Abstract
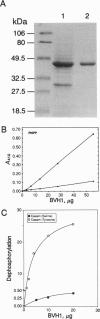
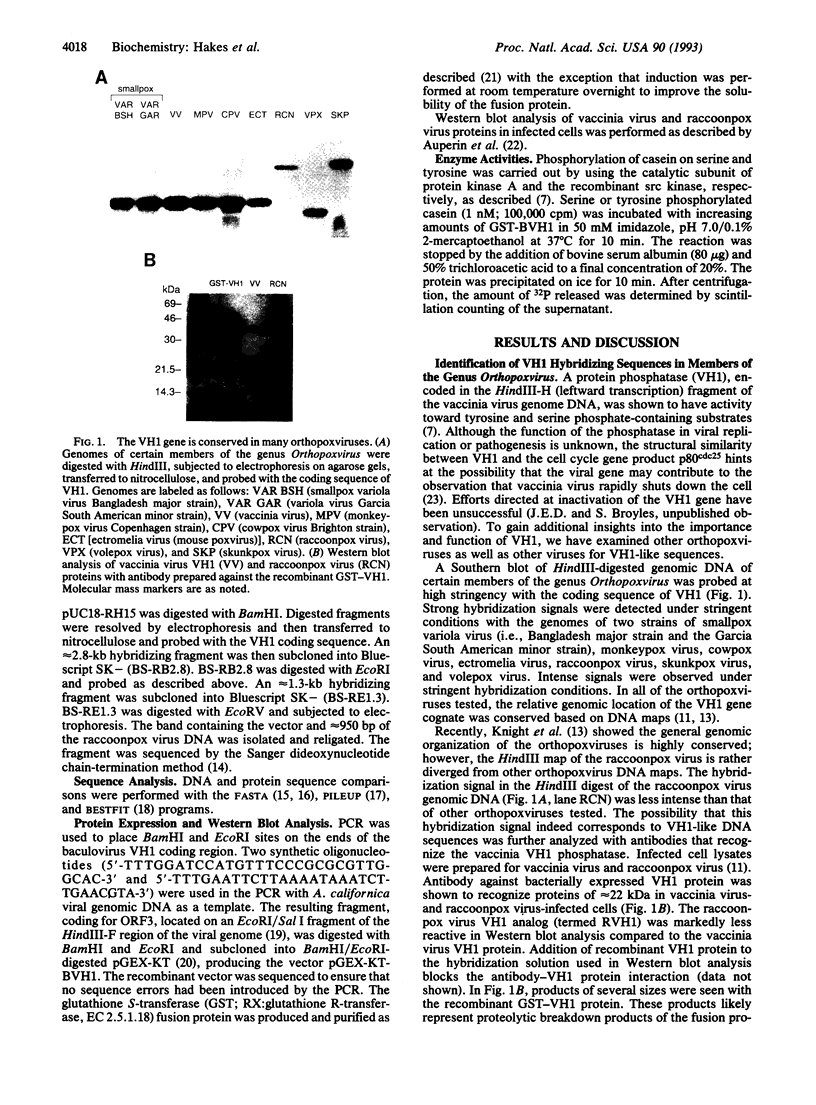
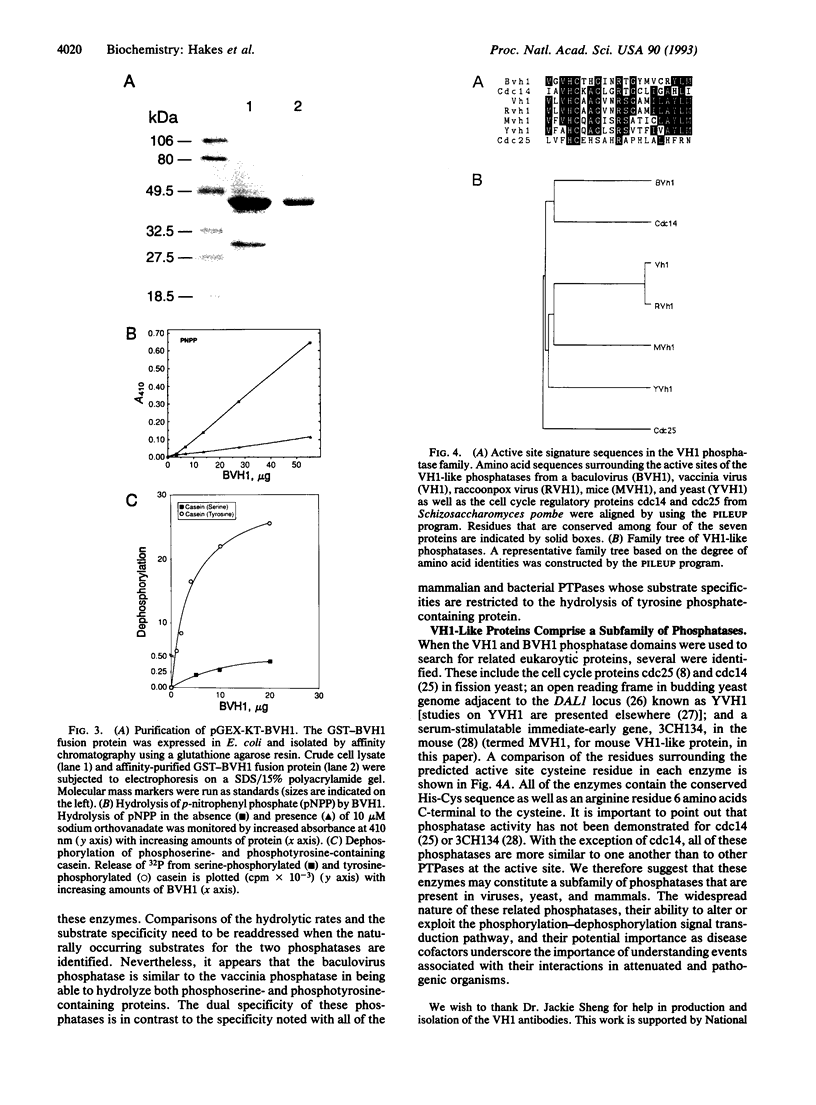
The vaccinia virus VH1 gene product is a dual specificity protein phosphatase with activity against both phosphoserine- and phosphotyrosine-containing substrates. We investigated the potential presence of VH1 analogs in other viruses. Hybridization and sequence data indicated that a phosphatase related to the VH1 phosphatase is highly conserved in the genomes of smallpox variola virus and other orthopoxviruses. The open reading frames from the raccoonpox virus and the smallpox variola virus Bangladesh major strain genomes encoding the VH1 analogs were sequenced and found to be highly conserved with the vaccinia virus VH1. An open reading frame from the baculovirus Autographa californica has sequence similarity to the VH1 phosphatase. The viral proteins appear to be structurally related to the cell cycle control protein p80cdc25. A recombinant phosphatase expressed from the baculovirus gene was found to share with the VH1 phosphatase the ability to hydrolyze substrates that contained both phosphoserine and phosphotyrosine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Esposito J. J., Lange J. V., Bauer S. P., Knight J., Sasso D. R., McCormick J. B. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988 Feb;9(2-3):233–248. doi: 10.1016/0168-1702(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholz R. G., Cooper T. G. The allantoinase (DAL1) gene of Saccharomyces cerevisiae. Yeast. 1991 Dec;7(9):913–923. doi: 10.1002/yea.320070903. [DOI] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Cavallaro K. F., Esposito J. J. Sequences of the raccoon poxvirus hemagglutinin protein. Virology. 1992 Sep;190(1):434–439. doi: 10.1016/0042-6822(92)91229-n. [DOI] [PubMed] [Google Scholar]
- Charles C. H., Abler A. S., Lau L. F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992 Jan;7(1):187–190. [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Knight J. C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985 May;143(1):230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Broyles S. S., Dixon J. E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991 Mar 28;350(6316):359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- Guan K., Hakes D. J., Wang Y., Park H. D., Cooper T. G., Dixon J. E. A yeast protein phosphatase related to the vaccinia virus VH1 phosphatase is induced by nitrogen starvation. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12175–12179. doi: 10.1073/pnas.89.24.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakes D. J., Dixon J. E. New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem. 1992 May 1;202(2):293–298. doi: 10.1016/0003-2697(92)90108-j. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Annu Rev Microbiol. 1968;22:359–390. doi: 10.1146/annurev.mi.22.100168.002043. [DOI] [PubMed] [Google Scholar]
- Knight J. C., Goldsmith C. S., Tamin A., Regnery R. L., Regnery D. C., Esposito J. J. Further analyses of the orthopoxviruses volepox virus and raccoon poxvirus. Virology. 1992 Sep;190(1):423–433. doi: 10.1016/0042-6822(92)91228-m. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Clues to action of cdc25 protein. Nature. 1991 May 16;351(6323):194–194. doi: 10.1038/351194b0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne N., Hardin S. E., Weaver R. F. Nucleotide sequence and transcript mapping of the HindIII F region of the Autographa californica nuclear polyhedrosis virus genome. J Gen Virol. 1991 Feb;72(Pt 2):285–291. doi: 10.1099/0022-1317-72-2-285. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Wan J., Xu H., Grunstein M. CDC14 of Saccharomyces cerevisiae. Cloning, sequence analysis, and transcription during the cell cycle. J Biol Chem. 1992 Jun 5;267(16):11274–11280. [PubMed] [Google Scholar]