Abstract
Introduction
Prostrate cancer (PC) is one of the most common malignancies and is frequently treated with an 8-week course of radiotherapy. CyberKnife (CK) based radioablation enables completion of therapy within 5-9 days. The aim of this study is an evaluation of the effectiveness and tolerance of CyberKnife-based radioablation in prostate cancer patients.
Material and methods
200 PC patients (94 low risk [LR], 106 intermediate risk [IR]) underwent CK irradiation every other day (fraction dose [fd] 7.25 Gy, total dose [TD] 36.25 Gy, time 9 days). PSA varied from 1.1 to 19.5 (median 7.7) and T stage from T1c to T2c. The percentage of patients with Androgen Deprivation Therapy (ADT), GI (gastrointestinal) and GU (genitourinary) toxicity (EORTC/RTOG scale), and PSA were checked at 1, 4 and 8 months, and thereafter every 6 months – up to a total of 26 months – post-treatment.
Results
The percentage of patients without ADT increased from 47.5% to 94.1% after 26 months. The maximum percentage of acute G3 adverse effects was 0.6% for GI, 1% for GU and G2 – 2.1% for GI and 8.5% for GU. No late G3 toxicity was observed. The maximum percentage of late G2 toxicity was 0.7% for GI and 3.4% for GU. Median PSA decreased from 7.7 to 0.1 ng/ml during FU. One patient relapsed and was treated with salvage brachytherapy.
Conclusions
We conclude that CK-based radioablation in low and intermediate risk PC patients is an effective treatment modality enabling OTT reduction and presents a very low percentage of adverse effects.
Keywords: prostate cancer radiotherapy, CyberKnife, radioablation, ultrahypofractionation, prostate cancer radiation treatment
INTRODUCTION
Prostate cancer (PC) is one of the most common cancers at present in the male population. The number of diagnosed cases has risen predominantly due to the development of health care and the improvement of prevention and diagnostic methods, and, furthermore, presents an increasing tendency in Poland. The number of registered cases in the Silesian Voivodeship in 2011 (with a decreasing population – 9000 inhabitants fewer in 2012 compared to 2011 [1]) was 1260 (14.1% of cancers registered among men) [2]. In 2012, these numbers were 1482 and 15.7% respectively [3]. These patients can be treated radically with surgery or radiotherapy (RT), i.e. external beam radiation therapy (EBRT) and brachytherapy (BT).
Brachytherapy is a relatively short treatment, but EBRT is time-consuming – in the vast majority of cases the overall treatment time (OTT) is approximately 8 weeks (the most common EBRT schedule is based on conventional irradiation – specifically the delivery of a fraction dose (fd) of 1.8–2.0 Gy to a total dose – varying usually from 76 to 81 Gy). Taking this into account, along with the increasing incidence of PC, the rationale for the numerous attempts at OTT reduction is clear. This goal is achievable by enlarging the fd value (hypofractionation). The choice of such a treatment modality is also supported by some radiobiological data suggesting a low value of PC alfa/beta coefficient – 1.5 [4, 5]. On the other hand, this fractionation schedule is clearly correlated with a higher risk of late adverse effects in healthy vital organs (rectum, bladder). The key to resolving this issue involves high precision beam delivery and limited, narrow margins around the prostate. This condition can be accomplished using modern RT units such as the CyberKnife (CK).
The aim of the study was an evaluation of the effectiveness and tolerance of CyberKnife-based radioablation in low and intermediate risk prostate cancer patients.
MATERIAL AND METHODS
The analyzed material comprised 200 prostate cancer patients aged 53 to 83 (mean age 69) treated between 2011-2014 with CyberKnife-based radioablation. There were 94 low (LR) and 106 intermediate risk (IR) patients.
According to our protocol of CK-based PC radioablation, we treated patients from LR and IR (except Gleason 4+3) groups with a maximal prostate dimension smaller or equal to 50 mm. All patients are referred to radiotherapy by urologists (vast majority), or a patient may come directly to our institution (we do not have a urology ward). Each patient and case is analysed and consulted on by our urologists or is sent to a local urologist to discuss the possibility of an operation.
In total we have two subgroups of patients treated with CK: patients who cannot be operated on due to medical reasons and patients who refuse surgery.
The Gleason score varied from 2 to 7 (3+4 only). 3 patients had Gleason 2, 1 Gleason 3, 19 Gleason 4, 66 Gleason 5, 95 Gleason 6 and 16 a Gleason score of 7. At our center prostate biopsies are done transrectally by interventional radiologists. At least eight cores have to be taken (four from each lobe). The topography of each core is described in detail. One core is placed in one container whereas the next is put separately into paraffin. Six slices are obtained from one core and examined. As of the beginning of 2013, the minimal Gleason score diagnosed on the basis of core biopsy is 3+2. In doubtful cases (i.e. as in all cases with a Gleason score less than six) immunohistochemical examinations (AMCR and P63) are performed. Prostate cancer is diagnosed only if the AMCR expression is positive and the P63 expression is negative.
As we are an oncology center and do not have a urology ward, the vast majority of biopsies are done at other (sometimes remote) centers. In the group of patients analyzed, 38 out of 89 cases with a Gleason score less than 6 were diagnosed prior to 2013. Of the remaining 51 cases, only three were diagnosed at our institution (in all three cases Gleason score 3+2).
The maximum PSA concentration varied from 1.05 to 19.53 ng/ml (mean 8.28, median 7.68). 97 patients presented with T1c, 45 with T2a, 40 with T2b and 18 with T2c stage respectively. The mean prostate dimensions were 42.5 x 37.6 x 40.4 mm in the X, Y and Z axes respectively.
Only 65 patients from the analyzed group were free from comorbidities. 123 patients suffered from hypertension and/or coronary disease, 25 from diabetes, 12 from asthma or obstructive pulmonary disease, 2 from anemia and 1 from polycythemia, rheumatoid arthritis and hyperthyroidism.
64 patients were asymptomatic. 104 had nycturia, 67 polyuria, 41 urination difficulties, 10 dysuria, 3 erectile dysfunction, 2 hematuria, 2 anal bleeding and 1 case diarrhea.
On the first day of radiotherapy the initial PSA concentration varied from 0.008 to 15.24 ng/ml (mean 4.05, median 3.61). Directly before the start of RT, 52.5% of patients were using ADT (androgen deprivation therapy) (81 a combination of LHRH analogs and flutamide, 16 LHRH analogs alone, 7 flutamide alone and 1 bicalutamide). The duration of ADT varied from 1 to 24 months (mean 5.4). According to our standards, there is no justification for ADT in the LR group. However, nearly 100% of our prostate cancer patients are referred for radiotherapy by urologists from other centers (in the analyzed group 47 LR patients started ADT before irradiation). In a substantial percentage of cases, the patients had started ADT before radiotherapy. We endeavour to discuss this with leading urologists and, in a number of cases, have convinced them to withdraw ADT.
All patients were irradiated using CyberKnife – a linear accelerator generating a 6 MV photon beam, installed on a 6-degrees-of-freedom robotic arm, integrated with a 6-degrees-of-freedom robotic couch. For prostate cancer patients, one of the options of the Multiplan treatment planning system, the Prostate Template Path, specifically designed for these clinical situations, was used. The tracking of implanted fiducials, ensuring the highest treatment precision, was continuously used during the treatment session [6].
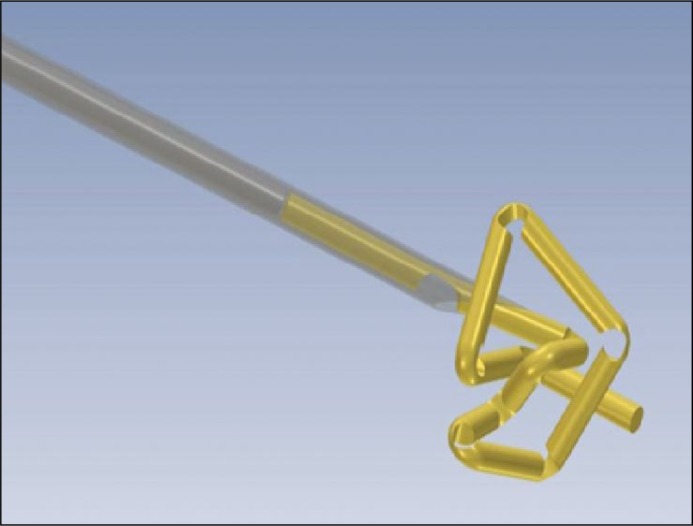
All patients underwent implantation with 3 fiducials – 20 mm in length, 0.3 mm in diameter, incised each 2 mm golden wires, forming a stable, compressed form (Gold Anchors™) during implantation (Figure 1) [7].
Figure 1.
Compressed form of marker (Gold Anchor).
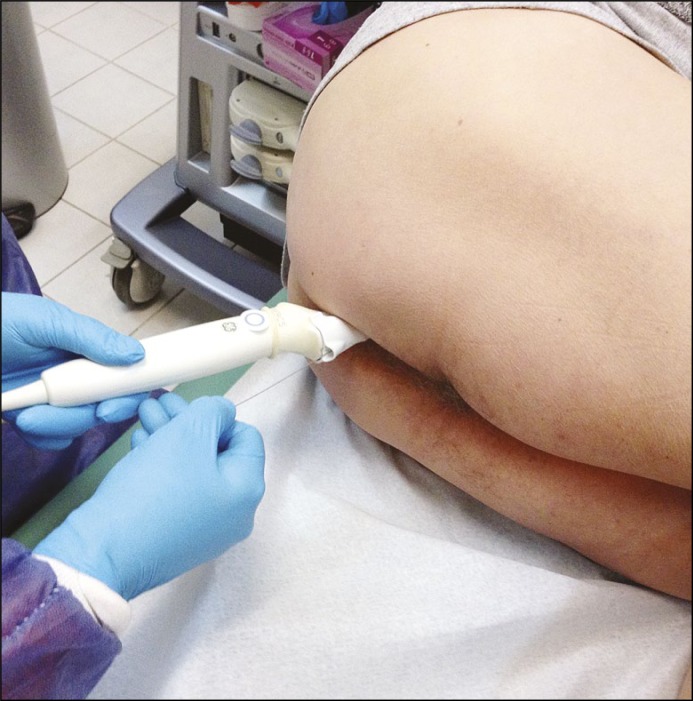
The implantation was performed transrectally, using an ultrasound head with a specially-designed guide and a 203 mm long needle (diameter 0.71 mm) (Figured 2 and 3).
Figure 2.
Marker implantation.
Figure 3.
Needle used for marker implantation.
Markers were implanted in a triangular-like configuration to ensure the possibility of potential prostate movement as well as its rotation, evaluation, and, furthermore, its correction (tracking). As one CK fraction delivery takes quite a long time (40-65 min.), the prostate can change its position in the pelvis as a result of bladder and rectum filling. For this reason, the prostate position has to be checked periodically and additionally, the position of the patient and the beam inlet geometry should be corrected. This procedure is fully automatic and can be done every 5 to 150 seconds. At the beginning of the fractional dose delivery, the prostate position is checked frequently; if it is stable/unaltered the frequency of checking may be decreased.
Patients were irradiated every other day (overall treatment time [OTT] 9 days) using a fraction dose (fd) of 7.25 Gy to the total dose (TD) of 36.25 Gy [8]. TD was delivered to the planning target volume (PTV), comprised of the clinical target volume (CTV – prostate with proximal 1 cm of seminal vesicles) and an additional margin (3 mm in the posterior and 5 mm in the other directions respectively). The maximal accepted dose in the target (PTV) was 120% of the planned (36,25 Gy) dose. The constraints for healthy tissues respected/observed during treatment planning are presented in Table 1.
Table 1.
Constraints for organ at risk
| Rectum | Bladder | ||
|---|---|---|---|
| Dose [Gy] | Volume [%] | Dose [Gy] | Volume [%] |
| 18.0 | 50 | 18.0 | 55 |
| 29.0 | 20 | 29.0 | 25 |
| 32.6 | 10 | 32.6 | 15 |
| 36.25 | 5 | 36.25 | 10 |
For femoral heads, 25 Gy was accepted for 45% of their volume. 120% of the TD was accepted for the urethra.
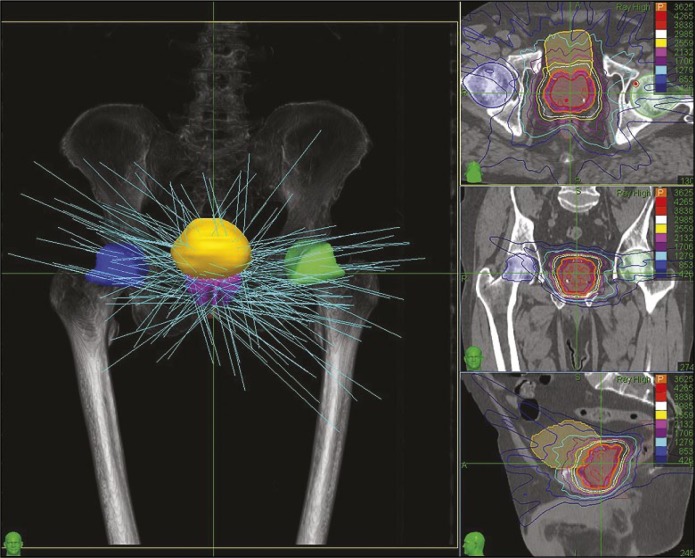
Radiotherapy planning (inverse) was performed on the basis of the CT and MRI fusion using the Multiplan system. Planning, as well as irradiation, was non-isocentric. Typically, between 180 to 250 beams were used (Figure 4).
Figure 4.
Beams’ configuration and the dose distribution.
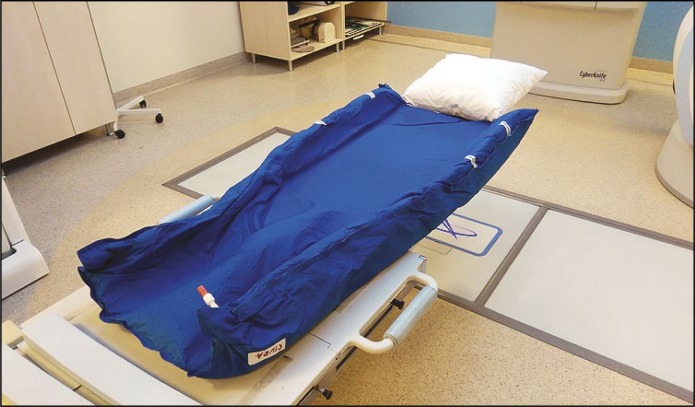
In all cases, the same immobilization vacuum system was used (Figure 5).
Figure 5.
Vacuum immobilization system.
Patients were controlled at the radiotherapy end, and subsequently at 1, 4, 8, 14, 20 and 26 months, after treatment completion. The gastrointestinal (GI) and genitourinary (GU) acute adverse effects according to the EORTC/RTOG scale [9] were monitored at the end of treatment and, moreover, 1 and 4 months thereafter. Next, the late GI and GU reactions in the EORTC/RTOG scale [10] were evaluated. During follow-up (FU) the percentage of patients without hormonal drugs and PSA concentration were checked additionally.
RESULTS
Detailed specifications of the results obtained are presented in Table 2.
Table 2.
The percentage of evaluated patients without ADT, GI and GU adverse effects, and their PSA concentrations during FU
| RT end | 1 month | 4 m. | 8 m. | 14 m. | 20 m. | 26 m. | |
|---|---|---|---|---|---|---|---|
| N of pts. | 200 | 142 | 167 | 149 | 117 | 48 | 18 |
| No ADT [%] | 46 | 66.2 | 76.1 | 81.2 | 86.3 | 85.4 | 94.1 |
| GI 0 [%] | 86.4 | 89.4 | 91.5 | 92.0 | 97.5 | 100.0 | 100.0 |
| GI 1 [%] | 13.1 | 8.5 | 6.7 | 7.3 | 2.5 | – | – |
| GI 2 [%] | 0.5 | 2.1 | 1.2 | 0.7 | – | – | – |
| GI 3 [%] | – | – | 0.6 | – | – | – | – |
| GU 0 [%] | 70.4 | 62.7 | 87.9 | 93.3 | 88.2 | 98.0 | 94.4 |
| GU 1 [%] | 20.1 | 32.4 | 10.3 | 6.0 | 8.4 | 2.0 | 5.6 |
| GU 2 [%] | 8.5 | 4.2 | 1.8 | 0.7 | 3.4 | – | – |
| GU 3 [%] | 1.0 | 0.7 | – | – | – | – | – |
| PSA range [ng/ml] | 0.008-15,2 | 0.008-16.3 | 0.02-5.7 | 0.00-6.4 | 0.002-6.7 | 0.008-6.3 | 0.01-10.0 |
| PSA mean | 4.1 | 2.1 | 1.1 | 0.7 | 0.5 | 0.5 | 0.9 |
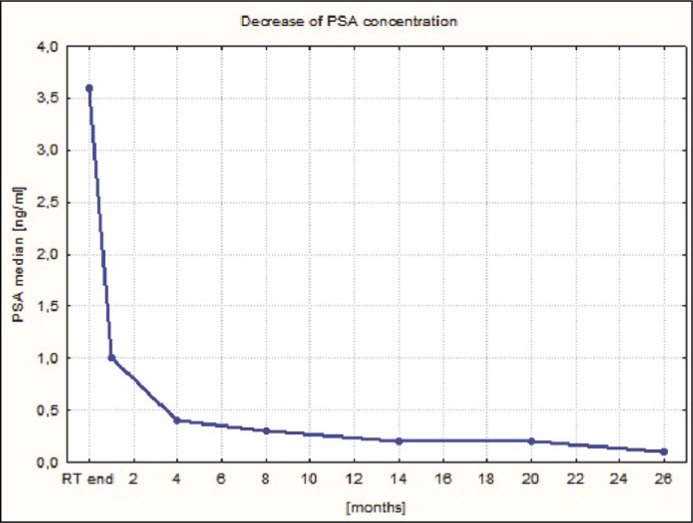
| PSA median | 3.6 | 1.0 | 0.4 | 0.3 | 0.2 | 0.2 | 0.1 |
GI – gastrointestinal adverse effect (grade), GU – genitourinary adverse effect (grade), m – month, N – number, ADT – androgen deprivation therapy, RT – radiotherapy
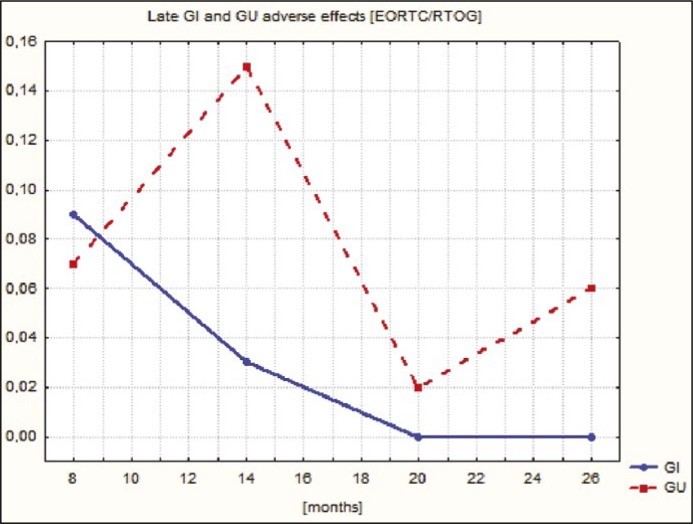
Graphical illustrations of PSA concentration decrease and the course of acute and late GI and GU adverse effects during follow-up are presented in Figures 6, 7 and 8.
Figure 6.
The course of PSA concentration during follow-up.
Figure 7.
The course of acute GI (gastrointestinal) and GU (genitourinary) adverse effects during follow-up.
Figure 8.
The course of late GI (gastrointestinal) and GU (genitourinary) adverse effects during follow-up.
During FU one patient had a confirmed relapse (18 months after RT) – (positive Phoenix criterion + biopsy) and was treated with salvage brachytherapy. Four patients, during follow-up, developed a second malignancy: one bladder cancer, one squamous cell lung cancer, one colon cancer and one papillary cancer of the thyroid gland.
DISCUSSION
The literature concerning CK-based radioablation of PC patients is quite broad. The vast majority of publications present very good results. One of the largest patient groups (1100) is presented in a metaanalysis by King et al. [11]. All patients were treated with a fd of 7.25 Gy to a TD of 36.25 Gy (median FU 36 months). The 5-year biochemical failure-free survival rate (bFFS) was 93% for the whole group, 95% for low risk (LR), 83% for intermediate risk (IR) and 78% for high risk (HR) cancer patients. The largest single center group was described by Katz et al. [12]. 515 PC patients treated to a TD of 35-36.25 Gy are reported (median FU 72 months). The bFFS for LR, IR and HR were 95.8%, 89.3% and 68.5% respectively. Concerning the group of 477 LR and IR patients (TD of 35-36.25 Gy) reported by Katz, PSA decreased from 5.3 to 0.11 ng/ml [13].
In the aforementioned study, Grade 2 acute adverse effects (GU and GI) affected 5% of patients. Late G2 GU and GI reactions were reported in 9.1% and 4% of patients respectively [12]. In another study (a 6-year report), Katz also describes late G3 adverse effects – 2% and 6% for GU and GI respectively [14]. A rather substantial percentage of GU late adverse effects is also described by Chen et al. on the basis of 204 patients (TD of 35-36.25 Gy) – three years after treatment, 5.7% reported leaking more frequently than once a day, 6.4% required pad usage and 10.8% indicated frequent dribbling [15]. Another large review of 1472 patients shows a very low percentage of severe acute effects after CK-based treatment of PC patients (G2 – 5–42% GU and 0–27% GI; G3 – 0.5% GU and 0% GI) [16]. The same authors report 0-29% of GU G2 and 1.3% of GU G3 late effects. Joh et al. report 11.5% of moderate and 8.5% of severe acute GI reactions (diarrhea) and only 1.5% of late GI events (2 years after treatment) in the group of 269 patients irradiated to a TD of 35.0–36.25 Gy [17]. However, Bhathasalli et al. describe 14.5% of GU late effects in a group of 228 patients with a minimum FU of 24 months [18]. In the group of 100 patients irradiated to 35 Gy (5 fractions), Bolzicco et al. describe 12% of G2 GU, 18% GI acute and 3% of G2 and 1% G3 GU late reactions [19].
Similarly, as in metaanalyses and in the aforementioned large single center patients’ group, the authors also report very good results in the smaller single center series. Bernetich et al., in a group of 142 patients irradiated to a TD of 35-36.25 Gy in 5 fractions, present a 5-year 100% success rate bFFS for very low risk (VLR), 91.7% for LR, 90% for IR and 86.7% for HR patients [20]. Fuller et al. report a decrease in PSA median from 5.4 before treatment (4 x 9.5 Gy) to 0.05 ng/ml 5 years later (bFFS 96.2%) in the group of 79 LR and IR patients [21]. In spite of a very aggressive radioablation schedule, acceptable percentages of G2 acute and G3 late GU adverse events were observed (10% and 6% respectively). A survey published by Oliai et al. (70 patients) showed, during a 3-year-long observation, 100%, 95% and 77.1% of bFFS for LR, IR and HR patients respectively irradiated to a TD of 35–36.25 Gy delivered in five fractions [22]. In the smallest discussed study by Janowski et al., concerning the irradiation of 57 patients (LR, IR, HR [9 patients]) with a large prostate (>50 ccm) to 35–36.25 Gy (5 fractions), a 2-year observation revealed a decrease in PSA median from 6.5 to 0.4 ng/ml and a relatively high percentage of late GU G2 (49.1%) adverse effects [23].
It is very difficult to compare our results (longest observation period 26 months) to those mentioned above – with a substantially longer FU. Moreover, considering the analysis of percentages of late (lack of G3, the maximum observed percentage of G2 – 0.7% for GI and 3.4% for GU) and acute (the maximum observed percentage of G3 – 0.6% for GI and 1% for GU; 2.1% GI and 8.5% GU of G2) adverse effects observed in the analyzed group, these are even lower than those reported by other publications.
CONCLUSIONS
We conclude from the above results and discussion that CyberKnife-based radioablation in low and intermediate risk prostate cancer patients is an effective and safe radiation modality, enabling the achievement of a very low percentage of biochemical failures (0.5%) and adverse effects during a 26-month-long follow-up. The final effects require a longer observation of the treated group.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Urząd Statystyczny w Katowicach [Statistical Office in Katowice] http://katowice.stat.gov.pl/dane-o-wojewodztwie/wojewodztwo-1071/ludnosc-wyznania-religijne/ [Google Scholar]
- 2.Kołosza Z, Banasik TR. Nowotwory złośliwe w województwie śląskim w 2011 roku. (Cancer in Silesian Voivodeship in 2011); Gliwice branch, Poland: Department of Epidemiology and Silesia Cancer Registry, M. Skłodowska-Curie m. Cancer Center and Institute of Oncology; [Google Scholar]
- 3.Kołosza Z, Banasik TR, Tukiendorf A. Nowotwory złośliwe w województwie śląskim w 2012 roku (Cancer in Silesian Voivodeship in 2012); Gliwice branch, Poland: Department of Epidemiology and Silesia Cancer Registry, M. Skłodowska-Curie m. Cancer Center and Institute of Oncology; [Google Scholar]
- 4.Fowler JF. The radiobiology of prostate cancer, including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 5.Fowler JF, Toma-Dasu I, Dasu A. Is the α/β ratio for prostate tumours really low and does it vary with the level of risk at diagnosis? Anticancer Res. 2013;33:1009–1011. [PubMed] [Google Scholar]
- 6.Głowacki G, Bodusz D, Majewski W, et al. Frakcjonowana radioterapia stereotaktyczna CyberKnife chorych na raka gruczołu krokowego – prezentacja metody. (Fractionated CyberKnifeTM stereotactic radiotherapy for patients with prostate cancer: presentation of the method) Nowotwory – Journal of Oncology. 2012;62:188–196. [Google Scholar]
- 7.Bodusz D, Głowacki G, Leszczyński W, Miśta W, Miszczyk L. Ocena migracji znaczników śródtkankowych GoldAnchorTM w czasie planowania leczenia promieniami chorych na raka gruczołu krokowego (Evaluation of GoldAnchorTM fiducial marker migration during the planning of radiation treatment for patients with prostate cancer) Przegl Lek. 2013;70:11–14. [PubMed] [Google Scholar]
- 8.Pawlicki T, Cotrutz C, King C. Prostate cancer therapy with stereotactic body radiation therapy. Front Radiat Ther Oncol. 2007;40:395–406. doi: 10.1159/000106049. [DOI] [PubMed] [Google Scholar]
- 9.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 10.RTOG/EORTC Late Radiation Morbidity Scoring Schema; http://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx. [Google Scholar]
- 11.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiat Oncol. 2013;109:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Katz AJ, Kang J. Quality of Life and Toxicity after SBRT for Organ-Confined Prostate Cancer, a 7-Year Study. Front Oncol. 2014;4:301. doi: 10.3389/fonc.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol. 2014;4:240. doi: 10.3389/fonc.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. doi: 10.1186/1748-717X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LN, Suy S, Wang H, et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol. 2014;9:148. doi: 10.1186/1748-717X-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan TJ, Siva S, Foroudi F, Gill S. Stereotactic body radiotherapy for primary prostate cancer: a systematic review. Med Imaging Radiat Oncol. 2014;58:601–611. doi: 10.1111/1754-9485.12213. [DOI] [PubMed] [Google Scholar]
- 17.Joh DY, Chen LN, Porter G, et al. Proctitis following stereotactic body radiation therapy for prostate cancer. Radiat Oncol. 2014;9:277. doi: 10.1186/s13014-014-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattasali O, Chen LN, Woo J, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol. 2014;9:52. doi: 10.1186/1748-717X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolzicco G, Favretto MS, Satariano N, Scremin E, Tambone C, Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013;13:49. doi: 10.1186/1471-2490-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernetich M, Oliai C, Lanciano R, et al. SBRT for the Primary Treatment of Localized Prostate Cancer: The Effect of Gleason Score, Dose and Heterogeneity of Intermediate Risk on Outcome Utilizing 2.2014 NCCN Risk Stratification Guidelines. Front Oncol. 2014;4:312. doi: 10.3389/fonc.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations. Front Oncol. 2014;4:321. doi: 10.3389/fonc.2014.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliai C, Lanciano R, Sprandio B, et al. Stereotactic body radiation therapy for the primary treatment of localized prostate cancer. J Radiat Oncol. 2013;2:63–70. doi: 10.1007/s13566-012-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janowski E, Chen LN, Kim JS, et al. Stereotactic body radiation therapy (SBRT) for prostate cancer in men with large prostates (>50 cm3) Radiat Oncol. 2014;15:241. doi: 10.1186/s13014-014-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]