domains I and II are shown in orange and blue, respectively. A. In the off position, P1 of domain II is not paired, allowing the formation of a terminator at the 3′ end. Upon glycine binding to the riboswitch, a conformational change brings the apical loop of domain I to P1 of domain II, and the apical loop of domain II binds P1 of domain I. Interdomain interactions facilitates glycine binding and stabilize the structure, facilitating formation of the anti-terminator at the 3′ end, and allowing gene transcription to take place. B. A ribbon representation of the X-ray structure (PDB ID 3P49) [43] of the two tandem domains of the glycine riboswitch, with nucleotides stabilizing interactions between domains shown in dark grey.
Glycine riboswitch cooperativity has been independently verified [53] and further explored by small-angle X-ray scattering (SAXS) [54, 55], hydroxyl radical cleavage [54, 56], nucleotide analog interference mapping (NAIM) [53], and native gel electrophoresis [56]. These studies suggested that ligand binding induces global conformational rearrangement and close packing of the RNA domains, both stabilized by long-range tertiary interactions between several regions of the glycine-binding domains (reviewed in [57]). The crystal structure of domain II from the V. cholerae glycine riboswitch revealed a three-way junctional architecture [44]. The glycine-binding pocket, located slightly above the junction, communicates with the junction via a nucleotide extruded from the pocket in the ligand-bound conformation. The structure also demonstrated close packing of domain II in the crystallographic riboswitch dimer through tertiary interactions [44] which were later revealed in detail in the crystal structure of the natural tandem V. cholerae riboswitch (Fig. 4B) [43]. These interactions include base-pairing between looped out nucleotides and two pairs of pseudo-symmetrical contacts formed by packing of a loop region from one domain with the minor grove of a helix from the second domain. Since domain I is shorter than domain II, interdomain interactions are formed by an apical loop in domain I and an internal loop in domain II. Although the mechanism of cooperative glycine binding was not fully understood, the structures and biochemical data suggested that structural rearrangements induced by glycine binding in one pocket are translated into conformational changes that enhance ligand binding in the other pocket through the observed interdomain interfaces.
Recently, cooperative glycine binding in the tandem glycine riboswitch has been challenged by two independent works [58, 59]. These studies identified base-pairing between an extended 5′ end and the linker of the riboswitch domains and predicted that this leader-linker pairing (P0 in Fig. 4A) forms a stable structural element termed a kink-turn motif. When incorporated into the tandem riboswitch, this energetically favorable RNA element increases glycine binding affinity and ensures synergistic action between glycine-binding and interaptamer interactions during global folding of the RNA. Unexpectedly, riboswitch constructs with leader-linker interactions did not display cooperative glycine binding and ITC experiments even raised concerns about double-site glycine binding to the extended riboswitch [60]. These studies have reopened the debate about the purpose for the tandem arrangement of glycine riboswitches.
Because the tandem architecture has been preserved during evolution and was not reduced to a single aptamer riboswitch, it likely provides some benefit in riboswitch function, either in ligand binding affinity or kinetics of the genetic response. Most recent structure-guided mutagenesis study [61] confirmed double-site occupancy in the extended tandem riboswitch and showed that such occupancy is not necessary for high affinity glycine binding. Instead, aptamer dimerization is energetically linked to ligand binding, particularly in domain I. The emerging model of the riboswitch mechanism [61] suggests that glycine binding requires dimerization of the aptamers and that stable helix P1 of domain I acts as a scaffold for dimeriaztion. Dimerization and ligand binding stabilize regulatory helix P1 of domain II and affect folding of the expression platform, exerting a regulatory response to the presence of glycine.
Why does the truncated tandem riboswitch act as a cooperative system? The possible lack of a stable linker prevents efficient aptamer dimerization while ligand binding to one aptamer stabilizes aptamer architecture, facilitates dimerization, and potentiates glycine binding to the second aptamer. It is also possible that a region from the glycine-unbound domain I interacts with domain II and interferes with glycine binding to domain II. Alternatively, ligand binding to domain I would eliminate this interaction and enhance the binding to domain II. Although this allosteric inhibition is not pronounced in the tandem riboswitch, it is apparent in an RNA construct with shortened domain I and full-length domain II [62]. Interestingly, this modulation is rescued by base-pairing of the domain I with a complementary oligodeoxynucleotide, demonstrating the feasibility of developing a genetic circuit that senses both the glycine and DNA signal [62].
6. Double ligand binding to tetrahydrofolate riboswitch: cooperativity at last
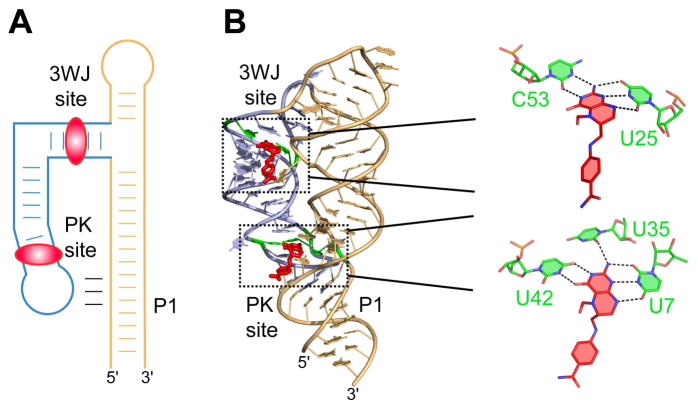
Unlike many other riboswitch structures, the X-ray structure of the ligand-bound tetrahydrofolate (THF) riboswitch has, surprisingly, revealed two ligand molecules bound to a single aptamer [63]. One molecule binds closely to the remote three-way junction while the second molecule binds in the grove of the long-distance pseudoknot (Fig. 5A). The pseudoknot is formed by base-pairing between the internal loop about the regulatory helix P1 and the apical loop of the stem-loop structure emerging from the three-way junction [63, 64]. The ligands are specifically recognized by evolutionarily conserved nucleotides in similar, but not identical, ligand-binding pockets. The long edge of both ligands is base-paired with a uridine while the Watson-Crick edges are recognized by a cytosine in the site about the three-way junction (3WJ site) and by a uridines in the site near the pseudoknot (PK site) (Fig. 5B). Although both ligands bind to the riboswitch in solution, binding characteristics of the riboswitch depend on the concentrations of divalent cations. The binding curves suggest non-cooperative ligand binding at an elevated (6 mM) MgCl2 concentration, and cooperative binding, with a Hill coefficient of 1.8, at the physiological (0.5 mM) MgCl2 concentration. Ablation of binding by mutagenesis in one site decreases binding affinity to the second site by approximately one order of magnitude [63]. This data suggests that the binding sites are not fully structured at low MgCl2 concentrations and binding to one site facilitates ligand interactions with the other site.
Fig. 5.
Two THF molecules bind one riboswitch aptamer. A. Ribbon representation of the X-ray structure of the THF riboswitch (PDB ID 4LVV) [63], with tetrahydrofolate depicted in red and conserved nucleotides stabilizing both ligands highlighted in purple. B. A closer view of both ligand binding sites. Hydrogen bonds depicted by dashed lines.
The ligand binding sites, however, are not equivalent in regulation, despite similarity in ligand recognition and binding affinities [63]. The mutant that knocks out the 3WJ site showed a modest ~3-fold decrease in transcription termination efficiency, demonstrating that the riboswitch is capable of regulation without the ligand bound to the junction. Conversely, mutation within the PK site resulted in very low regulatory activity, suggesting that this site is most important for genetic control by the THF riboswitch. The strong disparity between the ability of the two sites to modulate gene expression indicates that they have separate roles in the riboswitch function. The ligand bound to the 3WJ site likely stabilizes the junction and orients a stem-loop structure for the formation of the long-distance pseudoknot. In turn, the PK site stabilizes the pseudoknot and the regulatory helix P1, ensuring regulatory response. Interestingly, rare THF riboswitches that bear an altered junctional site bind only one equivalent of the ligand [65], emphasizing that the pseudoknot site is an essential feature of the THF riboswitch family and that cooperativity of ligand binding is characteristic for the vast majority of THF-sensing RNAs.
7. Double ligand binding to c-di-AMP riboswitch: a candidate for cooperativity
Typically riboswitches modulate expression of genes involved in metabolism and transport of their cognate ligands or their derivatives. The most prominent exceptions from this rule are riboswitches responding to second messengers cyclic dinucleotides c-di-GMP and c-di-AMP [66–68]. The c-di-AMP riboswitch represents an unusual regulatory systems responding to binding of two ligands [67, 68].
Three independent structural studies resulted in the determination of c-di-AMP riboswitch structures from four different bacterial species [69–71]. Unexpectedly, the structures revealed that an aptamer domain is composed of two pseudosymmetrical ligand-bound modules arranged such that the structure adopts a squared shape (Fig. 6A). Three corners of the structure feature either three three-way junctions or two junctions and a turn, while the fourth corner is built by a long-distance pseudoknot connecting the middle and 3′ region of the aptamer. Four helices connect the corners and form the sides of the square. Two molecules of c-di-AMP are positioned along two sides of the square with adenosyl moieties oriented towards the corners. Each adenosyl moiety of the ligands is recognized in an almost identical fashion so that the riboswitch displays double two-fold pseudo-symmetry (Fig. 6B) and shows amazing overall resemblance to the four-subunit structure of cooperative hemoglobin.
Fig. 6.
Pseudosymmetrical ligand binding of the c-di-AMP riboswitch. C-di-AMP ligands are shown in red. A. Schematic showing the c-di-AMP riboswitch having a square-like appearance. The riboswitch is bound to two c-di-AMP molecules. B. A ribbon representation of the X-ray structure of the c-di-AMP riboswitch (PDB ID 4QK8) [69]. Zoomed-in views highlight the similarity of AMP moiety binding to RNA.
The high ligand binding affinity and partial misfolding of the RNA in ITC experiments [69–71], however, make estimation of cooperativity extremely difficult and in-line probing data available before the structural elucidation were treated with a 1-to-1 stoichiometry of RNA-ligand binding [68]. Mutagenesis studies showed that RNA elements involved in the tertiary structure formation of each module are important for ligand binding [70, 71] although each binding modules may not be equally important. For instance, simultaneous conversion of ligand-recognizing C-G to G-C base pairs in both subsites of the 5′ module significantly reduces binding to the 3′ module, while the same mutations in the 3′ module modestly affect binding to the 5′ module [69]. These data suggest that formation of the 5′ module is required for subsequent folding of the 3′ module and the pseudoknot base-pairing that occludes the ribosome entry site and inhibits translation of the gene. Such interdependence between the binding sites hints at cooperativity of ligand binding and justifies future experiments to address how double-ligand binding relates to genetic regulation by the riboswitch.
8. Allosteric control in the c-di-GMP ridoswitch-ribozyme
The tandem c-di-GMP riboswitch-ribozyme, residing in the 5′ UTR of the mRNA for a putative virulence gene in the pathogenic bacterium Clostridium difficile, represents one of the most spectacular allosteric regulatory systems in the RNA world [67, 72]. This sensory system is composed of the c-di-GMP sensor connected to a self-splicing group I intron. C-di-GMP binding to the riboswitch stabilizes the domain-closing helix P1 that in turn facilitates the formation of the downstream ribozyme core allowing it to bind a GTP molecule and excise itself from the mRNA. Such mRNA splicing in the presence of GTP and c-di-GMP unmasks the start codon and reveals a perfect ribosome binding site for efficient translation of the protein. In the absence of c-di-GMP, the aptamer of the riboswitch does not adopt a stable conformation and its 3′ terminus engages in alternative base-pairing that leads to the creation of an atypical GTP-binding site in the ribozyme. The alternatively folded ribozyme makes a single cleavage and generates the mRNA trimmed of the nucleotides that would otherwise serve as a ribosome binding site. Such a truncated mRNA product disfavors translation resulting in the repression of protein biosynthesis. Although GTP is unlikely to be a limiting factor in the regulation, the finding of the allosteric RNA requiring two compounds to promote splicing and gene expression modulation hints that allosteric multi-sensory RNA systems comparable in complexity with proteins may have evolved in nature.
9. Concluding remarks
Several examples of metabolite-sensing regulatory RNAs discussed in this review show that natural RNAs are capable of using basic biological principles, such as allostery and cooperativity, in the modulation of gene expression. Although these principles are key in riboswitch folding and genetic switching and are therefore applicable to all riboswitches, the proportion of riboswitches that employ such principles in their classical definition, for dual or multiple ligand binding and modulation of function, is rather small. A number of riboswitches synergistically bind metabolites and metal cations; such binding is mostly an adaptation evolved to compensate the electronegative nature of RNA and allow efficient interactions with RNA. Only a couple riboswitches may be considered as allosteric systems reminiscent of proteins, and only one or two RNAs may be paralleled with cooperative oxygen sensing by hemoglobins. These differences between protein- and RNA-mediated genetic regulators are likely attributed to higher versatility and longer lifetime of proteins. Riboswitches typically act in cis and must fold and respond to the cognate ligand within a short time frame, in most cases co-transcriptionally. Therefore, they are less likely to evolve into larger dual ligand sensors. As demonstrated by tandem riboswitches, full-length riboswitches of different specificities can simply adjoin in the same RNA to ensure response to more than one signal if the protein must be synthesized when more than one physiological condition has to be met. It is not surprising that the glycine and c-di-GMP riboswitch-ribozyme are built by joining two RNA “blocks”, either two sensors or a sensor and enzyme. It is, however, intriguing how the THF riboswitch evolved with double-ligand binding capability. The evolution of the c-di-AMP riboswitch is even more fascinating since the two ligand-binding modules are connected by a long-range pseudoknot and are sufficiently different to imagine ancestry from a duplicated sequence. Undoubtedly, deciphering complex relationships in such regulatory RNAs represents an interesting problem. Further analysis of riboswitches could provide more intriguing results. For instance, poorly understood glutamine-specific riboswitches in many cases form double and sometimes even triple arrangements of sensing domains connected by conserved linkers [52]. These riboswitches however do not show cooperative ligand binding and the biological significance of such complex RNA structures is not clear at the moment. Careful elucidation of such systems could present a new avenue in developing designer genetic networks for synthetic biology and other applications.
An outstanding question is how regulatory RNAs employ allostery and cooperativity.
Metallosensing riboswitches can bind metal cations cooperatively.
Several riboswitches possibly employ cooperativity for small metabolite binding.
Tetrahydrofolate riboswitch has been shown to bind small metabolites cooperatively.
Acknowledgments
This work was supported by the New York University Medical Center funds (A.S.) and by the NIH 5T32GM088118-05 (A.P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Royer WE, Jr, Knapp JE, Strand K, Heaslet HA. Cooperative hemoglobins: conserved fold, diverse quaternary assemblies and allosteric mechanisms. Trends Biochem Sci. 2001;26:297–304. doi: 10.1016/s0968-0004(01)01811-4. [DOI] [PubMed] [Google Scholar]
- 2.Williamson JR. Cooperativity in macromolecular assembly. Nat Chem Biol. 2008;4:458–465. doi: 10.1038/nchembio.102. [DOI] [PubMed] [Google Scholar]
- 3.Recht MI, Williamson JR. RNA tertiary structure and cooperative assembly of a large ribonucleoprotein complex. J Mol Biol. 2004;344:395–407. doi: 10.1016/j.jmb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Elliott MB, Gottlieb PA, Gollnick P. The mechanism of RNA binding to TRAP: initiation and cooperative interactions. RNA. 2001;7:85–93. doi: 10.1017/s135583820100173x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattin BD, Zhao W, Travers K, Chu S, Herschlag D. Direct measurement of tertiary contact cooperativity in RNA folding. J Am Chem Soc. 2008;130:6085–6087. doi: 10.1021/ja800919q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porta H, Lizardi PM. An allosteric hammerhead ribozyme. Biotechnology (N Y) 1995;13:161–164. doi: 10.1038/nbt0295-161. [DOI] [PubMed] [Google Scholar]
- 7.Rouleau SG, Jodoin R, Bisaillon M, Perreault JP. Programming a highly structured ribozyme into complex allostery using RNA oligonucleotides. ACS Chem Biol. 2012;7:1802–1806. doi: 10.1021/cb300319m. [DOI] [PubMed] [Google Scholar]
- 8.Robertson MP, Ellington AD. Design and optimization of effector-activated ribozyme ligases. Nucleic Acids Res. 2000;28:1751–1759. doi: 10.1093/nar/28.8.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu Y, Yamashita S, Kazama N, Nobuoka K, Ohtsuka E. Construction of new ribozymes requiring short regulator oligonucleotides as a cofactor. J Mol Biol. 2000;299:1231–1243. doi: 10.1006/jmbi.2000.3825. [DOI] [PubMed] [Google Scholar]
- 10.Gu H, Furukawa K, Breaker RR. Engineered allosteric ribozymes that sense the bacterial second messenger cyclic diguanosyl 5′-monophosphate. Anal Chem. 2012;84:4935–4941. doi: 10.1021/ac300415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jose AM, Soukup GA, Breaker RR. Cooperative binding of effectors by an allosteric ribozyme. Nucleic Acids Res. 2001;29:1631–1637. doi: 10.1093/nar/29.7.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batey RT. Structures of regulatory elements in mRNAs. Curr Opin Struct Biol. 2006;16:299–306. doi: 10.1016/j.sbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Zengel JM, Lindahl L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1994;47:331–370. doi: 10.1016/s0079-6603(08)60256-1. [DOI] [PubMed] [Google Scholar]
- 14.Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breaker RR. Prospects for riboswitch discovery and analysis. Mol Cell. 2011;43:867–879. doi: 10.1016/j.molcel.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler WC, Dann CE., 3rd RNA allostery glimpsed. Nat Struct Mol Biol. 2006;13:569–571. doi: 10.1038/nsmb0706-569. [DOI] [PubMed] [Google Scholar]
- 17.Serganov A, Patel DJ. Metabolite recognition principles and molecular mechanisms underlying riboswitch function. Annu Rev Biophys. 2012;41:343–370. doi: 10.1146/annurev-biophys-101211-113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montange RK, Batey RT. Riboswitches: emerging themes in RNA structure and function. Annu Rev Biophys. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 19.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 20.Hennelly SP, Novikova IV, Sanbonmatsu KY. The expression platform and the aptamer: cooperativity between Mg2+ and ligand in the SAM-I riboswitch. Nucleic Acids Res. 2013;41:1922–1935. doi: 10.1093/nar/gks978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferre-D’Amare AR, Winkler WC. The roles of metal ions in regulation by riboswitches. Met Ions Life Sci. 2011;9:141–173. doi: 10.1039/9781849732512-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serganov A, Yuan YR, Pikovskaya O, Polonskaia A, Malinina L, Phan AT, Hobartner C, Micura R, Breaker RR, Patel DJ. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem Biol. 2004;11:1729–1741. doi: 10.1016/j.chembiol.2004.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noeske J, Richter C, Grundl MA, Nasiri HR, Schwalbe H, Wohnert J. An intermolecular base triple as the basis of ligand specificity and affinity in the guanine- and adenine-sensing riboswitch RNAs. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1372–1377. doi: 10.1073/pnas.0406347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serganov A, Huang L, Patel DJ. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;455:1263–1267. doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicens Q, Mondragon E, Batey RT. Molecular sensing by the aptamer domain of the FMN riboswitch: a general model for ligand binding by conformational selection. Nucleic Acids Res. 2011;39:8586–8598. doi: 10.1093/nar/gkr565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dann CE, 3rd, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 27.Wakeman CA, Ramesh A, Winkler WC. Multiple metal-binding cores are required for metalloregulation by M-box riboswitch RNAs. J Mol Biol. 2009;392:723–735. doi: 10.1016/j.jmb.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramesh A, Wakeman CA, Winkler WC. Insights into metalloregulation by M-box riboswitch RNAs via structural analysis of manganese-bound complexes. J Mol Biol. 2011;407:556–570. doi: 10.1016/j.jmb.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. Journal of bacteriology. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veyrier FJ, Boneca IG, Cellier MF, Taha MK. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS pathogens. 2011;7:e1002261. doi: 10.1371/journal.ppat.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price IR, Gaballa A, Ding F, Helmann JD, Ke A. Mn2+-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol Cell. 2015;57:1110–1123. doi: 10.1016/j.molcel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell. 2015;57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Li X, Gegenheimer P. Ribonuclease P catalysis requires Mg2+ coordinated to the pro-RP oxygen of the scissile bond. Biochemistry. 1997;36:2425–2438. doi: 10.1021/bi9620464. [DOI] [PubMed] [Google Scholar]
- 35.Harding MM, Hsin KY. Mespeus--a database of metal interactions with proteins. Methods Mol Biol. 2014;1091:333–342. doi: 10.1007/978-1-62703-691-7_23. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa K, Ramesh A, Zhou Z, Weinberg Z, Vallery T, Winkler WC, Breaker RR. Bacterial riboswitches cooperatively bind Ni2+ or Co2+ ions and control expression of heavy metal transporters. Mol Cell. 2015;57:1088–1098. doi: 10.1016/j.molcel.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thore S, Leibundgut M, Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- 39.Edwards TE, Ferre-D’Amare AR. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Serganov A, Huang L, Patel DJ. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature. 2009;458:233–237. doi: 10.1038/nature07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulshina N, Baird NJ, Ferre-D’Amare AR. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat Struct Mol Biol. 2009;16:1212–1217. doi: 10.1038/nsmb.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR, Strobel SA. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16:1218–1223. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler EB, Xiong Y, Wang J, Strobel SA. Structural basis of cooperative ligand binding by the glycine riboswitch. Chem Biol. 2011;18:293–298. doi: 10.1016/j.chembiol.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L, Serganov A, Patel DJ. Structural insights into ligand recognition by a sensing domain of the cooperative glycine riboswitch. Mol Cell. 2010;40:774–786. doi: 10.1016/j.molcel.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren A, Rajashankar KR, Patel DJ. Fluoride ion encapsulation by Mg2+ ions and phosphates in a fluoride riboswitch. Nature. 2012;486:85–89. doi: 10.1038/nature11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warner KD, Homan P, Weeks KM, Smith AG, Abell C, Ferre-D’Amare AR. Validating fragment-based drug discovery for biological RNAs: lead fragments bind and remodel the TPP riboswitch specifically. Chem Biol. 2014;21:591–595. doi: 10.1016/j.chembiol.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert SD, Reyes FE, Edwards AL, Batey RT. Adaptive ligand binding by the purine riboswitch in the recognition of guanine and adenine analogs. Structure. 2009;17:857–868. doi: 10.1016/j.str.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein DJ, Ferre-D’Amare AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 49.Cochrane JC, Lipchock SV, Strobel SA. Structural investigation of the GlmS ribozyme bound to its catalytic cofactor. Chem Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandal M, Lee M, Barrick JE, Weinberg Z, Emilsson GM, Ruzzo WL, Breaker RR. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science. 2004;306:275–279. doi: 10.1126/science.1100829. [DOI] [PubMed] [Google Scholar]
- 51.Sudarsan N, Hammond MC, Block KF, Welz R, Barrick JE, Roth A, Breaker RR. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- 52.Ames TD, Breaker RR. Bacterial aptamers that selectively bind glutamine. RNA Biol. 2011;8:82–89. doi: 10.4161/rna.8.1.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon M, Strobel SA. Chemical basis of glycine riboswitch cooperativity. RNA. 2008;14:25–34. doi: 10.1261/rna.771608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipfert J, Das R, Chu VB, Kudaravalli M, Boyd N, Herschlag D, Doniach S. Structural transitions and thermodynamics of a glycine-dependent riboswitch from Vibrio cholerae. J Mol Biol. 2007;365:1393–1406. doi: 10.1016/j.jmb.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipfert J, Sim AY, Herschlag D, Doniach S. Dissecting electrostatic screening, specific ion binding, and ligand binding in an energetic model for glycine riboswitch folding. RNA. 2010;16:708–719. doi: 10.1261/rna.1985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erion TV, Strobel SA. Identification of a tertiary interaction important for cooperative ligand binding by the glycine riboswitch. RNA. 2011;17:74–84. doi: 10.1261/rna.2271511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serganov A, Patel DJ. Amino acid recognition and gene regulation by riboswitches. Biochim Biophys Acta. 2009;1789:592–611. doi: 10.1016/j.bbagrm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherman EM, Esquiaqui J, Elsayed G, Ye JD. An energetically beneficial leader-linker interaction abolishes ligand-binding cooperativity in glycine riboswitches. RNA. 2012;18:496–507. doi: 10.1261/rna.031286.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kladwang W, Chou FC, Das R. Automated RNA structure prediction uncovers a kink-turn linker in double glycine riboswitches. J Am Chem Soc. 2012;134:1404–1407. doi: 10.1021/ja2093508. [DOI] [PubMed] [Google Scholar]
- 60.Baird NJ, Ferre-D’Amare AR. Modulation of quaternary structure and enhancement of ligand binding by the K-turn of tandem glycine riboswitches. RNA. 2013;19:167–176. doi: 10.1261/rna.036269.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruff KM, Strobel SA. Ligand binding by the tandem glycine riboswitch depends on aptamer dimerization but not double ligand occupancy. RNA. 2014;20:1775–1788. doi: 10.1261/rna.047266.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherman EM, Elsayed G, Esquiaqui JM, Elsayed M, Brinda B, Ye JD. DNA-rescuable allosteric inhibition of aptamer II ligand affinity by aptamer I element in the shortened Vibrio cholerae glycine riboswitch. J Biochem. 2014;156:323–331. doi: 10.1093/jb/mvu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trausch JJ, Ceres P, Reyes FE, Batey RT. The structure of a tetrahydrofolate-sensing riboswitch reveals two ligand binding sites in a single aptamer. Structure. 2011;19:1413–1423. doi: 10.1016/j.str.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang L, Ishibe-Murakami S, Patel DJ, Serganov A. Long-range pseudoknot interactions dictate the regulatory response in the tetrahydrofolate riboswitch. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14801–14806. doi: 10.1073/pnas.1111701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trausch JJ, Batey RT. A disconnect between high-affinity binding and efficient regulation by antifolates and purines in the tetrahydrofolate riboswitch. Chem Biol. 2014;21:205–216. doi: 10.1016/j.chembiol.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science. 2010;329:845–848. doi: 10.1126/science.1190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat Chem Biol. 2013;9:834–839. doi: 10.1038/nchembio.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao A, Serganov A. Structural insights into recognition of c-di-AMP by the ydaO riboswitch. Nat Chem Biol. 2014;10:787–792. doi: 10.1038/nchembio.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones CP, Ferre-D’Amare AR. Crystal structure of a c-di-AMP riboswitch reveals an internally pseudo-dimeric RNA. EMBO J. 2014;33:2692–2703. doi: 10.15252/embj.201489209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren A, Patel DJ. c-di-AMP binds the ydaO riboswitch in two pseudo-symmetry-related pockets. Nat Chem Biol. 2014;10:780–786. doi: 10.1038/nchembio.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen AG, Sudarsan N, Breaker RR. Mechanism for gene control by a natural allosteric group I ribozyme. RNA. 2011;17:1967–1972. doi: 10.1261/rna.2757311. [DOI] [PMC free article] [PubMed] [Google Scholar]