Abstract
Introduction
There is no well-defined follow-up scheme available to reliably detect persistent or recurrent vesicoureteric reflux (VUR) after endoscopic therapy (ET), but also to reduce postoperative invasive diagnostics in these children.
Our aim was the evaluation of possible predictors of persistence and recurrence of VUR, in order to elaborate and test a risk-adapted follow-up regimen.
Material and methods
92 patients (85/92%f, 7/8%m, age 2.99y) underwent direct isotope cystography (DIC) three months after ET. Persistent or recurrent VUR, scarring on dimercaptosuccinic acid (DMSA) scans and further fUTIs after therapy (follow-up 24.6 m) were documented and analysed.
Results
VUR persistence 3 months after ET was found in 11 (11.9%) patients; recurrent VUR in 4 (4.3%) patients. Scarring on preoperative DMSA and dilating VUR (°III and °IV) were significantly associated with recurrent VUR. If only children with preoperative positive DMSA scan or dilating VUR would have undergone DIC, only 58/92 DICs (64%) would have been necessary. Only 45.5% of otherwise detected VURs would have been identified using this risk-adapted strategy.
Conclusions
Limiting invasive follow-up diagnostics (VCUG) and, therewith, the radiation burden in a predefined group of patients at risk for persistence or recurrence of VUR is not recommended, due to the significant chance of missing persistent or new onset contralateral VUR. Therefore, we recommend a routine follow-up VCUG after ET. Further prospective scientific efforts to evaluate new, alternative factors influencing persistence and recurrence of VUR, in order to establish an effective follow-up strategy, are warranted.
Keywords: pediatric urology, vesicoureteric reflux, endoscopic reflux therapy, direct isotope cystography, follow-up
INTRODUCTION
Vesicoureteric reflux (VUR) is present in 1–3% of children in Europe and Northern America [1]. The link between VUR, urinary tract infections (UTI) and kidney damage by acute pyelonephritis has been understood since the mid to late 20th century [2, 3]. In the 1980s, endoscopic reflux therapy (ET) was introduced as a less invasive, alternative treatment for VUR [4, 5]. ET is now commonly used in the majority of patients with low grade VUR but also in selected high grade reflux patients [6, 7]. After a defined learning curve [8], success rates for reflux resolution, particularly with regard to a second endoscopic procedure [6] and with the combined HIT/STING technique [9, 10], may have almost caught up with those of ureteral reimplantation [11]. According to some publications, however, the efficacy of ET is hampered by a success rate far from 100% and late recurrences after therapy [7, 8]. There are reports of recurrence rates as high as 53.9% after two years, including an important part with recurrence of VUR 1 year after treatment (39/150, 26%) [12]. In contrast, a recent publication comparing ureteral reimplantation to endoscopic therapy found only two (6.2%) of 32 patients treated endoscopically, presenting with recurrent reflux after five years [6].
In consequence, the significance and indication of a postoperative voiding cystourethrography (VCUG) after ET, involving considerable invasivity, have been disputed in the literature [8, 13]. Possible risk factors for persistent or recurrent VUR are not very well defined, especially not in a manner helping to plan an individual follow-up strategy [14, 15].
Among all modalities for postoperative VCUG, direct isotope cystography (DIC) confers all information needed for objective follow-up after ET. High sensitivity as well as reproducibility of DIC compared with conventional VCUG have already been demonstrated [16, 17]. Additionally, the radiation dose is, depending on the technique used, possibly lower, as documented in the respective literature [18, 19]. At our institution, however, the radiation dose proved to be lower for conventional VCUG in comparison to DIC [20].
In this paper we analyse the clinical and objective risk factors for persistent VUR as assessed by a postoperative DIC performed 3 months after every ET of VUR and recurrent VUR, and discuss the efficacy of a possible risk-adapted follow-up.
MATERIAL AND METHODS
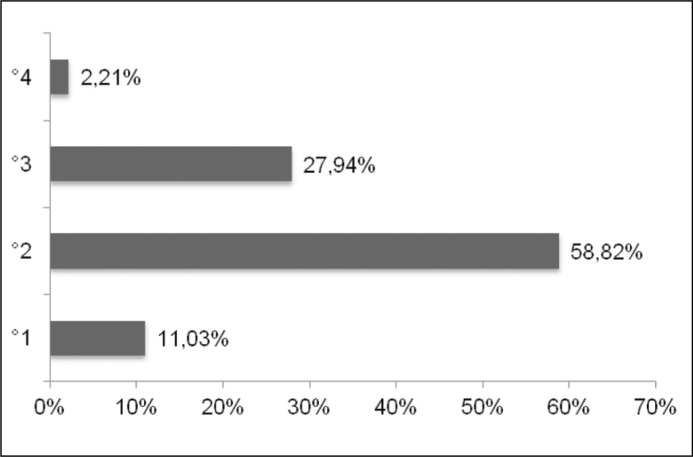
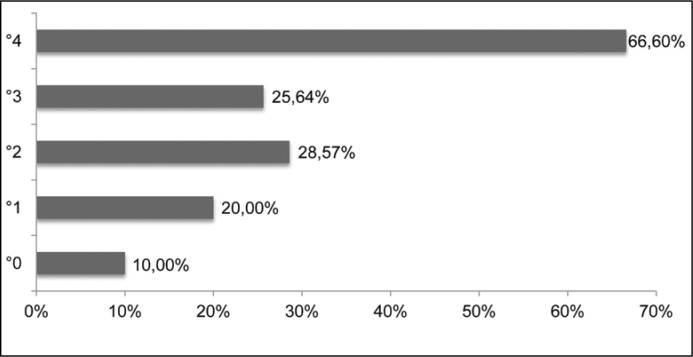
We retrospectively analysed a cohort of 92 patients (85/92% female, 7/8% male, mean age 2.99y) who underwent direct isotope cystography (DIC) after ET of VUR with dextranomer/hyaluronic acid (Dx/HA) at our institution between 2008 and 2013. Patients were referred to DIC for follow-up 3 months after ET of VUR. Patient characteristics and reflux grades are displayed in Table 1 and Figure 1A. Preoperatively all patients were evaluated by conventional VCUG and dimercaptosuccinic acid (DMSA) renal scan to evaluate differential function and possible scar formation. 70% of our patients had low grade (II) VUR (Figure 1A). A DMSA scan revealed postpyelonephritic changes in 39 (42.3%) patients (4 bilateral, 9 right, 26 left). Recurrent febrile urinary tract infections or breakthrough infections were the indications for endoscopic treatment. The probability of having a positive DMSA scan/system (n = 43/136) preoperatively was 10.6% (5/47) for systems without VUR (contralateral), 20% (3/15) for VUR °I, 28.75% (23/80) for VUR °II, 25.64% (10/39) for VUR °III and 66.6% (2/3) for VUR °IV (Figure 1B). Complications and further non-febrile and febrile UTIs were recorded. A VCUG was performed during follow-up of febrile UTIs to detect eventual recurrent VUR.
Table 1.
Patient characteristics n = 92
| Female | Male | ||
| Sex | 85 (92%) | 7 (8%) | |
| Mean | Median | Range | |
| Age | 2.99 | 2.21 | 0.92-13.58 |
| Right | Left | Bilateral | |
| Double system | 7 | 1 | 1 |
| VUR side (patients) | 14 (15.2%) | 32 (34.7%) | 46 (50%) |
| Reflux grade (systems) | Right | Left | |
| °I (contralateral) | 7 | 8 | |
| °II | 47 | 33 | |
| °III | 22 | 16 | |
| °IV | 1 | 2 | |
| Left | Right | Bilateral | |
| Scarring on DMSA scan (patients) | 26 | 9 | 4 |
| Left | Right | Total | |
| Number of refluxive systems diagnosed preoperatively (systems) | 77 | 59 | 136 |
Figure 1A.
VUR grades, systems (%).
Figure 1B.
Scarring on DMSA preoperatively/VUR (vesicoureteral reflux) grade.
Voiding and bowel dysfunction were addressed in all patients prior to and after ET. Median age at endoscopic therapy, however, was 2.21; only 22 patients were ≥4 years of age. Consequently, many patients were not accessible for a meaningful assessment of neither voiding dysfunction nor its successful therapy.
All patients received antibiotic prophylaxis initially.
Endoscopic treatment for vesicoureteric reflux (ET for VUR)
Endoscopic treatment was performed according to institutional standards, using Dx/HA (Deflux®) in a combined HIT/STING technique as described by Cerwinka et al. [10]. We used a mean of 1.4cc (median 1.5cc) of Dx/HA per patient, corresponding to 0.83cc (median 0.85cc) per ureteral orifice. 10 days after endoscopic injection of Dx/HA, antibiotic prophylaxis was discontinued. All patients were evaluated by ultrasound for relevant postoperative hydronephrosis at day 1 and 3 months postoperatively.
Direct isotope cystography (DIC)
Direct isotope cystography was carried out after catheterisation with a 6-8F urethral catheter, allowing bladder filling with a standardized volume ((age + 2)*30) of physiological saline (0.9%), which was charged with an age normalized load of 99metastable Technetium (99mTc) as detailed in the literature [21, 22]. Thereafter, the catheter was withdrawn. One frame per 10 seconds was recorded until spontaneous micturition returned. A Siemens Symbia® True Point™ SPECT CT gamma camera was used for acquisition. VUR was graded as per nuclear medicine standards [16] into mild, moderate and severe. Every picture series was evaluated by a nuclear medicine specialist and discussed in an interdisciplinary meeting with pediatric urologists. All patients received one dose of antibiotics to prevent iatrogenic UTI.
Data acquisition and analysis
Patient data were acquired retrospectively using the documentation in the local hospital information system (SAP®). Data were fed into a Microsoft® Excel® spreadsheet and descriptive statistical analysis with calculation of median and mean values was carried out using the same software. Statistical testing using chisquare tests was performed by the online statistics and biometry tool of the University of Muenster, Germany.
The hypothetical model designed after analysis of risk factors was applied to our patient cohort in order to test for reliability of detection of persistent or recurrent VUR.
RESULTS
In total, 157 ureterorenal systems were treated using the combined HIT and STING technique with Dx/HA. Thereof, 136 were diagnosed preoperatively by VCUG. The remaining 21 (13%) ureters were treated simultaneously on the contralateral side due to relevant hydrodistension of the ureteric orifice (≥HITII as described by Kirsch et al. [23]).
We observed no relevant obstructions or complications directly related to the intervention, such as bleeding or urethral or bladder injury.
Persistent VUR
Persistent VUR, defined as VUR 3 months after ET, was detected by DIC in 11 patients (12 ureterorenal units) accounting for a “radiological” success rate of 91.2% (systems) and 88.1% (patients) respectively. Additionally in 3 (3.2%) patients with primarily negative VCUG on that side, contralateral VUR was diagnosed by DIC (Table 2).
Table 2.
Overview of follow-up data
| Mean | Median | Range | ||
| Follow-up (months) | 24.63 | 19.0 | 3-64 | |
| Number of UTIs | 1 | 2 | 0 | |
| Febrile UTI in follow-up | 6 patients | 3 patients | 83 | |
| Non febrile UTI in follow-up | 3 patients | 0 patients | 89 | |
| Reflux grade (systems) | Right | Left | Bilateral | Total |
| Positive DIC | 3 | 10 | 1 | 14 |
| Thereof new contralateral refluxes | 2 | 1 | 3 | |
| Persistent refluxes | 1 | 9 | 1(2) | 11(12) |
| Treated systems | Treated patients | |||
| Deflux® success rate as seen in DIC at 3 months | 91.17% (124/136) | 88.05% (11/92) | ||
| intervention due to positive DIC (patients) | 10 (10.8%) | |||
| reflux after negative DIC (patients) | 4 (4.3%) | |||
| thereof treated | 4 (100%) |
In the patients with persistent or newly diagnosed VUR either ET was repeated (n = 10.71%) or open reflux surgery was performed (n = 4.29%). Further follow-up in these patients was uneventful with no further non-febrile or febrile UTIs.
Persistent VUR of the lower grade (°II, contralateral °I) was detected in 8%, whereas 14.2% presented with higher grade (°III–IV) VUR (p = 0.6). Of the patients with persistent VUR 27% (3/11) had a positive preoperative DMSA scan compared to 42.3% in the whole group (p = 0.67).
Recurrent VUR
We did not perform routine VCUG during follow-up after negative DIC at 3 months. We did, however, perform VCUG controls in patients suffering from fUTIs during further follow-up.
After a mean follow-up of 24.6 months, 8 patients with negative DIC postoperatively suffered fUTIs, accounting for a clinical success rate, defined as absence of fUTIs after therapy, of 91.3% (84/92). All 8 patients were girls. 4 (50%) of these patients were proven to have recurrent reflux after negative DIC by a repeated conventional VCUG. The mean follow-up in these four patients was 32.1 months. All had positive DMSA scans preoperatively and were treated by ureteral reimplantation with an uneventful clinical follow-up thereafter. Three of the remaining four patients that suffered fUTIs after a negative postoperative DIC, had a negative VCUG and no other fUTI during the further follow-up. One child (aged 3.75 years) was found to have voiding dysfunction, which was successfully addressed by urotherapy. For the remaining girl we performed another DMSA scan that did not reveal any new scars instead of a VCUG. There were no additional problems or further fUTIs during follow-up in this patient. The proportion of patients with recurrent VUR who suffered an additional fUTI after negative DIC 3 months after ET was found to be 57% (4/7, no statistical analysis due to low number of patients).
62.5% (5/8) patients with fUTIs during follow-up had a positive DMSA scan preoperatively compared to 40.47% (34/84) with an uneventful follow-up and no further fUTIs (p <0.01).
VUR recurrence occurred in 5.7% of patients with VUR° ≥III vs. 3.7% with VUR °II (p = 0.61 non-significant); further fUTIs after endoscopic therapy in 3.1% of patients with VUR °I-II and 13.9% with VUR °III-IV (p <0.03).
Voiding dysfunction
Of all toilet trained children (n = 39/92, 42.3%), 8 (20.5%) had voiding dysfunction (defined as residual urine, incontinence, urge, rare voiding/underactive bladder).
Of these 3 girls that had a febrile UTI after ET and a negative VCUG, one (aged 3.75 y) was found to have voiding dysfunction, which was addressed by urotherapy. None of the three patients had another UTI.
1/4 patients (25%) with recurrent VUR and 1/11 patients (9.0%) with persistent VUR had voiding dysfunction.
DISCUSSION
Endoscopic therapy by injecting bulking agents is a safe and reliable option for treating VUR. The ideal follow-up after endoscopic reflux therapy in children remains unknown and has not been standardised yet. For patient follow-up after ET of VUR, DIC seems to be a sensitive and secure examination method. The rate of persistent VUR detected in our patients (91.17% radiological success rate at 3 months) is comparable to success rates in the literature [8, 9, 24]. The rate of postoperative fUTIs was low, accounting for a clinical success rate (no further fUTIs) of 91.3%.
Whether and how patients after ET of VUR should be followed up by VCUG to determine the success rate has been controversial, and consequently questioned by most authors in the literature [8]. Given the high success rates of VCUG exams, despite their invasiveness and radiation burden, the temptation of not routinely indicating a follow-up VCUG is great. However, having made the decision to treat VUR in children and taking into account the possible risks involved with further fUTIs, follow-up seems mandatory. Whilst minimizing non-efficient invasive diagnostic measures is important, persistent VUR puts the patients at risk for further UTIs and possible kidney damage. Nonetheless, it is important to bear in mind that persistence or recurrence of VUR is a complication requiring another intervention under general anesthesia, which carries its own commonly known risks (Clavien Dindo Group IIIb) [25].
In our patients 92 DICs had to be undertaken to identify persistent VUR, defined as VUR present 3 months after ET, in 11 patients (11%) and contralateral, previously undetected VUR in 3 patients. In consequence, 78 of the follow-up DICs (85%) were of no direct value to the patients. However, their inclusion assured quality control and documentation of a high treatment success rate. We aimed at tailoring a risk-adapted follow-up protocol and therefore analyzed our patient group in more detail.
Although there were no statistically significant risk factors for VUR persistence, persistent VUR was more likely in patients with higher reflux grade (°III-IV, 14.2%) than in those with lower reflux grades (°II or contralateral °I, 8%) (p = 0.6 non-significant). DMSA changes were not linked to a higher probability of persistent VUR.
Four of our patients suffered fUTIs and were diagnosed with late recurrence of VUR after a mean follow-up of 32.1 months, whilst having had a negative DIC at 3 months. A longer follow-up increases the probability of reflux reappearance, as described in related publications [7]. Preoperative DMSA changes were predictors for recurrent VUR and fUTIs (p <0.01) at follow-up of our patients, as described in the literature [14, 26]. Patients with dilating VUR (≥°III) carried a higher risk (5.7 vs. 3.7%, p = 0.61 n.s.). Higher VUR grade, however, is linked to a increased risk of recurrent fUTI postoperatively, even if follow-up DIC is negative and there is no recurrent VUR (p <0.03). 4 of the patients who had suffered a subsequent fUTI after negative DIC at 3 months were not proven to have a recurrent VUR and did not suffer from further fUTIs thereafter.
Voiding dysfunction is known to be associated with persistent or recurrent VUR as well as renal damage [27]. Therefore it seems presumptuous, that in all children where this can be assessed and treated, but at least in toilet trained children, voiding dysfunction could be a valuable risk factor to help in stratifying patients for follow-up VCUGs. Our patients, however, were mostly not toilet trained and only 8 (20.5% of all toilet trained patients n = 39) reported symptoms of voiding dysfunction that were addressed by urotherapy. Compared to the findings of the Swedish Reflux Study (34%) [27], this number seems low; however, our patients mostly had lower grade VUR which, as a criterion other than patients age, might explain the difference. These low numbers precluded the use of voiding dysfunction as a risk factor.
We then tried to simulate whether a risk-adapted follow-up at three months, only involving a VCUG in patients at risk for later problems (positive DMSA, high-grade VUR, recurrent preoperative fUTIs), could have spared examinations, whilst being efficient in finding persistent VUR.
If only the children with preoperative positive DMSA or VUR °III-°IV were subjected to follow-up DIC, 58 procedures would have been performed, meaning a 37% reduction in post-operative VCUG. Thereof 51 (87%) would have been negative. 6 of 11 persistent VURs and 1 of 3 previously unknown contralateral VURs would have been detected. This accounts to 45.5% (50% including the previously unknown VURs) the efficacy of the former follow-up protocol in detecting persistent VUR.
A detection rate of only 45.5% for persistent VURs seems too low. Following the decision to treat VUR, it appears logical to evaluate success in order to protect those, in whom the treatment failed, from further UTIs. Neither the risk factors among our patients nor those from the literature seem reliably suited to identify persistent reflux. Therefore, a routine follow-up exam would be the only way to assure patient security and quality control, including “low-risk” VUR patients as our cohort.
A risk-adapted follow-up, therefore, seems not to be an efficient strategy in our group of patients. Possible future efforts should be directed at identifying children with persistent or recurrent VUR without the use of invasive exams.
In the long run and when a high success rate is assured, however, the decision whether or not to perform follow-up VCUG should be individualized and subject to the individual child's situation in order to prevent over diagnostics. Whereas it seems clear, also in view of our data, that a girl with dilating VUR and DMSA changes is at a relatively high risk for recurrence even 2-3 years after ET, a boy with low grade VUR and no DMSA change is at low risk. In children with UTIs after ET, further VUR diagnostics should be performed to rule out the possibility of a persistent or recurrent VUR.
The evaluation of the position of Deflux® mounds after ET by 4D sonography could be a promising aid in helping to identify children who could profit from a VCUG control after endoscopic therapy of VUR. Pichler et al. [28] showed in a study of 178 children after ET that shifting of the Deflux® depot correlated with a higher risk for postoperative UTIs. Additionally, in the children with shifted depots as seen in 4D US, 66.7% of VCUGs were positive.
CONCLUSIONS
Significant risk factors could be identified for recurrence of VUR after ET, whereas there were no reliable risk factors for persistence. Limiting follow-up examination to “high-risk” patients would not improve efficiency, leading to the same proportion of negative examinations and not detecting 50% of persistent or previously unknown contralateral VURs.
We conclude that the only way of reliably identifying persistent VUR is to perform a routine follow-up exam. Indications depend on individual success rates that have to be assessed by each center and should be subject to individual patient characteristics in order to minimize the amount of invasive diagnostics.
As for recurrence of VUR, children with preoperative DMSA changes and higher VUR grades (>°III) are, despite negative follow-up DIC, at higher risk and should be followed more closely clinically. In case of subsequent fUTI a VCUG should be carried out, which involves a 57% chance of finding a recurrent VUR. Additional parameters such as 4D sonographic localization of Deflux® mounds have to be closely evaluated in future studies with a view to establishing a rational follow-up regimen for children after ET of VUR.
Future, prospective studies involving multivariate analysis of other variables, such as the position of the Deflux® mounds affecting persistence or recurrence of VUR after ET, are warranted in order to define an effective follow-up strategy.
This study has been approved by the institutional review board and the ethics committee of the Hospital of the Sisters of Charity, Linz, Austria.
ACKNOWLEDGEMENTS
We would like to thank David Oswald for critical reading of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Wheeler D, Vimalachandra D, Hodson EM, Roy LP, Smith G, Craig JC. Antibiotics and surgery for vesicoureteric reflux: A meta-analysis of randomised controlled trials. Arch Dis Child. 2003;88:688–694. doi: 10.1136/adc.88.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RR. The relationship of vesico-ureteric reflux to urinary tract infection and chronic pyelonephritis-reflux nephropathy. Clin Nephrol. 1973;1:132–141. [PubMed] [Google Scholar]
- 3.Smellie JM, Ransley PG, Normand IC, Prescod N, Edwards D. Development of new renal scars: a collaborative study. Br Med J (Clin Res Ed) 1985;290:1957–1960. doi: 10.1136/bmj.290.6486.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matouschek E. Treatment of vesicoureteric reflux by transurethral teflon-injection (author's transl) Urologe A. 1981;20:263–264. [PubMed] [Google Scholar]
- 5.Oswald J, Riccabona M, Lusuardi L, Bartsch G, Radmayr C. Prospective comparison and 1-year follow-up of a single endoscopic subureteral polydimethylsiloxane versus dextrano-mer/hyaluronic acid copolymer injection for treatment of vesicoureteral reflux in children. Urology. 2002;60:894–897. doi: 10.1016/s0090-4295(02)01903-9. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aparicio L, Rovira J, Blazquez-Gomez E, et al. Randomized clinical trial comparing endoscopic treatment with dextranomer hyaluronic acid copolymer and cohen's ureteral reimplantation for vesicoureteral reflux: Long-term results. J Pediatr Urol. 2013;9:483–487. doi: 10.1016/j.jpurol.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Holmdahl G, Brandström P, Läckgren G, et al. The swedish reflux trial in children: II. Vesicoureteral reflux outcome. J Urol. 2010;184:280–285. doi: 10.1016/j.juro.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 8.Kaye JD, Srinivasan AK, Delaney C, et al. Clinical and radiographic results of endoscopic injection for vesicoureteral reflux: Defining measures of success. J Pediatr Urol. 2012;8:297–303. doi: 10.1016/j.jpurol.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Kirsch AJ, Perez-Brayfield M, Smith EA, Scherz HC. The modified sting procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the in-tramural ureter. J Urol. 2004;171:2413–2416. doi: 10.1097/01.ju.0000127754.79866.7f. [DOI] [PubMed] [Google Scholar]
- 10.Cerwinka WH, Scherz HC, Kirsch AJ. Dynamic hydrodistention classification of the ureter and the double hit method to correct vesicoureteral reflux. Arch Esp Urol. 2008;61:882–887. doi: 10.4321/s0004-06142008000800005. [DOI] [PubMed] [Google Scholar]
- 11.Politano VA, Leadbetter WF. An operative technique for the correction of vesicoureteral reflux. J Urol. 1958;79:932–941. doi: 10.1016/S0022-5347(17)66369-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee EK, Gatti JM, Demarco RT, Murphy JP. Long-term followup of dextranomer/hyaluronic acid injection for vesicoureteral reflux: Late failure warrants continued fol-lowup. J Urol. 2009;181:1869–1874. doi: 10.1016/j.juro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Bennett SD, Foot LM, Abel EJ. Is there a learning curve for subureteric injection of dextranomer/hyaluronic acid in the treatment of vesicoureteral reflux? J Pediatr Urol. 2010;6:122–124. doi: 10.1016/j.jpurol.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Sedberry-Ross S, Rice DC, Pohl HG, Belman AB, Majd M, Rushton HG. Febrile urinary tract infections in children with an early negative voiding cystourethrogram after treatment of vesicoureteral reflux with dextranomer/hyaluronic acid. J Urol. 2008;(4 suppl):1605–1609. doi: 10.1016/j.juro.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 15.Chi A, Gupta A, Snodgrass W. Urinary tract infection following successful dextrano-mer/hyaluronic acid injection for vesicoureteral reflux. J Urol. 2008;179:1966–1969. doi: 10.1016/j.juro.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Unver T, Alpay H, Biyikli NK, Ones T. Comparison of direct radionuclide cystography and voiding cystourethrography in detecting vesicoureteral reflux. Pediatr Int. 2006;48:287–291. doi: 10.1111/j.1442-200X.2006.02206.x. [DOI] [PubMed] [Google Scholar]
- 17.Margarit Mallol J, Vallejo Aparicio S, et al. Value of the direct cystoscintigraphy in the diagnosis of vesicoureteral reflux in patients with prenatal hydronephrosis. Cir Pediatr. 2011;24:174–178. [PubMed] [Google Scholar]
- 18.Schultz FW, Geleijns J, Holscher HC, Weststrate J, Zonderland HM, Zoetelief J. Radia-tion burden to paediatric patients due to micturating cystourethrography examinations in a Dutch children's hospital. Br J Radiol. 1999;72:763–772. doi: 10.1259/bjr.72.860.10624342. [DOI] [PubMed] [Google Scholar]
- 19.Stabin MG, Gelfand MJ. Dosimetry of pediatric nuclear medicine procedures. Q J Nucl Med. 1998;42:93–112. [PubMed] [Google Scholar]
- 20.Haid B, Becker T, Koen M, et al. Lower radiation burden in state of the art fluoroscopic cystography compared to direct isotope cystography in children. J Ped Urol. 2015;11:35.e1–6. doi: 10.1016/j.jpurol.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal V, Rangarajan V, Kamath T, Borwankar SS. Vesicoureteric reflux: Evaluation by bladder volume graded direct radionuclide cystogram. J Indian Assoc Pediatr Surg. 2009;14:15–18. doi: 10.4103/0971-9261.45360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandell GA, Eggli DF, Gilday DL, et al. Procedure guideline for radionuclide cystography in children. Society of nuclear medicine. J Nucl Med. 1997;38:1650–1654. [PubMed] [Google Scholar]
- 23.Kirsch AJ, Kaye JD, Cerwinka WH, et al. Dynamic hydrodistention of the ureteral orifice: A novel grading system with high interobserver concordance and correlation with vesicoureteral reflux grade. J Urol. 2009;182:1688–1692. doi: 10.1016/j.juro.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 24.Kirsch A, Hensle T, Scherz H, Koyle M. Injection therapy: Advancing the treatment of vesicoureteral reflux. J Pediatr Urol. 2006;2:539–544. doi: 10.1016/j.jpurol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traxel E, DeFoor W, Reddy P, Sheldon C, Minevich E. Risk factors for urinary tract infection after dextranomer/hyaluronic acid endoscopic injection. J Urol. 2009;182:1708–1712. doi: 10.1016/j.juro.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 27.Sillén U, Brandström P, Jodal U, et al. The swedish reflux trial in children: v. Bladder dysfunction. J Urol. 2010;184:298–304. doi: 10.1016/j.juro.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 28.Pichler R, Buttazzoni A, Bektic J, et al. Endoscopic treatment of vesicoureteral reflux using dextranomer/hyaluronic acid copolymer in children: results of postoperative follow-up with real-time 3D sonography. Urol Int. 2011;87:192–198. doi: 10.1159/000327609. [DOI] [PubMed] [Google Scholar]