Abstract
Introduction
To evaluate the efficacy and durability of Urolastic, a new urethral bulking agent in women with stress urinary incontinence (SUI), after a follow-up of 24-months.
Material and methods
A follow-up study of women with SUI who received a Urolastic injection and successfully passed the 12-month follow-up. Assessment included the Stamey Grade, 1-h Pad weight test, and the International quality of life (I-QoL) score.
Results
Nineteen women who completed the 12-month follow-up were invited for the 24-month follow-up study. One patient did not respond to the correspondence. Four of the 18 patients who responded to the correspondence reported removal of the Urolastic implant at another facility, based on their desire. The explanation for this removal was painful intercourse (n = 1) or less than optimal dryness (n = 3). The overall objective improvement in continence status at 24-months was 66% compared to the 89% at the 12-month follow-up, while in addition the 1-h pad weight test showed >50% reduction in pad weight in 66% of patients compared to 84% at the 12-month follow-up. Adverse events reported were urinary tract infection (n = 1), local genital infection with erosion into the vagina (n = 1), painful intercourse (n = 2), and urgency (n = 4).
Conclusions
Urolastic is comparable to other bulking agents in terms of durability, efficacy, and complications.
Keywords: urinary incontinence, stress, urethral bulking agent, urolastic
INTRODUCTION
Stress urinary incontinence (SUI) is the most common variant of urinary incontinence in the European community [1]. Although a sub-urethral tape could be an option for surgical correction of SUI, a less invasive urethral bulking agent is still favored by a great sector of patients [2]. In a previous publication, we presented the 12-month follow-up results of the Urolastic injection in the treatment of women with SUI. Urolastic (Urogyn B.V., Nijmegen, The Netherlands) consists of polydimethylsiloxane (PDMS) [3].
The 12-month follow-up study showed an 89% improvement in Stamey grade. The current study aims to address the durability and efficacy of Urolastic, in women with SUI after a 24-month follow-up.
MATERIAL AND METHODS
Between November 2011 and November 2013, 20 wo-men with SUI were included in a prospective, cohort study. Inclusion criteria were: women aged >18 y who had a urodynamic SUI with a Stamey grade of 1-2. Patients should have a bladder capacity of ≥300 ml and a postvoid residual of <100 ml. Exclusion criteria were: women with mixed urinary incontinence where urgency incontinence is the predominant component, a urodynamically proven detrusor overactivity (DO), pelvic organ prolapse (POP), women with a neurogenic bladder or women using an indwelling catheter on a chronic basis. Pregnant women or women planning to conceive within 2 years following the surgery were also excluded. The study was approved by the local ethical committee and signed informed consents were obtained from all participants.
Urolastic injection packet
The injectable implant, Urolastic, was available in a sterile prefilled dual container (2 syringes x 2.5 ml) that contained a static mixer for adequate premixing of the syringe content at time of injection.
Peri-urethral Injection of Urolastic
The Urolastic was injected under local anaesthesia (1% lidocaine) in lithotomy position. First, an applicator was introduced into the urethra to facilitate and guide the peri-urethral injection of Urolastic. Using an 18-gauge needle, Urolastic was injected periurethrally at 3 locations clockwise: 2, 6, and 10 o'clock. About 1.25 ml of Urolastic was injected at the 6 o'clock position, and 0.5 ml injected at the 2 and 10 o'clock positions. An average 2.09 ml of Urolastic was injected per patient. About 35% of the original study group underwent a second injection within 6 weeks. In these patients, the average volume of Urolastic injected (first + second injection) was 2.45 ml.
A cough test was then performed after filling the urinary bladder with 200 ml of saline. Patients received a systemic oral antibiotic (Ciprofloxacin 500 mg) for 5 days following the operation. When indicated, Urolastic was re-injected 6 weeks after the key treatment session.
Surgical outcome assessment
Efficacy
The following parameters were assessed in every patient at 6-weeks, 3-months, 12-months, and 24-months, postoperatively: Stamey Grade, 1-h pad test, number of pads used by the patient (averaged over 3 days before the day of visit to the clinic), number of incontinence episodes per 24-h and I-QoL questionnaire. Success was defined as an improvement in the Stamey Scale of 1 or more grades [4]. Other indicators of success were reports of >50% reduction in number of: incontinence episodes/day, number of pads/day, and weight of the 1-h pad weight test.
Safety reporting
During the regular follow-up visits, patients were clinically assessed for any potential adverse events related to the Urolastic injection procedure.
Data analysis
Wilcoxon signed- rank test was used for detecting differences between surgical outcomes of every visit compared to the baseline. The level of significance of the results was set at p <0.05.
RESULTS
In the original study, taking place in November 2011, 20 women with SUI, with a mean age 56 years (range 33-71) were included in the study. Three of the 20 patients had previous sub-urethral tape for the same condition. Thirteen of the 20 patients had 1 treatment session of Urolastic with an average of 2.1 ml of Urolastic injected in the peri-urethral tissue (0.47 ml at 2 o'clock, 1.1 ml at 6 o'clock, and 0.52 ml at 10 o'clock positions). Seven of the 20 (35%) had a second injection after 6-weeks with an extra average 0.35 ml of Urolastic injected. At the 6-weeks and 3-month follow-up visits, all 20 patients were available.
At the 12-months follow-up, the primary investigator lost contact with 1 patient. The 19 patients who successfully completed the 12 month follow-up were invited to complete the 24-month follow-up, only 18 out of the 19 patients responded. Fourteen out of the 18 patients (78%) still had the Urolastic peri-urethral implant in place. While 4 patients reported removal of the Urolastic implant at different facilities, based on their desire, due to painful intercourse (n = 1) or less than optimal dryness (n = 3).
Efficacy assessment at 24-months follow-up
There was a general decrease in the overall objective improvement in the continence status of the 18 patients available for the 24-month follow-up compared to the 12-month outcome; 66% of the patients had improved their continence status at the 24-month follow-up compared to the 89% at 12-month follow-up. About 45% of the patients became dry (Stamey = 0) at the 24-month follow-up compared to 68% at the 12-month follow-up. The 1-h pad-weight test showed more than 50% reduction in the average weight of pad in 12 out of 18 patients (66%) compared to 84% at the 12-month follow-up.
Safety Assessment at 24-months follow-up
During the course of the 24 month follow-up, one of 18 patients reported a urinary tract infection (UTI) which was treated by a short course of antibiotic. Another patient had a local genital infection with erosion into the vagina and planned for an implant removal. In this patient, the too superficial location of the Urolastic in the vaginal wall had been observed 3 months after the injection. However, complete protrusion of the Urolastic through the vaginal wall occurred after 24 months. On physical examination, the vaginal mucosa looked defective at the site of injection, and the whitish material of the implant could be seen through the defect in the mucosa. Two patients had complaints of moderate painful intercourse. Finally, four of the 18 patients reported urgency symptoms for which they receive a course of anticholinergics. Some of our patients were postmenopausal and diabetic. However, none of the patients in whom Urolastic was explanted had vaginal atrophy.
Table 1 summarizes the main features of the study at baseline, 12 months, and 24 months follow-up.
Table 1.
Summary of main study outcomes at the 12-month and 24-month follow-up
| Baseline | 12-month follow-up | 24-month follow-up | |
|---|---|---|---|
| Number of patients | 24 | 19 | 18 |
| Overall success* | – | 89% | 66% |
| Stamey grade = 0 | 0% | 68% | 45% |
| Urgency | 0% | 0% | 22% |
| Urinary retention | 0% | 15% | 0% |
| Erosion | 0% | 0% | 5% |
| Urinary tract infection | 0% | 15% | 5% |
Defined as a decrease in the Stamey Score by 1 grade compared to the baseline continence status.
Surgical outcome subgroup analysis
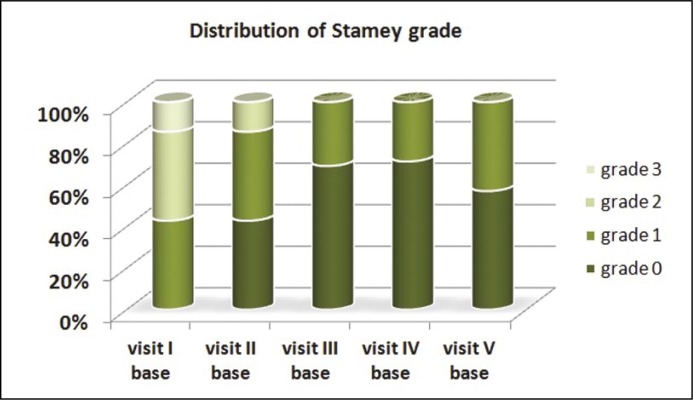
To evaluate the actual functionality of Urolastic, a subgroup analysis of the 14 patients who retained their implant in place at the 24-month visit was performed. The average Stamey grade significantly decreased from 1.8 (range 1-3) at baseline to 0.4 (range 0-1) at 24 months, p = 0.001. Figure 1 shows the distribution of various Stamey grades within the 14 patients at the 24-month follow-up.
Figure 1.
Distribution of Stamey grade within the study group over the 24-months (n = 14).
The average I-QoL score increased from 54.4 (range 23-81) at baseline to 74.6 (range 10-100) with a 37% increase, p = 0.02. at 24 months. The average pad weights also improved to be 1.4 g (range 0-6) at the 24-months compared to the 18.2 g (range 3-68) at baseline with a 94% reduction in pad weight, p = 0.001. The average number of incontinence episodes decreased from 21.4 (range 3-90) at baseline to be 4.8 (range 0-16) at 24-months with a 77% reduction, p = 0.003. The average number of pads decreased from 19.3 (range 3-49) at baseline to be 5.8 (range 0-20) at 24-months with a 70% reduction, p = 0.003.
DISCUSSION
The rationale of using bulking agents in the treatment of SUI is to enforce the proximal urethra. Injection of a bulking agent in the potential space between urethral mucosa and the surrounding muscle layer will make a local bulge of the mucosa. This leads to the sealing of the urethral lumen and prevention of urinary leaks [5, 6].
Surgical outcome
The current study presents the outcome of the first long term follow-up of the Urolastic urethral bulking agent (Urolastic®, Urogyn B.V., Nijmegen, The Netherlands) in treatment of women with SUI. The outcome of the previous 12-month follow-up of 20 women with SUI who received Urolastic, showed good surgical outcome with an 89% improvement in the Stamey grade (at least 1 grade reduction in the Stamey score). About 68% of these patients became dry (Stamey = 0) after the 12-month of follow-up. The mean Stamey grade was significantly reduced from 1.9 at baseline to be 0.4 at 12-months.
At the 24-month follow-up, 45% of the patients were still dry (Stamey = 0). The reason for this reduced number of dry patients is that 4 out of 18 patients had their Urolastic implants taken out at another facility. Interestingly, the 12-months records of these patients revealed that 2 of them had no change in their Stamey grade from baseline (Stamey = 3). One patient had an improvement in the Stamey score by 1 grade at the 12-months visit compared to the baseline. This patient reported removal of the Urolastic implant at another facility with then placement of sub-urethral tape 3 months before the date of the 24-month follow-up visit. The reason for placement was the worsening of the incontinence status. However, from the telephone discussion with the patient, we had the impression that the cause of her persistent incontinence was most probably due to a dominant overactivity component. The overall success rate in our series was 66% after a 24-month follow-up, this success rate was comparable to other urethral bulking agents reported in literature. Using the peri-urethral injection of collagen, Elsergany et al. [6] reported a success rate of 48.5% in 33 women with SUI, due to ISD, who were followed for a mean of 18 months (range 2-33). Two-year data obtained from 67 women who responded to treatment with the Macroplastique treatment were published by Ghoniem et al. [7]. The authors reported 84% sustained treatment success with 67% of them being dry (Stamey = 0). This analysis is limited because it included only 67 out of the 122 patients who were originally randomized to receive the Macoplastique treatment with no data provided about the patients in the comparison group. Monga et al. [8] reported 48% objective cure in patients with SUI who received a peri-urethral collagen injection at the 24-month follow-up. Toozs-Hobson et al. reported 64% subjective success in 116 patients who were followed for 24-months after the peri-urethral injection of polyacrylamide hydrogel (Bulkamid®) for the treatment of the SUI [9]. However, it is worth mentioning that it is difficult to hold a firm comparison between these agents in light of available literature due to the heterogeneity in methodologies and outcomes. Therefore, in most of the cases, selection of the bulking agent is dependent on its availability and on the surgeon's experience [10, 11].
Subgroup analysis
It was important to run a subgroup analysis of the data obtained from the 14 patients who retained their Urolastic implant till the 24-month visit. This helps to determine the long term functionality of this new urethral bulking agent.
There was a 37% increase in the average I-QoL score at 24-months compared to the baseline (49% increase at 12-months) which still gives an impression of fair improvement in the quality of life perception in these patients. There was a 94% reduction of pad weight at the 24-month visit compared to baseline (61% at 12 months visit). The average number of incontinence episodes showed a 77% reduction at the 24-month visit compared to baseline (73% at 12-months). The average number of pads showed a 70% reduction at the 24-month visit compared to baseline (68% at 12-months visit). These results indicate that the Urolastic implant is durable and sustains efficacy on long term follow-up.
Safety report
In the 2 year experience with Urolastic, we can say that the adverse events reported in our series go in line with those reported in the literature [6, 7, 9, 12] for other urethral bulking agents and none of these adverse events could be directly related to the Urolastic material. Four of the 18 patients (22%) in our series reported complaints of urgency. A UTI was reported by 1 out of the 18 patients and a local genital infection with erosion into the vagina occurred in 1 out of the 18 patients. Painful intercourse was reported by 2 out of the 18 patients, while 1 of them had her implant taken out based on her desire. Ghoneim et al. [7] reported the occurrence of UTI in 4 patients, vaginal infection in 1, and bladder overactivity in 1, out of the 67 patients who were followed for 2 years after the macroplastique injection therapy.
The current study included a relatively small number of patients. However, it can still give a clue to the efficacy and durability of Urolastic as a new urethral bulking agent. This study might be of value for researchers working on developing an optimal agent that is non-antigenic, biocompatible, and cellular [13] and not yet in existence as currently none of the available agents has proven to be the ideal [14].
CONCLUSIONS
Urolastic is durable and comparable to other bulking agents in terms of durability, efficacy and complications. This might be explained by its biocompatibility and its characteristic as a non-biodegradable agent with moderate adverse events.
ACKNOWLEDGEMENTS
This study was supported by Urogyn B.V., The Netherlands.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93:324–330. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Anders K, Cardozo L, et al. What do women want?: Interpretation of the concept of cure. J Pelvic Med Surg. 2003;9:273–277. [Google Scholar]
- 3.Zajda J, Farag F. Urolastic - a new bulking agent for the treatment of women with stress urinary incontinence: outcome of 12 months follow up. Adv Urol. 2013:724082. doi: 10.1155/2013/724082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamey TA. Endoscopic suspension of the vesical neck for urinary incontinence. Surg Gynecol Obstet. 1973;136:547–554. [PubMed] [Google Scholar]
- 5.Radley SC, Chapple CR, Lee JA. Transurethral implantation of silicone polymer for stress incontinence: evaluation of a porcine model and mechanism of action in vivo. BJU Int. 2000;85:646–650. doi: 10.1046/j.1464-410x.2000.00515.x. [DOI] [PubMed] [Google Scholar]
- 6.Elsergany R, Elgamasy AN, Ghoniem GM. Transurethral collagen injection for female stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:13–18. doi: 10.1007/BF01900535. [DOI] [PubMed] [Google Scholar]
- 7.Ghoniem G, Corcos J, Comiter C, Westney OL, Herschorn S. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol. 2010;183:1444–1449. doi: 10.1016/j.juro.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Monga AK, Robinson D, Stanton SL. Periurethral collagen injections for genuine stress incontinence: a 2-year follow-up. Br J Urol. 1995;76:156–160. doi: 10.1111/j.1464-410x.1995.tb07664.x. [DOI] [PubMed] [Google Scholar]
- 9.Toozs-Hobson P, Al-Singary W, Fynes M, Tegerstedt G, Lose G. Two-year follow-up of an open-label multicenter study of polyacrylamide hydrogel (Bulkamid®) for female stress and stress-predominant mixed incontinence. Int Urogynecol J. 2012;23:1373–1278. doi: 10.1007/s00192-012-1761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapple CR, Wein AJ, Brubaker L, et al. Stress incontinence injection therapy: what is best for our patients? Eur Urol. 2005;48:552–565. doi: 10.1016/j.eururo.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Mohr S, Siegenthaler M, Mueller MD, Kuhn A. Bulking agents: an analysis of 500 cases and review of the literature. Int Urogynecol J. 2013;24:241–247. doi: 10.1007/s00192-012-1834-8. [DOI] [PubMed] [Google Scholar]
- 12.Monga AK, Robinson D, Stanton SL. Periurethral collagen injections for genuine stress-incontinence - a 2-year follow-up. Br J Urol. 1995;76:156–160. doi: 10.1111/j.1464-410x.1995.tb07664.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis NF, Kheradmand F, Creagh T. Injectable biomaterials for the treatment of stress urinary incontinence: their potential and pitfalls as urethral bulking agents. Int Urogynecol J. 2013;24:913–919. doi: 10.1007/s00192-012-2011-9. [DOI] [PubMed] [Google Scholar]
- 14.Lucas MG, Bosch RJ, Burkhard FC, et al. EAU guidelines on surgical treatment of urinary incontinence. Actas Urol Esp. 2013;37:459–472. doi: 10.1016/j.acuro.2013.02.002. [DOI] [PubMed] [Google Scholar]